Abstract
The adverse effects of excessive use of artificial light at night (ALAN) are becoming increasingly evident and associated with several health problems including cancer. Results of epidemiological studies revealed that the increase in breast cancer incidents co-distribute with ALAN worldwide. There is compiling evidence that suggests that melatonin suppression is linked to ALAN-induced cancer risks, but the specific genetic mechanism linking environmental exposure and the development of disease is not well known. Here we propose a possible genetic link between environmental exposure and tumorigenesis processes. We discuss evidence related to the relationship between epigenetic remodelling and oncogene expression. In breast cancer, enhanced global hypomethylation is expected in oncogenes, whereas in tumour suppressor genes local hypermethylation is recognized in the promoter CpG chains. A putative mechanism of action involving epigenetic modifications mediated by pineal melatonin is discussed in relation to cancer prevalence. Taking into account that ALAN-induced epigenetic modifications are reversible, early detection of cancer development is of great significance in the treatment of the disease. Therefore, new biomarkers for circadian disruption need to be developed to prevent ALAN damage.
Keywords: melatonin, epigenetic modifications, light pollution, breast cancer
1. Introduction
Light pollution has become a global concern, a fact substantiated by a recent resolution of the American Medical Association (AMA) 2 years ago asserting that light at night is a source of environmental pollution because it disrupts daily rhythms and suppresses nocturnal melatonin production by the pineal gland [1]. Artificial light at night (ALAN) exposures have been reported to be associated with serious ecological consequences and health risks including cancer [2–4]. Recently, the impact of light pollution as a new environmental risk factor and its relation to human breast and prostate cancers was discussed [5]. Breast cancer incidence has been increasing worldwide for the past few decades, being higher in developed countries than in undeveloped countries, suggesting that changes in lifestyle could account for the increase in breast cancer rates [6]. While much attention has been given to spatial variables in regard to lifestyle, less attention has been given to the impact of temporal variables on disruption of our biological clock, which is entrained by light/dark cycles. Unfortunately, in urbanized societies these reliable cycles are disappearing and losing their power of rhythmicity because of the extensive use of ALAN.
The cellular mechanisms underlying temporal organization of physiological processes and the consequence of their disruption are not fully understood; therefore, it is difficult to pinpoint the relations between health risks and disruption of temporal organization. Stevens et al. [7] raised a possible link between electrical power in breast cancer incidence and function of the pineal gland. Later, Stevens & Davis [8] raised the ‘melatonin hypothesis’ where the hormone indole is suggested to mediate the effect of ALAN exposure on breast cancer development. Melatonin production by the pineal gland occurs mainly during the dark period and ALAN exposure abrogates the nocturnal enzymatic activity responsible for the production of the hormone [9]. The exact mechanism involved is not known, but it may be that the abolished nocturnal levels of melatonin increase circulating oestrogen levels and/or cause upregulation of oestrogen receptor-α positive breast cancer and eventually cause increased breast endothelial cell proliferation [10,11]. Other potential physiological and/or molecular dysfunctions mediated by disruption of the typical nocturnal melatonin rhythm such as metabolic, endocrine and epigenetic abnormalities have also been suggested [12,13].
Accumulated evidence has demonstrated that epigenetic modifications are involved in the pathogenesis of several malignant diseases, including prostate, gastric, lung and breast cancers [14–17]. In general, epigenetic mechanisms involve activation of oncogenes and deactivation of cancer suppressor genes, in which the gene expression in both cases is affected by chromatin remodelling of its binding sites of transcriptional factors [18]. Epigenetic regulation in breast cancer is increasingly being reported in both human and animal models. In the present review, we consider the evidence for association between ALAN and breast cancer incidences mediated by the suppression of melatonin production. We also review recent advances on the molecular regulation of epigenetic modifications in breast cancer, including aberrant DNA methylation and histone acetylation of related oncogenes and cancer suppressor genes. Finally, epigenetic modifications are also discussed here as a possible genetic basis for mediating the malignant effects of ALAN exposure.
2. Artificial light at night as a risk factor for breast cancer
In the past decade, several epidemiological studies have investigated the co-distribution between breast cancer incidence and light pollution [19–22]. These studies and others find compelling evidence supporting a potential role of electric light at night on breast cancer risk. In Israel, a significant positive correlation between night illumination levels (collected in the framework of the US Air Force Defense Meteorological Satellite Program) and breast cancer incidence (National Cancer Registry, Israel) was revealed, but not with lung cancer as a negative control [23]. In another study, Kloog et al. [24] revealed that breast cancer co-distributes with light pollution worldwide, whereas the correlation analysis failed to establish any significant relation between light at night and negative controls, colorectal, larynx and liver cancers.
Typically, bedroom spectral and irradiance compositions originating from indoor or outdoor illumination (light filtering through windows) are expected to be characterized by both low wavelength frequencies and low irradiance levels, particularly during the duration of nocturnal sleep. Indoor dim light signals during nocturnal sleep time can still penetrate throughout closed eyelids and be detected via sensitive retinal photoreceptors to regulate circadian functions. Supportively, the ‘blind’ mole rat Spalax ehernbergi, can detect light of both short and long wavelengths (479 nm and 697 nm, respectively) under increasing light intensities (73–498 µW cm−2), though the eyes of the species are severely degenerate and the vestigial retina is completely concealed by a thick integument of skin and fur [25–27]. Exposure to blue wavelength light (λmax = 450 nm) from light-emitting diodes delivered through closed eyelids has also been shown to robustly suppress nocturnal melatonin levels and delay the melatonin onset phase [28,29]. Although the eyelids can filter optical signals and show an inverse relation between transmittance and wavelength frequency, light signals can still be detected on the retina and modulate circadian regulation [30].
In a longitudinal study, Verkasalo et al. [31] investigated the correlation between the degree of visual impairment (five categories ranging from moderate to total blindness) and breast cancer incidences in Finland. In their study, they cross-linked 17-year data from the Finnish Register of Visual Impairment and from the Finnish Cancer Registry to include 10 935 women with different visual impairment contributing to 56 000 person-years at risk. The analyses showed a significant inverse dose-dependent relation between the degree of visual impairment and cancer risk, whereby a 50% decrease in breast cancer incidence was estimated among totally blind women. Furthermore, the results of this study suggest a potential role for light in breast cancer prevalence mediated by suppression of melatonin production by the pineal gland.
In mammals, retinal photoreceptors serve a dual function of reacting to light stimulus for conscious sight and circadian entrainment. These functions are suggested to be supported by two distinct classes of retinal photoreceptors. Light detection for conscious sight is mediated by the conventional image-forming photoreceptors, rods and cones expressed in the outer retina. At the circadian level, the light signals are mediated by non-image-forming photoreceptors located in the inner retina and intrinsically photosensitive [32–34]. In gene-targeted studies, mice lacking the intrinsically photosensitive non-image-forming photoreceptors displayed intact visual perception and were incapable of detecting light for circadian responses, indicating that these photoreceptors may possibly be a central component in the circadian system, but not obligatory for visual perception [35]. Studies in totally blind humans demonstrated that light exposure can suppress melatonin production and phase shift its circadian rhythm [36,37]. These studies in human and non-human animals suggest a functional separation between image-forming and non-image-forming photoreceptors. Nevertheless, several studies in transgenic mice with dysfunction non-image-forming photoreceptors showed that these mice retain sufficient retinal photosensitivity to derive some responses [38–40]. Furthermore, blind individuals with low vision perception [41] and totally blind animals [42] display functional photo-entrained circadian responses such as circadian shifting, melatonin suppression and thermoregulation. Overall, these studies suggest that both the image- forming and non-image-forming photoreceptor pathways work simultaneously to provide complete circadian perception.
Generally, the literature suggests that women have longer habitual sleep time with 7–9 h in bed [43–46], higher melatonin amplitude and earlier melatonin peak levels compared with counterpart men [47]. Furthermore, tendencies for reduced sleep efficiencies and sleep difficulties of women have been also reported recently [48]. Taking into account the sleeping patterns described for women and the fact that light can certainly penetrate closed eyelids and efficiently alter circadian responses, bedroom ALAN should be of concern to women, particularly in modern societies.
In a comparative case–control study, the relations between night illumination conditions (emerging from outdoor and indoor lighting) in the sleeping habitat and breast cancer risk were assessed for 1679 Israeli women (794 breast cancer patients and 885 controls) [49]. The results revealed a significant association between breast cancer incidence and ALAN coming from inside (light and/or TV on) and outside (impinging from street lamps, billboards, sports or shopping centres) the bedroom. To the best of authors’ knowledge, this is the first study to show that not only are shift workers in a high risk group for breast cancer but also women sleeping in illuminated habitats. The effect of ALAN on breast cancer development is suggested to be mediated by the night-time suppression of the typical daily rhythm of melatonin, because the pineal hormone is produced in the dark phase of the 24 h cycle [50,51].
3. Melatonin suppression
The neuro-hormone melatonin (N-acetyl-methoxytryptamine) produced and secreted from the pineal gland under dark conditions (known as the ‘hormone of darkness’) is considered a ‘Jack of all trades’ as it is involved in many of our body functions [51,52]. Melatonin production in the pineal gland is sensitive to light and it was shown that even exposures of low intensity [53] and short duration [54] will suppress its production. Short wavelengths are very effective in suppressing melatonin production, which is wavelength dependent [55,56]. In human [57,58] and animals [59,60], pineal melatonin production is highly photosensitive to light of short wavelength (i.e. blue light). More recently, nocturnal production levels of melatonin have been shown to be inversely correlated with irradiances of narrowband blue LED light (peak λ = 469 nm; half peak bandwidth = 26 nm) and the suppression impact of this narrow spectrum was more severe than that of 4000k white florescent light at twice the energy of the former [56,61]. Interestingly, blue-shifted photosensitivity of the retinal non-visual photoreceptors is postulated to be a conserved adapted feature of all vertebrates, which initially was adapted to the local blue-rich spectral composition of oceanic environments and selected later to be a unique ocular feature of all terrestrial vertebrates [62]. ALAN pollution has become a major problem for both environment stability and human health. The association between high levels of ALAN and cancer incidence is one of the most discussed health issues in industrialized civilization [63]. The unfavourable impacts of ALAN exposure on tumorigenesis are expected to be associated with the suppression of nocturnal melatonin production [12]. One promising mechanism of action of melatonin-induced oncostatic effects is based on extensive research showing prominent epigenetic modifications in cancer cells [64]. Melatonin is suggested to be potentially effective in the treatment of breast cancer progression by modulating epigenetic markers [65].
4. Epigenetics
It was the British biologist Conrad H. Waddington [66] who defined epigenetics as the study of the way in which environment influences modification of traits (phenotypes) without a change in DNA sequence. He used this term for describing the interactions between genes and their products. In general, epigenetic modifications can be divided into two categories, DNA methylation and post-transcriptional histone modifications. In mammals, methylation of promoter CpG sites by DNA methyltransferase plays a key role in the regulation of gene expression [67]. CpG methylation inhibits gene expression by preventing transcriptional factors binding to the gene promoter, whereas DNA hypomethylation increases the transcriptional activity of the gene [68,69]. Moreover, the N-terminal of histone proteins can undergo variant structure remodelling including methylation, acetylation, phosphorylation and other reactions. The most common histone remodelling is the acetylation of a lysine residue of the N-terminal ends. This modification increases gene expression by chromatin opening which makes it more accessible for transcriptional factors [70,71]. In contrast to DNA methylation, histone modifications can either lead to chromatin opening or closing, resulting in increasing or decreasing transcriptional activity of genes. The flexible regulation of gene expression by histone remodelling depends on the specific type of modification and the exact modified residue [71,72]. These epigenetic DNA modifications play a pivotal role in regulation of physiological, pathological, homeostatic and developmental mechanisms for survival [73,74].
As epigenetic modifications can occur in the adult mammal, they offer an alternative view in regard to the molecular mechanism involved. For example, DNA methylation of CpG sites will increase the rate of mutations of methylated cytosines by an order of magnitude [75]. It was also demonstrated that when DNA methylation patterns are altered owing to an environmental stimulus, these CpG sites will be more vulnerable to mutation than non-methylated sites [76].
Endocrine disruptors are environmental chemicals that affect the function of the endocrine system by mimicking or blocking the actions of hormones, altering hormone signalling or disrupting hormone production [76]. Endocrine disruption can have profound consequences owing to the crucial role of hormones in development and health [77]. The mechanism of action mediating genetic effects of environmental factors is not clear. However, several studies suggest that epigenetics plays a definite role in regulating environmentally induced genetic responses [78,79]. DNA methylation and histone proteins have been demonstrated to mediate a wide range of pathological conditions such as diabetes [80], reproductive disorders [81], autoimmune diseases [82], neurodegenerative diseases [83] and cancer [84] to environmental factors. A recent review, which has focused on the complex interplay between environmental factors and endocrine-related disorders, concluded that epigenetic modifications act as flexible mediators between environmental changes and endocrine disorders, and these flexible mediators show great potential for explaining endocrine flexibility in regulating developmental responses of individuals over their lifespan [85]. Therefore, the elucidation of the role of epigenetics in endocrine disruptor actions and in the aetiology of disease will definitely provide an insight into diagnosis and therapy of diseases resulting from environmental exposure [86]. Furthermore, it is likely that it will also be critical to consider epigenetic modifications in cases of normal endocrinology and metabolic events.
5. Artificial light at night, melatonin, epigenetics and breast cancer nexus
Disruption of the biological rhythms by ALAN has been increasingly associated with different types of malignant tumours, particularly breast cancer. Temporal disruptions from ALAN have been suggested to increase breast cancer risk among women in response to shift work and sleep deprivation [87]. Furthermore, a dose–response meta-analysis of the malignant effect of night shift work provided clear evidence supporting a direct dose-dependent response for breast cancer with increasing years of night shift work [88]. According to several epidemiological screening studies, exposure to ALAN increases breast cancer risk by abolishing the distinctive night-time production of melatonin by the pineal gland [89]. Supportively, a high risk of breast cancer was reported in women with sleep deprivation during the sensitive night period when melatonin levels are expected to be at their highest [90]. In mice, ALAN exposure has been shown to stimulate breast and prostate cancer growth and development of metastases; these adverse effects of ALAN were lessened by exogenous melatonin treatment [91,92]. Furthermore, results of several studies demonstrate anti-oncogenic activity of melatonin in breast and prostate cancers [65,93]. The mechanism of action underlying the anti-oncogenic activity of melatonin has not been completely established, but the involvement of melatonin in regulation of epigenetic responses through DNA modifications has been suggested [65,91].
Currently, it is well recognized that epigenetics is an important aetiological factor of tumours, including breast cancer. Principally, the genome of cancer tissues presents both global hypomethylation and promoter hypermethylation of tumour suppressor genes [94]. In breast cancer, global hypomethylation is frequently linked with genomic instability and upregulation of oncogenes [95]. Hpermethylation within CpG sites promotes epigenetic chromatin closing and further results in silencing of tumour suppressor genes, cell proliferation and tumour development [96]. Finally, promoter hypermethylation of breast cancer oncogenes has been suggested to silence growth regulatory genes resulting in anomalous cell proliferation, where global hypomethylation stimulates the expression of metastatic genes required for cancer cells dissemination [97]. While these epigenetic aberrations are well documented in tumour processes, little is known about how these DNA methylation abnormalities are initiated and controlled. One proposal envisages a role for melatonin in mediating environmental epigenetic effects.
Epigenetic modifications of malignant processes by melatonin are still unclear. However, melatonin can regulate epigenetic modifications in cancer cells by both DNA methylation and histone protein remodelling. In breast cancer, melatonin is involved in downregulation of related oncogenes either by methylation of the Aromatase gene (CYP19) or deacetylation of CYP19 histones [98]. In melatonin-treated human breast cancer cell lines, the increased DNA methylation corresponded with downregulation of the oncogenes EGR3 and POU4F2/Brn-3b and upregulation of the tumour suppressor gene GPC3 [99]. Furthermore, melatonin treatment can suppresses human breast cancer cell proliferation by deacetylation of oncogenes, resulting in chromatin closing and thus inhibition of the binding of transcriptional factor required for triggering the expression of oncogenes [100].
In 4T1 breast cancer inoculated, short-day acclimated BALB/c female mice, ALAN exposure of short wavelength illumination (450 lux and 469 nm) resulted in significant global DNA hypomethylation and this epigenetic effect was inhibited by exogenous melatonin during the night time when light interference was applied [91]. Therefore, it is of prime importance to evaluate whether exogenous melatonin can counteract blue ALAN-induced aberrant DNA methylation and moderate epigenetic cancer development. Taken together, the association between epigenetic effects of melatonin, tumorigenesis and the suppressive action of ALAN on nocturnal melatonin production by the pineal gland, support the nexus between ALAN exposure, melatonin suppression and breast cancer progression. The connection between ALAN and cancer-related epigenetic pathways, particularly global DNA methylation, is presented in figure 1. Accordingly, the light signals generated in retinal non-image-forming photoreceptors that are intrinsically photosensitive [32] regulate melatonin production by the pineal gland which in turn mediates the anti-cancer effect of aberrant epigenetic modifications such as DNA methylation.
Figure 1.
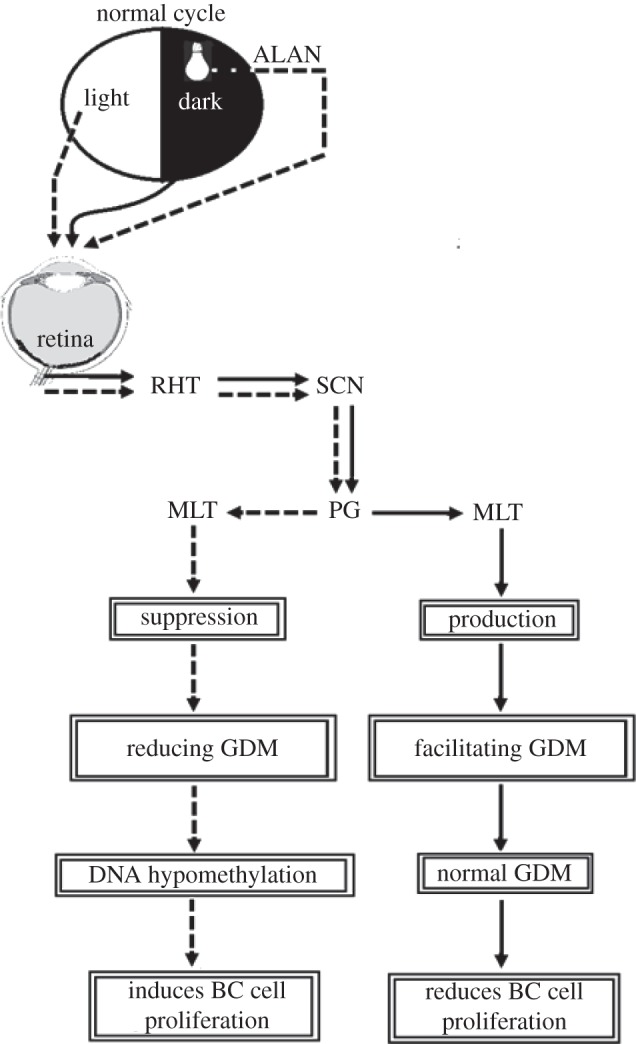
Link between environmental illumination conditions and epigenetic-induced breast cancer (BC). Light signals are the most proximate cues affecting the master circadian clock in the suprachiasmatic nucleus (SCN). Light signals are conveyed from the retina to the SCN through the retino-hypothalamic tract (RHT) and thereafter to the pineal gland (PG). Melatonin (MLT) synthesis takes place in the PG stimulated by darkness during the dark phase and inhibited during both the normal light phase and artificial light at night (ALAN) mainly of short wavelength. MLT regulates epigenetic cancer-related pathways such as global DNA methylation (GDM), in this case preventing the loss of methyl groups.
Although, several studies have been conducted in human and non-human animals regarding the ecological and physiological impacts of ALAN, the threshold characteristics of ALAN for triggering malignant diseases such as breast cancer remain to be defined. To assess clinical relevance, initial in vivo studies in animal models and in vitro human cell lines are warranted to characterize spectral, irradiance and duration thresholds of ALAN from different illumination sources, particularly new lighting technologies such as the blue light-emitting diodes. In these studies, melatonin suppression and epigenetic responses, particularly global and local DNA methylation of oncogenes and cancer suppressor genes, can be used as biomarkers for constructing the threshold exposure to ALAN. In the area of epidemiology, studies should be carried out to examine the relation between ALAN and cancer incidences more fully, in which bedroom light characteristics and habitual sleeping with ALAN should be the main focus. In the area of early detection and treatment, studies should be conducted to explore the role of melatonin and epigenetic modifications as biomarkers for early detection, prevention and treatment of ALAN related cancers. Finally, the combined results from these studies are expected to increase awareness, knowledge and behavioural changes towards developing new outdoor and indoor light pollution standards that would comprise power efficiency, energy saving and, above all, less risk to human health than the current standards.
6. Conclusion
Light pollution is an increasing problem compromising the timing of pivotal biological activities. Cancer incidence, particularly breast cancer, is among the most challenging health problems resulting from light pollution in our modern lifestyle. Epigenetic modifications are imperative for gene regulation and expression, and have robust effects on development of diseases such as tumorigenesis. Melatonin can provide the missing link between environmental disruption of biological rhythms and the epigenetic molecular machinery which regulates global DNA hypomethylation in oncogenes and local DNA hypermethylation in tumour suppressor genes. Although substantial and significant progress has been made in understanding the molecular basis of epigenetic-induced tumorigenesis, more research is still warranted, particularly for characterizing the spectral and irradiance sensitivity of the biological clock to ALAN, a novel source of pollution. Furthermore, the exact relation between circadian disruption, melatonin suppression, and aberrant DNA methylation and histone acetylation requires further research. Understanding the interplay between environmental factors and epigenetic remodelling of certain oncogenes would be of great interest for both establishing sustainable illumination and discovering novel biomarkers for cancer. DNA methylation and melatonin level profiling in high risk groups, particularly night shift workers, are important for the prevention, early detection and treatment of developing cancers.
Conflict of interests
We have no competing interests.
References
- 1.AMA. 2012. AMA adopts new public health policies at annual meeting 2012. See http://www.cisionwire.com/american-medical-association/r/ama-adopts-new-public-health-policies-at-annual-meeting,c9275938 .
- 2.Gaston KJ, Bennie J, Davies TW, Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. Camb. Phil. Soc. 88, 912–927. [DOI] [PubMed] [Google Scholar]
- 3.Bedrosian TA, Nelson RJ. 2013. Influence of the modern light environment on mood. Mol. Psychiatry 18, 75 175–75 177. [DOI] [PubMed] [Google Scholar]
- 4.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. 2014. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 64, 207–218. ( 10.3322/caac.21218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haim A, Portnov BA. 2013. Light pollution as a new risk factor for human breast and prostate cancers. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 6.Boyle P, Levin B. 2008. World cancer report http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/index.php.
- 7.Stevens RG, Davis S, Thomas DB, Anderson LE, Wilson BW. 1992. Electric power, pineal function, and the risk of breast cancer. FASEB J. 6, 853–860. [DOI] [PubMed] [Google Scholar]
- 8.Stevens RG, Davis S. 1996. The melatonin hypothesis: electric power and breast cancer. Environ. Health Perspect. 104(Suppl. 1), 135–140. ( 10.1289/ehp.96104s1135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendt J. 2005. Melatonin: characteristics, concerns, and prospects. J. Biol. Rhythms 20, 291–303. ( 10.1177/0748730405277492) [DOI] [PubMed] [Google Scholar]
- 10.Cos S, González A, Martínez-Campa C, Mediavilla MD, Alonso-González C, Sánchez-Barceló EJ. 2006. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect. Prevent. 30, 118–128. ( 10.1016/j.cdp.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 11.Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT, Dauchy EM, Frasch T, Duplesis T. 2011. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J. Mammary Gland Biol. Neoplasia 16, 235–245. ( 10.1007/s10911-011-9222-4) [DOI] [PubMed] [Google Scholar]
- 12.Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, Mao L, Dauchy E, Sauer LA. 2011. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J. Pineal Res. 51, 259–269. ( 10.1111/j.1600-079X.2011.00888.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SE, Kim SJ, Yoon HJ, Yu SY, Yang H, Jeong SI, Hwang SY, Park CS, Park YS. 2012. Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J. Pineal Res. 54, 80–88. [DOI] [PubMed] [Google Scholar]
- 14.Jerónimo C, Bastian PJ, Bjartell A, Carbone GM, Catto JW, Clark SJ, Henrique R, Nelson WG, Shariat SF. 2011. Epigenetics in prostate cancer: biologic and clinical relevance. Eur. Urol. 60, 753–766. ( 10.1016/j.eururo.2011.06.035) [DOI] [PubMed] [Google Scholar]
- 15.Qu Y, Dang S, Hou P. 2013. Gene methylation in gastric cancer. Clin. Chim. Acta 424, 53–65. ( 10.1016/j.cca.2013.05.002) [DOI] [PubMed] [Google Scholar]
- 16.Balgkouranidou I, Liloglou T, Lianidou ES. 2013. Lung cancer epigenetics: emerging biomarkers. Biomark Med. 7, 49–58. ( 10.2217/bmm.12.111) [DOI] [PubMed] [Google Scholar]
- 17.Nowsheen S, Aziz K, Tran PT, Gorgoulis VG, Yang ES, Georgakilas AG. 2014. Epigenetic inactivation of DNA repair in breast cancer. Cancer Lett. 342, 213–222. ( 10.1016/j.canlet.2012.05.015) [DOI] [PubMed] [Google Scholar]
- 18.Choi JD, Lee JS. 2013. Interplay between epigenetics and genetics in cancer. Genomics Inform. 11, 164–173. ( 10.5808/GI.2013.11.4.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. 2006. Night work and risk of breast cancer. Epidemiology 17, 108–111. ( 10.1097/01.ede.0000190539.03500.c1) [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M. 2010. Light at night and breast cancer risk: results from a population-based case-control study in Connecticut, USA. Cancer Causes Control 21, 2281–2285. ( 10.1007/s10552-010-9653-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE. 2013. A case-referent study: light at night and breast cancer risk in Georgia. Int. J. Health Geogr. 12, 23–33. ( 10.1186/1476-072X-12-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, Reynolds P. 2014. Light at night and breast cancer risk among California teachers. Epidemiology 25, 697–706. ( 10.1097/EDE.0000000000000137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. 2008. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol. Int. 25, 65–81. ( 10.1080/07420520801921572) [DOI] [PubMed] [Google Scholar]
- 24.Kloog I, Stevens RG, Haim A, Portnov BA. 2010. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21, 2059–2068. ( 10.1007/s10552-010-9624-4) [DOI] [PubMed] [Google Scholar]
- 25.Zubidat AE, Nelson RJ, Haim A. 2010. Photoentrainment in blind and sighted rodent species: responses to photophase light with different wavelengths. J. Exp. Biol. 213, 4213–4222. ( 10.1242/jeb.048629) [DOI] [PubMed] [Google Scholar]
- 26.Zubidat AE, Nelson RJ, Haim A. 2010. Differential effects of photophase irradiance on metabolic and urinary stress hormone concentrations in blind and sighted rodents. Chronobiol Int. 27, 487–516. ( 10.3109/07420521003678577) [DOI] [PubMed] [Google Scholar]
- 27.Sanyal S, Jansen HG, de Grip WJ, Nevo E, de Jong WW. 1990. The eye of the blind mole rat, Spalax ehrenbergi. Rudiment with hidden function? Invest. Ophthalmol. Vis. Sci. 31, 1398–1404. [PubMed] [Google Scholar]
- 28.Figueiro MG, Bierman A, Rea MS. 2013. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat. Sci. Sleep 5, 133–141. ( 10.2147/NSS.S52203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeitzer JM, Fisicaro RA, Ruby NF, Heller HC. 2014. Millisecond flashes of light phase delay the human circadian clock during sleep. J. Biol. Rhythms 29, 370–376. [Epub ahead of print] ( 10.1177/0748730414546532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bierman A, Figueiro MG, Rea MS. 2011. Measuring and predicting eyelid spectral transmittance. J. Biomed. Opt. 16, 067 011–067 019. ( 10.1117/1.3593151) [DOI] [PubMed] [Google Scholar]
- 31.Verkasalo PK, Pukkala E, Stevens RG, Ojamo M, Rudanko SL. 1999. Inverse association between breast cancer incidence and degree of visual impairment in Finland. Br. J. Cancer 80, 1459–1460. ( 10.1038/sj.bjc.6690544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas RJ, Lall GS, Allen AE, Brown TM. 2012. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog. Brain Res. 199, 1–18. ( 10.1016/B978-0-444-59427-3.00001-0) [DOI] [PubMed] [Google Scholar]
- 33.Hughes S, Hankins MW, Foster RG, Peirson SN. 2012. Melanopsin phototransduction: slowly emerging from the dark. Prog. Brain Res. 199, 19–40. ( 10.1016/B978-0-444-59427-3.00002-2) [DOI] [PubMed] [Google Scholar]
- 34.Lamb TD. 2013. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog. Retin. Eye Res. 36, 52–119. ( 10.1016/j.preteyeres.2013.06.001) [DOI] [PubMed] [Google Scholar]
- 35.Güler AD, et al. 2008. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453, 102–105. ( 10.1038/nature06829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III 1995. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 332, 6–11. ( 10.1056/NEJM199501053320102) [DOI] [PubMed] [Google Scholar]
- 37.Klerman EB, Shanahan TL, Brotman DJ, Rimmer DW, Emens JS, Rizzo JF, III, Czeisler CA. 2002. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J. Biol. Rhythms 17, 548–555. ( 10.1177/0748730402238237) [DOI] [PubMed] [Google Scholar]
- 38.Hattar S, et al. 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81. ( 10.1038/nature01761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mrosovsky N, Hattar S. 2003. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol. Int. 20, 989–999. ( 10.1081/CBI-120026043) [DOI] [PubMed] [Google Scholar]
- 40.Panda S, et al. 2003. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527. ( 10.1126/science.1086179) [DOI] [PubMed] [Google Scholar]
- 41.Lockley SW, Arendt J, Skene DJ. 2007. Visual impairment and circadian rhythm disorders. Dialogues Clin. Neurosci. 9, 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haim A, Heth G, Pratt H, Nevo E. 1983. Photoperiodic effects on thermoregulation in a ‘blind’ subterranean mammal. J. Exp. Biol. 107, 59–64. [DOI] [PubMed] [Google Scholar]
- 43.Ohayon MM. 2004. Interactions between sleep normative data and sociocultural characteristics in the elderly. J. Psychosom. Res. 56, 479–486. ( 10.1016/j.psychores.2004.04.365) [DOI] [PubMed] [Google Scholar]
- 44.Ursin R, Bjorvatn B, Holsten F. 2005. Sleep duration, subjective sleep need, and sleep habits of 40- to 45-year-olds in the Hordaland Health Study. Sleep 28, 1260–1269. [DOI] [PubMed] [Google Scholar]
- 45.Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF. 2007. American time use survey: sleep time and its relationship to waking activities. Sleep 30, 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KA, Gay CL. 2010. Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Res. Nurs. Health 34, 7–19. ( 10.1002/nur.20413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA, Duffy JF. 2010. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms 25, 288–296. ( 10.1177/0748730410374943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng Y, Wainwright NW, Cappuccio FP, Surtees PG, Luben R, Wareham N, Brayne C, Khaw KT. 2014. Self-reported sleep patterns in a British population cohort. Sleep Med. 15, 295–302. ( 10.1016/j.sleep.2013.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kloog I, Portnov BA, Rennert HS, Haim A. 2011. Does the modern urbanized sleeping habitat pose a breast cancer risk? Chronobiol. Int. 28, 76–80. ( 10.3109/07420528.2010.531490) [DOI] [PubMed] [Google Scholar]
- 50.Blask DE, et al. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 65, 11 174–11 184. ( 10.1158/0008-5472.CAN-05-1945) [DOI] [PubMed] [Google Scholar]
- 51.Reiter RJ, Fraschini F. 1969. Endocrine aspects of the mammalian pineal gland: a review. Neuroendocrinology 5, 219–255. ( 10.1159/000121862) [DOI] [PubMed] [Google Scholar]
- 52.Reiter RJ. 2003. Melatonin: clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 17, 273–285. ( 10.1016/S1521-690X(03)00016-2) [DOI] [PubMed] [Google Scholar]
- 53.Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, Orgül S, Wirz-Justice A. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 90, 1311–1316. ( 10.1210/jc.2004-0957) [DOI] [PubMed] [Google Scholar]
- 54.Thapan K, Arendt J, Skene DJ. 2001. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 535, 261–267. ( 10.1111/j.1469-7793.2001.t01-1-00261.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erren TC, Pape HG, Reiter RJ, Piekarski C. 2008. Chronodisruption and cancer. Naturwissenschaften 95, 367–382. ( 10.1007/s00114-007-0335-y) [DOI] [PubMed] [Google Scholar]
- 56.Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. 2011. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage. 92, 2714–2722. ( 10.1016/j.jenvman.2011.06.029) [DOI] [PubMed] [Google Scholar]
- 57.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2, 31ra33 ( 10.1126/scitranslmed.3000741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chellappa SL, et al. 2012. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J. Clin. Endocrinol. Metab. 97, E433–E437. ( 10.1210/jc.2011-2391) [DOI] [PubMed] [Google Scholar]
- 59.Peichl L. 2005. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 287, 1001–1012. ( 10.1002/ar.a.20262) [DOI] [PubMed] [Google Scholar]
- 60.Bullough JD, Rea MS, Figueiro MG. 2006. Of mice and women: light as a circadian stimulus in breast cancer research. Cancer Causes Control 17, 375–383. ( 10.1007/s10552-005-0574-1) [DOI] [PubMed] [Google Scholar]
- 61.West KE, et al. 2011. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J. Appl. Physiol. 110, 619–626. ( 10.1152/japplphysiol.01413.2009) [DOI] [PubMed] [Google Scholar]
- 62.Erren TC, Erren M, Lerchl A, Meyer-Rochow VB. 2008. Clockwork blue: on the evolution of non-image-forming retinal photoreceptors in marine and terrestrial vertebrates. Naturwissenschaften 95, 273–279. ( 10.1007/s00114-007-0315-2) [DOI] [PubMed] [Google Scholar]
- 63.Haus EL, Smolensky MH. 2013. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 17, 273–284. ( 10.1016/j.smrv.2012.08.003) [DOI] [PubMed] [Google Scholar]
- 64.Mikeska T, Bock C, Do H, Dobrovic A. 2012. DNA methylation biomarkers in cancer: progress towards clinical implementation. Expert Rev. Mol. Diagn. 12, 473–487. ( 10.1586/erm.12.45) [DOI] [PubMed] [Google Scholar]
- 65.Korkmaz A, Reiter RJ. 2008. Epigenetic regulation: a new research area for melatonin? J. Pineal Res. 44, 41–44. [DOI] [PubMed] [Google Scholar]
- 66.Waddington CH. 1940. Organisers and genes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 67.Mazzio EA, Soliman KF. 2012. Basic concepts of epigenetics: impact of environmental signals on gene expression. Epigenetics 7, 119–130. ( 10.4161/epi.7.2.18764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deaton AM, Bird A. 2011. CpG islands and the regulation of transcription. Genes Dev. 25, 1010–1022. ( 10.1101/gad.2037511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones PA. 2012. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492. ( 10.1038/nrg3230) [DOI] [PubMed] [Google Scholar]
- 70.Cheung P, Lau P. 2005. Epigenetic regulation by histone methylation and histone variants. Mol. Endocrinol. 19, 563–573. ( 10.1210/me.2004-0496) [DOI] [PubMed] [Google Scholar]
- 71.Cosgrove MS. 2007. Histone proteomics and the epigenetic regulation of nucleosome mobility. Expert Rev. Proteomics 4, 465–478. ( 10.1586/14789450.4.4.465) [DOI] [PubMed] [Google Scholar]
- 72.Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein TA. 2004. Silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 18, 1251–1262. ( 10.1101/gad.300704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischle W, Kiermer V, Dequiedt F, Verdin E. 2001. The emerging role of class II histone deacetylases. Biochem. Cell Biol. 79, 337–348. ( 10.1139/o01-116) [DOI] [PubMed] [Google Scholar]
- 74.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370, 737–749. ( 10.1042/BJ20021321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sved J, Bird A. 1990. The expected equilibrium of the CpG dinucleotide in vertebrate genomes under a mutation model. Proc. Natl Acad. Sci. USA 87, 4692–4696. ( 10.1073/pnas.87.12.4692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skinner MK. 2014. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell Endocrinol. 7207, 00 223–00 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crisp TM, et al. 1998. Environmental endocrine disruption: an effects assessment and analysis. Environ. Health Perspect. 106(Suppl 1), 11–56. ( 10.1289/ehp.98106s111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaenisch R, Bird A. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254. ( 10.1038/ng1089) [DOI] [PubMed] [Google Scholar]
- 79.Wilson AG. 2008. Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J. Periodontol. 79, 1514–1519. ( 10.1902/jop.2008.080172) [DOI] [PubMed] [Google Scholar]
- 80.Gilbert ER, Liu D. 2012. Epigenetics: the missing link to understanding β-cell dysfunction in the pathogenesis of type 2 diabetes. Epigenetics 7, 841–852. ( 10.4161/epi.21238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernández AF, Toraño EG, Urdinguio RG, Lana AG, Fernández IA, Fraga MF. 2014. The epigenetic basis of adaptation and responses to environmental change: perspective on human reproduction. Adv. Exp. Med. Biol. 753, 97–117. ( 10.1007/978-1-4939-0820-2_6) [DOI] [PubMed] [Google Scholar]
- 82.Martino D, Kesper DA, Amarasekera M, Harb H, Renz H, Prescott S. 2014. Epigenetics in immune development and in allergic and autoimmune diseases. J. Reprod. Immunol. 104–105, 43–48. ( 10.1016/j.jri.2014.05.003) [DOI] [PubMed] [Google Scholar]
- 83.Modgil S, Lahiri DK, Sharma VL, Anand A. 2014. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl. Neurodegener. 3, 9–23. ( 10.1186/2047-9158-3-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL. 2005. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc. Natl Acad. Sci. USA 102, 8644–8649. ( 10.1073/pnas.0503218102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Ho SM. 2011. Epigenetics meets endocrinology. J. Mol. Endocrinol. 46, R11–R32. ( 10.1677/JME-10-0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skinner MK, Manikkam M, Guerrero-Bosagna C. 2010. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21, 214–222. ( 10.1016/j.tem.2009.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He C, Anand ST, Ebell MH, Vena JE, Robb SW. In press Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int. Arch. Occup. Environ. Health. ( 10.1007/s00420-014-0986-x) [DOI] [PubMed] [Google Scholar]
- 88.Wang F, et al. 2013. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 24, 2724–2732. ( 10.1093/annonc/mdt283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leonardi GC, Rapisarda V, Marconi A, Scalisi A, Catalano F, Proietti L, Travali S, Libra M, Fenga C. 2012. Correlation of the risk of breast cancer and disruption of the circadian rhythm. Oncol. Rep. 28, 418–428. [DOI] [PubMed] [Google Scholar]
- 90.Davis S, Mirick DK, Stevens RG. 2001. Night shift work, light at night, and risk of breast cancer. J. Natl Cancer Inst. 93, 1557–1562. ( 10.1093/jnci/93.20.1557) [DOI] [PubMed] [Google Scholar]
- 91.Schwimmer H, Metzer A, Pilosof Y, Szyf M, Machnes ZM, Fares F, Harel O, Haim A. 2014. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol. Int. 31, 44–150. ( 10.3109/07420528.2013.842925) [DOI] [PubMed] [Google Scholar]
- 92.Haim A, Yukler A, Harel O, Schwimmer H, Fares F. 2010. Effects of chronobiology on prostate cancer cells growth in vivo. Sleep Sci. 3, 32–35. [Google Scholar]
- 93.Blask DE, Sauer LA, Dauchy RT. 2002. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr. Top. Med. Chem. 2, 113–132. ( 10.2174/1568026023394407) [DOI] [PubMed] [Google Scholar]
- 94.Dworkin AM, Huang TH, Toland AE. 2009. Epigenetic alterations in the breast: Implications for breast cancer detection, prognosis and treatment. Semin. Cancer Biol. 19, 165–171. ( 10.1016/j.semcancer.2009.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Byler S, Goldgar S, Heerboth S, Leary M, Housman G, Moulton K, Sarkar S. 2014. Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer Res. 34, 1071–1077. [PubMed] [Google Scholar]
- 96.Xiang TX, Yuan Y, Li LL, Wang ZH, Dan LY, Chen Y, Ren GS, Tao Q. 2013. Aberrant promoter CpG methylation and its translational applications in breast cancer. Chin J. Cancer. 32, 12–20. ( 10.5732/cjc.011.10344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szyf M, Pakneshan P, Rabbani SA. 2004. DNA methylation and breast cancer. Biochem. Pharmacol. 68, 1187–1197. ( 10.1016/j.bcp.2004.04.030) [DOI] [PubMed] [Google Scholar]
- 98.Korkmaz A, Sanchez-Barcelo EJ, Tan DX, Reiter RJ. 2009. Role of melatonin in the epigenetic regulation of breast cancer. Breast Cancer Res. Treat. 115, 13–27. ( 10.1007/s10549-008-0103-5) [DOI] [PubMed] [Google Scholar]
- 99.Lee SE, Kim SJ, Yoon HJ, Yu SY, Yang H, Jeong SI, Hwang SY, Park CS, Park YS. 2013. Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J. Pineal Res. 54, 80–88. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, et al. 2012. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J. Pineal Res. 53, 77–90. ( 10.1111/j.1600-079X.2012.00973.x) [DOI] [PubMed] [Google Scholar]


