Abstract
Over the past 3 billion years, an endogenous circadian rhythmicity has developed in almost all life forms in which daily oscillations in physiology occur. This allows for anticipation of sunrise and sunset. This physiological rhythmicity is kept at precisely 24 h by the daily cycle of sunlight and dark. However, since the introduction of electric lighting, there has been inadequate light during the day inside buildings for a robust resetting of the human endogenous circadian rhythmicity, and too much light at night for a true dark to be detected; this results in circadian disruption and alters sleep/wake cycle, core body temperature, hormone regulation and release, and patterns of gene expression throughout the body. The question is the extent to which circadian disruption compromises human health, and can account for a portion of the modern pandemics of breast and prostate cancers, obesity, diabetes and depression. As societies modernize (i.e. electrify) these conditions increase in prevalence. There are a number of promising leads on putative mechanisms, and epidemiological findings supporting an aetiologic role for electric lighting in disease causation. These include melatonin suppression, circadian gene expression, and connection of circadian rhythmicity to metabolism in part affected by haem iron intake and distribution.
Keywords: circadian disruption, breast cancer, circadian genes, light at night, iron
1. Primordial source
The Sun, our primordial source, provides bright light during the day, and virtually no light at night. For several billion years, the solar signal has moulded an endogenous circadian rhythmicity in almost all life forms; for mammals this includes sleep–wake, core body temperature, metabolism and oscillations in gene expression and hormone production throughout the body. This endogenous rhythmicity has allowed for a physiological anticipation of the onset of day and the onset of night, a distinct competitive advantage in a dangerous world. From time immemorial, these circadian rhythms have been reset each day to precisely 24 h by exposure to the Sun.
Electric light, by contrast, is dim and ill-timed, disrupting all aspects of our endogenous circadian rhythmicity; its intensity and spectral content are often not adequate during the day for proper circadian resetting, and are too much during the night for a true ‘dark’ to be detected [1]. This can lead to ‘circadian disruption’ compromising general well-being and perhaps increasing risk of a variety of specific diseases [2–5].
The first proposal for an association of electric lighting at night and a disease was for breast cancer [6]. In the beginning, this was based, perhaps simplistically, on a nocturnal light-induced suppression of melatonin, and the observed oncostatic action of melatonin on human breast cancer cells in vitro [7] and in rodent models of breast cancer [8,9]. As the mysteries of the circadian system are being revealed, it is evident that circadian disruption from electric lighting might also play a role in some other major maladies including obesity, diabetes, and depression and affective disorders [2,10,11], all diseases that are on the increase in the industrialized societies of the world, as well as growing problems in the developing world.
2. Diseases of modern life
There is now an abundance of experimental evidence in humans that electric light during the night and altered sleep can disrupt circadian rhythmicity in hormones, circadian gene expression, markers of metabolism and many other physiological parameters (e.g. [12–19]).
It must be noted that the studies of ‘sleep deprivation’ do not isolate disrupted sleep from exposure to light at night; in fact, light during the night, even at very low levels, may help cause disrupted sleep. For example, the study conducted by Ackerman et al. [15] used a protocol in which 12 young males were examined over a 48 h period in a sleep laboratory. For nights 1 and 2 (N2) there was an 8-h period of total dark, whereas for night 3 (N3) light level was less than 5 lx throughout the night; the subjects were instructed to remain awake during this entire night as they lay recumbent in bed. The authors found significant differences between N3 and N2 in BMAL1 (now known as ARNTL) expression (lowered after N3) and heat shock protein (HSPA1B) expression (elevated after N3). For N3, dim-light melatonin onset (DLMO) was the same as N2, whereas melatonin acrophase was delayed in N3 but total production was increased compared with N2. They interpret their findings as due to sleep deprivation as opposed to light at night. This study was very carefully conducted by accomplished researchers. However, why did the authors not use total dark for N3? Probably because the subjects could not stay awake in total dark; so, electric light may enable sleep deprivation, even if too low to directly suppress melatonin production. In this sense, their results are also due to ‘light-at-night’. In addition, the study of Moller-Levet et al. [16] does not separate effects of light exposure from effects of short sleep at all, as described in Stevens et al. [5].
Disentangling sleep disruption from light-induced circadian disruption experimentally is difficult if not impossible. Although the differences are scientifically interesting, from the health perspective it may be moot: electric light at night, even at low levels, may lead to circadian disruption directly and/or sleep disruption indirectly, either of which may result in adverse health consequences for human beings.
The physiological effects of light at night and sleep disruption have been ‘proven’ in the sense that there is general acceptance in the scientific community of its truth; i.e. a consensus of experts. What has not been ‘proven’ is that electric light-at-night causally increases risk of cancer, or obesity, or diabetes, or depression. These connections are each plausible because many of the established physiological effects of circadian disruption and/or disturbed sleep due to light at night are also implicated in disease pathogenesis; for example, cell-cycle regulation [20] and DNA damage response [21] for cancer, altered leptin and ghrelin for obesity [22], and loss of glycaemic control for diabetes [23]. However, the direct evidence is circumstantial (i.e. observational epidemiology), as it must be because experiments in humans (randomized clinical trials) are unethical for any agent suspected of causing harm.
So what does this circumstantial evidence look like?
The first prediction based on the light-at-night theory for breast cancer causation was that women who work non-day shifts would be at higher risk [5]; this was originally based on a suppression of melatonin leading to a rise in circulating oestradiol, a known risk factor for breast cancer [24]. Because almost all people in modern societies are exposed to electric light during at least part of the night, day working women would not be unexposed making detection of a real effect of light-at-night difficult. Nonetheless, it was reasoned that women who worked at night would have even more exposure [25]. Results have been mixed but on balance support an association of night work with increased risk of breast cancer [26]. There have been only a few studies of physiological differences between day working women and shift working women that might explain a higher risk. In the most recent, Bracci et al. [27] found striking differences in expression of circadian genes in lymphocytes (see §4), and significantly higher circulating oestradiol in shift working women. They compared rotating shift nurses to day working nurses, and obtained a blood and urine sample in the morning before a day shift that was after a day off from work for both groups.
Can observational epidemiology lead to proof of causation? The answer is yes, depending on the definitions of the words ‘cause’ and ‘proof’. It has been proven that cigarette smoking causes lung cancer based solely on observational epidemiology; ‘proof’ meaning a consensus of experts and ‘causes' meaning causally increases risk. The International Agency for Research on Cancer (IARC) has formalized this process admirably in its monograph series of workshops to assess specific agents suspected of causally increasing risk of cancer. The IARC ‘Preamble’ provides a detailed guide for the structure of this activity [28]. There are five levels of confidence in the evidence which a panel of invited experts ponders: 1, ‘human carcinogen’; 2a, ‘probable carcinogen’; 2b, ‘possible carcinogen’; 3, ‘inadequate evidence’; 4, ‘probably not a carcinogen’. Meetings are held in Lyon, include a couple of dozen experts, and last 10 days.
Evidence includes human cancer studies (observational epidemiology), animal cancer studies (toxicology) and mechanistic data (biomolecular effects of the agent that might lead to cancer). If the epidemiology, in the opinion of the expert panel, provides ‘sufficient’ evidence of association that cannot otherwise be explained by confounding or bias, then the agent is classified as 1: ‘human carcinogen’ regardless of whether or not there is an animal model or an accepted biological mechanism. In other words, when the epidemiological studies accumulate to the point where a reasonable expert concludes that there is no other viable explanation for the results, then the only remaining explanation is that the agent causes cancer. If the epidemiology is not considered to be at that point, then the results of animal cancer studies play an important role in the classification. And finally, basic mechanistic studies may augment confidence in a classification among the expert panel.
Agents assessed since 1975 and classified as 1 ‘human carcinogen’ include smoking, ionizing radiation, hepatitis B virus, benzene, polychlorinated biphenyls and 98 others. Class 2a is just short of class 1 in weight of evidence, and much stronger than the 2b classification. In 2007, the IARC classified ‘shiftwork that involves circadian disruption’ as a class 2a [29] so it joined anabolic steroids, vinyl fluoride, nitrogen mustard and 62 other agents. The shift work classification was based on a compelling animal model, strong mechanistic data and ‘limited’ epidemiological studies: the epidemiology was consistent with a causal relationship, but bias or confounding could not be entirely ruled out as possible explanations for the results.
Other predictions have also been made based on the ‘light-at-night’ theory with varying degrees of support [30]: blind women would be at lower risk of breast cancer [31], and reported sleep duration would be inversely associated with risk [32]. Finally, on the population level, if light at night accounts for any sizeable proportion of the breast cancer burden in society, then ambient light level as measured by satellite at night should be correlated with breast cancer risk across communities [33]. This has been tested and confirmed within Israel [34], and among 164 countries of the world [35].
Recently, epidemiological evidence has been published on the association of ambient bedroom light level at night during the sleep period (either measured or self-reported) and risk of obesity [36,37], and of depression [38]. Of course, it is unclear how accurate a self-report might be, but these findings are intriguing and analogous to the few case–control studies to examine risk of breast cancer [39,40] in which women were asked to rank the typical light levels in their bedrooms at night from ‘totally dark’ to ‘can see end of bed’ to ‘can read a newspaper’. If these reported associations are causal, then there would be obvious and easy interventions such as to use black-out shades and elimination of all light sources in the bedroom no matter how minute; if night lights are needed, a dim red light would be the least disruptive to the circadian system.
3. Circadian light detection
The mechanism by which the circadian system perceives light is one of the more interesting discoveries in modern biology. Only in 1998 with the discovery of melanopsin [41] did the details begin to become elucidated. Then in 2002, the first new photoreceptive cell in the retina to be discovered in 150 years was described [42–44]; these cells are called ipRCGs (intrinsically photoreceptive retinal ganglion cells), contain melanopsin, and are maximally sensitive to light of wavelength about 480 nm, which is not the peak sensitivity of the visual photoreceptors. Why would the photoreceptor for the circadian system be tuned to 480 nm? Although skylight through most of the day contains high irradiance at all wavelengths, the maximum is at 480 nm and is perceived by human vision as that beautiful blue on a clear day at mid-morning. Perhaps this is the best wavelength to signal to an organism that it is day as opposed to night, and thus melanopsin evolved, teleologically speaking, for that purpose. The visual system with rods and cones has a very different duty than the ipRCGs; vision is about image formation and navigating the environment, whereas ipRCGs must help keep the physiology of the organism on a strict 24 h schedule in synchrony with the Sun.
Most people in the industrialized world must use electric light for work and domestic life. As a result, the amount of electrical illumination of the human environment has grown dramatically in the past 50 years [45], with large geographical variation [46]. The question is how to use that light to maximum benefit. No use of electric light may result in the most robust and synchronized circadian rhythmicity, but obviously ignores the requirements of a modern life. At the other end, constant bright light would be tantamount to torture. Ideally, use of light should optimize work, home life and entertainment, yet minimally influence circadian physiology.
In a unique approach to demonstrating the impact of electric light on circadian rhythmicity in people in the modern world, Wright et al. [47] recruited eight participants (two female; average age 30) who lived and worked in and around Boulder, Colorado. Each subject completed a two-week study in July in which they were first assessed for circadian markers in their work and home environments, and then spent a week camping in the mountains with no use of electric lights at all. Among the eight subjects were a range of chronotypes (lark/owl; morning types/evening types) with a wide range of habitual bedtimes. Using a salivary measure, the mean melatonin onset was about 2 h after sunset in the baseline, modern life, condition with a high inter-person variability; melatonin offset was a little more than 2 h after sunrise. Melatonin onset and offset are regarded as the beginning and end of biological night for humans. Sleep in the baseline condition began around midnight and ended before the melatonin offset. After a week camping, among these same subjects, melatonin onset was very close to sunset and melatonin offset very close to sunrise, and there was far less variability among the subjects; the larks and owls were both aligned closely to the duration of natural daylight. Start of sleep also shifted to an earlier time and there was much less variability among the subjects. Interestingly, the duration of light exposure under baseline was greater than while camping, as would be expected, but total light exposure was far greater while camping. Wright et al. [47] conclude: ‘Increased exposure to sunlight may help to reduce the physiological, cognitive and health consequences of circadian disruption’. They also point out: ‘Natural sunlight is a stronger environmental zeitgeber or time cue for the internal circadian clock than is electrical lighting in the constructed environment’. In other words, people in the modern world not only get light during the night, they get far less light during the day inside electrically lit buildings. This can lead to circadian confusion and de-synchronization of the rhythms throughout the body.
There are a number of aspects of the impact of light-at-night on human circadian rhythmicity that have been elucidated: (i) blue light is most effective, red the least [12]; (ii) there is a dose–response [48,49]; (iii) light exposure during the day influences night-time sensitivity [50]; (iv) there are differences among individuals in sensitivity [51,52]; and (v) even through closed eyelids, a very bright light can suppress melatonin [53]. These are important findings for continued fruitful study of the health effects of exposure to light-at-night, and thereby what intervention and mitigation strategies might be most effective in the future.
Lucas et al. [54] address the question of optimal lighting for the modern life and circadian health. They describe the physiology of the non-visual light detection pathway, the fact that it is most sensitive to wavelength 480 nm, and they advocate an additional light exposure metric based on this biology. The standard illumination unit is ‘photopic lux’ or usually shortened to ‘lux’. Meters to measure illumination, used by photographers for example, are calibrated to weight the wavelength of the incident light to yield a single number reflecting the best colour (or daytime) vision in human beings, the peak wavelength for which is 555 nm. However, the peak circadian effective wavelength is, as mentioned above, 480 nm, so that the standard lux meter may not give a good indication of exposure to ‘circadian effective’ light. Lucas et al. [54] point out that circadian effective light at night disrupts circadian rhythmicity, yet is also optimal for an alerting response, important for many work-related requirements of the job. This presents a conundrum for lighting the built environment at night for work: bright, short wavelength light optimizes performance, but compromises circadian rhythmicity. For leisure, however, the message is to keep light as dim as comfortable, and shifted toward longer wavelengths.
4. Molecular epidemiology: circadian genes and disease
If circadian disruption compromises health, then changes in circadian gene function should have an impact [55,56]. There is a large and growing literature on the effects of circadian gene knockouts (KO) in mice, and polymorphisms in humans, on disease risk [57–63]. These studies suggest the possibility that absent or altered function of circadian genes may increase the risk of some diseases in people. From this line of evidence comes the obvious question of whether epigenetic mechanisms from environmental exposures, such as light-at-night, could also alter function of ‘normal’ or wild-type circadian genes in such a way as also to increase disease risk and/or severity.
Emerging areas of interest for circadian and cancer researchers are the roles of the core circadian genes in maintaining proper gene expression profiles, with a particular focus on their influence over cancer-related transcripts. So far, there have been ten core circadian genes identified in humans: CLOCK [64], casein kinase I, epsilon (CSNK1E) [65], cryptochrome 1 (CRY1), cryptochrome 2 (CRY2) [66], period 1 (PER1), period 2 (PER2), period 3 (PER3) [67,68], neuronal PAS domain protein 2 (NPAS2) [69], TIMELESS [70] and aryl hydrocarbon receptor nuclear translocator-like (ARNTL) (also referred to as brain and muscle Arnt-like protein-1 (BMAL1)) [71,72]. It has been estimated that 2–10% of all mammalian genes are clock-controlled, indicating extensive circadian gene regulation [73]. Biological clocks controlled by circadian genes provide organisms with a survival advantage by organizing their behaviour and physiology around predictable cyclic changes in the environment. These core circadian genes have also been shown to play critical roles in many cancer-related biological pathways including cell-cycle regulation, DNA repair and apoptosis [74,75].
Some pioneering work in the field of molecular cancer epidemiology has demonstrated that genetic variants in the circadian genes may be biomarkers associated with breast cancer risk. In the first molecular epidemiologic study of a circadian gene and risk of human cancer, a structural variant in the circadian gene PER3 was detected to be significantly associated with increased risk of breast cancer [76]. This clock–cancer connection was confirmed in later studies, which showed genetic associations between the circadian genes NPAS2 [77], CRY2 [78] and CLOCK [79] and breast cancer risk.
Epigenetic changes may be equally important as genetic mutations for the multi-step process of cancer development and progression. Epigenetic changes, including DNA methylation, have recently been identified as important contributors to gene regulation, perhaps accounting for even more of the variability in gene expression than is attributable to structural genetic variations. The epigenetic association analyses of circadian genes and breast cancer further found significantly hypomethylated promoter of CLOCK [79] and hypermethylated promoter of CRY2 [78] in breast cancer cases compared with controls. These detected epigenetic changes are consistent with their expression patterns in breast tumour tissues compared to adjacent normal tissues: high expression level of CLOCK as a putative ‘oncogene’ and low expression level of CRY2 as a possible ‘tumour suppressor’. These findings, together with the previously identified clock–cancer connection in breast cancer, warrant a more comprehensive investigation of methylation status, especially in the promoters of circadian genes, and an analysis of the impact of these epigenetic changes on breast cancer risk in a larger population.
A variety of environmental agents, ranging from brassica vegetables to nitrous oxide, might conceivably modify DNA methylation in humans. However, relatively little is known about the effects of circadian disruption caused by shiftwork on the epigenomic architecture.
The first genome-wide methylation analysis investigating epigenetic impact of circadian disruption revealed that the methylation patterns of more than 5000 CpG sites (a cytosine separated by only one phosphate from a guanine in the DNA sequence) were altered in long-term shift workers [80]. Interestingly, nearly twice as many CpG sites were hypermethylated in shift workers than were hypomethylated. These data are consistent with the general consensus that most tumours undergo widespread loss of methylation at the global level, but exhibit hypermethylation at CpG-rich, gene-associated regions such as those included on the array chip [81]. Notably, many of the differentially methylated CpG sites were located near the promoter sequences of methylation related and cancer-relevant genes. Further analysis also found that 50 CpG loci corresponding to 31 miRNAs were differentially methylated in night shift workers compared with day workers, including the circadian-relevant miR-219, the expression of which has been implicated in several cancers [82]. MicroRNAs (miRNAs) are a class of endogenous small non-coding RNAs that negatively regulate gene expression by inducing degradation or translational inhibition of target mRNAs. miRNAs are involved in the control of many cellular processes altered in cancer, including proliferation, differentiation and apoptosis [83].
These findings support the hypothesis that long-term exposure to shift work can alter epigenetic patterns. This hypothesis was further tested on two cancer-relevant circadian genes, CLOCK and CRY2, as previous findings had demonstrated that breast cancer patients tended to have low levels of CLOCK promoter methylation [79] and high levels of CRY2 promoter methylation [78]. Significant methylation changes in these genes were noted in this study of shift workers [80], and for both genes, the direction of the changes in shift workers was identical to that observed in breast cancer patients. This finding has been supported and extended by Bracci et al. [27] who reported higher expression (based on transcript levels in peripheral blood lymphocytes) of CLOCK and lower CRY2 among shift working women compared with day working women. Bracci et al. [27] also reported higher expression of Rev-Erb-Alpha in shift working women, a finding of potential importance to a link with metabolism (see §5).
As transcriptional regulators, circadian genes mediate the expression of many cancer-related genes and play various roles in cancer-relevant pathways such as DNA repair [84,85]. As such, the epigenetic impact of shift work on the activity and function of core circadian regulators may provide a missing link in the relationship between breast cancer and night shift work.
Among the many association studies in humans and functional studies in mice of the circadian genes, ARNTL may be one of the more interesting. ARNTL is the only circadian gene for which its knockout alone leads to arrhythmia in mice [86]; the knockout of any of the other circadian genes does not by itself obliterate circadian rhythmicity because there is redundancy in the system (e.g. three Period genes and two Cryptochrome genes). [ARNTL does have a paralog, ARNTL2, but it is under direct ARNTL control unlike other circadian gene paralog groups]. ARNTL may be a key link from the circadian system to metabolism, possibly through its control by Rev-erb-alpha and ROR (see §5). Deficiency of ARNTL in mice has been reported to accelerate overall ageing and cellular senescence in tissues, implicating ARNTL in DNA damage response and in response to oxidative stress [87].
5. Iron and haem
There has been a recent surge in interest in the interrelationships of metabolism with circadian rhythmicity [88]. For many years, growing epidemiological evidence has implicated short sleep duration and/or sleep disturbance with higher weight and with risk of type 2 diabetes [89]. The mechanisms for this have been opaque until the recent molecular biological insights into the interactions of circadian genes with genes implicated in control of metabolism. Sahar & Sassone-Corsi [90] suggested that since CLOCK is a histone acetyl transferase, its disruption might play a role in breast cancer risk by altering expression of key cell-cycle regulators known to be involved in breast cancer pathogenesis such as cyclin D1. More broadly, CLOCK and SIRT1 interact in control of metabolism [91], and this may thereby influence oxidative balance in cells and tissues which itself might reset circadian rhythmicity. Tamaru et al. [92] reported that oxidative stress at high enough levels accomplishes this circadian resetting via ARNTL transcription factor; the stressor they used was H2O2, which itself is not a free radical, but when it encounters iron, it breaks down to hydroxyl radical, a potent mediator of biomolecular damage [93,94]. Another intriguing finding is that the ligand for rev-erb-a is haem [95]. Rev-erb-a and ROR form a secondary circadian loop that interacts with the primary negative feedback loop of CLOCK/ARNTL and PERs/CRYs in which Rev-erb-a represses the expression of ARNTL, whereas ROR stimulates expression [96]. Thus, iron seems to be emerging as playing a central role in the connections among nutritional state, timing of food intake and circadian rhythmicity.
6. Colon cancer and prostate cancer
Although the most evidence to date is on circadian disruption and breast cancer, there is also a rationale for an interest in cancer of the colon. Two epidemiological studies have reported significantly elevated risk of colon cancer in shift working women [97] and men [98]. Other evidence includes an observation that short sleep duration was associated with an elevated risk of colorectal adenoma [99], and reported associations of circadian gene polymorphisms and colon cancer risk [100]. Red meat intake as a risk factor for colon cancer is one of the few consistent findings of epidemiological studies [101]. Haem iron content of red meat is a prime suspect in the aetiology of this increased risk [102].
Prostate cancer risk may also be affected by circadian disruption for reasons similar to those for breast cancer [103]. The evidence base is far smaller than for breast cancer, but there are some interesting data on circadian gene variants and risk [104], and also epidemiologic data which report a higher risk in shift workers [98].
7. The need for sleep and the need for dark
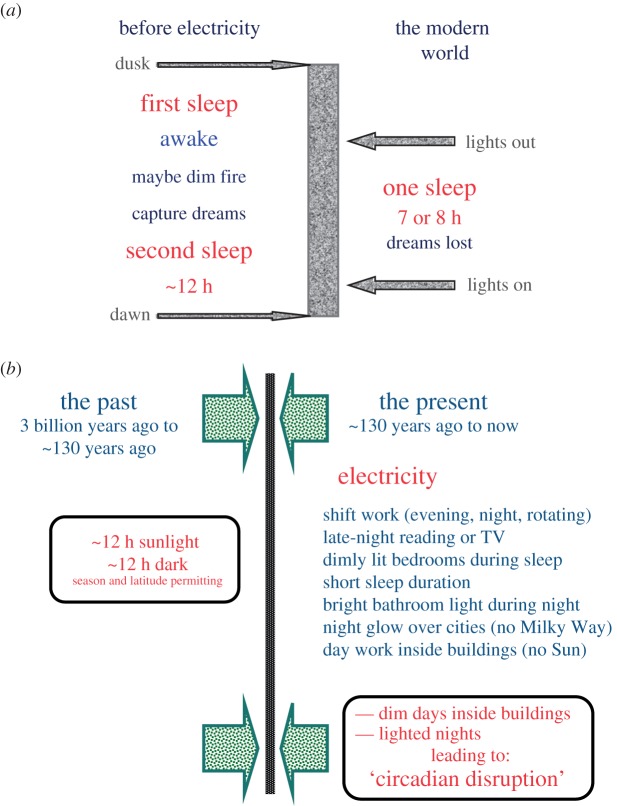
Since the introduction of electricity, there has been a change in what is considered ‘normal’ sleep (figure 1a). Historical evidence indicates that sleep before electricity was biphasic [105]; there was a ‘first sleep’ which lasted several hours, then a period of wakefulness either in the dark or with a small fire, followed by a second sleep. Ekirch [105] argues that this biphasic sleep evolved over a very long time period and has been lost in the contemporary world. The modern compacted sleep is typically confined to a 7 or 8 h period of relative dimness (few people sleep in a completely dark bedroom environment). On this basis, Ekirch also speculates that one of the losses due to our compacted sleep is that of dream consolidation, and its potential impact on psychological health. Figure 1b shows some of the myriad societal changes brought with electric lighting.
Figure 1.
(a) Before electricity, sleep occurred in the dark and was biphasic; in the modern world, sleep is compacted to a short 7 or 8 h period of dimness encapsulated by electric light. (b) Electricity has changed society in many ways. (Online version in colour.)
During the night, light from a fire is very different from that from an electric light: camp fire and candles are far dimmer than electric lighting and the wavelength is strongly skewed toward the red end of the spectrum; as a result, fire-light has much less impact on circadian rhythmicity than electric light.
As a modern recreation of sleep in times past, Wehr [106] conducted experiments in human subjects which demonstrated biphasic sleep in a laboratory setting. Subjects on an 8 h dark schedule slept for about 7.5 h (by polysomnography), and their elevated night-time melatonin production was limited to the dark phase, as expected. The same subjects on a 14 h dark period slept for an average of about 8.5 h in two episodes separated by a period of ‘quiet wakefulness' in between. The term ‘quiet wakefulness' reflects the fact that in this laboratory setting, the subjects were required to remain in bed in the dark. Melatonin production, however, showed no dip in the middle of the night and remained high for close to 12 h. Although dark is required for pineal melatonin production, sleep is not.
The point of emphasis to all this is that while sleep is deeply important to well-being, so too is exposure at night to dark. The importance of sleep has finally entered mainstream thinking and practice; however, the importance of dark is still greatly underappreciated. Without dark, sleep is difficult and compromised. Without dark, circadian rhythmicity, as reflected in nocturnal melatonin production, is disrupted. Both sleep disruption and circadian disruption have been shown to have profound effects on physiology. Absence of dark at night can lead to both, which may then have negative effects on long-term health in a vast array of maladies.
8. Conclusion
The question posed in the title, ‘Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem?’ cannot yet be answered with assurance, but is important to ask. It must be stressed that there is ample evidence for the disruptive effect of electric light on physiology in short-term experiments in humans. There is some epidemiologic evidence on the long-term impact on disease but this evidence is not yet adequate to render a verdict. It is an urgent issue given the increasing pervasiveness of electric lighting in our built environment that heretofore has been designed without any consideration of circadian health in mind.
Excessive lighting of the night sky is as important an Earth issue as climate change. The impact on life, including plant, insect and animal, is now beginning to be appreciated as large. Disruption of circadian rhythmicity has profound effects on physiology, many aspects of which are deleterious. Neonates and small children, even beginning in utero, may be at particular risk because of their rapidly developing physiology [107].
In addition to the impact on life forms, generation of light at night also contributes directly to greenhouse gas emissions from fossil fuel combustion to produce the electricity needed: almost 20% of electricity consumption worldwide is for lighting, and at least 30% of this light is wasted [108,109].
As an environmental issue, lighting of the day inside buildings impacts only humans (and maybe cockroaches), whereas lighting the night-time affects all life including humans. Just as the technology to artificially light the night exploded in use during the twentieth century, newer technology (a field called ‘photonics') is now making it possible to generate, direct and manage light at night for improved visual acuity, more efficiency and less waste, and to better accommodate circadian physiology of life forms in general, but notably of the species directly responsible for the pervasiveness of light at night, which would be us.
Authors contributions
R.G.S. and Y.Z. contributed to conceptualizing and writing of this manuscript.
Funding statement
The authors acknowledge funds from the University of Connecticut and Yale University.
Conflict of interests
The authors have no competing interests.
References
- 1.Stevens RG. 2009. Electric light causes cancer? Surely you're joking, Mr. Stevens. Mutat. Res. Rev. 682, 1–6. ( 10.1016/j.mrrev.2009.01.003) [DOI] [PubMed] [Google Scholar]
- 2.Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provecio I, Rea MS, Reinlib L. 2007. Meeting report: the role of envrionmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect. 115, 1357–1362. ( 10.1289/ehp.10200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi JS, Hong HK, Ko CH, McDearmon EL. 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775. ( 10.1038/nrg2430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. 2013. Adverse health effects of nighttime lighting: comments on American Medical Association Policy Statement. Am. J. Prevent. Med. 45, 343–346. ( 10.1016/j.amepre.2013.04.011) [DOI] [PubMed] [Google Scholar]
- 5.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. 2014. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 64, 207–218. ( 10.3322/caac.21218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens RG. 1987. Review and commentary: electric power use and breast cancer: a hypothesis. Am. J. Epidemiol. 125, 556–561. [DOI] [PubMed] [Google Scholar]
- 7.Hill SM, Blask DE. 1988. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 48, 6121–6126. [PubMed] [Google Scholar]
- 8.Shah PN, Mhatre MC, Kothari LS. 1984. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 44, 3403–3407. [PubMed] [Google Scholar]
- 9.Blask DE, et al. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 65, 11 174–11 184. ( 10.1158/0008-5472.CAN-05-1945) [DOI] [PubMed] [Google Scholar]
- 10.Wirz-Justice A. 2007. Chronobiology and psychiatry. Sleep Med. Rev. 11, 423–427. ( 10.1016/j.smrv.2007.08.003) [DOI] [PubMed] [Google Scholar]
- 11.Evans JA, Davidson AJ. 2013. Health consequences of circadian disruption in humans and animal models. Prog. Mol. Biol. Transl. Sci. 119, 283–323. ( 10.1016/B978-0-12-396971-2.00010-5) [DOI] [PubMed] [Google Scholar]
- 12.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J. Neurosci. 21, 6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockley SW, Brainard GC, Czeisler CA. 2003. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 88, 4502–4505. ( 10.1210/jc.2003-030570) [DOI] [PubMed] [Google Scholar]
- 14.Wirz-Justice A, Kräuchi K, Cajochen C, Danilenko KV, Renz C, Weber JM. 2004. Evening melatonin and bright light administration induce additive phase shifts in dim light melatonin onset. J. Pineal Res. 36, 192–194. ( 10.1111/j.1600-079X.2004.00117.x) [DOI] [PubMed] [Google Scholar]
- 15.Ackermann K, Plomp R, Lao O, Middleton B, Revell VL, Skene DJ, Kayser M. 2013. Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiol. Int. 30, 901–909. ( 10.3109/07420528.2013.784773) [DOI] [PubMed] [Google Scholar]
- 16.Möller-Levet CS, et al. 2013. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc. Natl Acad. Sci. USA 110, E1132–E1141. ( 10.1073/pnas.1217154110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer SN, et al. 2014. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl Acad. Sci. USA 111, E682–E691. ( 10.1073/pnas.1316335111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leproult R, Holmbäck U, Van Cauter E. 2014. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869. ( 10.2337/db13-1546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyon A, Balbo M, Morselli LL, Tasali E, Leproult R, L'Hermite-Balériaux M, Van Cauter E, Spiegel K. 2014. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J. Clin. Endocrinol. Metab. 13, jc20134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt T, Sassone-Corsi P. 2007. Riding tandem: circadian clocks and the cell cycle. Cell 129, 461–464. ( 10.1016/j.cell.2007.04.015) [DOI] [PubMed] [Google Scholar]
- 21.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. 2010. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 584, 2618–2625. ( 10.1016/j.febslet.2010.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taheri S, Lin L, Austin D, Young T, Mignot E. 2004. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 1, e62 ( 10.1371/journal.pmed.0010062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel K, Tasali E, Leproult R, Van Cauter E. 2009. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat. Rev. Endocrinol. 5, 253–261. ( 10.1038/nrendo.2009.23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key TJ. 2011. Endogenous oestrogens and breast cancer risk in premenopausal and postmenopausal women. Steroids 76, 812–815. ( 10.1016/j.steroids.2011.02.029) [DOI] [PubMed] [Google Scholar]
- 25.Stevens RG, et al. 2011. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup. Environ. Med. 68, 154–162. ( 10.1136/oem.2009.053512) [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, Huang S, Chen J. 2013. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 37, 197–206. ( 10.1016/j.canep.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 27.Bracci M, et al. 2014. Rotating-shift nurses after a day off: peripheral clock gene expression, urinary melatonin, and serum 17-β-estradiol levels. Scand. J. Work Environ. Health 40, 295–304. ( 10.5271/sjweh.3414) [DOI] [PubMed] [Google Scholar]
- 28.International Agency for Research on Cancer. Preamble. See http://monographs.iarc.fr/ENG/Preamble/index.php.
- 29.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V. 2007. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 8, 1065–1066. ( 10.1016/S1470-2045(07)70373-X) [DOI] [PubMed] [Google Scholar]
- 30.Stevens RG. 2009. Light at night, circadian disruption, and breast cancer: assessment of existing evidence. Int. J. Epidemiol. 38, 963–970. ( 10.1093/ije/dyp178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn RA. 1991. Profound bilateral blindness and the incidence of breast cancer. Epidemiology 2, 208–210. ( 10.1097/00001648-199105000-00008) [DOI] [PubMed] [Google Scholar]
- 32.Verkasalo PK, Lillberg K, Stevens RG, Hublin C, Partinen M, Koskenvuo M, Kaprio J. 2005. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 65, 9595–9600. ( 10.1158/0008-5472.CAN-05-2138) [DOI] [PubMed] [Google Scholar]
- 33.Stevens RG. 2011. Testing the light-at-night (LAN) theory for breast cancer causation. Chronobiol. Int. 28, 653–656. ( 10.3109/07420528.2011.606945) [DOI] [PubMed] [Google Scholar]
- 34.Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. 2008. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol. Int. 25, 65–81. ( 10.1080/07420520801921572) [DOI] [PubMed] [Google Scholar]
- 35.Kloog I, Stevens RG, Haim A, Portnov BA. 2010. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21, 2059–2068. ( 10.1007/s10552-010-9624-4) [DOI] [PubMed] [Google Scholar]
- 36.Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. 2013. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 98, 337–344. ( 10.1210/jc.2012-2874) [DOI] [PubMed] [Google Scholar]
- 37.McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. 2014. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the breakthrough generations study. Am. J. Epidemiol. 180, 245–250. ( 10.1093/aje/kwu117) [DOI] [PubMed] [Google Scholar]
- 38.Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. 2013. Exposure to light at night and risk of depression in the elderly. J. Affect. Disord. 151, 331–336. ( 10.1016/j.jad.2013.06.018) [DOI] [PubMed] [Google Scholar]
- 39.Davis S, Mirick DK, Stevens RG. 2001. Night shift work, light at night, and risk of breast cancer. J. Natl Cancer Inst. 93, 1557–1562. ( 10.1093/jnci/93.20.1557) [DOI] [PubMed] [Google Scholar]
- 40.O'Leary ES, Schoenfeld ER, Stevens RG, Kabat GC, Henderson K, Grimson R, Gammon MD, Leske MC. 2006. Electromagnetic fields and breast cancer on Long Island study group. Shift work, light at night, and breast cancer on Long Island, New York. Am. J. Epidemiol. 164, 358–366. ( 10.1093/aje/kwj211) [DOI] [PubMed] [Google Scholar]
- 41.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. 1998. Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA 95, 340–345. ( 10.1073/pnas.95.1.340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. ( 10.1126/science.1067262) [DOI] [PubMed] [Google Scholar]
- 43.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070. ( 10.1126/science.1069609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt TM, Chen SK, Hattar S. 2011. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34, 572–580. ( 10.1016/j.tins.2011.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cinzano P, Falchi F. 2014. Quantifying light pollution. J. Quant. Spectrosc. Radiat. Transf. 139, 13–20. ( 10.1016/j.jqsrt.2013.11.020) [DOI] [Google Scholar]
- 46.Hölker F, et al. 2010. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc. 15, 13. [Google Scholar]
- 47.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. 2013. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23, 1554–1558. ( 10.1016/j.cub.2013.06.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brainard GC, Lewy AJ, Menaker M, Fredrickson RH, Miller LS, Weleber RG, Cassone V, Hudson D. 1988. Dose-response relationship between light irradiance and the suppression of melatonin in human volunteers. Brain Res. 454, 212–218. ( 10.1016/0006-8993(88)90820-7) [DOI] [PubMed] [Google Scholar]
- 49.West KE, et al. 2011. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J. Appl. Physiol. 110, 619–626. ( 10.1152/japplphysiol.01413.2009) [DOI] [PubMed] [Google Scholar]
- 50.Hébert M, Martin SK, Lee C, Eastman CI. 2002. The effects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res. 33, 198–203. ( 10.1034/j.1600-079X.2002.01885.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monteleone P, Esposito G, La Rocca A, Maj M. 1995. Does bright light suppress nocturnal melatonin secretion more in women than men? J. Neural Transm. 102, 75–80. ( 10.1007/BF01276567) [DOI] [PubMed] [Google Scholar]
- 52.Chellappa SL, et al. 2012. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J. Clin. Endocrinol. Metab. 97, E433–E437. ( 10.1210/jc.2011-2391) [DOI] [PubMed] [Google Scholar]
- 53.Figueiro MG, Bierman A, Rea MS. 2013. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat. Sci. Sleep 5, 133–141. ( 10.2147/NSS.S52203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucas RJ, et al. 2014. Measuring and using light in the melanopsin age. Trends Neurosci. 37, 1–9. ( 10.1016/j.tins.2013.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens RG, Rea MS. 2001. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control 12, 279–287. ( 10.1023/A:1011237000609) [DOI] [PubMed] [Google Scholar]
- 56.Stevens RG. 2005. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology 16, 254–258. ( 10.1097/01.ede.0000152525.21924.54) [DOI] [PubMed] [Google Scholar]
- 57.Kettner NM, Katchy CA, Fu L. 2014. Circadian gene variants in cancer. Ann. Med. 46, 208–220. ( 10.3109/07853890.2014.914808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fonken LK, Nelson RJ. 2014. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 35, 648–670. ( 10.1210/er.2013-1051) [DOI] [PubMed] [Google Scholar]
- 59.Garaulet M, Smith CE, Gomez-Abellán P, Ordovás-Montañés M, Lee YC, Parnell LD, Arnett DK, Ordovás JM. 2014. REV-ERB-ALPHA circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol. Nutr. Food Res. 58, 821–829. ( 10.1002/mnfr.201300361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dashti HS, Smith CE, Lee YC, Parnell LD, Lai CQ, Arnett DK, Ordovás JM, Garaulet M. 2014. CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol. Int. 31, 660–667. ( 10.3109/07420528.2014.886587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hua P, et al. 2014. Cry1 and Tef gene polymorphisms are associated with major depressive disorder in the Chinese population. J. Affect. Disord. 157, 100–103. ( 10.1016/j.jad.2013.11.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reutrakul S, Van Cauter E. 2014. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann. NY Acad. Sci. 1311, 151–173. ( 10.1111/nyas.12355) [DOI] [PubMed] [Google Scholar]
- 63.Kovanen L, Kaunisto M, Donner K, Saarikoski ST, Partonen T. 2013. CRY2 genetic variants associate with dysthymia. PLoS ONE 8, e71450 ( 10.1371/journal.pone.0071450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King DP, et al. 1997. Positional cloning of the mouse circadian clock gene. Cell 89, 641–653. ( 10.1016/S0092-8674(00)80245-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki T, Ishikawa A, Yoshimura T, Namikawa T, Abe H, Honma S, Honma K, Ebihara S. 2001. Quantitative trait locus analysis of abnormal circadian period in CS mice. Mamm. Genome 12, 272–277. ( 10.1007/s003350010280) [DOI] [PubMed] [Google Scholar]
- 66.Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang RP, Todo T, Wei YF, Sancar A. 1996. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry 35, 13 871–13 877. ( 10.1021/bi962209o) [DOI] [PubMed] [Google Scholar]
- 67.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. 1997. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269. ( 10.1016/S0896-6273(00)80417-1) [DOI] [PubMed] [Google Scholar]
- 68.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. 1997. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389, 512–516. ( 10.1038/39086) [DOI] [PubMed] [Google Scholar]
- 69.Reick M, Garcia JA, Dudley C, McKnight SL. 2001. NPAS2: an analog of clock operative in the mammalian forebrain. Science 293, 506–509. ( 10.1126/science.1060699) [DOI] [PubMed] [Google Scholar]
- 70.Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A. 2005. Coupling of human circadian and cell cycles by the Timeless protein. Mol. Cell Biol. 25, 3109–3116. ( 10.1128/MCB.25.8.3109-3116.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cermakian N, Whitmore D, Foulkes NS, Sassone-Corsi P. 2000. Asynchronous oscillations of two zebrafish CLOCK partners reveal differential clock control and function. Proc. Natl Acad. Sci. USA 97, 4339–4344. ( 10.1073/pnas.97.8.4339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu W, Ikeda M, Abe H, Honma S, Ebisawa T, Yamauchi T, Honma K, Nomura M. 1999. Characterization of three splice variants and genomic organization of the mouse BMAL1 gene. Biochem. Biophys. Res. Commun. 260, 760–767. ( 10.1006/bbrc.1999.0970) [DOI] [PubMed] [Google Scholar]
- 73.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. 2002. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83. ( 10.1038/nature744) [DOI] [PubMed] [Google Scholar]
- 74.Fu L, Lee CC. 2003. The circadian clock: pacemaker and tumour suppressor. Nat. Rev. Cancer 3, 350–361. ( 10.1038/nrc1072) [DOI] [PubMed] [Google Scholar]
- 75.Sahar S, Sassone-Corsi P. 2009. Metabolism and cancer: the circadian clock connection. Nat. Rev. Cancer 9, 886–896. ( 10.1038/nrc2747) [DOI] [PubMed] [Google Scholar]
- 76.Zhu Y, Brown HN, Zhang Y, Stevens RG, Zheng T. 2005. Period3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol. Biomark. Prev. 14, 268–270. [PubMed] [Google Scholar]
- 77.Zhu Y, Stevens RG, Leaderer D, Hoffman A, Holford T, Zhang Y, Brown HN, Zheng T. 2008. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res. Treat. 107, 421–425. ( 10.1007/s10549-007-9565-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffman AE, et al. 2010. The core circadian gene Cryptochrome 2 influences breast cancer risk, possibly by mediating hormone signaling. Cancer Prev. Res. 3, 539–548. ( 10.1158/1940-6207.CAPR-09-0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffman AE, et al. 2010. CLOCK in breast tumorigenesis: genetic, epigenetic, and transcriptional profiling analyses. Cancer Res. 70, 1459–1468. ( 10.1158/0008-5472.CAN-09-3798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y, Stevens RG, Hoffman AE, Tjonneland A, Vogel UB, Zheng T, Hansen J. 2011. Epigenetic impact of long-term shiftwork: pilot evidence from circadian genes and whole-genome methylation analysis. Chronobiol. Int. 28, 852–861. ( 10.3109/07420528.2011.618896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruike Y, Imanaka Y, Sato F, Shimizu K, Tsujimoto G. 2010. Genome-wide analysis of aberrant methylation in human breast cancer cells using methyl-DNA immunoprecipitation combined with high-throughput sequencing. BMC Genomics 11, 137 ( 10.1186/1471-2164-11-137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi F, Chen X, Fu A, Hansen J, Stevens R, Tjonneland A, Vogel UB, Zheng T, Zhu Y. 2013. Aberrant DNA methylation of miR-219 promoter in long-term night shiftworkers. Environ. Mol. Mutagen 54, 406–413. ( 10.1002/em.21790) [DOI] [PubMed] [Google Scholar]
- 83.Croce CM, Calin GA. 2005. miRNAs, cancer, and stem cell division. Cell 122, 6–7. ( 10.1016/j.cell.2005.06.036) [DOI] [PubMed] [Google Scholar]
- 84.Yi CH, Zheng T, Leaderer D, Hoffman A, Zhu Y. 2009. Cancer-related transcriptional targets of the circadian gene NPAS2 identified by genome-wide ChIP-on-chip analysis. Cancer Lett. 284, 149–156. ( 10.1016/j.canlet.2009.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A. 2010. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc. Natl Acad. Sci. USA 107, 4890–4895. ( 10.1073/pnas.0915085107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. 2010. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr. Biol. 20, 316–321. ( 10.1016/j.cub.2009.12.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khapre RV, Kondratova AA, Susova O, Kondratov RV. 2011. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 10, 4162–4169. ( 10.4161/cc.10.23.18381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bellet MM, Sassone-Corsi P. 2010. Mammalian circadian clock and metabolism—the epigenetic link. J. Cell Sci. 123, 3837–3848. ( 10.1242/jcs.051649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gangwisch JE. 2009. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes. Rev. 10(Suppl. 2), 37–45. ( 10.1111/j.1467-789X.2009.00663.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sahar S, Sassone-Corsi P. 2007. Circadian clock and breast cancer: a molecular link. Cell Cycle 6, 1329–1331. ( 10.4161/cc.6.11.4295) [DOI] [PubMed] [Google Scholar]
- 91.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. 2008. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340. ( 10.1016/j.cell.2008.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamaru T, et al. 2013. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS ONE 8, e82006 ( 10.1371/journal.pone.0082006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevens RG, Kalkwarf DR. 1990. Iron, radiation, and cancer. Environ. Health Perspect. 87, 291–300. ( 10.1289/ehp.9087291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galaris D, Skiada V, Barbouti A. 2008. Redox signaling and cancer: the role of ‘labile’ iron. Cancer Lett. 266, 21–29. ( 10.1016/j.canlet.2008.02.038) [DOI] [PubMed] [Google Scholar]
- 95.Raghuram S, et al. 2007. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 14, 1207–1213. ( 10.1038/nsmb1344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kojetin DJ, Burris TP. 2014. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 13, 197–216. ( 10.1038/nrd4100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. 2003. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl Cancer Inst. 95, 825–828. ( 10.1093/jnci/95.11.825) [DOI] [PubMed] [Google Scholar]
- 98.Parent MÉ, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. 2012. Night work and the risk of cancer among men. Am. J. Epidemiol. 176, 751–759. ( 10.1093/aje/kws318) [DOI] [PubMed] [Google Scholar]
- 99.Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. 2011. Short duration of sleep increases risk of colorectal adenoma. Cancer 117, 841–847. ( 10.1002/cncr.25507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karantanos T, Theodoropoulos G, Pektasides D, Gazouli M. 2014. Clock genes: their role in colorectal cancer. World J. Gastroenterol. 20, 1986–1992. ( 10.3748/wjg.v20.i8.1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norat T, et al. 2005. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J. Natl Cancer Inst. 97, 906–916. ( 10.1093/jnci/dji164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corpet DE. 2011. Red meat and colon cancer: should we become vegetarians, or can we make meat safer? Meat Sci. 89, 310–316. ( 10.1016/j.meatsci.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 103.Zhu Y, Zheng T, Stevens RG, Zhang Y, Boyle P. 2006. Does ‘clock’ matter in prostate cancer? Cancer Epidemiol. Biomark. Prev. 15, 3–5. ( 10.1158/1055-9965.EPI-05-0631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu Y, et al. 2009. Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 69, 9315–9322. ( 10.1158/0008-5472.CAN-09-0648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ekirch AR. 2006. At day's close: night in times past. New York, NY: W. W. Norton & Company. [Google Scholar]
- 106.Wehr TA. 1992. In short photoperiods, human sleep is biphasic. J. Sleep Res. 1, 103–107. ( 10.1111/j.1365-2869.1992.tb00019.x) [DOI] [PubMed] [Google Scholar]
- 107.Stevens RG. 2012. Does electric light stimulate cancer development in children? Cancer Epidemiol. Biomark. Prev. 21, 701–704. ( 10.1158/1055-9965.EPI-12-0015) [DOI] [PubMed] [Google Scholar]
- 108.OECD/IEA (Organization for Economic Co-operation and Development)/International Energy Agency). 2006. Light's labour's lost policies for energy-efficient lighting. Paris, France: OECD/IEA. [Google Scholar]
- 109.IDA (International Dark-Sky Association). 2014. Economic issues in wasted and inefficient outdoor lighting. Information Sheet no. 26. Tucson, AZ: IDA.