Abstract
We report final event-driven analysis data on the immunogenicity and efficacy of the human papillomavirus 16 and 18 ((HPV-16/18) AS04-adjuvanted vaccine in young women aged 15 to 25 years from the PApilloma TRIal against Cancer In young Adults (PATRICIA). The total vaccinated cohort (TVC) included all randomized participants who received at least one vaccine dose (vaccine, n = 9,319; control, n = 9,325) at months 0, 1, and/or 6. The TVC-naive (vaccine, n = 5,822; control, n = 5,819) had no evidence of high-risk HPV infection at baseline, approximating adolescent girls targeted by most HPV vaccination programs. Mean follow-up was approximately 39 months after the first vaccine dose in each cohort. At baseline, 26% of women in the TVC had evidence of past and/or current HPV-16/18 infection. HPV-16 and HPV-18 antibody titers postvaccination tended to be higher among 15- to 17-year-olds than among 18- to 25-year-olds. In the TVC, vaccine efficacy (VE) against cervical intraepithelial neoplasia grade 1 or greater (CIN1+), CIN2+, and CIN3+ associated with HPV-16/18 was 55.5% (96.1% confidence interval [CI], 43.2, 65.3), 52.8% (37.5, 64.7), and 33.6% (−1.1, 56.9). VE against CIN1+, CIN2+, and CIN3+ irrespective of HPV DNA was 21.7% (10.7, 31.4), 30.4% (16.4, 42.1), and 33.4% (9.1, 51.5) and was consistently significant only in 15- to 17-year-old women (27.4% [10.8, 40.9], 41.8% [22.3, 56.7], and 55.8% [19.2, 76.9]). In the TVC-naive, VE against CIN1+, CIN2+, and CIN3+ associated with HPV-16/18 was 96.5% (89.0, 99.4), 98.4% (90.4, 100), and 100% (64.7, 100), and irrespective of HPV DNA it was 50.1% (35.9, 61.4), 70.2% (54.7, 80.9), and 87.0% (54.9, 97.7). VE against 12-month persistent infection with HPV-16/18 was 89.9% (84.0, 94.0), and that against HPV-31/33/45/51 was 49.0% (34.7, 60.3). In conclusion, vaccinating adolescents before sexual debut has a substantial impact on the overall incidence of high-grade cervical abnormalities, and catch-up vaccination up to 18 years of age is most likely effective. (This study has been registered at ClinicalTrials.gov under registration no. NCT001226810.)
INTRODUCTION
Cervical cancer is the fourth most common cancer among women, with estimates from 2012 indicating that there are 528,000 new cases and 266,000 deaths each year worldwide (1). It is now established that persistent infection (PI) with human papillomavirus (HPV) is a prerequisite for cervical cancer (2). Approximately 70% of cervical cancer cases are attributable to high-risk (hr) HPV-16 and -18, with HPV-31, -33, -35, -45, -51, -52, and -58 contributing to an additional 20% of cases (3).
The GSK group of companies have developed a prophylactic vaccine against HPV types 16 and 18, formulated with the AS04 adjuvant system (containing aluminum hydroxide and 3-O-desacyl-4′ monophosphoryl lipid A). This vaccine is immunogenic and efficacious and has a clinically acceptable safety profile (4–11). In the end-of-study analysis of the according-to-protocol cohort from a large randomized, double-blind, controlled study (the PApilloma TRIal against Cancer In young Adults [PATRICIA]; registration no. NCT001226810), high vaccine efficacy (VE) was shown against PIs and high-grade cervical intraepithelial neoplasia (CIN) associated with HPV-16 and/or HPV-18 (12). Cross-protective efficacy was also shown against some phylogenetically related and nonrelated nonvaccine hr HPV types (13).
The risk of HPV infection starts from the onset of sexual activity, and the rate of acquisition of infection is highest in adolescents (14, 15). Therefore, the target population for current organized public health vaccination programs is adolescent girls before sexual debut, although a number of countries have also initiated catch-up vaccination programs up to 26 years of age (16–19). In this article, we provide data on the impact of the HPV-16/18 AS04-adjuvanted vaccine in a cohort of adolescent girls and young women from PATRICIA (8), who at baseline had no DNA detected for 14 hr HPV types, were seronegative for HPV-16 and HPV-18, and who had normal cytology results. This total vaccinated, HPV-naive cohort (TVC-naive) immunologically and virologically approximates the target population of current vaccination programs in terms of exposure to and acquisition of HPV types. To approximate the potential impact of catch-up vaccination, we also report results for the total vaccinated cohort (TVC), which includes all women who received at least one vaccine dose irrespective of their baseline cytological, serological, or HPV DNA status and approximates the population of women targeted by catch-up HPV vaccination programs.
The data summarized here are from the final event-driven analysis of PATRICIA and include approximately 39 months of follow-up. Data from a prespecified, descriptive end-of-study analysis, which include follow-up to month 48, have been published previously (12, 13). However, the final event-driven data from the prespecified conclusive analysis, for which type I error was controlled, are included in the vaccine prescribing information in many countries and help to illustrate the high overall efficacy of the HPV-16/18 AS04-adjuvanted vaccine. Here, we report age-stratified VE against all grades of CIN associated with HPV-16 and/or -18 and irrespective of HPV type in the lesion, VE against PIs with hr HPV types, and immunogenicity.
MATERIALS AND METHODS
Data are derived from the final event-driven analysis of PATRICIA, which was a phase III, randomized, double-blind, controlled efficacy study (8, 12). The design of PATRICIA has been reported previously, and the event-driven analysis was the prespecified primary analysis (8).
Participants.
Healthy women aged 15 to 25 years at the time of first vaccination who reported no more than six lifetime sexual partners before study enrollment (this criterion was not applied to subjects aged 15 to 17 years in Finland) were enrolled regardless of their HPV DNA status, HPV serostatus, or cytology at baseline. Since women were not asked to specify the precise number of total lifetime sexual partners, this overall HPV exposure variable was not ascertained. Written informed consent/assent was obtained from all participants and/or their parents. The protocol and other materials were approved by independent ethics committees or institutional review boards.
Procedures.
Women were randomized in a 1:1 ratio to receive either the HPV-16/18 AS04-adjuvanted vaccine (Cervarix; GSK group of companies) or a control hepatitis A vaccine at 0, 1, and 6 months (8, 12). The study protocol prescribed that both groups were to be unblinded following the month 48 visit and offered the crossover vaccine. Further follow-up of subjects enrolled in Finland is ongoing (20). Cervical sample collection, HPV DNA testing, gynecological and cytopathological examinations, assessment of cytology, and testing of cervical and biopsy samples for the presence of DNA from 14 hr HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), using the broad-spectrum PCR SPF10 LiPA25 system (version 1 based on licensed Innogenetics SPF10 technology; Labo Biomedical Products, Rijswijk, Netherlands), and type-specific PCR for HPV-16 and HPV-18 were performed as described previously (8, 12, 21). HPV DNA detected in the tissue biopsy specimens was regarded as associated with the lesion.
Antibodies against HPV-16 and HPV-18 were assessed by enzyme-linked immunosorbent assay (ELISA) in a subset of women from selected study sites (22). Seropositivity was defined as an antibody titer greater than or equal to the assay cutoff: 8 ELISA units (EU)/ml for HPV-16 and 7 EU/ml for HPV-18.
Management of abnormal cytology results and colposcopy referral.
A prespecified clinical management algorithm for abnormal cytology results and colposcopy referral was employed. The colposcopy referral algorithm was designed to capture the most clinically relevant lesions, i.e., those that were most likely to persist. Subjects with a normal Pap smear underwent yearly scheduled cytological examinations. For a single observation of abnormal low-grade cytology, such as atypical squamous cells of undetermined significance (ASC-US) with HPV DNA Hybrid Capture II (HCII)-positive results (referred to as high-risk HPV probe positive or HR+) or low-grade squamous cell intraepithelial lesion (LSIL), the cytology was to be repeated at the next scheduled study visit (6 months later). Two observations (consecutive or intermittent) of low-grade cytology led to a referral for colposcopy. Subjects with a single observation of high-grade abnormal cytology (atypical squamous cells in which high-grade squamous intraepithelial lesions could not be excluded [ASC-H], atypical glandular cells of undetermined significance [AGC-US], or high-grade squamous intraepithelial lesion [HSIL] or greater) were referred for immediate colposcopy with cervical biopsy and, if appropriate, endocervical specimen collection and further medical follow-up. In a protocol amendment, the algorithm was updated to adapt to the evolution of standard medical practices (23) to allow for women with ASC-US (HR+ or with testing not performed) or LSIL to be immediately referred for colposcopic evaluation at the discretion of the investigator.
Prespecified colposcopy management algorithms were employed in which cytology and/or colposcopy had to be repeated for some outcomes at the next scheduled study visit (6 months later). If the biopsy or endocervical specimen results were negative or ≤CIN grade 1 (CIN1) or if the cytology result were ≤LSIL, the cytology and colposcopy were to be repeated at 6 months. If the biopsy or the endocervical specimen result was CIN grade 2 or greater ([CIN2+], defined as CIN grade 2 [CIN2], CIN grade 3 [CIN3], adenocarcinoma in situ [AIS], or invasive carcinoma) or the cytology result was ≥HSIL, a loop electrosurgical excision procedure or cone biopsy was to be performed. Further management was performed according to local medical practice.
Statistical analysis.
This was an event-driven analysis study with a fixed sample size. The final analysis was triggered when a prespecified number of endpoints was reached (at least 36 cases of CIN2+ associated with HPV-16/18, including at least 15 cases of CIN2+ associated with HPV-18) in the according-to-protocol cohort for efficacy, as defined previously (8). The TVC included all women who received at least one vaccine dose and were evaluable for efficacy (i.e., had a baseline PCR or cytology sample and one further sample available). The TVC-naive included women who received at least one vaccine dose, were DNA negative for all 14 hr HPV types investigated and seronegative for HPV-16 and HPV-18, and had normal cytology at baseline. Women infected with low-risk HPV types only were not excluded. Follow-up for each woman started on the day after administration of the first dose of study vaccine. Any lesions diagnosed as a result of abnormal cytology or any infections detected at the first visit were included in the outcome analysis. Follow-up time for each analysis ended (i) at the time of an event (e.g., detection of CIN2+ or start of PI), (ii) for those who did not have an event and who completed the study, at 48 months after administration of the first vaccine dose, or (iii) for those who did not have an event and who were active in the study at the time this present final event-driven analysis was performed, at the date of the last visit for which a biopsy, cytology, or PCR sample was available.
Histopathological and virological efficacy outcomes were evaluated as described previously (8, 12). We evaluated VE against CIN1+, CIN2+, and CIN3+ associated with HPV-16 or HPV-18 DNA in the lesion and against CIN1+, CIN2+, and CIN3+ irrespective of HPV DNA (this included all lesions regardless of whether an HPV type was detected). We also evaluated VE against 6-month and 12-month PIs associated with vaccine HPV types (HPV-16/18), common nonvaccine hr HPV types (HPV-31/33/45/51), and any hr HPV type (HPV-16/18/31/33/35/39/45/51/52/56/58/59/66/68). Additionally, we stratified efficacy analyses by age (15 to 17, 18 to 20, or 21 to 25 years). Analyses were prespecified, except for cross-protective efficacy against PIs with the combination of nonvaccine HPV types 31/33/45/51, against which consistent cross-protection against virological and clinical endpoints was shown in the 4-year end-of-study analysis (12, 13).
VE was calculated with a conditional exact method (see the supplemental material for details). For all endpoints, the overall alpha of 0.05 was divided into 0.021 for the interim analysis (97.9% confidence interval [CI]) and 0.039 for the final analysis (96.1% CI). The 96.1% CIs presented could be interpreted as 95% CIs to limit the overall type one error to 5%. For the final analysis, significance was defined when the lower limit of the 96.1% CI for VE was greater than 30.0% for CIN2+ associated with HPV-16/18 and greater than zero for all other endpoints. Event rates were calculated as the number of cases divided by the sum of the follow-up period in years for each group and are expressed per 100 woman-years.
HPV-16 and HPV-18 geometric mean antibody titers (GMTs) with 95% CI were calculated for the vaccine group. In an exploratory post hoc analysis, we stratified GMT data by age (15 to 17 or 18 to 25 years) and by number of reported sexual partners in the year prior to study (0, 1 or 2, or ≥3 partners). For the GMT calculations, seronegative women were assigned a value of half the assay cutoff level.
For VE against 6-month and 12-month PI, we performed an additional exploratory analysis which excluded those women in the TVC-naive at baseline. This cohort (TVC baseline positive), represents women who had evidence of past and/or current HPV infection or lesions at baseline.
Statistical analyses were done with Statistical Analysis System (SAS) 9.2 and Proc StatXact-7.
RESULTS
Study population.
Totals of 11,641 and 18,644 women were included in the TVC-naive and TVC, respectively. Demographic and baseline characteristics for these cohorts are shown in Table 1, together with characteristics by age strata (36% and 32% were aged 15 to 17 years, 21% and 22% aged 18 to 20 years, and 43% and 46% aged 21 to 25 years in the TVC-naive and TVC, respectively). The mean ages at first vaccination were 19.8 years (TVC-naive) and 20.0 years (TVC). Compliance with completion of the three-dose vaccination schedule was high (92% in each group). Approximately 8% and 10% of subjects were withdrawn from the TVC-naive and TVC, respectively, at the time of the final event-triggered analysis. The numbers of subjects who did not complete the study were balanced between the vaccine and control groups. At the time of the final event-driven analysis, the mean durations of follow-up for the TVC-naive and TVC were 39.5 (standard deviation [SD], 9.0) and 39.4 (SD, 9.7) months, respectively.
TABLE 1.
Demographic and baseline characteristics
| Cohort and parametera | Valueb for group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total cohortc |
15–17 yr |
18–20 yr |
21–25 yr |
|||||
| Vaccine | Control | Vaccine | Control | Vaccine | Control | Vaccine | Control | |
| TVC-naive | ||||||||
| No. of subjects | 5,822 | 5,819 | 2,063 | 2,081 | 1,206 | 1,257 | 2,543 | 2,476 |
| Mean age (SD), yr | 19.9 (3.2) | 19.8 (3.1) | 16.4 (0.6) | 16.4 (0.6) | 19.1 (0.8) | 19.1 (0.8) | 23.0 (1.4) | 23.0 (1.4) |
| Region | ||||||||
| Asia Pacific | 2,203 (37.8) | 2,134 (36.7) | 121 (5.9) | 115 (5.5) | 486 (40.3) | 516 (41.1) | 1,588 (62.4) | 1,498 (60.5) |
| Europe | 2,173 (37.3) | 2,209 (38.0) | 1,719 (83.3) | 1,738 (83.5) | 184 (15.3) | 195 (15.5) | 269 (10.6) | 276 (11.1) |
| North America | 772 (13.3) | 786 (13.5) | 132 (6.4) | 129 (6.2) | 340 (28.2) | 356 (28.3) | 299 (11.8) | 301 (12.2) |
| Latin America | 674 (11.6) | 690 (11.9) | 91 (4.4) | 99 (4.8) | 196 (16.3) | 190 (15.1) | 387 (15.2) | 401 (16.2) |
| Ever had sexual intercourse | ||||||||
| Yes | 4,655 (82.0) | 4,674 (82.1) | 1,352 (66.0) | 1,357 (65.6) | 996 (85.6) | 1,056 (86.2) | 2,298 (93.6) | 2,256 (94.2) |
| No | 1,020 (18.0) | 1,017 (17.9) | 695 (34.0) | 711 (34.4) | 167 (14.4) | 168 (13.7) | 157 (6.4) | 138 (5.8) |
| No data | 147 | 128 | 16 | 13 | 43 | 33 | 88 | 82 |
| No. of sexual partners in last year | ||||||||
| 0 | 210 (4.5) | 208 (4.5) | 57 (4.2) | 50 (3.7) | 48 (4.8) | 53 (5.0) | 105 (4.6) | 105 (4.7) |
| 1 | 3,665 (78.9) | 3,655 (78.4) | 898 (66.6) | 915 (67.6) | 767 (77.2) | 800 (76.2) | 1,993 (87.0) | 1,935 (85.9) |
| 2 | 530 (11.4) | 552 (11.8) | 257 (19.1) | 248 (18.3) | 121 (12.2) | 145 (13.8) | 150 (6.5) | 159 (7.1) |
| ≥3 | 238 (5.1) | 245 (5.3) | 137 (10.2) | 140 (10.3) | 57 (5.7) | 52 (5.0) | 44 (1.9) | 53 (2.4) |
| Not applicabled | 1,020 | 1,017 | 695 | 711 | 167 | 168 | 157 | 138 |
| No data | 159 | 142 | 19 | 17 | 46 | 39 | 94 | 86 |
| Chlamydia trachomatise | ||||||||
| Negative | 5,225 (96.5) | 5,224 (96.5) | 1,943 (98.7) | 1,955 (98.8) | 1,046 (94.8) | 1,124 (95.5) | 2,230 (95.4) | 2,140 (94.9) |
| Positive | 191 (3.5) | 191 (3.5) | 25 (1.3) | 24 (1.2) | 57 (5.2) | 53 (4.5) | 107 (4.6) | 114 (5.1) |
| No data | 406 | 404 | 95 | 102 | 103 | 80 | 206 | 222 |
| Contraceptive usef | ||||||||
| Hormonal | 3,107 (53.4) | 3,236 (55.6) | 862 (41.8) | 895 (43.0) | 723 (60.0) | 792 (63.0) | 1,515 (59.6) | 1,547 (62.5) |
| Intrauterine device | 311 (5.3) | 259 (4.5) | 5 (0.2) | 4 (0.2) | 47 (3.9) | 43 (3.4) | 259 (10.2) | 211 (8.5) |
| Sterilized | 59 (1.0) | 48 (0.8) | 1 (<0.1) | 1 (<0.1) | 5 (0.4) | 3 (0.2) | 52 (2.0) | 44 (1.8) |
| Smoking status | ||||||||
| Never smoked or smoked for ≤6 mo | 4,253 (74.9) | 4,221 (74.1) | 1,395 (68.1) | 1,403 (67.8) | 900 (77.4) | 935 (76.3) | 1,950 (79.4) | 1,880 (78.5) |
| Smoker for ≥6 mo (current or past) | 1,422 (25.1) | 1,472 (25.9) | 652 (31.9) | 666 (32.2) | 263 (22.6) | 290 (23.7) | 505 (20.6) | 514 (21.5) |
| No data | 147 | 126 | 16 | 12 | 43 | 32 | 88 | 82 |
| TVC | ||||||||
| No. of subjects | 9,319 | 9,325 | 2,973 | 2,984 | 2,065 | 2,095 | 4,269 | 4,236 |
| Mean age (SD), yr | 20.0 (3.1) | 20.0 (3.1) | 16.4 (0.6) | 16.4 (0.6) | 19.1 (0.8) | 19.1 (0.8) | 23.0 (1.4) | 23.0 (1.4) |
| Region | ||||||||
| Asia Pacific | 3,175 (34.1) | 3,177 (34.1) | 164 (5.5) | 179 (6.0) | 743 (36.0) | 760 (36.3) | 2,258 (52.9) | 2,229 (52.6) |
| Europe | 3,224 (34.6) | 3,224 (34.6) | 2,448 (82.3) | 2,451 (82.1) | 310 (15.0) | 309 (14.7) | 465 (10.9) | 464 (11.0) |
| North America | 1,532 (16.4) | 1,538 (16.5) | 201 (6.8) | 196 (6.6) | 617 (29·9) | 646 (30.8) | 713 (16·7) | 695 (16.4) |
| Latin America | 1,388 (14.9) | 1,386 (14.9) | 160 (5.4) | 158 (5.3) | 395 (19.1) | 380 (18.1) | 833 (19.5) | 848 (20.0) |
| Ever had sexual intercourse | ||||||||
| Yes | 7,924 (87.0) | 7,936 (87.1) | 2,152 (72.9) | 2,142 (72.3) | 1,810 (90.3) | 1,835 (90.2) | 3,951 (95.5) | 3,949 (96.2) |
| No | 1,183 (13.0) | 1,176 (12.9) | 800 (27.1) | 820 (27.7) | 194 (9.7) | 198 (9.7) | 188 (4.5) | 158 (3.8) |
| No data | 212 | 213 | 21 | 22 | 61 | 62 | 130 | 129 |
| No. of sexual partners in last year | ||||||||
| 0 | 294 (3.7) | 292 (3.7) | 73 (3.4) | 59 (2.8) | 68 (3.8) | 73 (4.0) | 153 (3.9) | 160 (4.1) |
| 1 | 5,862 (74.1) | 5,869 (74.1) | 1,291 (60.1) | 1,288 (60.2) | 1,300 (72.0) | 1,324 (72.5) | 3,262 (82.8) | 3,248 (82.4) |
| 2 | 1,114 (14.1) | 1,161 (14.7) | 433 (20.1) | 462 (21.6) | 285 (15.8) | 302 (16.5) | 394 (10.0) | 397 (10.1) |
| ≥3 | 636 (8.0) | 595 (7.5) | 352 (16.4) | 329 (15.4) | 153 (8.5) | 128 (7.0) | 131 (3.3) | 137 (3.5) |
| Not applicabled | 1,183 | 1,176 | 800 | 820 | 194 | 198 | 188 | 158 |
| No data | 230 | 232 | 24 | 26 | 65 | 70 | 141 | 136 |
| Chlamydia trachomatise | ||||||||
| Negative | 8,155 (94.5) | 8,188 (94.5) | 2,748 (97.3) | 2,758 (97.4) | 1,740 (91.7) | 1,817 (92.9) | 3,659 (93.8) | 3,604 (93.3) |
| Positive | 478 (5.5) | 475 (5.5) | 76 (2.7) | 75 (2.6) | 157 (8.3) | 139 (7.1) | 243 (6.2) | 260 (6.7) |
| No data | 686 | 662 | 149 | 151 | 168 | 139 | 367 | 372 |
| Contraceptive usef | ||||||||
| Hormonal | 5,544 (59.5) | 5,662 (60.7) | 1,416 (47.6) | 1,452 (48.7) | 1,357 (65.7) | 1,410 (67.3) | 2,763 (64.7) | 2,795 (66.0) |
| Intrauterine device | 501 (5.4) | 472 (5.1) | 7 (0.2) | 6 (0.2) | 83 (4.0) | 86 (4.1) | 411 (9.6) | 379 (8.9) |
| Sterilized | 105 (1.1) | 96 (1.0) | 1 (0.0) | 2 (0.1) | 6 (0.3) | 4 (0.2) | 96 (2.2) | 90 (2.1) |
| Smoking status | ||||||||
| Never smoked or smoked for ≤6 mo | 6,401 (70.3) | 6,388 (70.1) | 1,840 (62.3) | 1,867 (63.0) | 1,471 (73.4) | 1,469 (72.2) | 3,080 (74.4) | 3,405 (74.1) |
| Smoker for ≥6 mo (current or past) | 2,706 (29.7) | 2,726 (29.9) | 1,112 (37.7) | 1,096 (37.0) | 533 (26.6) | 565 (27.8) | 1,059 (25.6) | 1,062 (25.9) |
| No data | 212 | 211 | 21 | 21 | 61 | 61 | 130 | 129 |
TVC, total vaccinated cohort. TVC-naive, total vaccinated cohort of women who at baseline had no DNA detected for 14 high-risk HPV types, were seronegative for HPV-16 and HPV-18, and had normal cytology results.
Data are number of subjects (percentage) unless indicated otherwise. Where data were missing, percentages were calculated from data available.
Twenty-two subjects in the TVC and 15 subjects in the TVC-naive were aged <15 years or >25 years at time of first vaccination and are not included in an age stratum.
Responded “no” to the question, “Have you ever had sexual intercourse?”
Chlamydia trachomatis detected in cervical samples by PCR (Cobas Amplicor, Roche).
Women may have used more than one method of contraception or a method that is not listed.
Overall, subjects were predominantly from Europe and Asia Pacific (35% and 34%, respectively, in the TVC), with some differences among the age groups in the geographical distribution of participants. Most notably, 80% of 15- to 17-year-olds were from Finland. Finnish participants were recruited exclusively at schools, as described previously (20). Thirty-six percent of all 18- to 20-year-olds and 53% of all 21- to 25-year-olds were from Asia Pacific.
At baseline, the overall study population (TVC) was predominantly sexually active, and only 13% reported that they had never had sexual intercourse (defined as penetrative or genital-to-genital sexual contact) (Table 1). Only 4% of those who reported prior sexual activity indicated no sexual partner in the year prior to the study, while 74% indicated one sexual partner. More 15- to 17-year-olds (27%) than 18- to 20-year-olds (10%) or 21- to 25-year-olds (4%) reported that they had never had sexual intercourse, but a greater proportion of the 15- to 17-year-olds who did report sexual intercourse had more than one partner in the year prior to the study, compared to 18- to 20-year-olds or 21- to 25-year-olds (21%, 16%, and 10% for two partners and 16%, 8%, and 3% for three partners, respectively). In the TVC-naive, a larger proportion of 15- to 17-year-olds than of 18- to 20- or 21- to 25-year-olds reported that they had never had sexual intercourse (34%, 14%, and 6%, respectively). Of those baseline negative women with reported data regarding sexual intercourse, a larger proportion of 15- to 17-year-olds than of 18- to 20- or 21- to 25-year-olds reported at least three sexual partners in the last year (10%, 5%, and 2%, respectively), and a smaller proportion reported only one sexual partner (67%, 77%, and 86%, respectively).
In both cohorts, a smaller proportion of 15- to 17-year-olds than of 18- to 20- or 21- to 25-year-olds reported that they had never smoked or had smoked for ≤6 months. Chlamydia trachomatis positivity was approximately 2 to 4 times higher in the older age groups than in the 15- to 17-year-olds.
Approximately one-quarter of women in the TVC (26%) had evidence of past and/or current HPV-16 and/or -18 infection (seropositive and/or HPV DNA positive for at least one of the vaccine HPV types) at baseline (see Table S1 in the supplemental material). When stratified by age, a higher proportion of 18- to 20-year-olds (27%) and 21- to 25-year-olds (30%) had evidence of past and/or current HPV-16/18 infection compared with 15- to 17-year-olds (20%). Approximately 5% of these women were HPV-16 DNA positive and approximately 2% were HPV-18 DNA positive at baseline, with fewer than 1% positive for both HPV types (data not shown). Approximately 20% of women were DNA positive for at least one hr HPV type at baseline. Baseline seropositivity was 17% for HPV-16 and 12% for HPV-18. At baseline, over 90% of the women had normal cytology, 9% had ASC-US (regardless of HCII result) or LSIL and 0.5% had high-grade cytology (HSIL, ASC-H AGC) (see Table S2 in the supplemental material). Baseline cytology status was similar in each stratum. A total of 7,003 (38%) women had evidence of past and/or current HPV infection or lesions at baseline (i.e., DNA positive for at least one of the 14 hr HPV types, seropositive for HPV-16 and/or HPV-18, or abnormal cytology at baseline).
Immunogenicity.
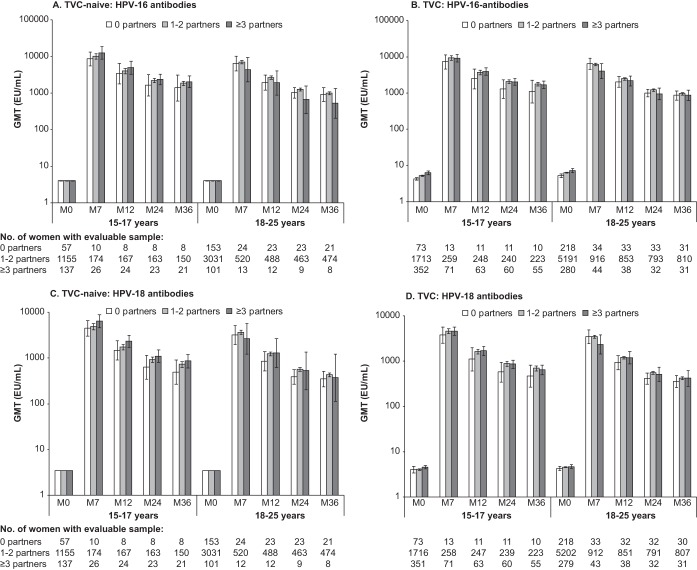
In both cohorts, following an initial peak at month 7, HPV-16 and HPV-18 GMTs were sustained throughout 36 months of follow-up (Fig. 1). In the TVC-naive for women aged 15 to 17 years, HPV-16 and HPV-18 peak GMTs at month 7 tended to increase by number of sexual partners in the year prior to study at all postvaccination time points, although 95% CIs for GMTs overlapped for all groups. In contrast, in the TVC, among adult women aged 18 to 25 years, peak HPV-16 and -18 GMTs at month 7 tended to be the lowest in individuals with higher numbers of sexual partners in the year prior to vaccination. However, the 95% CIs overlapped for all groups, and these tendencies were not statistically significant even in the population of TVC women who had evidence of past and/or current HPV infection at baseline (HPV DNA positive and/or seropositive for HPV-16 or HPV-18 and/or abnormal cytology at baseline) (data not shown).
FIG 1.
Geometric mean antibody titers measured by ELISA in the vaccine group, according to age and reported number of sexual partners in the year prior to study, against HPV-16 and HPV-18 in the TVC-naive (A and C, respectively) and the TVC (B and D, respectively). Bars show log10 geometric mean antibody titer (GMT) and 95% confidence interval. For the GMT calculation, seronegative women were assigned a value of half the assay cutoff level. M, month.
Efficacy against PI.
VE against persistent infection (PI) with vaccine HPV-16/18 was higher than that against nonvaccine hr HPV types (Table 2). However, in the TVC-naive we still observed statistically significant cross-protective VE against persistent 6-month and 12-month infections, both with a combination of nonvaccine HPV-31/33/45/51 (42.5% [96.1% CI, 32.9 to 50.9] and 49.0% [34.7 to 60.3], respectively) and with any hr HPV type (33.5% [26.9 to 39.6] and 36.7% [27.4 to 44.9], respectively). With the exception of VE against 12-month PI with HPV-31/33/45/51 in 18- to 20-year-old women, this was true for all age groups in the TVC-naive, albeit at a lower level than corresponding VE against PIs with HPV-16/18.
TABLE 2.
Efficacy against 6-month and 12-month persistent infections stratified by agea
| Age stratum | Endpoint | HPV type | Group | TVC-naive |
TVC-baseline positive |
TVC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | No. of cases | Rate | Efficacy (96.1% CI) | n | No. of cases | Rate | Efficacy (96.1% CI) | n | No. of cases | Rate | Efficacy (96.1% CI) | ||||
| All | 6 mo | HPV-16/18 | Vaccine | 5,406 | 32 | 0.20 | 93.0 (89.7, 95.3) | 3,450 | 466 | 5.74 | 31.7 (22.5, 39.8) | 8,856 | 498 | 2.08 | 56.4 (51.3, 61.1) |
| Control | 5,375 | 435 | 2.87 | 3,484 | 668 | 8.40 | 8,859 | 1,103 | 4.77 | ||||||
| HPV-31/33/45/51 | Vaccine | 5,406 | 287 | 1.85 | 42.5 (32.9, 50.9) | 3,450 | 612 | 7.71 | 8.1 (−3.3, 18.2) | 8,856 | 899 | 3.84 | 23.5 (16.1, 30.3) | ||
| Control | 5,375 | 487 | 3.22 | 3,484 | 673 | 8.39 | 8,859 | 1,160 | 5.02 | ||||||
| Any hr HPV | Vaccine | 5,406 | 809 | 5.51 | 33.5 (26.9, 39.6) | 3,450 | 1,561 | 28.47 | 2.9 (−4.6, 9.8) | 8,856 | 2,370 | 11.75 | 17.5 (12.6, 22.2) | ||
| Control | 5,375 | 1,163 | 8.29 | 3,484 | 1,627 | 29.31 | 8,859 | 2,790 | 14.25 | ||||||
| 12 mo | HPV-16/18 | Vaccine | 5,331 | 22 | 0.14 | 89.9 (84.0, 94.0) | 3,294 | 305 | 3.58 | 23.3 (10.0, 34.7) | 8,625 | 327 | 1.35 | 47.3 (39.2, 54.4) | |
| Control | 5,291 | 212 | 1.38 | 3,357 | 398 | 4.67 | 8,648 | 610 | 2.55 | ||||||
| HPV-31/33/45/51 | Vaccine | 5,331 | 110 | 0.70 | 49.0 (34.7, 60.3) | 3,294 | 344 | 4.05 | 7.7 (−7.9, 21.1) | 8,625 | 454 | 1.88 | 23.5 (12.8, 32.9) | ||
| Control | 5,291 | 212 | 1.38 | 3,357 | 378 | 4.39 | 8,648 | 590 | 2.46 | ||||||
| Any hr HPV | Vaccine | 5,331 | 383 | 2.53 | 36.7 (27.4, 44.9) | 3,294 | 1,002 | 14.77 | 4.6 (−4.6, 13.0) | 8,625 | 1,385 | 6.32 | 17.4 (10.9, 23.4) | ||
| Control | 5,291 | 587 | 3.99 | 3,357 | 1,060 | 15.48 | 8,648 | 1,647 | 7.65 | ||||||
| 15–17 yr | 6 mo | HPV-16/18 | Vaccine | 1,988 | 12 | 0.20 | 95.4 (91.6, 97.7) | 928 | 150 | 6.57 | 50.0 (38.1, 59.8) | 2,916 | 162 | 1.94 | 70.5 (64.5, 75.7) |
| Control | 2,020 | 249 | 4.29 | 899 | 268 | 13.15 | 2,919 | 517 | 6.59 | ||||||
| HPV-31/33/45/51 | Vaccine | 1,988 | 170 | 2.92 | 42.0 (29.0, 52.8) | 928 | 230 | 10.62 | 19.4 (2.7, 33.3) | 2,916 | 400 | 5.01 | 30.2 (20.0, 39.2) | ||
| Control | 2,020 | 290 | 5.04 | 899 | 271 | 13.17 | 2,919 | 561 | 7.18 | ||||||
| Any hr HPV | Vaccine | 1,988 | 421 | 7.75 | 32.2 (22.5, 40.7) | 928 | 492 | 33.54 | 12.9 (0.6, 23.7) | 2,916 | 913 | 13.24 | 22.0 (14.4, 29.0) | ||
| Control | 2,020 | 600 | 11.43 | 899 | 521 | 38.50 | 2,919 | 1,121 | 16.98 | ||||||
| 12 mo | HPV-16/18 | Vaccine | 1,971 | 6 | 0.10 | 95.4 (89.4, 98.5) | 906 | 99 | 4.07 | 46.0 (29.6, 58.9) | 2,877 | 105 | 1.24 | 66.1 (57.0, 73.4) | |
| Control | 2,002 | 129 | 2.17 | 888 | 170 | 7.55 | 2,890 | 299 | 3.65 | ||||||
| HPV-31/33/45/51 | Vaccine | 1,971 | 65 | 1.09 | 49.1 (29.8, 63.4) | 906 | 124 | 5.16 | 14.3 (−11.2, 34.1) | 2,877 | 189 | 2.26 | 30.1 (14.7, 42.9) | ||
| Control | 2,002 | 128 | 2.15 | 888 | 141 | 6.02 | 2,890 | 269 | 3.24 | ||||||
| Any hr HPV | Vaccine | 1,971 | 200 | 3.50 | 38.3 (25.5, 49.1) | 906 | 320 | 16.94 | 8.6 (−7.7, 22.4) | 2,877 | 520 | 6.84 | 22.5 (12.4, 31.5) | ||
| Control | 2,002 | 319 | 5.68 | 888 | 338 | 18.52 | 2,890 | 657 | 8.83 | ||||||
| 18–20 yr | 6 mo | HPV-16/18 | Vaccine | 1,084 | 8 | 0.26 | 91.6 (82.2, 96.7) | 838 | 134 | 7.22 | 18.2 (−4.7, 36.2) | 1,922 | 142 | 2.87 | 45.1 (31.6, 56.1) |
| Control | 1,114 | 95 | 3.08 | 844 | 162 | 8.83 | 1,958 | 257 | 5.22 | ||||||
| HPV-31/33/45/51 | Vaccine | 1,084 | 57 | 1.89 | 32.2 (2.4, 53.2) | 838 | 160 | 8.71 | 4.5 (−20.6, 24.5) | 1,922 | 217 | 4.47 | 13.3 (−5.4, 28.7) | ||
| Control | 1,114 | 86 | 2.78 | 844 | 168 | 9.12 | 1,958 | 254 | 5.15 | ||||||
| Any hr HPV | Vaccine | 1,084 | 165 | 5.81 | 31.3 (15.1, 44.6) | 838 | 399 | 32.54 | 3.6 (−11.7, 16.8) | 1,922 | 564 | 13.88 | 13.6 (2.5, 23.4) | ||
| Control | 1,114 | 241 | 8.47 | 844 | 412 | 33.75 | 1,958 | 653 | 16.06 | ||||||
| 12 mo | HPV-16/18 | Vaccine | 1,061 | 6 | 0.20 | 86.1 (66.2, 95.5) | 790 | 92 | 4.70 | −4.1 (−43.1, 24.3) | 1,851 | 98 | 1.95 | 25.4 (1.1, 43.9) | |
| Control | 1,093 | 44 | 1.41 | 795 | 89 | 4.51 | 1,888 | 133 | 2.61 | ||||||
| HPV-31/33/45/51 | Vaccine | 1,061 | 23 | 0.76 | 28.0 (−29.9, 60.8) | 790 | 84 | 4.23 | 10.2 (−23.9, 35.0) | 1,851 | 107 | 2.13 | 13.6 (−14.3, 34.8) | ||
| Control | 1,093 | 33 | 1.05 | 795 | 93 | 4.71 | 1,888 | 126 | 2.47 | ||||||
| Any hr HPV | Vaccine | 1,061 | 68 | 2.31 | 37.7 (13.7, 55.4) | 790 | 247 | 15.85 | 10.3 (−8.0, 25.5) | 1,851 | 315 | 6.99 | 16.9 (2.5, 29.2) | ||
| Control | 1,093 | 111 | 3.70 | 795 | 269 | 17.67 | 1,888 | 380 | 8.41 | ||||||
| 21–25 yr | 6 mo | HPV-16/18 | Vaccine | 2,327 | 12 | 0.18 | 87.6 (76.7, 94.1) | 1,681 | 182 | 4.58 | 21.8 (3.8, 36.5) | 4,008 | 194 | 1.82 | 42.8 (30.8, 52.8) |
| Control | 2,237 | 91 | 1.45 | 1,736 | 238 | 5.85 | 3,973 | 329 | 3.18 | ||||||
| HPV-31/33/45/51 | Vaccine | 2,327 | 60 | 0.90 | 49.1 (28.6, 64.1) | 1,681 | 222 | 5.66 | 0.7 (−21.0, 18.6) | 4,008 | 282 | 2.67 | 19.9 (5.1, 32.3) | ||
| Control | 2,237 | 111 | 1.78 | 1,736 | 234 | 5.70 | 3,973 | 345 | 3.33 | ||||||
| Any hr HPV | Vaccine | 2,327 | 222 | 3.47 | 36.1 (23.3, 46.9) | 1,681 | 670 | 24.07 | −3.0 (−15.3, 8.1) | 4,008 | 892 | 9.72 | 14.9 (6.3, 22.7) | ||
| Control | 2,237 | 322 | 5.44 | 1,736 | 693 | 23.37 | 3,973 | 1,015 | 11.42 | ||||||
| 12 mo | HPV-16/18 | Vaccine | 2,292 | 10 | 0.15 | 75.7 (48.9, 89.7) | 1,596 | 114 | 2.77 | 14.8 (−11.4, 34.9) | 3,888 | 124 | 1.15 | 31.6 (12.4, 46.7) | |
| Control | 2,192 | 39 | 0.62 | 1,669 | 139 | 3.25 | 3,861 | 178 | 1.68 | ||||||
| HPV-31/33/45/51 | Vaccine | 2,292 | 22 | 0.33 | 59.2 (29.8, 77.1) | 1,596 | 136 | 3.32 | 1.3 (−27.2, 23.5) | 3,888 | 158 | 1.47 | 20.3 (0.1, 36.5) | ||
| Control | 2,192 | 51 | 0.81 | 1,669 | 144 | 3.36 | 3,861 | 195 | 1.85 | ||||||
| Any hr HPV | Vaccine | 2,292 | 115 | 1.78 | 31.2 (10.8, 47.1) | 1,596 | 435 | 13.06 | −0.5 (−15.7, 12.7) | 3,888 | 550 | 5.62 | 12.0 (0.5, 22.2) | ||
| Control | 2,192 | 157 | 2.59 | 1,669 | 453 | 13.00 | 3,861 | 610 | 6.38 | ||||||
Any hr HPV, any high-risk HPV (HPV-16/18/31/33/35/39/45/51/52/56/58/59/66/68); n, number of evaluable women in each group; no. of cases, number of evaluable women reporting at least one event; rate, number of cases divided by sum of follow-up period (per 100 person-years), where follow-up period started on the day after the first vaccine dose; TVC, total vaccinated cohort; TVC-naive, total vaccinated cohort of women who at baseline had no DNA detected for 14 high-risk HPV types, were seronegative for HPV-16 and HPV-18, and had normal cytology results. The TVC-baseline-positive population excluded those women in the TVC who were HPV naive at baseline (i.e., women who were DNA negative for all 14 high-risk HPV types, were seronegative for HPV-16 and HPV-18, and had normal cytology at baseline).
In the TVC, VE against 6-month and 12-month PIs with vaccine or nonvaccine hr HPV types was highest for women aged 15 to 17 years (Table 2). In women aged 18 to 20 years and 21 to 25 years, statistically significant VE was shown against PIs with HPV-16/18 and with any hr HPV type but was not consistently shown for PIs with nonvaccine HPV-31/33/45/51. In the baseline positive subset of women in the TVC, who had evidence of past and/or current HPV infection, no cross-protective VE was observed against 6-month or 12-month PIs with HPV-31/33/45/51 or any hr HPV type in women aged 18 to 25 years (Table 2).
Efficacy against cervical intraepithelial neoplasia.
In the TVC-naive, a total of 88 CIN1+ cases associated with HPV-16 and/or HPV-18 were identified (including 64 CIN2+ and 13 CIN3+, of which 3 were adenocarcinoma in situ [AIS]) during the follow-up period (Table 3). VE against CIN1+, CIN2+, and CIN3+ associated with HPV-16 and/or -18 was 96.5% (89.0 to 99.4), 98.4% (90.4 to 100), and 100% (64.7 to 100), respectively. In the TVC, a total of 347 CIN1+ cases associated with HPV-16 and/or HPV-18 were identified (including 256 CIN2+ and 108 CIN3+, of which 5 were AIS [all in the control group]) (Table 3). The majority of the CIN2+ lesions were associated with HPV-16 (227/256, 87%). In 28.6% of the 256 CIN2+ cases associated with HPV-16 and/or HPV-18, DNA from nonvaccine hr HPV types was also detected. VE against CIN1+, CIN2+, and CIN3+ associated with HPV-16 and/or -18 was 55.5% (43.2 to 65.3), 52.8% (37.5 to 64.7), and 33.6% (−1.1 to 56.9), respectively.
TABLE 3.
Efficacy against CIN1+, CIN2+, and CIN3+ associated with HPV-16 and/or -18, stratified by agea
| Age stratum | Endpoint | HPV typeb | Group | TVC-naive |
TVC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | No. of cases | Rate | Efficacy (96.1% CI) | n | No. of cases | Rate | Efficacy (96.1% CI) | ||||
| All | CIN1+ | HPV-16/18 | Vaccine | 5,449 | 3 | 0.02 | 96.5 (89.0, 99.4) | 8,667 | 107 | 0.43 | 55.5 (43.2, 65.3) |
| Control | 5,436 | 85 | 0.54 | 8,682 | 240 | 0.97 | |||||
| HPV-16 | Vaccine | 5,449 | 2 | 0.01 | 97.3 (89.3, 99.7) | 8,667 | 90 | 0.36 | 54.5 (40.6, 65.4) | ||
| Control | 5,436 | 73 | 0.46 | 8,682 | 198 | 0.80 | |||||
| HPV-18 | Vaccine | 5,449 | 1 | 0.01 | 94.5 (62.8, 99.9) | 8,667 | 18 | 0.07 | 70.4 (48.0, 84.1) | ||
| Control | 5,436 | 18 | 0.11 | 8,682 | 61 | 0.24 | |||||
| CIN2+ | HPV-16/18 | Vaccine | 5,449 | 1c | 0.01 | 98.4 (90.4, 100) | 8,667 | 82 | 0.33 | 52.8 (37.5, 64.7) | |
| Control | 5,436 | 63 | 0.40 | 8,682 | 174 | 0.70 | |||||
| HPV-16 | Vaccine | 5,449 | 1c | 0.01 | 98.2 (89.1, 100) | 8,667 | 75 | 0.30 | 50.6 (33.5, 63.6) | ||
| Control | 5,436 | 56 | 0.36 | 8,682 | 152 | 0.61 | |||||
| HPV-18 | Vaccine | 5,449 | 0 | 0.00 | 100 (61.3, 100) | 8,667 | 8 | 0.03 | 75.7 (44.4, 90.8) | ||
| Control | 5,436 | 12 | 0.08 | 8,682 | 33 | 0.13 | |||||
| CIN3+ | HPV-16/18 | Vaccine | 5,449 | 0 | 0.00 | 100 (64.7, 100) | 8,667 | 43 | 0.17 | 33.6 (−1.1, 56.9) | |
| Control | 5,436 | 13 | 0.08 | 8,682 | 65 | 0.26 | |||||
| HPV-16 | Vaccine | 5,449 | 0 | 0.00 | 100 (57.1, 100) | 8,667 | 41 | 0.16 | 31.4 (−5.9, 56.0) | ||
| Control | 5,436 | 11 | 0.07 | 8,682 | 60 | 0.24 | |||||
| HPV-18 | Vaccine | 5,449 | 0 | 0.00 | 100 (−170.8, 100) | 8,667 | 2 | 0.01 | 77.7 (−14.7, 98.0) | ||
| Control | 5,436 | 3 | 0.02 | 8,682 | 9 | 0.04 | |||||
| 15–17 yr | CIN1+ | HPV-16/18 | Vaccine | 1,996 | 1 | 0.02 | 97.6 (85.0, 100) | 2,880 | 28 | 0.32 | 73.6 (58.8, 83.7) |
| Control | 2,022 | 42 | 0.68 | 2,891 | 106 | 1.22 | |||||
| HPV-16 | Vaccine | 1,996 | 1 | 0.02 | 97.0 (81.2, 99.9) | 2,880 | 25 | 0.29 | 71.2 (53.7, 82.8) | ||
| Control | 2,022 | 34 | 0.55 | 2,891 | 87 | 1.00 | |||||
| HPV-18 | Vaccine | 1,996 | 0 | 0.00 | 100 (64.1, 100) | 2,880 | 4 | 0.05 | 87.5 (63.1, 97.0) | ||
| Control | 2,022 | 13 | 0.21 | 2,891 | 32 | 0.36 | |||||
| CIN2+ | HPV-16/18 | Vaccine | 1,996 | 1c | 0.02 | 96.6 (78.5, 99.9) | 2,880 | 19 | 0.22 | 72.8 (53.2, 85.0) | |
| Control | 2,022 | 30 | 0.49 | 2,891 | 70 | 0.80 | |||||
| HPV-16 | Vaccine | 1,996 | 1c | 0.02 | 95.8 (72.5, 99.9) | 2,880 | 18 | 0.21 | 69.9 (47.0, 83.8) | ||
| Control | 2,022 | 24 | 0.39 | 2,891 | 60 | 0.69 | |||||
| HPV-18 | Vaccine | 1,996 | 0 | 0.00 | 100 (51.0, 100) | 2,880 | 2 | 0.02 | 87.4 (43.5, 98.8) | ||
| Control | 2,022 | 10 | 0.16 | 2,891 | 16 | 0.18 | |||||
| CIN3+ | HPV-16/18 | Vaccine | 1,996 | 0 | 0.00 | 100 (23.2, 100) | 2,880 | 6 | 0.07 | 74.9 (34.3, 92.1) | |
| Control | 2,022 | 7 | 0.11 | 2,891 | 24 | 0.27 | |||||
| HPV-16 | Vaccine | 1,996 | 0 | 0.00 | 100 (5.7, 100) | 2,880 | 6 | 0.07 | 72.6 (27.3, 91.4) | ||
| Control | 2,022 | 6 | 0.10 | 2,891 | 22 | 0.25 | |||||
| HPV-18 | Vaccine | 1,996 | 0 | 0.00 | 100 (−527.0, 100) | 2,880 | 0 | 0.00 | 100 (−68.8, 100) | ||
| Control | 2,022 | 2 | 0.03 | 2,891 | 4 | 0.05 | |||||
| 18–20 yr | CIN1+ | HPV-16/18 | Vaccine | 1,090 | 0 | 0.00 | 100 (83.0, 100) | 1,862 | 30 | 0.58 | 55.7 (29.6, 72.8) |
| Control | 1,139 | 26 | 0.81 | 1,902 | 69 | 1.31 | |||||
| HPV-16 | Vaccine | 1,090 | 0 | 0.00 | 100 (81.5, 100) | 1,862 | 23 | 0.44 | 60.3 (33.1, 77.2) | ||
| Control | 1,139 | 24 | 0.75 | 1,902 | 59 | 1.12 | |||||
| HPV-18 | Vaccine | 1,090 | 0 | 0.00 | 100 (−183.7, 100) | 1,862 | 7 | 0.13 | 52.3 (−29.8, 84.5) | ||
| Control | 1,139 | 3 | 0.09 | 1,902 | 15 | 0.28 | |||||
| CIN2+ | HPV-16/18 | Vaccine | 1,090 | 0 | 0.00 | 100 (78.5, 100) | 1,862 | 21 | 0.40 | 61.9 (34.4, 78.7) | |
| Control | 1,139 | 21 | 0.66 | 1,902 | 56 | 1.06 | |||||
| HPV-16 | Vaccine | 1,090 | 0 | 0.00 | 100 (77.3, 100) | 1,862 | 18 | 0.35 | 63.3 (34.3, 80.5) | ||
| Control | 1,139 | 20 | 0.63 | 1,902 | 50 | 0.94 | |||||
| HPV-18 | Vaccine | 1,090 | 0 | 0.00 | 100 (−544.0, 100) | 1,862 | 3 | 0.06 | 69.4 (−26.1, 95.1) | ||
| Control | 1,139 | 2 | 0.06 | 1,902 | 10 | 0.19 | |||||
| CIN3+ | HPV-16/18 | Vaccine | 1,090 | 0 | 0.00 | 100 (−75.1, 100) | 1,862 | 10 | 0.19 | 51.4 (−11.9, 80.5) | |
| Control | 1,139 | 4 | 0.12 | 1,902 | 21 | 0.40 | |||||
| HPV-16 | Vaccine | 1,090 | 0 | 0.00 | 100 (−183.7, 100) | 1,862 | 9 | 0.17 | 51.7 (−16.6, 81.7) | ||
| Control | 1,139 | 3 | 0.09 | 1,902 | 19 | 0.36 | |||||
| HPV-18 | Vaccine | 1,090 | 0 | 0.00 | 100 (−5,157.1, 100) | 1,862 | 1 | 0.02 | 65.9 (−372.9, 99.5) | ||
| Control | 1,139 | 1 | 0.03 | 1,902 | 3 | 0.06 | |||||
| 21–25 yr | CIN1+ | HPV-16/18 | Vaccine | 2,356 | 2 | 0.03 | 88.8 (50.0, 98.9) | 3,916 | 49 | 0.45 | 25.3 (−12.0, 50.6) |
| Control | 2,271 | 17 | 0.27 | 3,880 | 65 | 0.60 | |||||
| HPV-16 | Vaccine | 2,356 | 1 | 0.02 | 93.6 (55.9, 99.9) | 3,916 | 42 | 0.39 | 19.9 (−25.3, 49.1) | ||
| Control | 2,271 | 15 | 0.24 | 3,880 | 52 | 0.48 | |||||
| HPV-18 | Vaccine | 2,356 | 1 | 0.02 | 52.1 (−959.2, 99.4) | 3,916 | 7 | 0.06 | 50.5 (−37.2, 84.0) | ||
| Control | 2,271 | 2 | 0.03 | 3,880 | 14 | 0.13 | |||||
| CIN2+ | HPV-16/18 | Vaccine | 2,356 | 0 | 0.00 | 100 (62.9, 100) | 3,916 | 42 | 0.39 | 13.2 (−37.1, 45.3) | |
| Control | 2,271 | 12 | 0.19 | 3,880 | 48 | 0.44 | |||||
| HPV-16 | Vaccine | 2,356 | 0 | 0.00 | 100 (62.9, 100) | 3,916 | 39 | 0.36 | 7.9 (−49.4, 43.3) | ||
| Control | 2,271 | 12 | 0.19 | 3,880 | 42 | 0.39 | |||||
| HPV-18 | Vaccine | 2,356 | 0 | 0.00 | 3,916 | 3 | 0.03 | 57.5 (−98.7, 93.6) | |||
| Control | 2,271 | 0 | 0.00 | 3,880 | 7 | 0.06 | |||||
| CIN3+ | HPV-16/18 | Vaccine | 2,536 | 0 | 0.00 | 100 (−490.4, 100) | 3,916 | 27 | 0.25 | −34.1 (−160.6, 29.7) | |
| Control | 2,271 | 2 | 0.12 | 3,880 | 20 | 0.18 | |||||
| HPV-16 | Vaccine | 2,536 | 0 | 0.00 | 100 (−490.4, 100) | 3,916 | 26 | 0.24 | −35.9 (−168.6, 29.8) | ||
| Control | 2,271 | 2 | 0.12 | 3,880 | 19 | 0.17 | |||||
| HPV-18 | Vaccine | 2,536 | 0 | 0.00 | 3,916 | 1 | 0.01 | 50.4 (−996.4, 99.4) | |||
| Control | 2,271 | 0 | 0.00 | 3,880 | 2 | 0.02 | |||||
n, number of evaluable women in each group; no. of cases, number of evaluable women reporting at least one event; rate, number of cases divided by sum of follow-up period (per 100 person-years), where follow-up period started on the day after the first vaccine dose; TVC, total vaccinated cohort; TVC-naive, total vaccinated cohort of women who at baseline had no DNA detected for 14 high-risk HPV types, were seronegative for HPV-16 and HPV-18, and had normal cytology results.
Women were infected with one or both HPV types (thus, the number of women with an HPV-16-associated lesion and the number with an HPV-18-associated lesion might not equal number with an HPV-16/18-associated lesion).
This young woman acquired the HPV-16 responsible for development of the lesion prior to completion of the full three-dose series (HPV-16 DNA was detected at months 6, 12, and 18; the CIN2 lesion was detected at month 21, and HPV-16 DNA was the only type in the lesion).
In age-stratified analyses for baseline negative women (TVC-naive), VE against HPV-16/18-associated CIN2+ was 96.6% (78.5 to 99.9) for women aged 15 to 17 years, 100% (78.5 to 100) for women aged 18 to 20 years, and 100% (62.9 to 100) for women aged 21 to 25 years (Table 3). In the TVC, VE against HPV-16/18-associated CIN2+ was 72.8% (53.2 to 85.0) for women aged 15 to 17 years and 61.9% (34.4 to 78.7) for women aged 18 to 20 years but was negligible for women aged 21 to 25 years (13.2% [−37.1 to 45.3]).
To assess the potential public health benefit of the vaccine, we also evaluated efficacy against CIN, irrespective of HPV DNA type in the lesion (Table 4). In the TVC-naive, a total of 317 CIN1+ cases, irrespective of HPV DNA, were identified (including 143 CIN2+ and 26 CIN3+, of which 3 were AIS). VE increased with increasing lesion severity: 50.1% (35.9 to 61.4) for CIN1+, 70.2% (54.7 to 80.9) for CIN2+, and 87.0% (54.9 to 97.7) for CIN3+. In the age-stratified analysis, estimates of VE were similar in the three age strata and statistically significant against all grades of CIN in women aged 15 to 17 years. Cases of CIN were limited in numbers in the older age groups, and statistically significant VE was not attained against CIN3+ in women aged 18 to 20 years or against CIN2+ or CIN3+ in women aged 21 to 25 years, although point estimates of vaccine efficacy ranged from 57 to 100% (Table 4).
TABLE 4.
Efficacy against CIN1+, CIN2+, and CIN3+, stratified by age, irrespective of HPV type in the lesiona
| Age stratum | Endpoint | Group | TVC-naive |
TVC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | No. of cases | Rate | Efficacy (96.1% CI) | n | No. of cases | Rate | Efficacy (96.1% CI) | |||
| All | CIN1+ | Vaccine | 5,449 | 106 | 0.67 | 50.1 (35.9, 61.4) | 8,667 | 451 | 1.85 | 21.7 (10.7, 31.4) |
| Control | 5,436 | 211 | 1.35 | 8,682 | 577 | 2.37 | ||||
| CIN2+ | Vaccine | 5,449 | 33 | 0.21 | 70.2 (54.7, 80.9) | 8,667 | 224 | 0.91 | 30.4 (16.4, 42.1) | |
| Control | 5,436 | 110 | 0.70 | 8,682 | 322 | 1.31 | ||||
| CIN3+ | Vaccine | 5,449 | 3b | 0.02 | 87.0 (54.9, 97.7) | 8,667 | 77 | 0.31 | 33.4 (9.1, 51.5) | |
| Control | 5,436 | 23 | 0.15 | 8,682 | 116 | 0.47 | ||||
| 15–17 yr | CIN1+ | Vaccine | 1,996 | 51 | 0.85 | 52.2 (31.5, 67.0) | 2,880 | 182 | 2.15 | 27.4 (10.8, 40.9) |
| Control | 2,022 | 108 | 1.77 | 2,891 | 252 | 2.96 | ||||
| CIN2+ | Vaccine | 1,996 | 20 | 0.33 | 67.8 (44.7, 82.1) | 2,880 | 84 | 0.98 | 41.8 (22.3, 56.7) | |
| Control | 2,022 | 63 | 1.03 | 2,891 | 145 | 1.68 | ||||
| CIN3+ | Vaccine | 1,996 | 2 | 0.03 | 85.5 (33.1, 98.6) | 2,880 | 18 | 0.21 | 55.8 (19.2, 76.9) | |
| Control | 2,022 | 14 | 0.23 | 2,891 | 41 | 0.47 | ||||
| 18–20 yr | CIN1+ | Vaccine | 1,090 | 25 | 0.82 | 46.9 (10.2, 69.4) | 1,862 | 110 | 2.17 | 23.6 (0.2, 41.6) |
| Control | 1,139 | 49 | 1.55 | 1,902 | 147 | 2.84 | ||||
| CIN2+ | Vaccine | 1,090 | 5 | 0.16 | 82.1 (51.2, 94.9) | 1,862 | 46 | 0.89 | 44.2 (17.7, 62.7) | |
| Control | 1,139 | 29 | 0.91 | 1,902 | 84 | 1.60 | ||||
| CIN3+ | Vaccine | 1,090 | 1 | 0.03 | 82.6 (−55.0, 99.7) | 1,862 | 19 | 0.21 | 43.0 (−5.8, 70.3) | |
| Control | 1,139 | 6 | 0.19 | 1,902 | 34 | 0.44 | ||||
| 21–25 yr | CIN1+ | Vaccine | 2,356 | 30 | 0.45 | 46.9 (13.6, 68.0) | 3,916 | 159 | 1.48 | 11.7 (−11.3, 30.0) |
| Control | 2,271 | 54 | 0.85 | 3,880 | 178 | 1.67 | ||||
| CIN2+ | Vaccine | 2,536 | 8 | 0.12 | 57.5 (−7.1, 84.8) | 3,916 | 94 | 0.87 | −0.2 (−37.0, 26.7) | |
| Control | 2,271 | 18 | 0.28 | 3,880 | 93 | 0.87 | ||||
| CIN3+ | Vaccine | 2,356 | 0 | 0.00 | 100 (−160.1, 100) | 3,916 | 40 | 0.37 | 3.2 (−57.1, 40.4) | |
| Control | 2,271 | 3 | 0.05 | 3,880 | 41 | 0.38 | ||||
n, number of evaluable women in each group; no. of cases, number of evaluable women reporting at least one event; rate, number of cases divided by sum of follow-up period (per 100 person-years), where follow-up period started on day after first vaccine dose; TVC, total vaccinated cohort; TVC-naive, total vaccinated cohort of women who at baseline had no DNA detected for 14 high-risk HPV types, were seronegative for HPV-16 and HPV-18, and had normal cytology results.
Two CIN3 cases were associated with HPV-33, and one subject had a CIN3 lesion which could not be associated with a high-risk HPV type according to the rules prespecified by the Endpoint Committee. This subject had a 6-month persistent infection with HPV-58 and a CIN2 lesion associated with HPV-58 preceding the CIN3 diagnosis, suggesting that HPV-58 might have been involved in the development of the lesion.
In the TVC a total of 1028 CIN1+ cases irrespective of HPV DNA in the lesion were identified (including 546 CIN2+ and 193 CIN3+, of which 9 were AIS [2 in the vaccine group and 7 in the control group]) (Table 4). VE irrespective of HPV DNA in the lesion was 30.4% (16.4 to 42.1) against CIN2+ and 33.4% (9.1 to 51.5) against CIN3+. As previously noted for HPV-16/18-associated lesions, low or negligible efficacy was shown in women aged 21 to 25 years against CIN2+ (−0.2% [−37.0 to 26.7]) or CIN3+ (3.2% [−57.1 to 40.4]) irrespective of DNA in the lesion.
DISCUSSION
We examined the impact of the HPV-16/18 AS04-adjuvanted vaccine on women aged 15 to 25 years from PATRICIA, either those who at baseline had no evidence of hr HPV infection (TVC-naive), approximating the population of adolescent girls targeted by HPV vaccination programs, or all vaccinated women in the study (TVC). We build on previously published findings (7, 8, 12, 13) by reporting data from the final, prespecified event-driven analysis regarding age-stratified immunogenicity and efficacy against persistent hr HPV infections and all grades of CIN associated with HPV-16 and/or -18 and also irrespective of HPV type.
In the TVC-naive, we confirmed the high efficacy of the HPV-16/18 AS04-adjuvanted vaccine in preventing lesions associated with HPV-16 and/or HPV-18. Efficacy was 96.5% against CIN1+, 98.4% against CIN2+, and 100% against the immediate precursor of invasive cervical cancer, CIN3+. These results are generally in line with published data regarding efficacy due to vaccine types for the licensed quadrivalent HPV-6/11/16/18 vaccine in a similar cohort of women, despite there being differences in methodologies between these studies (24, 25).
In the TVC, the vaccine prevented approximately 56% and 53% of CIN1+ and CIN2+ associated with vaccine types HPV-16 and/or -18, respectively, compared with the control. Although direct comparison of studies with differences in methodology and populations can be subject to a variety of issues, the estimate against CIN2+ is within the same range as previously reported in studies with the licensed quadrivalent HPV vaccine in an equivalent population (24, 26–28). Efficacy against CIN3+ associated with HPV-16 and/or -18 was 34% across all ages but was not significant. Uniform, statistically significant VE against HPV-16/18-associated CIN1+, CIN2+, and CIN3+ was observed in the 15- to 17-year-old stratum only (74%, 73%, and 75%, respectively).
An important public health aspect of our analysis was to evaluate overall efficacy of the HPV-16/18 AS04-adjuvanted vaccine against clinical lesions without taking into consideration HPV DNA testing results, as nonvaccine hr HPV types also contribute to at least 20% of the burden of cervical disease (3). The overall VE in the TVC-naive, irrespective of HPV DNA results, was 50%, 70%, and 87% against CIN1+, CIN2+, and CIN3+, respectively. In the TVC, irrespective of HPV DNA in the lesion, the vaccine prevented 30% and 33% of high-grade cervical lesions (CIN2+ and CIN3+), respectively. Reductions of 42% and 44% were observed in 15- to 17-year-olds and in 18- to 20-year-olds, respectively, but none was observed for 21- to 25-year-olds. The lower efficacy in the oldest age group could be due to a larger proportion of women in this group with prevalent infections at baseline, which the vaccine does not impact (29). The higher efficacy estimates in CIN2+ and CIN3+ reflect the increasing relative prevalence of HPV-16 and -18 compared to some other hr HPV types with increasing lesion severity (30–32) but also the consistent cross-protective efficacy of the HPV-16/18 AS04-adjuvanted vaccine against HPV-31, -33, -45, and -51 (8, 13), which may extend even further (33, 34).
To obtain the greatest benefit from prophylaxis, most HPV vaccination programs target young adolescents with the aim of immunizing before HPV exposure through sexual contact (15–17). As there are no clinical efficacy studies with HPV vaccines in the target population, the TVC-naive cohort from PATRICIA was used as an approximation of HPV-naive adolescents, by including only study participants who at baseline had no evidence of exposure to any of 14 hr HPV types detected by PCR, seronegativity to HPV-16/18, and no evidence of cytological abnormalities. We chose the age strata to reflect the variability in currently implemented HPV vaccination programs, some of which (assuming high compliance to the three-dose schedule) recommend catch-up vaccination to 17 years of age and others through 25 to 26 years of age. In modeling and cost-benefit analyses, 18 years is generally considered to be the upper age limit at which HPV vaccines are considered to be cost-effective (35, 36). The incidences of CIN lesions associated with HPV-16/18, or irrespective of HPV DNA, were consistently higher in women 15 to 17 years than in women 21 to 25 years, but observed estimates of VE against CIN and persistent hr HPV infections were similar in each age group.
At present, no conventional method exists to assign lesion causality when several HPV types are detected. The complexities of evaluating cross-protective efficacy against CIN lesions are addressed in more detail in an article by Wheeler and colleagues (13). Virological endpoints have the advantage of not being complicated by infection with multiple HPV types. In young women aged 15 to 17 years, VE against 6-month and 12-month PIs with HPV-16 and/or -18 was 71% and 66%, respectively, which reflects the 73% to 75% efficacy observed against CIN1+, CIN2+, and CIN3+ associated with HPV-16 and/or -18 in this age group in the TVC. We also observed some cross-protection in 15- to 17-year-olds against PIs with the combination of nonvaccine hr types HPV-31/33/45/51. No cross-protection was observed in older age groups with evidence of past and/or current HPV infection at baseline (baseline positive cohort).
Multiple infections were commonly associated with CIN2+ lesions in the control group of the TVC-naive. Estimates of the proportion of lesions associated with HPV-16/18 in unvaccinated women ranged from 31.6% (lesions with only HPV-16 and/or -18 present) to 64.3% (lesions with HPV-16 and/or -18 plus at least one additional HPV type) for CIN2+ and 31.6% to 73.7% for CIN3+. Yet, in the baseline negative subjects, the vaccine provided high and very high efficacy against both CIN2+ and CIN3+ lesions, irrespective of HPV type.
Approximating a sexually naive population has several limitations. HPV serology is not a perfect marker of prior exposure to HPV infection. Previous studies have shown that approximately 30 to 40% of women with incident HPV-16 infection never have detectable antibodies (37, 38). Furthermore, serological testing was not performed for cross-neutralizing antibodies (39). Thus, cross-protective immunogenicity and prior exposure to nonvaccine HPV types were unknown. Similarly, although the PCR used in this study detected 14 of the most relevant hr HPV types, women in the TVC-naive could have been infected with other high-risk types not detected by the specific PCR-based HPV DNA assays. Therefore, the TVC-naive may have included some young women who were not truly HPV naive, which would result in an underestimation of the expected vaccine impact in naive young adolescents. On the other hand, even among the target population of young adolescents, some might already be infected with HPV, for example, through sexual abuse. Other factors which may limit the extrapolation of the study results were the enrollment of 80% of 15- to 17-year-olds from a single country (Finland) and the enrollment of approximately half of the older age groups from Asia Pacific, although recent data from Scotland have shown the impact of the HPV-16/18 AS04-adjuvanted vaccine in other populations in a real-world setting (40). Additionally, compliance with the three-dose vaccination schedule was >90% in this study, which is higher than that achieved in most catch-up campaigns, particularly for older cohorts (19, 41, 42).
In conclusion, this study confirms the widely held view that targeting young adolescent girls before sexual debut for prophylactic HPV vaccination could have a substantial impact on the incidence of high-grade cervical abnormalities. Modeling data predict that anti-HPV-16 and -18 antibodies elicited by the HPV-16/18 AS04-adjuvanted vaccine will persist for many years after vaccination, suggesting that vaccinated young girls may be protected for the most of their sexually active lives (43). Inconsistent or negligible efficacy was observed for women aged 21 to 25 years, likely due to a higher proportion of women with prevalent infections at baseline. This is in line with analyses suggesting reduced or negligible cost-effectiveness of vaccination programs in women aged 21 years and above (36, 44). For women aged 18 to 25 years, unfortunately neither the models nor our data are conclusive. There may, however, be individual benefit for vaccination of HPV-negative women aged 18 years and older.
ACKNOWLEDGMENTS
The study was funded by GlaxoSmithKline Biologicals SA, which designed the trial in collaboration with investigators and coordinated gathering, analysis, and interpretation of data. The development of the manuscript was supported and coordinated by GlaxoSmithKline Biologicals SA.
We thank all study participants and their families. We gratefully acknowledge the work of the central and local study coordinators and of staff members of the sites that participated in this study. The analysis was done by an external statistician to maintain the trial blinding until the completion of 48 months of follow-up for all subjects. Investigators from the HPV PATRICIA Study Group gathered data for the trial and cared for the women. Contributions to statistical support were provided by T. Zahaf, M.-P. David (GSK Vaccines, Wavre, Belgium), L. Declerck (S-Clinica), J. Dupin (4Clinics), and J.-L. Maroye (Modern Solutions for Business). Writing and editorial assistance were provided by J. Taylor (freelance medical writers, United Kingdom, on behalf of GSK Vaccines, Wavre Belgium); editorial assistance and manuscript coordination were provided by J. Andersson (Cromsource Ltd., United Kingdom, on behalf of GSK Vaccines, Wavre, Belgium).
D.D., G.D., F.S., K.H., and T.Z. are employed by the GSK group of companies. D.D. owns stock in the GSK group of companies, F.S. and G.D. own stock shares and stock options in the GSK group of companies, and G.D. holds relevant patents. All investigators at study clinical sites were funded through their institutions to do the study protocol. B.R., C.M.W., D.A., F.Y.A., J.C.T., J.P., J.S., M.L., P.N., S.M.G., S.R.S., U.J., and X.C. have received funding to do other HPV vaccine studies for GlaxoSmithKline Biologicals SA; M.L., J.P., J.S., and S.M.G. have received research funding, via their institutions, from Merck Sharp & Dohme, and J.S. has also received research funding from Qiagen; C.M.W. has received through her institution funding for HPV vaccine trials from Merck Sharp & Dohme and reagents and equipment from Roche Molecular Systems; X.C. has received research funding, via his institution, from Merck Sharp & Dohme, Sanofi Pasteur MSD; T.F.S. has received personal fees from GlaxoSmithKline Biologicals SA; S.R.S. has also received funding through her institution from CSL Ltd.; S.M.G. has received consulting fees from GlaxoSmithKline Biologicals SA, CSL Ltd., and Merck Sharp & Dohme; B.R. has received honoraria via her professional corporation from GlaxoSmithKline Biologicals SA; X.C. has received honoraria from GlaxoSmithKline Biologicals SA and Merck Sharp & Dohme, Sanofi Pasteur MSD; S.M.G. has received payment from Merck Sharp & Dohme for board membership and has received payment from GlaxoSmithKline Biologicals SA, Merck Sharp & Dohme, and CSL Ltd. for lectures, including service on a speakers' bureau; F.X.B. has received payment from GlaxoSmithKline Biologicals SA for lectures, including service on a speakers' bureau, and S.M.G. and S.R.S. have received payment from GlaxoSmithKline Biologicals SA for the development of educational presentations; S.R.S. received honoraria from GlaxoSmithKline Biologicals SA via her institution for membership on advisory boards and for presentation at educational seminars; F.Y.A. has received payment from GlaxoSmithKline Biologicals SA and Merck Sharp & Dohme for the development of educational presentations; B.R., C.M.W., S.R.S., and U.J. have received travel reimbursements from GlaxoSmithKline Biologicals SA; C.M.W. and F.X.B. have received travel reimbursements from Merck Sharp & Dohme, Sanofi Pasteur MSD; and S.M.G. has received travel reimbursements from GlaxoSmithKline Biologicals SA, Merck Sharp & Dohme, and CSL Ltd. A.M., G.L., H.C.K., P.D.S., S.N.C., and W.A.J.P. declare that they have no conflicts of interest.
D.A., J.P., M.L., S.M.G., X.B., D.D., F.S., C.M.W., A.M., X.C., S.R.S., and G.D. formed the core writing team for the report. D.A. and the core writing team had full access to all the trial data, including existing analyses, and had final responsibility for the decision to submit for publication. All authors reviewed and commented upon a draft of the manuscript and gave final approval to submit for publication.
The principal investigators and coinvestigators of the HPV PATRICIA Study Group are as follows: in Australia, I. Denham, S. M. Garland, A. Mindel, M. O'Sullivan, and S. R. Skinner; in Brazil, P. Naud and J. C. Teixeira; in Belgium, P. De Sutter and W. A. J. Poppe; in Canada, F. Y. Aoki, P. H. Orr, F. Diaz-Mitoma, M. Dionne, L. Ferguson, M. Miller, P. M. Orr, K. Papp, B. Ramjattan, B. Romanowski, and R. Somani; in Finland, D. Apter, T. Karppa, N. Kudjoi, M. Kupari, M. Lehtinen, L. Niemi, J. Paavonen, J. Palmroth, T. Petaja, U. Romppanen, S. Salmivesi, M. Siitari-Mattila, S. Svartsjo, and L. Tuomivaara; in Germany, K. H. Belling, T. Gent, F. Gieseking, T. Grubert, W. Harlfinger, W. D. Hopker, S. Jensen-El Tobgui, K. Peters, S. Schoenian, K. Schulze, and T. Schwarz; in Italy, C. Liverani and G. Mojana; in Mexico, J. Salmeron; in Philippines, G. Benitez, C. Crisostomo, R. Del Rosario-Raymundo, M. J. Germar, G. Limson, J. Raymundo, M. C. Remollino, G. Villanueva, S. Villanueva, J. D. Zamora, and L. Zamora; in Spain, J. Bajo, J. Bayas, M. Campins, X. Castellsague, M. Castro, C. Centeno, L. Rodriguez, A. Torne, and J. A. Vidart; in Taiwan, S. N. Chow, M. H. Yu, C. C. Yuan, and T.-Y. Chu; in Thailand, S. Angsuwathana, U. Jaisamrarn, and K. Wilawan; in the United Kingdom, E. Abdulhakim, M. Cruickshank, S. Govindraj, H. Kitchener, D. Lewis, I. Pavel, R. Pawa, and A. Szarewski (deceased); and in the United States, R. Ackerman, K. Ault, N. Bennett, M. Caldwell, C. Chambers, A. Chatterjee, L. Demars, T. Quinn De Santis, L. Downs, D. Ferris, P. Fine, D. Harper, J. Hedrick, W. Herzig, W. Huh, C. Hutchinson, L. Kamemoto, T. Klein, K. P. Klugman, W. Koltun, A. Kong, J. Lalezari, P. Lee, L. Leeman, S. Luber, M. Martens, C. Peterson, K. Pitts, J. Rosen, W. Rosenfeld, M. Scutella, L. Seidman, M. Sperling, M. Stager, J. T. Stapleton, K. Swenson, C. Thoming, L. Twiggs, S. Tyring, A. Waldbaum, C. M. Wheeler, and E. Zbella. Other contributors are as follows: Endpoint Committee, N. Kiviat, K. P. Klugman, and P. Nieminen; Independent Data Monitoring Committee, C. Bergeron, E. Eisenstein, R. Marks, T. Nolan, and S. K. Tay; GSK clinical study support, S. Albers, P. Bollaerts, A. Camier, B. Colau, A. De Breyne, S. Genevrois, N. Martens, P. Peeters, N. Smoes, B. Spiessens, F. Tavares, A. Meurée, N. Houard, A. Tonglet, S. Vanden-Dunghen, A. S. Vilain, and K. R. Ward; laboratory contributions, E. Alt, B. Iskaros, A. Limaye, X. Liu-Jarin, R. D. Luff, M. McNeeley, and C. Provenzano (Quest Diagnostics Clinical Trials, Teterboro, NJ, USA), and A. Molijn, W. Quint, L. Struijk, M. Van de Sandt, and L. J. Van Doorn (DDL Diagnostic Laboratory, Voorburg, Netherlands).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00591-14.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2012. GLOBOCAN v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. International Agency for Research on Cancer, Lyon, France: http://globocan.iarc.fr Accessed 1 September 2014. [Google Scholar]
- 2.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. 2002. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Sanjosé S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menendez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andujar M, Castellsague X, Sanchez GI, Nowakowski AM, Bornstein J, Munoz N, Bosch FX, Retrospective International Survey and HPV Time Trends Study Group. 2010. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 4.Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. 2009. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 5:332–340. doi: 10.4161/hv.5.5.7211. [DOI] [PubMed] [Google Scholar]
- 5.Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. 2008. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 26:6630–6638. doi: 10.1016/j.vaccine.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, Dubin G, HPV Vaccine Adolescent Study Investigators Network. 2007. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 40:564–571. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsagué X, De Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G, HPV PATRICIA Study Group. 2007. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 8.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, HPV PATRICIA Study Group. 2009. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 9.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G, HPV GlaxoSmithKline Vaccine Study Group. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 10.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G, HPV Vaccine Study Group. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 11.GlaxoSmithKline Vaccine HPV-007 Study Group. 2009. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 374:1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 12.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, Chow SN, Kitchener H, Teixeira JC, Hedrick J, Limson G, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, De Carvalho NS, Germar MJ, Peters K, Mindel A, De Sutter P, Bosch FX, David MP, Descamps D, Struyf F, Dubin G, HPV PATRICIA Study Group. 2012. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, Teixeira JC, Skinner SR, Jaisamrarn U, Limson G, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Harper DM, Huh W, Hardt K, Zahaf T, Descamps D, Struyf F, Dubin G, Lehtinen M, HPV PATRICIA Study Group. 2012. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 14.Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, Juliar BE, Breen TE, Fortenberry JD. 2005. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 191:182–192. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscicki AB. 2007. HPV infections in adolescents. Dis Markers 23:229–234. doi: 10.1155/2007/136906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koulova A, Tsui J, Irwin K, Van DP, Biellik R, Aguado MT. 2008. Country recommendations on the inclusion of HPV vaccines in national immunization programmes among high-income countries, June 2006-January 2008. Vaccine 26:6529–6541. doi: 10.1016/j.vaccine.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 17.Agosti JM, Goldie SJ. 2007. Introducing HPV vaccine in developing countries—key challenges and issues. N Engl J Med 356:1908–1910. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 18.Wright TC Jr, Huh WK, Monk BJ, Smith JS, Ault K, Herzog TJ. 2008. Age considerations when vaccinating against HPV. Gynecol Oncol 109(Suppl 2):S40–S47. doi: 10.1016/j.ygyno.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Garland SM. 2014. The Australian experience with the human papillomavirus vaccine. Clin Ther 36:17–23. doi: 10.1016/j.clinthera.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Lehtinen M, Apter D, Dubin G, Kosunen E, Isaksson R, Korpivaara EL, Kyhä-Osterlund L, Lunnas T, Luostarinen T, Niemi L, Palmroth J, Petäjä T, Rekonen S, Salmivesi S, Siitari-Mattila M, Svartsjö S, Tuomivaara L, Vilkki M, Pukkala E, Paavonen J. 2006. Enrolment of 22,000 adolescent women to cancer registry follow-up for long-term human papillomavirus vaccine efficacy: guarding against guessing. Int J STD AIDS 17:517–521. doi: 10.1258/095646206778145550. [DOI] [PubMed] [Google Scholar]
- 21.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. 2006. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol 44:3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. 2008. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 4:425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 23.American Society for Colposcopy and Cervical Pathology. 2014. Guidelines and resources. http://www.igcs.org/professionalEducation/treatmentResources/ASCCP/ASCCPGuidelines.html Accessed 19 August 2014.
- 24.Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia PJ, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Huh WK, Joura EA, Kurman RJ, Majewski S, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan JT, Lupinacci LC, Giacoletti KE, Sings HL, James MK, Hesley TM, Barr E, Haupt RM. 2010. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 25.Dillner J, Kjaer S, Wheeler C, Sigurdsson K, Iversen O, Hernandez-Avila M, Perez G, Brown D, Koutsky LA, Tay EH, García P, Ault KA, Garland S, Leodolter S, Olsson S, Tang G, Ferris D, Paavonen J, Lehtinen M, Steben M, Bosch X, Joura EA, Kurman R, Majewski S, Muñoz N, Myers E, Villa L, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Maanson R, Lu S, Vuocolo S, Hesley T, Barr E, Haupt R. 2010. Four year efficacy of prophylactic human papillomavirus (types 6/11/16/18) L1 virus-like particle vaccine against low-grade cervical, vulvar, and vaginal intraepithelial neoplasia and condylomata accuminata. BMJ 341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA, Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. 2007. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 27.FUTURE II Study Group. 2007. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 28.Ault KA, Future II Study Group. 2007. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 29.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR, HPV Costa Rican Vaccine Trial Group. 2007. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among women with preexisting infection: a randomized trial. JAMA 298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 30.Kitchener HC, Almonte M, Wheeler P, Desai M, Gilham C, Bailey A, Sargent A, Peto J, ARTISTIC Trial Study Group. 2006. HPV testing in routine cervical screening: cross sectional data from the ARTISTIC trial. Br J Cancer 95:56–61. doi: 10.1038/sj.bjc.6603210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briolat J, Dalstein V, Saunier M, Joseph K, Caudroy S, Prétet JL, Birembaut P, Clavel C. 2007. HPV prevalence, viral load and physical state of HPV-16 in cervical smears of patients with different grades of CIN. Int J Cancer 121:2198–2204. doi: 10.1002/ijc.22959. [DOI] [PubMed] [Google Scholar]
- 32.Kjaer SK, Breugelmans G, Munk C, Junge J, Watson M, Iftner T. 2008. Population-based prevalence, type- and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer 123:1864–1870. doi: 10.1002/ijc.23712. [DOI] [PubMed] [Google Scholar]
- 33.Szarewski A, Skinner R, Garland S, Romanowski B, Schwarz T, Apter D, Chow S-N, Paavonen J, Rosario-Raymundo M, Teixeira J, De Carvalho N, Castro-Sanchez M, Castellsagué X, Poppe W, De Sutter P, Huh W, Chatterjee A, Tjalma J, Ackerman R, Martens M, Papp K, Bajo-Arenas J, Harper D, Torné A, David M-P, Struyf F, Lehtinen M, Dubin G. 2013. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low risk HPV types (PATRICIA randomised trial): an unexpected observation. J Infect Dis 208:1391–1396. doi: 10.1093/infdis/jit360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehtinen M, Dillner J. 2013. Clinical HPV vaccine trials and beyond. Nature Rev Clin Oncol 10:400–410. doi: 10.1038/nrclinonc.2013.84. [DOI] [PubMed] [Google Scholar]
- 35.French KM, Barnabas RV, Lehtinen M, Kontula O, Pukkala E, Dillner J, Garnett GP. 2007. Strategies for the introduction of human papillomavirus vaccination: modelling the optimum age- and sex-specific pattern of vaccination in Finland. Br J Cancer 96:514–518. doi: 10.1038/sj.bjc.6603575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JJ, Goldie SJ. 2008. Health and economic implications of HPV vaccination in the United States. N Engl J Med 359:821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, Kiviat N, Galloway DA. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis 174:927–936. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 38.Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst 86:494–499. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Draper E, Bissett SJ, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S. 2013. A randomized, observer-blinded immunogenicity trial of Cervarix® and Gardasil® human papillomavirus vaccines in 12-15 year old girls. PLoS One 8:e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Donaghy M. 2014. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. BrJ Cancer 110:2804–2811. doi: 10.1038/bjc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.European Centre for Disease Prevention and Control. 2012. Introduction of HPV vaccines in EU countries—an update. ECDC, Stockholm, Sweden. doi: 10.2900/60814. [DOI] [Google Scholar]
- 42.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L, Immunization Services Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. 2014. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014—United States. Morb Mortal Wkly Rep 63:620–624. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6329a3.htm. [PMC free article] [PubMed] [Google Scholar]
- 43.David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, Van Damme P. 2009. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 115:S1–S6. doi: 10.1016/j.ygyno.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Liu PH, Hu FC, Lee PI, Chow SN, Huang CW, Wang JD. 2010. Cost-effectiveness of human papillomavirus vaccination for prevention of cervical cancer in Taiwan. BMC Health Serv Res 10:11. doi: 10.1186/1472-6963-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]