Abstract
Foot-and-mouth disease (FMD) is one of the most highly contagious and economically devastating diseases, and it severely constrains the international trade of animals. Vaccination against FMD is a key element in the control of FMD. However, vaccination of susceptible animals raises critical issues, such as the differentiation of infected animals from vaccinated animals. The current study developed a reliable and rapid test to detect antibodies against the conserved, nonstructural proteins (NSPs) of the FMD virus (FMDV) to distinguish infected animals from vaccinated animals. A monoclonal antibody (MAb) against the FMDV NSP 3B was produced. A competitive enzyme-linked immunosorbent assay (cELISA) for FMDV/NSP antibody detection was developed using a recombinant 3ABC protein as the antigen and the 3B-specific MAb. Sera collected from naive, FMDV experimentally infected, vaccinated carrier, and noncarrier animals were tested using the 3B cELISA. The diagnostic specificity was 99.4% for naive animals (cattle, pigs, and sheep) and 99.7% for vaccinated noncarrier animals. The diagnostic sensitivity was 100% for experimentally inoculated animals and 64% for vaccinated carrier animals. The performance of this 3B cELISA was compared to that of four commercial ELISA kits using a panel of serum samples established by the World Reference Laboratory for FMD at The Pirbright Institute, Pirbright, United Kingdom. The diagnostic sensitivity of the 3B cELISA for the panel of FMDV/NSP-positive bovine serum samples was 94%, which was comparable to or better than that of the commercially available NSP antibody detection kits. This 3B cELISA is a simple, reliable test to detect antibodies against FMDV nonstructural proteins.
INTRODUCTION
Foot-and-mouth disease (FMD) is one of the most highly contagious and economically devastating diseases of cloven-hoofed animals, and it severely constrains the international trade of animals and animal products. Vaccination against FMD, in addition to the slaughter and restriction of the movement of infected animals, is a key element in the control of FMD. However, countries that vaccinate in the event of an outbreak must reestablish their FMD-free status to the satisfaction of their trading partners (1, 2). Vaccination of susceptible animals raises critical issues, such as the differentiation of infected animals from vaccinated animals and the development of carrier status because of subclinical infection in vaccinated animals.
FMD is caused by the FMD virus (FMDV), which is a member of the genus Aphthovirus and the family Picornaviridae (3), and it exhibits seven serotypes, O, A, Asia 1, C, SAT 1,SAT 2, and SAT 3. FMDV has a positive-sense, single-stranded RNA genome of 8,400 nucleotides that code for 12 proteins. Four structural proteins (VP1, VP2, VP3, and VP4) compose the viral capsid, and eight proteins are nonstructural proteins (NSPs; L, 2A, 2B, 2C, 3A, 3B, 3C, and 3D). All 12 proteins allow the virus to replicate in infected cells (4–6). Antibodies to the 3ABC NSPs are a reliable indicator of infection, regardless of the FMDV serotype. Enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies against NSPs are widely used to differentiate vaccinated and infected animals because purified vaccines are free of NSPs and thus elicit antibodies only against structural proteins (7). However, not all manufacturers produce purified FMD vaccines, and the degree of purity among FMD vaccine manufacturers is not always identical (8).
Several tests for the detection of antibodies against NSPs were reported, and some of these tests were made into commercially available kits. The tests produced by Svanova, Bommeli, and UBI and several other published tests (9–15) are not ideal, because these tests require species-specific conjugated antibodies. Separate assays are required to test samples from different species (cattle, deer, goats, and sheep), and no reagents are available for wildlife (2, 16). A wide range of animal species are susceptible to FMDV. Therefore, a competitive ELISA (cELISA) would be advantageous because serum samples from different species could be tested without changing reagents (17). cELISAs are simple, easy to perform, and species independent. Numerous cELISAs for the detection of antibodies against NSPs were used to differentiate vaccinated animals from infected animals (1, 18). However, polyclonal antibodies were used as the competitor in these tests. The use of polyclonal antibodies cannot ensure consistent quality compared to the quality achieved by the use of monoclonal antibodies (MAbs) because of batch-to-batch variations. Sørensen et al. (17) produced a MAb against NSP 3B and developed a cELISA using the same MAb (L74D5) used as the capture and detector antibody in a blocking ELISA. The disadvantage of this ELISA system is that when the antigen binds to the capture antibody, the same epitope that is recognized by the polyclonal or competition antibodies might be hidden, which reduces the test sensitivity. The PrioCheck NS test uses a specific MAb against NSP and a recombinant NSP protein in a cELISA format. One study demonstrated that the PrioCheck NS test is sensitive and very specific in the buffalo populations of eastern Africa (16). However, the use of a commercial kit for regular diagnosis and surveillance can be costly. The development of an effective in-house test for the detection of antibodies against FMDV/NSP regardless of the species is necessary to make daily tests more affordable.
A MAb against a conserved epitope located on the 3B NSP was produced. A cELISA for FMD NSP 3B antibody detection was developed using this MAb and a recombinant 3ABC protein. The assay was validated using a panel of serum samples and samples collected from naive, experimentally inoculated, and vaccinated carrier and noncarrier animals. Development of a reliable NSP antibody assay would enable successful disease diagnosis, the establishment of disease-free zones, and the acceleration of global FMD control.
MATERIALS AND METHODS
Nonstructural protein production.
The cloning and expression of His-tagged recombinant 3ABC protein were performed as described previously (1, 19). Recombinant bacterial antigen was produced in cultures that were incubated for 2 to 3 h at 37°C with shaking until an optical density at 600 nm (OD600) of 0.6 to 0.7 was reached. Protein expression was induced by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM, and the solution was incubated for an additional 3 h at 37°C with shaking. The culture was pelleted using centrifugation at 8,000 × g for 15 min at 4°C. The cell pellet was completely resuspended at room temperature in BugBuster reagent (25 U/ml; Novagen, Darmstadt, Germany) to extract the recombinant 6×His-3ABC. Benzonase nuclease (Novagen) was added, and the suspension was incubated at room temperature with gentle shaking for 10 to 20 min. Insoluble cell debris was collected using centrifugation at 10,000 × g for 20 min at 4°C. The pellet was completely resuspended in the same volume of BugBuster reagent and centrifuged at 10,000 × g for 20 min at 4°C to collect the inclusion bodies. The final pellet was resuspended in 50 ml of 8 M urea, 0.1 M sodium phosphate buffer, 0.01 M Tris-HCl, pH 8.0, until the inclusion bodies (recombinant 3ABC protein) became soluble and the solution was transparent. Any insoluble material was removed using centrifugation at 10,000 × g for 20 min at 4°C. The collected supernatant containing the recombinant 3ABC NSP was aliquoted and stored at −70°C.
Peptides, mouse immunization, and monoclonal antibody production.
The peptide H2N-CGPYAGPLERQKPLK-OH (20), derived from 3B1, was synthesized for immunization, and its purity was assessed using high-pressure liquid chromatography (New England Peptide, MA, USA). The peptide was conjugated to the carrier protein keyhole limpet hemocyanin (KLH) by New England Peptide.
The Canadian Science Centre for Human and Animal Health Animal Care Committee approved the animal use and study procedures (animal use document C-02-002). The procedures for mouse immunization and MAb production were performed as previously described (21). Hybridoma supernatants were screened using the recombinant 3ABC NSP as the antigen in an indirect ELISA. The positive clone (F8-3Bp) was subcloned, and the isotype was determined using a mouse monoclonal antibody isotyping kit (Roche, Indianapolis, IN, USA).
Development of the 3B cELISA.
The optimal concentrations for recombinant 3ABC protein, MAb, and conjugated antibody dilutions were determined using checkerboard titrations of known strongly positive, weakly positive, and negative sera. The 3B cELISA was performed by coating microtiter plates (Immunoplate; Nunc, Roskilde, Denmark) with 100 μl/well of recombinant 3ABC antigen that was diluted on the basis of the titration of each batch in 0.06 M carbonate/bicarbonate buffer, pH 9.6, and the plates were incubated overnight at 4°C. After five washes with the washing buffer (0.05% Tween 20 in 0.01 M phosphate-buffered saline [PBS-T]), 100 μl of saturation buffer (50 mM Tris-HCl, 150 mM NaCl, 0.05% NaN3, 0.2% bovine serum albumin, 6% d-sorbitol) was added to each well and the plate was incubated overnight at 4°C. The saturation buffer was discarded, and the plates were dried at room temperature for 5 h, heat sealed under vacuum, and stored for up to 12 months at 4°C. For testing, the plates were washed 3 times with PBS-T, followed by the addition of 50 μl of heat-inactivated test serum (final dilution, 1:5) in duplicate and an equal volume of hybridoma culture supernatants (1:1,000) in PBS-T. The plates were incubated at 37°C for 1 h with agitation. After washing five times, 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:2,000; Jackson ImmunoResearch Laboratories Inc.) in PBS-T was added and the plates were incubated for 1 h at 37°C with subsequent washing. The 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB) substrate (Sigma-Aldrich, St. Louis, MO, USA) was added, and color development was stopped after 15 min with the addition of 50 μl/well of 2 M sulfuric acid. The OD450 was determined using an automated plate reader (Photometer Multiskan reader; Labsystems, Foster, VA, USA).
Results were calculated on the basis of the results for reference sera with strongly positive (Q1), weakly positive (Q2), and negative (Q3) results. Test results (the mean for the serum sample tested in duplicate wells) were derived using the following formula: percent inhibition (PI) = [(Q3 sample OD − test sample OD)/(Q3 sample OD − Q1 sample OD)] × 100.
Negative sera from cattle, pigs, and sheep.
Negative serum samples were collected from naive cattle (n = 503), pigs (n = 508), and sheep (n = 507) in Canada. Sera from naive cattle (n = 353) and sheep (n = 436) were also included in the evaluation in Mexico.
Raising of sera against the seven serotypes of FMDV in cattle, sheep, pigs, and deer.
The FMDV strains used in this study (O/UKG/11/2001, A22 Iraq, A24/Cruzeiro/Br/55, C1 Noville, Asia 1/Shamir, SAT 1/BOT 1/68, SAT 1 KEN/4/98, SAT 2 SAU 1/2000, and SAT 3 ZIM 4/81) are reference strains that were obtained from the World Reference Laboratory for FMD (WRLFMD) at The Pirbright Institute, Pirbright, United Kingdom. Baby hamster kidney clone 21 (BHK-21) cells or lamb kidney (LK) primary cells were used to prepare viruses of the seven FMDV serotypes. The cells were infected with FMDV in Glasgow's minimal essential medium supplemented with 2 mM l-glutamine and 50 μg/ml gentamicin. Viruses were harvested upon observation of a complete cytopathic effect (CPE) and clarified using centrifugation at 2,000 × g for 20 min.
All procedures involving experimental animal inoculations and care were performed according to the Canadian Council of Animal Care guidelines. The local animal care committee approved all animal procedures prior to initiation of the study. Cattle, sheep, pigs, and deer (1, 22) were inoculated with one of the FMDV strains. Cattle and sheep were infected via intradermal/subdermal injections on the dorsal aspect of the tongue and pigs were inoculated in their heel bulb with a total viral dose of 106 50% tissue culture infectious doses (TCID50) at five sites. Two white-tailed deer (animals D16 and D20) were inoculated intranasally with 104 TCID50 of FMDV O/UKG/11/2001 diluted in 5 ml of culture medium. Blood samples were collected before (0 days postinoculation [p.i.]) and after virus inoculation until 28 to 31 days p.i.
Panel of positive bovine serum samples for evaluation of the 3B cELISA.
The panel of bovine serum samples (23) was established by the WRLFMD at The Pirbright Institute, Pirbright, United Kingdom. This panel evaluates new tests and provides quality control for new batches of existing tests. Thirty-six bovine serum samples were selected from a series of vaccination-challenge experiments. The details of the experiments used to generate these samples were reported previously (23).
Sera from control, vaccinated, and carrier cattle.
Sera from control, vaccinated, and carrier cattle were raised by the WRLFMD at The Pirbright Institute. Cattle were vaccinated with O1/Manisa and challenged with O/UKG/34/2001 in two experiments, UV and UY (24, 25). The vaccination dose in the second experiment (UY) was increased 10-fold compared to that in the first experiment (UV). Cattle were grouped into control (nonvaccinated), vaccinated noncarrier, and vaccinated carrier groups. The carrier state was confirmed by either positive virus isolation or a positive quantitative reverse transcription-PCR (RT-PCR) result for the probang samples taken from individual animals (24, 25). Serum samples were collected from these animals 7 days before vaccination and at 7-day intervals until 168 days postchallenge (dpc) (26, 27). However, not every animal was sampled at all time points.
Statistical analysis.
Correlation analyses for comparisons of the two test methods (the 3B cELISA versus the PrioCheck NS test) were performed using Microsoft Excel software. Statistical significance was set at a P value of <0.05.
RESULTS
Production of a monoclonal antibody against the 3B peptide.
The KLH-conjugated FMDV 3B peptide (H2N-CGPYAGPLERQKPLK-OH) was used as the immunogen to produce the peptide-specific MAb (F8-3Bp). This MAb is an IgG1 isotype with kappa light chains. The MAb was examined for its reactivity and specificity against the recombinant 3ABC NSP and the 3B peptide using an indirect ELISA. Results indicated that the MAb reacted with the recombinant 3ABC protein and the 3B peptide in the ELISA with an OD higher than 1.0 after background subtraction. The MAb F8-3Bp competed with the serum from an FMDV-infected animal.
Development and evaluation of the 3B cELISA.
A cELISA to detect antibodies against FMDV NSP was developed using the newly generated MAb (F8-3Bp) and the recombinant 3ABC protein as the antigen. The optimal concentrations for the recombinant 3ABC protein, MAb, and conjugated antibody dilutions were optimized using checkerboard titrations of known strongly positive, weakly positive, and negative sera.
A total of 1,518 negative serum samples (n = 503 bovine, n = 508 porcine, and n = 507 ovine serum samples) were collected from Canada and tested using the 3B cELISA. The frequencies of the percent inhibition (PI) generated from these sera were normally distributed (Fig. 1a). The mean PIs for the negative sera were 4.43% (standard deviation [SD] = 14.03%), −8.63% (SD = 14.0%), and −9.6% (SD = 9.59%) for bovine, porcine, and ovine serum samples, respectively. Similar results were obtained using sera collected from Mexico (n = 353 bovine and n = 436 ovine serum samples) (Fig. 1b). The mean PIs were −6.4% (SD = 17.35%) and −15.76% (SD = 18.92%) for bovine and ovine serum samples, respectively. The negative cutoff values, which were calculated by the addition of 3 SDs to the mean PIs for the negative serum samples, equaled 19 to 46% inhibition for all animal species. Therefore, the cutoff value was set at <50% inhibition (28). Less than 1% of the tested negative samples collected from cattle, pigs, and sheep had values that exceeded this cutoff value, which produced a diagnostic specificity of 99.4%.
FIG 1.
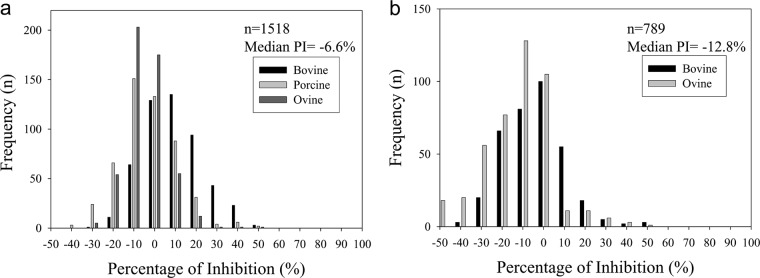
Frequency distribution of the negative sera tested using the 3B cELISA for serum samples collected from disease-free cattle (n = 503), pigs (n = 508), and sheep (n = 507) in Canada (a) and cattle (n = 353) and pigs (n = 436) in Mexico (b). Recombinant 3ABC protein was coated onto microtiter plates. Equal volumes of diluted sera and MAb (F8-3Bp) were added to the plates and allowed to compete with antibodies in the serum samples. Results are expressed as percent inhibition.
A panel of bovine serum samples for the evaluation of antibodies against FMDV NSP was tested using the 3B cELISA, and the results were compared with those of other commercially available tests. This serum sample panel (n = 36) was selected from a series of vaccination-challenge experiments (23, 29). All samples were seropositive by the virus neutralization test (VNT) (23). Comparison of the results of the 3B cELISA with those of commercially available tests indicated that the 3B cELISA detected the highest percentage of positive sera (94%) among the NSP antibody detection tests (Table 1). The PrioCheck NS test had a slightly lower percentage of positive results (92%) than the 3B cELISA. The Bommedi, UBI, and Svanova tests demonstrated rates of positive results ranging from 61 to 81%. The 3B cELISA detected the NSP antibody in 34 of 36 serum samples, and it failed to detect antibodies against NSP in 2 samples (from animals UV10 and UV19) that were also negative in the Bommedi, UBI, and Svanova tests. These two samples were from vaccinated (O1/Manisa) and challenged (O/UKG) carriers. Their VNT titers were 178 and 256, respectively. Three serum samples (two from the vaccinated carrier group, one from the vaccinated noncarrier group) with VNT titers of >512 exhibited a negative NSP antibody response in the PrioCheck NS test (29).
TABLE 1.
Comparison of test results with FMDV NSP antibody evaluation panele
| Animal group | No. of animals seropositive/total no. of animals tested (%) |
||||
|---|---|---|---|---|---|
| 3B cELISA | PrioCheck NSa | Bommedib | UBIc | Svanovad | |
| Vaccinated carrier | 18/20 | 18/20 | 14/20 | 11/20 | 10/20 |
| Vaccinated noncarrier | 4/4 | 3/4 | 3/4 | 2/4 | 2/4 |
| Control carrier | 7/7 | 7/7 | 7/7 | 6/7 | 6/7 |
| Control noncarrier | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 |
| Total | 34/36 (94) | 33/36 (92) | 29/36 (81) | 24/36 (67) | 22/36 (61) |
PrioCheck NS, Cedi Diagnostics B.V., Lelystad, The Netherlands.
Bommedi, Chekit-FMD-3ABC bo-ov, Bommeli Diagnostics, Liebefeld-Bern, Switzerland.
UBI, UBI FMDV NS ELISA (cattle), United Biomedical Inc., Hauppauge, NY.
Svanova, Svanovir FMDV 3ABC-Ab ELISA, Svanova Biotech AB, Uppsala, Sweden.
All animals (n = 36 animals from the vaccinated and control groups) were challenged and shown to be seropositive by VNT. The results of all tests except the 3B cELISA have previously been reported by Parida et al. (23).
Serum samples from vaccinated noncarriers in vaccination-challenge experiment 1 were tested using both the 3B cELISA and the PrioCheck NS test to evaluate the 3B cELISA as a test for differentiating vaccinated from infected animals (DIVA). The PrioCheck NS test is the test that is the most commonly used for FMD/NSP-specific antibody detection. Therefore, the 3B cELISA results were compared with those of the PrioCheck NS test. A total of 114 and 111 of 115 samples had percent inhibitions of <50% by the 3B cELISA and PrioCheck NS test, respectively. These results indicate that no or a few antibodies against NSP were present in these vaccinated noncarrier animals. One sample that was an exception (sample UV8), which was collected at 42 dpc, showed a positive antibody response with a PI of 60%, which produced a test specificity for DIVA of 99.1% for the 3B cELISA (Fig. 2a). Four samples demonstrated positive antibody responses in the PrioCheck NS test for a test specificity of 96.6% (Fig. 2b). All cattle in the control group (nonvaccinated, challenged animals) exhibited a PI of >50% from 14 dpc, which indicates the presence of antibodies to the 3B NSP. The 3B cELISA results demonstrated a good correlation with the PrioCheck NS test results, with correlation coefficients of 0.87 and 0.98 for the two experiments, respectively (Fig. 3). Moreover, the sera taken from vaccinated carrier cattle were tested using the 3B cELISA, and the results were compared with the previous PrioCheck NS test results (Fig. 4). Seven of 11 cattle showed positive seroconversion by both the 3B cELISA and the PrioCheck NS test after 14 dpc. However, two carrier cattle (animals UV2 and UV14), which were confirmed to be positive using reverse transcription-PCR, were negative by these two tests. The other two carrier cattle (animals UV5 and UY76) showed positive antibody responses against NSP in the PrioCheck NS test but negative antibody responses in the 3B cELISA. The correlation coefficient between the 3B cELISA and the PrioCheck NS test was 0.70 for both experiments, with P being <0.0001 (Fig. 4C).
FIG 2.
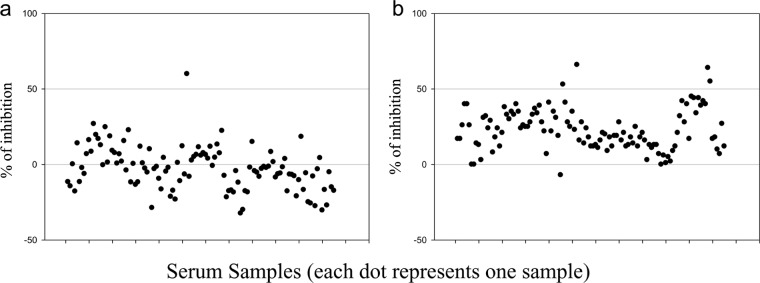
Evaluation of the 3B cELISA using sera from vaccinated noncarrier cattle. During the course vaccine experiment 1, serum samples were collected from vaccinated noncarrier cattle (animals UV3, UV4, UV6, UV7, UV8, UV12, UV15, UV16, UV18, UV20, and UV21) at 7-day intervals from −7 dpv to 168 dpc. Samples were tested using the 3B cELISA (a) and the PrioCheck NS test (b).
FIG 3.
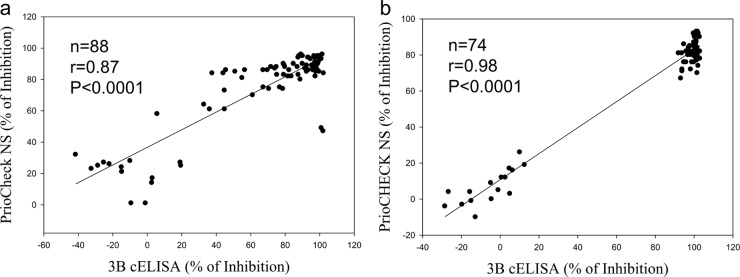
Correlations between the 3B cELISA and the PrioCheck NS test results for sera collected from the control group (unvaccinated, challenged animals). Correlation coefficients between the 3B cELISA and the PrioCheck NS test were determined for vaccine experiment 1 (a) and vaccine experiment 2 (b).
FIG 4.
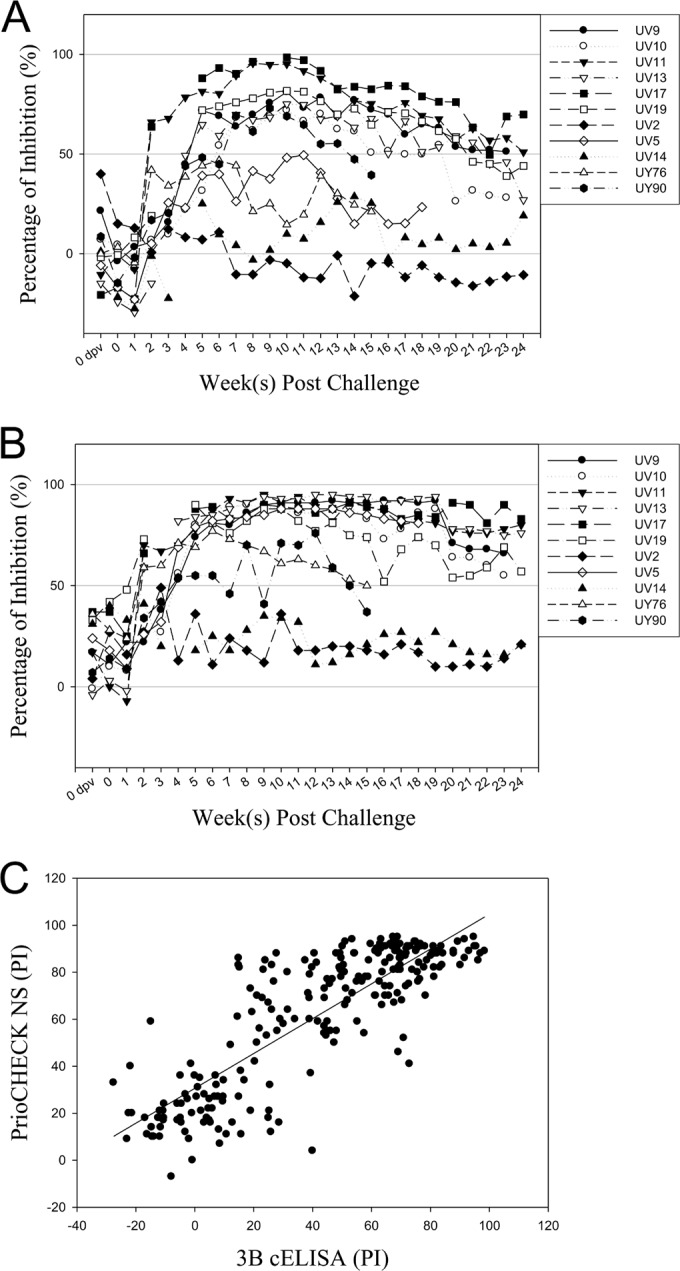
Results of the 3B cELISA and the PrioCheck NS test using sera collected from vaccinated carrier cattle. During the course of the two vaccination-challenge experiments, serum samples were collected from vaccinated carrier cattle at 7-day intervals from −7 dpv to 168 dpc and assayed by the 3B cELISA (A) and the PrioCheck NS test (B). (C) Correlations between the 3B cELISA and the PrioCheck NS test.
Detection of seroconversion in FMDV-inoculated animals using 3B cELISA.
The antibody responses to FMDV/NSP in experimentally inoculated animal species (15 cattle, 9 pigs, 18 sheep, and 2 deer) were examined using the 3B cELISA. Antibodies to NSP were undetectable from 0 to 5 days p.i. for swine, cattle, and deer (Table 2). One of 18 sheep showed a positive antibody response against NSP at 5 days p.i. A total of 89 to 100% of samples from all tested species demonstrated positive antibody responses after 9 days p.i. Two samples, one from a pig and one from a sheep, demonstrated positive NSP antibody responses at 9 days p.i. Therefore, seroconversions occurred mainly between 6 and 9 days p.i. Samples from all animals except one pig remained positive for antibody responses until the end of the experiment (28 days p.i.). These results indicate that the 3B cELISA detected antibodies against NSP in all tested animal species.
TABLE 2.
FMDV NSP-specific antibody responses in different animal species inoculated with different serotypes of FMDV
| Animal species | No. (%) positive at: |
|||
|---|---|---|---|---|
| 5 days p.i. | 7 days p.i. | 9 days p.i. | 28 days p.i. | |
| Pigs (n = 9) | 0 (0) | 1 (12.5) | 8 (89) | 8 (89) |
| Sheep (n = 18) | 1 (6) | 14 (82) | 17 (94) | 18 (100) |
| Cattle (n = 15) | 0 (0) | 8 (53) | 15 (100) | 15 (100) |
| Deer (n = 2) | 0 (0) | 2 (100) | 2 (100) | 2 (100) |
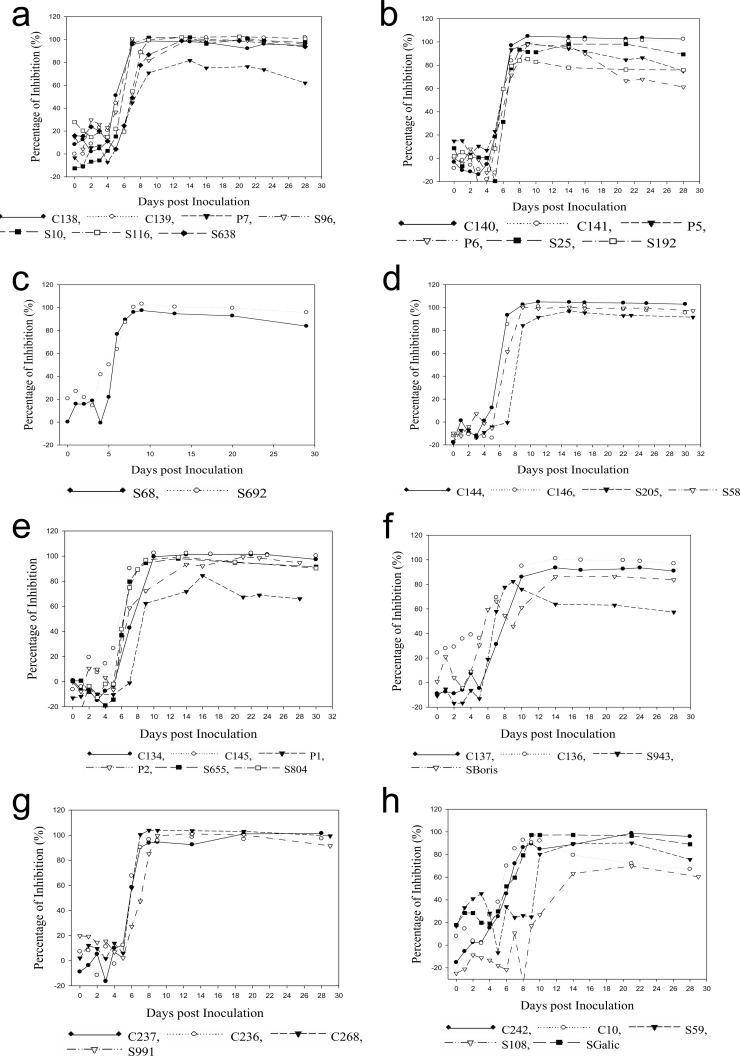
Serum samples from animals experimentally inoculated with all seven serotypes were tested to determine whether the 3B cELISA detected NSP antibodies in sera containing different serotypes. The results demonstrated positive antibody responses to NSP at 8 to 10 days p.i., and the responses remained positive until the end of the experiment (Fig. 5). Two serum samples from sheep experimentally inoculated with SAT 3 showed a positive seroconversion after 10 days p.i. and remained positive until 28 days p.i. These results showed that the 3B cELISA is serotype independent for NSP-specific antibody detection.
FIG 5.
Detection of FMDV/NSP antibodies in animals. Sera were collected from animals experimentally inoculated with the seven FMDV serotypes, O (a), A24 (b), A22 (c), C1 (d), Asia 1 (e), SAT 1 (f), SAT 2 (g), SAT 3 (h), and tested using the 3B cELISA. C, cattle; S, sheep; P, pig; D, deer.
DISCUSSION
The use of vaccines against FMD is an economic strategy to prevent and control this disease. The differentiation of infected and vaccinated animals is also important in surveillance efforts. An accurate test for detecting infection in susceptible animals is needed, especially for infections after vaccination (30). ELISA for the detection of antibodies against FMDV/NSP can distinguish between vaccinated animals and infected animals. A good MAb that recognizes strain-independent epitopes on FMDV/NSP is required to develop tests for FMDV/NSP antibody detection. Linear B-cell epitopes located in the FMDV NSP were identified (31, 32). Höhlich et al. (20) identified six linear B-cell epitopes in the NSPs. Three of six peptides were recognized by sera collected from all infected animals regardless of the infecting strains. In their experiments, none of the serum samples from vaccinated animals contained antibodies against these peptides. Therefore, our study used one (CGPYAGPLERQKPLK) of the 3B peptides (20) as the immunogen for MAb production, and the MAb produced against this peptide was used as the basis for the 3B cELISA to differentiate vaccinated from infected animals.
The MAbs that are raised by peptides may not recognize original or recombinant protein (33, 34). However, the MAb F8-3Bp that was produced in the current study reacted with the 3B peptide and recombinant 3ABC. Similar results were also obtained by Yang et al. (35), who successfully produced MAbs against FMDV/NSP 3D using immunization with recombinant 3D and a booster with a 3D peptide. Different species may recognize different epitopes. Sera from FMDV-infected pigs failed to react with any linear epitope located on the 3D protein (35). The peptide used for immunization in this study was recognized by sera from all infected animals independently of the animal species (20).
A 3B cELISA was developed using the recombinant 3ABC protein and the 3B-specific MAb. A cutoff value of 50% inhibition was established on the basis of the findings for 2,307 negative serum samples. This cutoff provided a clear distinction between positive and negative sera. Diagnostic specificities using this cutoff value were 99.4% for all tested animal species (cattle, pigs, and sheep). The diagnostic specificity of a previously developed 3ABC indirect ELISA for bovines was estimated to be 96.4% (15). Some previously developed tests were designed to detect NSP antibodies predominantly in cattle, but these tests are less useful in sheep and pigs. Sheep, in particular, may fail to develop detectable levels of these antibodies because of the frequently subclinical nature of FMD (30, 36). Immune responses to infection may vary within individuals, virus serotypes, and animal species, but our 3B cELISA detected antibodies against FMDV/NSP in cattle, swine, and sheep in a serotype-independent manner. However, more deer and buffalo samples may need to be tested.
Evaluations of the performance of a new test require comparisons of the performance with standard samples. A panel of serum samples (n = 36) collected from FMD-vaccinated and challenged animals (WRLFMD, Pirbright, United Kingdom) was used to evaluate the 3B cELISA. The test results indicated that the 3B cELISA detected the highest percentage of positive serum samples (94%) among the NSP antibody detection tests (the PrioCheck NS, Bommedi, UBI, and Svanova tests). The 3B cELISA failed to detect two positive samples (from animals UV10 and UV19) from this panel. These samples consisted of sera collected from cattle that were vaccinated and challenged by direct contact. The most reliable and consistent route of infection in experimental studies on FMD is generally via intradermal/subdermal injection of virus (2). Therefore, one possible explanation for this observation may be that vaccinated cattle challenged by contact with infected donors rather than by needle inoculation were generally subclinically infected and produced low levels of NSP antibody because of limited viral replication in the presence of high levels of structural antibodies produced against the vaccine (23).
The aim of this study was to develop a test for DIVA, and samples collected from vaccinated cattle in two vaccination-challenge experiments were analyzed. Theoretically, antibody responses against FMDV/NSP in the vaccinated population should be similar to those in the naive population. Serum samples collected from 11 vaccinated and challenged noncarrier cattle that demonstrated positive virus isolation results from probang samples that cleared by 28 days after initial contact (24, 25) were tested using the 3B cELISA and the PrioCheck NS test. A total of 114 of the 115 samples from these animals (collected from −7 days postvaccination [dpv] to 168 dpc) showed negative results in the 3B cELISA, which indicated that no antibodies against NSP were present in those serum samples. These cattle were challenged after vaccination, but there was little or no FMDV replication because of induction of the protective immune response. Therefore, no antibodies against NSP were detected.
Samples collected from control cattle (nonvaccinated, challenged animals) were also tested using the 3B cELISA, and the results were comparable to the PrioCheck NS test results (23). The PrioCheck NS test demonstrated the highest diagnostic specificity among the commercial ELISA kits in a comparative study (37, 38). The 3B cELISA results exhibited a good correlation with the PrioCheck NS test results, with correlation coefficients of 0.87 and 0.98 for experiments 1 and 2, respectively. Clearly, the 3B cELISA can be used to detect antibodies against FMDV/NSP and differentiate vaccinated from infected animals (39).
The use of vaccination in countries where FMD is endemic or during an outbreak situation results in a strong possibility that the clinical disease will be masked in animals with partial immunity that are exposed to live virus (36). The challenge for FMD diagnosis is to identify vaccinated animals that have had contact with live virus and become carriers. The sera from vaccinated carrier cattle in two vaccination-challenge experiments were tested using the 3B cELISA, and the results were compared with the PrioCheck NS test results. Seven of 11 cattle showed seroconversion in both the 3B cELISA and the PrioCheck NS test after 14 dpc. Two vaccinated carrier cattle (animals UV2 and UV14), which were confirmed to have positive results using RT-PCR, showed negative results in both the 3B cELISA and the PrioCheck NS test. The other two vaccinated carriers (animals UV5 and UY76) showed positive antibody responses against FMDV/NSP in the PrioCheck NS test but were negative by the 3B cELISA. The development of antibodies to NSP correlates with the extent of virus replication. Therefore, the immune response induced by vaccination may reduce virus replication and the amount of FMDV/NSP produced, which would result in a low anti-NSP antibody response (17, 26, 40). One outcome could be that the antibody response may fall below the detection limit of the 3B cELISA because of a decreased level of FMDV/NSPs. However, some vaccinated carrier animals fail to develop antibodies against the NSPs (11). Present tests for antibodies to NSPs cannot completely guarantee that a population of vaccinated animals exposed to live virus contains no carriers (2). Caution must be taken when analyzing data from vaccinated animals. The definitive identification of carrier status or subclinically infected animals requires the recovery of live FMDV from these animals or, potentially, the use of real-time RT-PCR (2, 36).
In conclusion, the 3B cELISA that was developed using a 3B-specific MAb and a recombinant 3ABC antigen is a simple and reliable diagnostic test for the detection of FMDV 3B-specific antibodies. The 3B cELISA exhibited performance characteristics comparable to those of the PrioCheck NS test, and it outperformed other FMDV/NSP antibody detection tests. The binding epitope of the MAb used in this 3B cELISA is well characterized and conserved among the seven FMDV serotypes. The test is serotype and species independent. The advantages of the 3B cELISA over the PrioCheck NS test include (i) a reduced assay time of under 4 h, using precoated plates, for the 3B cELISA compared to an assay time of 20 h for the PrioCheck NS test and (ii) a much lower cost for the 3B cELISA (<$1 per sample in duplicate) than the PrioCheck NS test ($5 to $6 per sample). The reduced time and the reduced cost required to detect antibodies against NSP while maintaining diagnostic sensitivity and specificity are critical for FMD diagnosis.
ACKNOWLEDGMENTS
We gratefully thank the animal care staff for expert animal services and Soren Alexandersen and Charles Nfon for critical review of the manuscript.
This work was supported by funds from the Canadian Food Inspection Agency and the Department for Environment, Food and Rural Affairs (DEFRA), United Kingdom, through grant SE 1125 at the Pirbright Institute.
Footnotes
This is a contribution from the National Centre for Foreign Animal Disease, Canada.
REFERENCES
- 1.Clavijo A, Zhou EM, Hole K, Galic B, Kitching P. 2004. Development and use of a biotinylated 3ABC recombinant protein in a solid-phase competitive ELISA for the detection of antibodies against foot-and-mouth disease virus. J Virol Methods 120:217–227. doi: 10.1016/j.jviromet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Alexandersen S, Zhang Z, Donaldson A, Garland A. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol 129:1–36. doi: 10.1016/S0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 3.Belsham GJ. 1993. Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Prog Biophys Mol Biol 60:241–260. doi: 10.1016/0079-6107(93)90016-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domingo E, Escarmis C, Baranowski E, Ruiz-Jarabo CM, Carrillo E, Nunez JI, Sobrino F. 2003. Evolution of foot-and-mouth disease virus. Virus Res 91:47–63. doi: 10.1016/S0168-1702(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 5.Mason PW, Pacheco JM, Zhao QZ, Knowles NJ. 2003. Comparisons of the complete genomes of Asian, African and European isolates of a recent foot-and-mouth disease virus type O pandemic strain (PanAsia). J Gen Virol 84:1583–1593. doi: 10.1099/vir.0.18669-0. [DOI] [PubMed] [Google Scholar]
- 6.Fry EE, Stuart DI, Rowlands DJ. 2005. The structure of foot-and-mouth disease virus. Curr Top Microbiol Immunol 288:71–101. [DOI] [PubMed] [Google Scholar]
- 7.Sørensen K, Madsen K, Madsen E, Salt J, Nqindi J, Mackay D. 1998. Differentiation of infection from vaccination in foot-and-mouth disease by the detection of antibodies to the non-structural proteins 3D, 3AB and 3ABC in ELISA using antigens expressed in baculovirus. Arch Virol 143:1461–1476. doi: 10.1007/s007050050390. [DOI] [PubMed] [Google Scholar]
- 8.Doel T. 2001. Repeated administration of maximum payload emergency vaccines made from inactivated purified antigen concentrates do not induce significant titles of antibodies against non-structural proteins of foot-and-mouth disease virus, p 88–92. Report of a session of the Research Group of the Standing Technical Committee of the European Commission for the Control of Foot-and-Mouth Disease. European Commission for the Control of Foot-and-Mouth Disease, Rome, Italy. [Google Scholar]
- 9.Bergmann IE, Malirat V, Neitzert E, Beck E, Panizzutti N, Sanchez C, Falczuk A. 2000. Improvement of a serodiagnostic strategy for foot-and-mouth disease virus surveillance in cattle under systematic vaccination: a combined system of an indirect ELISA-3ABC with an enzyme-linked immunoelectrotransfer blot assay. Arch Virol 145:473–489. doi: 10.1007/s007050050040. [DOI] [PubMed] [Google Scholar]
- 10.De Diego M, Brocchi E, Mackay D, De Simone F. 1997. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch Virol 142:2021–2033. doi: 10.1007/s007050050219. [DOI] [PubMed] [Google Scholar]
- 11.Mackay D, Forsyth M, Davies P, Berlinzani A, Belsham G, Flint M, Ryan M. 1998. Differentiating infection from vaccination in foot-and-mouth disease using a panel of recombinant, non-structural proteins in ELISA. Vaccine 16:446–459. doi: 10.1016/S0264-410X(97)00227-2. [DOI] [PubMed] [Google Scholar]
- 12.Shen F, Chen P, Walfield A, Ye J, House J, Brown F, Wang C. 1999. Differentiation of convalescent animals from those vaccinated against foot-and-mouth disease by a peptide ELISA. Vaccine 17:3039–3049. doi: 10.1016/S0264-410X(99)00148-6. [DOI] [PubMed] [Google Scholar]
- 13.Kweon CH, Ko YJ, Kim WI, Lee SY, Nah JJ, Lee KN, Sohn HJ, Choi KS, Hyun BH, Kang SW. 2003. Development of a foot-and-mouth disease NSP ELISA and its comparison with differential diagnostic methods. Vaccine 21:1409–1414. doi: 10.1016/S0264-410X(02)00684-9. [DOI] [PubMed] [Google Scholar]
- 14.Srisombundit V, Tungthumniyom N, Linchongsubongkoch W, Lekcharoensuk C, Sariya L, Ramasoota P, Lekcharoensuk P. 2013. Development of an inactivated 3Cpro-3ABC (mu3ABC) ELISA to differentiate cattle infected with foot and mouth disease virus from vaccinated cattle. J Virol Methods 188:161–167. doi: 10.1016/j.jviromet.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Mohapatra JK, Pandey LK, Sanyal A, Pattnaik B. 2011. Recombinant non-structural polyprotein 3AB-based serodiagnostic strategy for FMD surveillance in bovines irrespective of vaccination. J Virol Methods 177:184–192. doi: 10.1016/j.jviromet.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Bronsvoort BM, Parida S, Handel I, McFarland S, Fleming L, Hamblin P, Kock R. 2008. Serological survey for foot-and-mouth disease virus in wildlife in eastern Africa and estimation of test parameters of a nonstructural protein enzyme-linked immunosorbent assay for buffalo. Clin Vaccine Immunol 15:1003–1011. doi: 10.1128/CVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sørensen K, De Stricker K, Dyrting K, Grazioli S, Haas B. 2005. Differentiation of foot-and-mouth disease virus infected animals from vaccinated animals using a blocking ELISA based on baculovirus expressed FMDV 3ABC antigen and a 3ABC monoclonal antibody. Arch Virol 150:805–814. doi: 10.1007/s00705-004-0455-z. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez A, Dopazo J, Saiz J, Sobrino F. 1994. Immunogenicity of non-structural proteins of foot-and-mouth disease virus: differences between infected and vaccinated swine. Arch Virol 136:123–131. doi: 10.1007/BF01538822. [DOI] [PubMed] [Google Scholar]
- 19.Clavijo A, Lin M, Riva J, Mallory M, Lin F, Zhou E. 2001. Development of a competitive ELISA using a truncated E2 recombinant protein as antigen for detection of antibodies to classical swine fever virus. Res Vet Sci 70:1–7. doi: 10.1053/rvsc.2000.0434. [DOI] [PubMed] [Google Scholar]
- 20.Höhlich BJ, Wiesmuller KH, Schlapp T, Haas B, Pfaff E, Saalmuller A. 2003. Identification of foot-and-mouth disease virus-specific linear B-cell epitopes to differentiate between infected and vaccinated cattle. J Virol 77:8633–8639. doi: 10.1128/JVI.77.16.8633-8639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Clavijo A, Suarez-Banmann R, Avalo R. 2007. Production and characterization of two serotype independent monoclonal antibodies against foot-and-mouth disease virus. Vet Immunol Immunopathol 115:126–134. doi: 10.1016/j.vetimm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Moniwa M, Embury-Hyatt C, Zhang Z, Hole K, Clavijo A, Copps J, Alexandersen S. 2012. Experimental foot-and-mouth disease virus infection in white tailed deer. J Comp Pathol 147:330–342. doi: 10.1016/j.jcpa.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Parida S, Fleming L, Gibson D, Hamblin PA, Grazioli S, Brocchi E, Paton DJ. 2007. Bovine serum panel for evaluating foot-and-mouth disease virus nonstructural protein antibody tests. J Vet Diagn Invest 19:539–544. doi: 10.1177/104063870701900513. [DOI] [PubMed] [Google Scholar]
- 24.Cox SJ, Voyce C, Parida S, Reid SM, Hamblin PA, Paton DJ, Barnett PV. 2005. Protection against direct-contact challenge following emergency FMD vaccination of cattle and the effect on virus excretion from the oropharynx. Vaccine 23:1106–1113. doi: 10.1016/j.vaccine.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Cox SJ, Voyce C, Parida S, Reid SM, Hamblin PA, Hutchings G, Paton DJ, Barnett PV. 2006. Effect of emergency FMD vaccine antigen payload on protection, sub-clinical infection and persistence following direct contact challenge of cattle. Vaccine 24:3184–3190. doi: 10.1016/j.vaccine.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Parida S, Cox S, Reid S, Hamblin P, Barnett P, Inoue T, Anderson J, Paton D. 2005. The application of new techniques to the improved detection of persistently infected cattle after vaccination and contact exposure to foot-and-mouth disease. Vaccine 23:5186–5195. doi: 10.1016/j.vaccine.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Parida S, Anderson J, Cox SJ, Barnett PV, Paton DJ. 2006. Secretory IgA as an indicator of oro-pharyngeal foot-and-mouth disease virus replication and as a tool for post vaccination surveillance. Vaccine 24:1107–1116. doi: 10.1016/j.vaccine.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson RH. 1998. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech 17:469–526. [DOI] [PubMed] [Google Scholar]
- 29.Perkins J, Parida S, Clavijo A. 2007. Use of a standardized bovine serum panel to evaluate a multiplexed nonstructural protein antibody assay for serological surveillance of foot-and-mouth disease. Clin Vaccine Immunol 14:1472–1482. doi: 10.1128/CVI.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexandersen S, Zhang Z, Donaldson AI. 2002. Aspects of the persistence of foot-and-mouth disease virus in animals—the carrier problem. Microb Infect 4:1099–1110. doi: 10.1016/S1286-4579(02)01634-9. [DOI] [PubMed] [Google Scholar]
- 31.Sharma GK, Mohapatra JK, Pandey LK, Mahajan S, Mathapati BS, Sanyal A, Pattnaik B. 2012. Immunodiagnosis of foot-and-mouth disease using mutated recombinant 3ABC polyprotein in a competitive ELISA. J Virol Methods 185:52–60. doi: 10.1016/j.jviromet.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Uttenthal Å, Parida S, Rasmussen TB, Paton DJ, Haas B, Dundon WG. 2010. Strategies for differentiating infection in vaccinated animals (DIVA) for foot-and-mouth disease, classical swine fever and avian influenza. Expert Rev Vaccines 9:73–87. doi: 10.1586/erv.09.130. [DOI] [PubMed] [Google Scholar]
- 33.McCullough KC, Crowther JR, Butcher RN. 1985. A liquid-phase ELISA and its use in the identification of epitopes on foot-and-mouth disease virus antigens. J Virol Methods 11:329–338. doi: 10.1016/0166-0934(85)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Van Regenmortel M. 2001. Antigenicity and immunogenicity of synthetic peptides. Biologicals 29:209–213. doi: 10.1006/biol.2001.0308. [DOI] [PubMed] [Google Scholar]
- 35.Yang M, Clavijo A, Li M, Hole K, Holland H, Wang H, Deng MY. 2007. Identification of a major antibody binding epitope in the non-structural protein 3D of foot-and-mouth disease virus in cattle and the development of a monoclonal antibody with diagnostic applications. J Immunol Methods 321:174–181. doi: 10.1016/j.jim.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Kitching RP. 2002. Identification of foot and mouth disease virus carrier and subclinically infected animals and differentiation from vaccinated animals. Rev Sci Tech 21:531–535. [DOI] [PubMed] [Google Scholar]
- 37.Brocchi E, Bergmann I, Dekker A, Paton D, Sammin D, Greiner M, Grazioli S, De Simone F, Yadin H, Haas B. 2006. Comparative evaluation of six ELISAs for the detection of antibodies to the non-structural proteins of foot-and-mouth disease virus. Vaccine 24:6966–6979. doi: 10.1016/j.vaccine.2006.04.050. [DOI] [PubMed] [Google Scholar]
- 38.Kittelberger R, Mackereth G, Sewell M, Keall J, Clough R, Pigott C, O'Keefe J. 2008. Specificity of non-structural protein enzyme-linked immunosorbent assays for the detection of serum antibodies against foot-and-mouth disease virus in a target population in New Zealand. N Z Vet J 56:227–232. doi: 10.1080/00480169.2008.36838. [DOI] [PubMed] [Google Scholar]
- 39.Paton DJ, de Clercq K, Greiner M, Dekker A, Brocchi E, Bergmann I, Sammin DJ, Gubbins S, Parida S. 2006. Application of non-structural protein antibody tests in substantiating freedom from foot-and-mouth disease virus infection after emergency vaccination of cattle. Vaccine 24:6503–6512. doi: 10.1016/j.vaccine.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Parida S, Fleming L, Oh Y, Mahapatra M, Hamblin P, Gloster J, Paton DJ. 2008. Emergency vaccination of sheep against foot-and-mouth disease: significance and detection of subsequent sub-clinical infection. Vaccine 26:3469–3479. doi: 10.1016/j.vaccine.2008.04.026. [DOI] [PubMed] [Google Scholar]