Abstract
The mechanisms underpinning the ecological impacts of the presence of artificial night lighting remain elusive. One suspected underlying cause is that the presence of light at night (LAN) supresses nocturnal production of melatonin, a key driver of biological rhythm and a potent antioxidant with a proposed role in immune function. Here, we briefly review the evidence for melatonin as the link between LAN and changes in behaviour and physiology. We then present preliminary data supporting the potential for melatonin to act as a recovery agent mitigating the negative effects of LAN in an invertebrate. Adult crickets (Teleogryllus commodus), exposed to constant illumination, were provided with dietary melatonin (concentrations: 0, 10 or 100 µg ml−1) in their drinking water. We then compared survival, lifetime fecundity and, over a 4-week period, immune function (haemocyte concentration, lysozyme-like and phenoloxidase (PO) activity). Melatonin supplementation was able only partially to mitigate the detrimental effects of LAN: it did not improve survival or fecundity or PO activity, but it had a largely dose-dependent positive effect on haemocyte concentration and lysozyme-like activity. We discuss the implications of these relationships, as well as the usefulness of invertebrates as model species for future studies that explore the effects of LAN.
Keywords: melatonin, invertebrates, light at night, immunomodulation
1. Introduction
Exposure to even low-intensity night lighting (<0.3 lux—comparable with full moonlight on a clear night) may be a major disruptor of behavioural and physiological processes [1,2] and yet many species living in urban environments continually experience far greater intensities of light at night (LAN) [3]. The ecological consequences of the increasing presence of artificial LAN are well documented in animals although the evidence from natural populations, in particular, is frequently correlational [2,4,5]. Behaviourally, LAN is linked to an increase in the observed number of bird strikes and subsequent deaths [6,7] and changes in the foraging capacity and activity patterns of vertebrates [8–12] and invertebrates [5,13]. At the population level, animals living in well-lit urban environments are observed to shift the timing and structure of their mating calls [14–17] and the commencement of breeding [15,18]. Evidence documenting the physiological impact of LAN comes largely from correlational studies of vertebrate (often human) health. A recent focus has been on links between the intensity of LAN and an increased risk of cancer, immune suppression and heart disease observed in shift workers, a group with frequently chronic exposure to high-intensity night lighting [19–22]. Human studies are typically correlational, but similar carcinogenic and immunosuppressant effects of artificial night lighting have been observed from laboratory experiments on mice and rats [23–26], although immune-enhancing effects of dim night lighting have also been reported [27]. Convincing support for the link between LAN and significant modifications to both behaviour (activity patterns) and physiology (moult dates and reproductive status) also comes from a series of long-term studies combining field and experimental trials on European blackbirds (Turdus merula) [12,18,28,29]. However, despite the recent surge in research on the impacts of night lighting within the fields of ecology and medicine, we still lack broad-scale, cross-species experimental evidence of the negative effects of LAN (but see [30]). Data on wild populations that experimentally link LAN to decreased biological function are notably absent and the underlying mechanisms driving the observed changes remain both poorly understood and largely vertebrate focused.
(a). Melatonin as a possible driver of biological change
One suspected underlying cause for the observed variation in behaviour and physiology is that the presence of LAN disrupts endogenous circadian rhythms and specifically supresses nocturnal production of the indolamine melatonin (N-acetyl-5-methoxytryptamine). Melatonin is one of the key drivers of biological rhythm whose principal function is to relay information about changes in day-length [31,32]. Perhaps less well known, but of potentially considerable physiological importance, is the critical role melatonin plays as an antioxidant (for a recent review, see [33]). The major problem faced by species living in regions with artificial night lighting is that, in the presence of LAN, the potential for melatonin to act as either an antioxidant or a regulator of biological rhythm may be compromised because endogenous melatonin concentrations are reduced. Our focus in this article is threefold: to provide a brief overview of what is currently known about melatonin in both vertebrates and invertebrates (particularly in relation to LAN); to report preliminary data that support current hypotheses implicating circulating melatonin as a causal intermediary between LAN and changes to one aspect of invertebrate physiological function, immunity; and to highlight future areas of research required to determine the multiple roles of melatonin and its importance to phenotype fitness.
(b). Melatonin and circadian rhythm
First isolated from the bovine pineal gland [34], the indolamine melatonin has subsequently been identified in all higher taxonomic groups [33,35]. Its structure is thought to be highly conserved across taxa [33,35,36] and in animals its biosynthesis from tryptophan via serotonin (see figure 1) is believed to be comparable for vertebrates and invertebrates [35]. The primary site of endogenous melatonin synthesis in vertebrates is the pineal gland, but its presence has been documented in many other organs and tissues, including the retina, intestine, salivary gland, Hardian gland and leucocytes [33,37]. In invertebrates, endogenous melatonin is typically synthesized in the cerebral ganglia but it is similarly found in other tissues and organs, including the eyes and reproductive tissues [35,38–41].
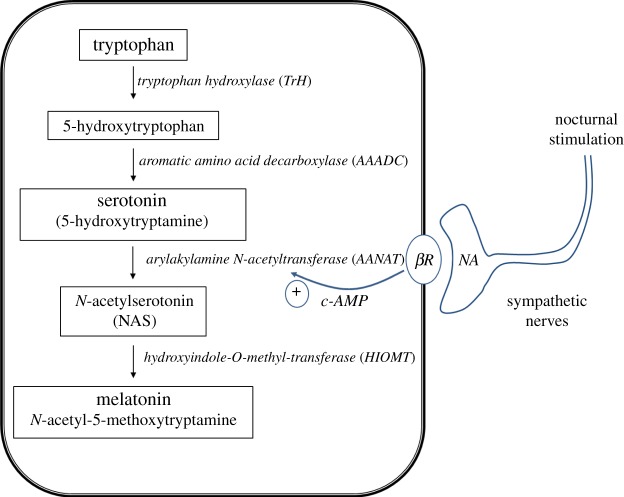
Figure 1.
Simplified schematic biosynthetic pathway depicting the production of melatonin in the pineal gland of vertebrates. Neural input is required for the rate-limiting step in the vertebrate pathway involving the conversion of serotonin to N-acetyl-serotonin (NAS). Enzymes shown in italics; c-AMP = cyclic AMP; βR, NA = β-adrenergic receptors. (Online version in colour.)
The role of melatonin as a chronobiotic is well established [31,32]. In vertebrates, circulating concentrations of pineal-derived melatonin vary throughout a 24 h cycle, typically reaching their highest concentrations during periods of natural darkness and their lowest concentrations during daylight hours [31–33]. Seasonal shifts in circulating melatonin concentrations are also evident, with lower concentrations observed in winter compared with those in summer [42]. Experimental manipulation of melatonin links variation in its presence with shifts in the behaviour and locomotor activity of vertebrates, including rats, birds and lizards [43–46]. In invertebrates, the relationship between circulating concentrations of melatonin and circadian rhythm is less clear [35,47]. Nocturnal peaks are observed in a range of species, including planarians [40,48], Drosophila [49–51], a cockroach [38] and a silkworm [39]. However, there are several exceptions to this general pattern: in the crustacea, many species exhibit diurnal peaks or no peak at all (for a comprehensive recent review, see [52]); in two species of hymenoptera, Apis mellifera and Apis ceranathe, circulating melatonin exhibits multiple daily peaks and troughs [53]; and in both the opisthobranch mollusc Aplysia californica [54] and the cricket Gryllus bimaculatus [55] melatonin concentrations vary across organs and tissues within the same individual (although the degree to which this is driven by biosynthesized melatonin is untested). Notwithstanding these species-specific differences in peak concentrations, variation in melatonin concentrations is linked to shifts in behaviour in a number of invertebrates, including Drosophila [56], crickets [57] and Daphnia [58]. The observed differences in the timing and location of peak melatonin concentrations in the invertebrates is perhaps not surprising given the relative breadth of taxonomic diversity compared with vertebrates, but nonetheless it raises some interesting questions regarding the ubiquitous nature of the hormone and reinforces the belief that functionally it is more than simply a signal of darkness [33].
(c) Melatonin, oxidative stress and immune function
Melatonin's function as a free radical scavenger and potent antioxidant has been comparatively recently recognized but is now generally accepted [33,59–61]. The antioxidant capacity of melatonin is derived in part because it is amphiphilic and is thus able freely to move intracellularly across membranes, including mitochondria, the organelle primarily responsible for cellular respiration and thus one of the main sites of free radical generation [62,63]. At the cellular level, endogenous melatonin has been directly linked to enhanced cell maintenance [61], mitochondrial activity [63,64] and increased antioxidant capacity of gametes in vertebrates [65,66].
The potential indirect antioxidant effects of melatonin on physiological processes, including immune function and survival, are less well understood mechanistically but nonetheless are proposed. Melatonin supplementation experiments generally reveal a positive effect of melatonin on survival in both vertebrates and invertebrates [67–70]. Similarly, a number of studies have found melatonin plays a fundamental role in immune-modulation, but this has been explored almost exclusively in vertebrates (for recent reviews see [71–73]). The immune-modulatory effect of melatonin on the adaptive vertebrate immune system is supported by the existence of specific melatonin receptors in immune organs as well as immunocompetent cells, with its role in innate immunity recently proposed [71]. This latter finding is of particular interest from the invertebrate perspective, because while invertebrates lack an adaptive immune response, their innate immune system, particularly that of the insects, is highly effective and has cellular recognition and defensive pathways that are comparable with vertebrates (for a comprehensive coverage of the discipline see [74]). Thus, the observed capacity for melatonin to modulate innate immunity in vertebrates indicates its possible role in invertebrate immune function. Melatonin's ubiquitous presence, conserved structure and functional versatility combined with the photosensitivity of its biosynthetic pathway [75] makes it a prime candidate to mediate shifts in biological function in the presence of LAN. Despite this, the critical three-way links between the presence of LAN, circulating melatonin concentration and behaviour or physiology remain largely undetermined and in invertebrates relatively unexplored.
(i). LAN, circulating melatonin concentrations and shifts in biological function
Early in vitro experiments in human cells [75] and subsequent experiments in vivo confirmed that exposure to LAN suppresses circulating melatonin in vertebrates [76–78]; however, the functional significance of these results is only now becoming a focal point for research exploring the ecological effects of LAN. Correlational studies provide good support for light suppression of melatonin as a physiological mechanism driving the biological change. For example, in the European starling (Sturnus vulgaris) locomotion and feeding rhythms were positively associated with plasma melatonin concentrations following short-term exposure to low-intensity lighting with steadily changing photoperiods [79]. Similarly, in tench (Tinca tinca) rhythmic variation in circulating melatonin concentrations and locomotor activity were affected by exposure to light pulses during periods of natural darkness [80]. Perhaps the most convincing support comes from a long-term experimental manipulation in the European blackbird (Turdus merula) which found that melatonin concentrations of birds raised in the presence of LAN were consistently lower and locomotor activity consistently higher when compared with birds raised with a nocturnal period of darkness [42]. However, such studies were unable to determine whether melatonin directly affected the behavioural change. Supplementation with dietary melatonin has provided the strongest physiological support to date. For example, in Japanese quail (Coturnix coturnix japonica) immune function was significantly lowered when birds were kept under constant illumination compared with birds maintained on either short- (8 h light) or long-day (16 h light) cycles, but supplementation with melatonin in the drinking water provided a positive dose-dependent immune-enhancing effect [81]. Using a comparable experimental design Vinogradova and Anisimov [82] found that melatonin provided in the drinking water was able to reduce the severity of a number of age-related metabolic disorders in rats exposed to constant illumination.
Several major gaps are apparent in the literature. First, comparable data linking either LAN and circulating concentrations of melatonin or LAN and changes in invertebrate behaviour or physiology are lacking (but see [40,48]). Second, while a few studies have explored the longitudinal effects of long-term exposure to LAN at the individual level in vertebrates [28,29], to our knowledge there are no comparable data for invertebrates. Finally, direct evidence of the links between LAN, circulating concentrations of melatonin and changes to immune function remain, with the exception of a few studies in vertebrates, untested. To begin to address some of these knowledge gaps, our research uses the nocturnal black field cricket Teleogryllus commodus as a model species to explore the effect of LAN on individual fitness and the ability of melatonin to negate the potential negative impacts of LAN. Research in a closely related cricket species, Gryllus bimaculatus, has revealed nocturnal peaks in circulating melatonin [55] and demonstrated the functional role of melatonin for egg development and hatching [41,83], as well as locomotor activity [57]. Our research on T. commodus indicates that the presence of night-time illumination during development correlates with reductions in immune function and concentrations of circulating melatonin. In brief, following an initial immune challenge crickets maintained under constant light (24 h, LL) were unable to mount an immune response following a wounding challenge, resulting in a lower haemocyte concentration and reduced lysozyme-like activity compared with crickets reared under a 12 h day 12 h night (LD) light regimen (mean haemocyte concentration post-challenge in LL crickets = 15.85 ± 2.03 cells ml−1 × 106; LD crickets 23.48 ± 2.06 cells ml−1 × 106; p = 0.01; lysozyme-like activity, measured as Δ absorbance, for LL crickets = 0.34 ± 0.02; LD crickets = 0.39 ± 0.02; p = 0.02; J Durrant, EB Michaelides, MP Green and TM Jones 2013, unpublished data). Moreover, circulating melatonin concentrations were significantly lower in adult LL crickets compared with melatonin concentrations of LD crickets (median [interquartile range] concentration of melatonin in LL crickets = 13.53 [9.4–27.86] pg ml−1; LD crickets = 23.78 [18.62–57.65] pg ml−1; p = 0.03; J Durrant, EB Michaelides, MP Green and TM Jones 2013, unpublished data). These data form the basis for the experimental design and scientific reasoning behind the current melatonin supplementation study. Here, we exposed adult crickets to an identical source of constant illumination but experimentally manipulated concentrations of circulating melatonin through addition of dietary melatonin in the drinking water. We predicted that, if melatonin is able to act as a recovery agent countering the potential negative impact of prolonged exposure to artificial LAN, then crickets supplemented with the highest concentrations of dietary melatonin should respond positively in terms of survival, fecundity and immune function.
2. Methods
Studies were undertaken using a laboratory population that originated from Victoria, Australia (37.56238 S, 145.31920 E) and were subsequently bred for four generations under standard conditions [84] in incubators (maintained at 28°C; under a 12 h light : 12 h dark artificial lighting regimen; LD). Experimental individuals were reared as juveniles in standard conditions but were transferred within a day of their final moult to individual containers and placed under a constant light regimen (maintained at 28°C; 24 h continuous illumination of 4000 lux; LL) until their death. The severe experimental situation of constant illumination at 4000 lux provides an ecological extreme but we assume that it will induce reductions in circulating melatonin and thus permit us to explore explicitly the role of melatonin in modulating immune function, survival and fecundity through the use of dietary supplementation. Adult crickets were assigned to one of the three melatonin treatments: 0 µg ml−1 melatonin (which represents the control group and is the group we predict will not be able to respond to immune challenges as found in our previous research—see §1c(i)), 10 µg ml−1 melatonin or 100 µg ml−1 melatonin (n = 14 females and 10 males per treatment). All individuals were provided with 4 ml of deionized water in a small Petri dish (diameter 35 mm; height 11 mm) for the first 2 days following their adult moult. This ensured that crickets were hydrated following their final moult and provided a baseline measure of immune function for all crickets (see §2c). After this initial 2-day period, individuals were transferred to their allocated treatment and provided with 4 ml of water containing their allocated melatonin treatment and this was changed thrice weekly until their death. All chemicals and reagents were obtained from Sigma-Aldrich, NSW, Australia.
(a). Supplementation with dietary melatonin
Dietary supplementation of melatonin was administered through drinking water (following [57]). Melatonin powder was dissolved in 100% ethanol at 50 mg ml−1 and stored in the dark at 4°C. Working dilutions were made fresh weekly from the stock using distilled water to give solutions of 0, 10 or 100 µg ml−1. Preliminary trials indicated that this method was effective in raising circulating concentrations of melatonin in adult T. commodus. The mean concentrations of circulating melatonin after 1 week of supplementation were 259 ± 79.8 ng ml−1 (0 μg ml−1 melatonin, n = 4 crickets), 401 ± 76.9 ng ml−1 (10 μg ml−1, n = 2 crickets) and 1812 ± 528 ng ml−1 (100 μg ml−1, n = 2 crickets)—melatonin concentrations were measured using high-performance liquid chromatography (modified from [85]).
(b). The relationship between melatonin concentration, survival and fecundity
To assess the impact of melatonin treatment on female fecundity, 2 weeks post the final moult, female crickets were mated to a male from the stock population (maintained under standard conditions) and were then provided with a Petri dish (diameter 45 mm; height 15 mm) filled with moist sand for oviposition. The sand-filled dish was changed twice weekly until the females died. The total number of eggs laid was counted to provide a measure of lifetime fecundity. For all individuals, body mass (g) was taken prior to any procedure; and average femur length (mm) was taken as a measure of body size. The residuals from a correlation between leg length and body mass were taken as a measure of adult body condition.
(c). The relationship between melatonin concentration and measures of immune function
We used three independent assays of immune function as these are known to vary both in the degree of response across invertebrate species and between the sexes [86]. (i) The concentration of circulating haemocytes was estimated as these are the cells within the haemolymph that form the core cellular response in invertebrate innate immunity [87]. (ii) Lysozyme-like activity was assessed as a measure of the potential for crickets to resist bacterial challenges as lysozymes are the group of enzymes principally involved in degrading bacterial cell walls [74]. (iii) Phenoloxidase (PO) activity was quantified as PO becomes activated upon cuticular wounding and infection, and is an important precursor instigating repair and encapsulation following infection or parasitism [88]. To determine simultaneously the longitudinal effects of melatonin supplementation, we collected haemolymph at four time periods: 2 days post the final moult and prior to placing crickets on their allocated melatonin treatment (week 0, baseline measure—see §3); 9 days post final moult (week 1); 16 days post final moult (week 2) and finally at 30 days post adult moult (week 4). For consistency, haemolymph extraction always took place 3 h prior to natural sunset. At each extraction event, we took a total of 8 μl of haemolymph by making a small puncture in the left side of the cricket abdomen using a sterile needle. The first 2 µl of haemolymph was placed into a 0.5 ml Eppendorf tube on ice with 16 µl of an anticoagulant solution and was immediately used to estimate haemocyte concentration; the remaining 6 µl was placed into a 0.5 ml Eppendorf tube with 80 µl of phosphate buffered saline (PBS; pH 7.4), snap frozen in liquid nitrogen and stored (−80°C) until analysis of lysozyme-like and PO activity.
(d). Immune function analyses
(i) To estimate the concentration of haemocytes, 10 µl of the haemolymph–anticoagulant solution was transferred to a haemocytometer and the total number of haemocyte cells per cubic millimetre was calculated (following [84]). (ii) To assess lysozyme-like activity, we added 10 µl of haemolymph–PBS solution to 80 µl of Micrococcus lutus (Lysodeikticus) bacteria solution (3 mg ml−1 PBS) and measured the change in absorbance at 490 nm using a plate reader (PerkinElmer Enspire 3.0 Multimode Plate Reader) over a 120 min, maintained at 30°C. (iii) To assess PO activity we transferred 5 µl of the haemolymph–PBS solution and 7 µl of 1.3 mg ml−1 bovine pancreas α-chymotrypsin to each well in a standard 98-well plate; incubated at room temperature for 20 min and then we added 90 µl of l-dihyroxyphenylalanine (l-DOPA). The change in absorbance was measured over the following 120 min (temperature 26°C; absorbance 490 nm). Control and quality control samples were included on every assay plate for lysozyme-like and PO assays.
(e). Statistics
All data exploration and analyses were performed in JMP (v. 11, SAS, NC, USA). Variables were assessed for normality prior to analysis. Variation in (i) survival was assessed using the Proportional Hazards survival platform, which incorporates a semi-parametric regression model where the survival time of each member of a population is assumed to follow its own hazard function (following [89]); (ii) fecundity was determined using standard least squares regression models; (iii) immune assays were assessed using general linear models (GLMs) with restricted maximum likelihood (REML), adding cricket identity as a random fixed effect to account for the multiple samples taken per individual. This method was chosen over a repeated-measures approach because variations in sample size across immune assays rendered the dataset incomplete for some individuals. Maximal models included dietary melatonin treatment, week (sampling week 1–4), sex and all interactions. Leg length, body condition, the number of eggs laid and survival were also added where appropriate. Each model was reduced using hierarchical removal of all terms with a significance of p > 0.1 (except our designated melatonin treatment). Unless otherwise stated, significant interactions were assessed using post-hoc Student's t-tests and data presented are means ± s.e. All tests were two-tailed with a significance level of p < 0.05.
3. Results
(a). Relationship between melatonin concentration, survival and fecundity
Females laid on average 292.6 ± 37.52 eggs. The number of eggs laid was unrelated to dietary melatonin treatment (table 1a); however, it was weakly positively related to female leg length (table 1a). Average adult lifespan was comparable for the three melatonin treatments (mean ± standard error number of days survived = 57.11 ± 3.32 days; table 1b); and was positively related to leg length (table 1b).
Table 1.
Models exploring variations in lifetime egg production, survival and three measures of immune function for male and female crickets exposed as adults to constant light but provided with dietary melatonin at one of three concentrations (0, 10 or 100 μg ml−1). Significant p-values are indicated in bold.
| models and parameters | slope (β ± SE) | statistic | p-value |
|---|---|---|---|
| (a) Lifetime egg production | |||
| melatonin concentration | F2,36 = 1.56 | 0.40 | |
| leg length | 0.43 ± 0.22 | F1,36 = 3.69 | 0.06 |
| (b) Number of days survived | |||
| melatonin concentration | 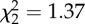 |
0.50 | |
| leg length | 0.13 ± 0.06 | 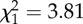 |
0.05 |
| (c) Haemocyte concentration (×106 cells ml−1) | |||
| melatonin concentration | F2,62 = 4.37 | 0.02 | |
| sex | F1,62 = 0.05 | 0.83 | |
| week | 0.59 ± 0.32 | F1,188 = 3.44 | 0.07 |
| melatonin concentration × sex | F2,62 = 2.75 | 0.07 | |
| melatonin concentration × week | F2,189 = 4.50 | 0.01 | |
| sex × week | F3,188 = 4.46 | 0.04 | |
| (d) Lysozyme-like activity (Δ absorbance) | |||
| melatonin concentration | F2,65 = 0.61 | 0.55 | |
| week | 0.02 ± 0.004 | F1,158 = 15.79 | 0.0001 |
| melatonin concentration × week | F2,158 = 3.53 | 0.03 | |
| (e) Phenoloxidase activity (Δ absorbance) | |||
| melatonin concentration | F2,67 = 0.04 | 0.96 | |
| sex | F1,65 = 66.93 | <0.0001 | |
| week | 0.14 ± 0.01 | F1,170 = 117.70 | <0.0001 |
| leg length | 0.05 ± 0.02 | F1,186 = 4.35 | 0.04 |
(b). Relationship between melatonin concentration and immune function
(i). Baseline measures
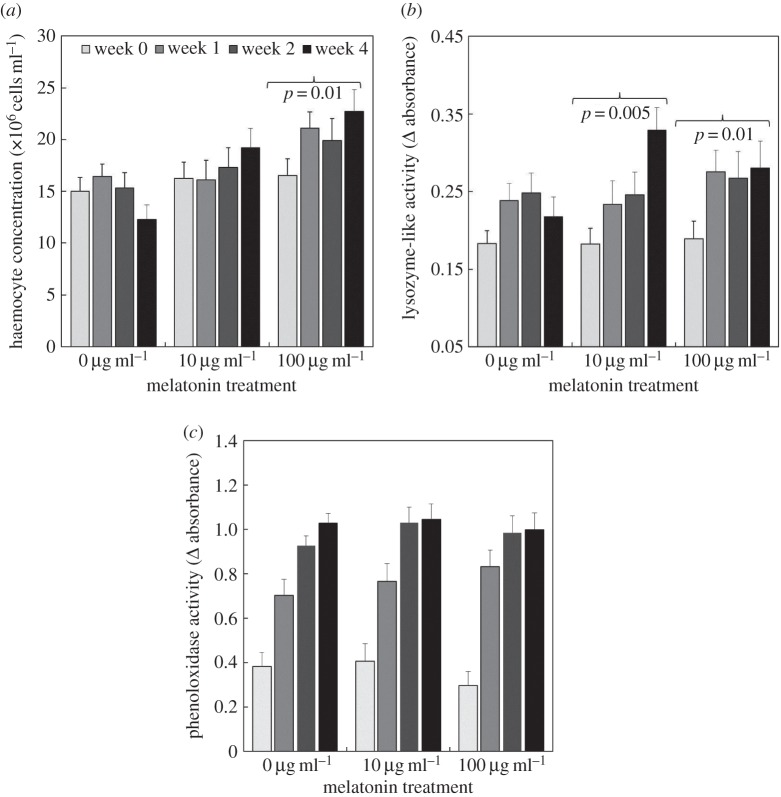
One-way ANOVAs confirmed that, within each sex, initial immune measures were comparable across the three melatonin treatments (at week 0, prior to individuals being placed on their allocated melatonin treatment): (i) haemocyte concentration in females: F2,38 = 0.86, p = 0.43; males: F2,21 = 0.44, p = 0.65; (ii) lysozyme-like activity in females: F2,26 = 1.31, p = 0.29; males: F2,15 = 1.13, p = 0.35; (iii) PO activity in females: F2,26 = 1.25, p = 0.30 males: F2,15 = 0.55, p = 0.59; see figure 2a–c. This provides confidence that subsequent differences across weeks arose as a result of the melatonin treatment rather than differences between individuals.
Figure 2.
Average (a) haemocyte concentration, (b) lysozyme-like activity and (c) phenoloxidase activity over four collection weeks for individual crickets reared on one of three concentrations of dietary melatonin (0, 10, 100 μg ml−1) added as a dietary supplement to the drinking water. Error bars represent standard errors about the mean. Brackets above columns for haemocyte concentration and lysozyme-like activity indicate significant increases across weeks (p-values indicate the level of significance) derived from models exploring variations within melatonin treatments (see §3 and table 1c–d).
(ii). Haemocyte concentration
The concentration of haemocytes detected in the haemolymph varied between the sexes, across weeks and with melatonin treatment (table 1c; figure 2a). To explore the nature of the interaction between melatonin treatment and week, we ran separate GLMs (that controlled for sex and cricket identity). These revealed that haemocyte concentration was comparable across weeks for crickets in the 0 μg ml−1 (slope ± s.e. across weeks: β = −0.74 ± 0.48; F1,68 = 2.38, p = 0.13) and 10 μg ml−1 melatonin treatments (slope ± SE across weeks: β = 0.92 ± 0.56; F1,58 = 2.72, p = 0.10), but haemocyte concentrations increased significantly across weeks for crickets provided with 100 μg ml−1 of dietary melatonin (slope ± s.e. across weeks: β = 1.68 ± 0.45; F1,60 = 6.61, p = 0.01). Similar post-hoc GLMs separated by sex (controlling for cricket identity and melatonin treatment) revealed that the significant interaction between sex and week (table 1c) arose because haemocyte concentration increased over the sampling period for males (slope ± s.e. across weeks: β = 1.29 ± 0.50; F1,72 = 6.77, p = 0.01) but not females (slope ± s.e. across weeks: β = −0.08 ± 0.41; F1,114 = 0.04, p = 0.84).
(iii). Lysozyme-like activity
Lysozyme-like activity varied significantly across weeks and with melatonin concentrations (table 1d; figure 2b). As in §3b(ii), to explore the nature of the interaction between melatonin treatment and week, we ran separate GLMs (that controlled for cricket identity). These revealed that lysozyme-like activity was comparable across weeks for crickets in the 0 μg/ml (slope ± s.e. across weeks: β = 0.002 ± 0.007; F1,57 = 0.12, p = 0.73) but increased significantly across weeks for crickets in the 10 μg ml−1 (slope ± s.e. across weeks: β = 0.03 ± 0.0.008; F1,47 = 14.09, p = 0.0005) and 100 μg ml−1 (slope ± s.e. across weeks: β = 0.02 ± 0.008; F1,53 = 6.34, p = 0.01) melatonin treatments.
(iv). PO activity
PO activity was comparable for all melatonin treatments (figure 2c; table 1e); however, it increased across the sampling weeks (table 1e; figure 2c), was significantly higher for females compared to males and was positively related to leg length (table 1e).
(c). Relationships among immune parameters
In females, lysozyme-like activity was correlated with haemocyte concentration (Pearson correlation: r = 0.60, p < 0.001, n = 128) and PO activity (Pearson correlation: r = 0.0.29, p = 0.001, n = 133). In contrast, there was no relationship between haemocyte concentration and PO activity (Pearson correlation: r = 0.12, p = 0.18, n = 128). In males, all measures of immune function were significantly correlated (Pearson correlations between haemocyte concentration and lysozyme-like activity: r = 0.60, p < 0.0001; haemocyte concentrations and PO activity: r = 0.48, p < 0.0001; lysozyme-like activity and PO activity: r = 0.55, p < 0.0001; n = 85).
4. Discussion
Our study provides evidence relating to the proposed links between LAN, concentrations of circulating melatonin and invertebrate immune function using the black field cricket, Teleogryllus commodus, as a model invertebrate. We demonstrate that even short-term supplementation with dietary melatonin through the adult phase of the life cycle modulated some aspects of immune function (but it provided no survival or fecundity advantage) for individuals exposed to constant illumination. The observed variation in response to supplementation with dietary melatonin across the three immune assays, and particularly the lack of response in PO activity, highlights the potential complexity of invertebrate immune function. Whilst our data suggest the potential for melatonin-mediated immune enhancement, further studies are required to identify whether this confers future fitness benefits, particularly for natural populations. We discuss our observations and highlight possible avenues for future research with a focus on invertebrate species.
(a). Identifying underlying mechanisms and future research areas
The link between circulating melatonin and immune function is rarely highlighted in any species other than vertebrates, and how melatonin modulates immune response remains a subject of debate [72,73,90]. The fact that crickets maintained on the 0 μg ml−1 melatonin treatment had comparable haemocyte concentrations and lysozyme-like activity across the majority of their adult lifespan supports previous findings that adult Teleogryllus commodus maintained under intense constant illumination may have compromised immunity and be less able to respond to immune challenges (J Durrant, EB Michaelides, MP Green and TM Jones 2013, unpublished data; see §1). This inability to respond to a challenge is likely a significant issue for nocturnal species such as T. commodus that not only inhabit both rural and urban environments but also have life history and mating strategies evolved to be optimal during periods of darkness [91]. The preliminary data presented here suggest that supplementation with dietary melatonin has a largely dose-dependent immune-enhancing effect on two of the three key measures of immune function: haemocyte concentration and lysozyme-like activity. This suggests that the core cellular response in invertebrate immunity [87] and the ability to resist bacterial infection may have the potential for recovery [74]. In contrast, melatonin supplementation had little effect on PO activity, despite its strong positive correlation with both haemocyte concentration and lysozyme activity and its consistent increased response over time. No firm conclusions can be drawn from the current experiment, but one possibility is that the invertebrate PO pathway, which is involved in repair, encapsulation and melanization [88] may either be less susceptible to variation in light or is able to trade off maintenance in cuticular melanization with investment in immune function [92].
Our experimental data also lend support to the idea that disruptions to endogenous levels of melatonin may be the underlying mechanism promoting variations in immune response observed in the presence of light. It remains to be tested whether such differences are observed at lower and potentially more ecologically relevant light intensities. However, recent evidence suggests that even relatively low light levels (5 lux) invoke compromised physiological function (including depressed immune function) [93,94]. Further investigation is also required to confirm definitively whether melatonin affects invertebrate immune function directly or indirectly via some other metabolic pathway. We did not determine exactly how immune function was modulated, but assessment of the effect of LAN on the antioxidant capacity of non-circulating melatonin, bound in tissues and cells such as the haemocytes themselves, may prove a valuable avenue for future research that aims to address directly the relative contribution made by melatonin as an antioxidant. A further point to note with respect to the current study is that adult crickets in this experiment were raised as juveniles in a relatively benign environment that had a semi-natural day–night cycle; the impact on life history trade-offs including immune function, reproduction and survival is likely to be considerably stronger when individuals are exposed to stress (including LAN) during the juvenile phase of their life cycle [94,95].
Evidence is mounting in support of the melatonin pathway as a potential source driving the observed behavioural and physiological changes induced by the presence of artificial light (for recent examples, see §1). Our research has broader species-level implications for invertebrate populations in urban environments. A reduced immune function may lead to reductions in parasite resistance and may ultimately have consequences for offspring and species fitness [74]. There is future potential to monitor egg hatching or offspring survival, two processes regulated through melatonin in the cricket, G. bimaculatus [41,83]. Cross-generational effects of artificial lighting are yet to be measured, but if females deposit melatonin in the eggs they lay, as is supposed the case for G. bimaculatus [41], then this might provide both a vital protective function and a circadian clock in early life which are disrupted by the presence of urban night lighting. The variation in female investment of melatonin may also explain the lack of observed variation in fecundity in the current experiment, as females were mated to males that were maintained under normal light conditions. Thus it is conceivable that male-derived melatonin transferred during mating was able to mitigate variation in female condition during egg-laying sensu the distasteful cantharidins transferred during mating by the male beetle, Neopyrochroa flabellate, which females then allocate to eggs, so providing them with protection from predation [96]. The fact that the effect of melatonin supplementation on immune function varied highlights the complexity of the underlying relationships between light, melatonin and life history traits. It also suggests that, regardless of taxa, caution should be exercised when making general inferences about the benefits of melatonin based on single trait assays because the relationship between melatonin concentration and immune function (for example) may not be comparable or indeed indicative of the relationship between melatonin and survival [82,93]. It is highly conceivable that, despite its ubiquity, species-level differences in life history and ontogeny will determine the relative importance of melatonin for circadian rhythm or immune function in varying lighting conditions (see also, [97]). A fuller understanding of the biosynthetic pathways for invertebrate melatonin, and confirmation and isolation of the receptors involved, will provide us with more predictive power to address these concerns [98].
Finally, an intriguing possibility, yet to be investigated, is the degree to which invertebrates and vertebrates are able to mitigate the negative effects of living in an environment that potentially compromises circulating concentrations of melatonin. The bioavailability of melatonin through dietary supplementation is thought to be varied, dependent on species and geography, but relatively low [99]; however, a number of laboratory studies, including this current study, have shown that supplementation with dietary melatonin can rapidly promote behavioural and physiological change [68,100,101]. This suggests that selective foraging of foods with high melatonin content may provide another means by which animals can mitigate the negative impact of living in brightly lit urban environments [102].
5. Conclusion
Ecological light pollution, in the form of LAN, is highly likely to remain a legacy of population expansion and urban growth. To minimize the biological impact of the presence of artificial LAN necessitates an understanding of underlying mechanisms. The complexity of the biological response requires an interdisciplinary approach that combines long-term field and experimental laboratory studies in behaviour, physiology and neurobiology. Such data are logistically challenging to obtain, particularly in vertebrates; however, given the ubiquitous nature and functionality of melatonin, invertebrates may provide a tractable avenue for future research in the area. Finally, despite the fact that species-specific (as well as taxon-wide) differences in response to LAN are already well documented, it is critical that research in the field includes studies on the myriad of invertebrates that may live in urban environments, particularly given their importance in maintaining the health of aquatic and terrestrial ecosystems.
Acknowledgements
The authors wish to thank the editors for the opportunity to write this paper; Dr Kirsty Turner (University of Melbourne) for assistance with immune assays; Drs Dedreia Tull and Thusitha Rupasinghe (Metabolomics Australia) for assistance with determining circulating concentrations of melatonin.
Authors' contributions
The data for this paper were collected by T.M.J. (survival and fecundity), J.D. (immune function and survival) and E.B.M. (survival and fecundity).
Funding statement
T.M.J. was funded by the University of Melbourne; M.P.G. is the Merck Serono Lecturer in Reproductive Biology and is partially funded by Merck Serono GmBH; J.D. and E.B.M. were supported by grants from the Holsworth Wildlife Research Endowment Fund.
Conflict of interests
M.P.G. is the Merck Serono Lecturer in Reproductive Biology.
References
- 1.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 2.Rich C, Longcore T. (eds). 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press. [Google Scholar]
- 3.Gaston KJ, Bennie J, Davies TW, Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 88, 912–927. [DOI] [PubMed] [Google Scholar]
- 4.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 43, 215–224. ( 10.1111/j.1600-079X.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 5.Eisenbeis G, Hanel A. 2009. Light pollution and the impact of artificial night lighting on insects. In Ecology of cities and towns (eds McDonnell MJ, Hahs AK, Breuste JH.), pp. 243–263. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Merkel FR, Johansen KL. 2011. Light-induced bird strikes on vessels in Southwest Greenland. Mar. Poll. Bull. 62, 2330–2336. ( 10.1016/j.marpolbul.2011.08.040) [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez A, Rodriguez B, Curbelo AJ, Perez A, Marrero S, Negro JJ. 2012. Factors affecting mortality of shearwaters stranded by light pollution. Anim. Cons. 15, 519–526. ( 10.1111/j.1469-1795.2012.00544.x) [DOI] [Google Scholar]
- 8.Freeman HJ. 1981. Alpine swifts feeding by artificial light at night. British Birds. 74, 149. [Google Scholar]
- 9.Polak T, Korine C, Yair S, Holderied MW. 2011. Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J. Zool. 285, 21–27. [Google Scholar]
- 10.Santos CD, Miranda AC, Granadeiro JP, Lourenco PM, Saraiva S, Palmeirim JM. 2010. Effects of artificial illumination on the nocturnal foraging of waders. Acta Oecol. 36, 166–172. ( 10.1016/j.actao.2009.11.008) [DOI] [Google Scholar]
- 11.Titulaer M, Spoelstra K, Lange C, Visser ME. 2012. Activity patterns during food provisioning are affected by artificial light in free living great tits (Parus major). PLoS ONE. 7, e37377 ( 10.1371/journal.pone.0037377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J. 2014. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J. Anim. Ecol. 83, 681–692. ( 10.1111/1365-2656.12150) [DOI] [PubMed] [Google Scholar]
- 13.Moore MV, Pierce SM, Walsh HM, Kvalvik SK, Lim JD. 2000. Urban light pollution alters the diel vertical migration of Daphnia. Verh. Int. Verein. Limnol. 27, 779–782. [Google Scholar]
- 14.Baker BJ, Richardson JML. 2006. The effect of artificial light on male breeding-season behaviour in green frogs, Rana clamitans melanota. Can. J. Zool. 84, 1528–1532. ( 10.1139/z06-142) [DOI] [Google Scholar]
- 15.Kempenaers B, Borgstrom P, Loes P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pairing success and lay-date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 16.Miller MW. 2006. Apparent effects of light pollution on singing behavior of American robins. Condor. 108, 130–139. ( 10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2) [DOI] [Google Scholar]
- 17.Montevecchi WA. 2006. Influences of artificial light on marine birds. In Ecological consequences of artificial night lighting (eds Rich C, Longcore R.), pp. 94–113. Washington, DC: Island Press. [Google Scholar]
- 18.Dominoni DM, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blask DE. 2009. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 13, 257–264. ( 10.1016/j.smrv.2008.07.007) [DOI] [PubMed] [Google Scholar]
- 20.Dickerman B, Liu JH. 2012. Does current scientific evidence support a link between light at night and breast cancer among female night-shift nurses? Review of evidence and implications for occupational and environmental health nurses . Workplace Health Saf. 60, 273–281. ( 10.3928/21650799-20120529-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauley SM. 2004. Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med. Hyp. 63, 588–596. ( 10.1016/j.mehy.2004.03.020) [DOI] [PubMed] [Google Scholar]
- 22.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. 2003. Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. J. Nat. Canc. Inst. 95, 825–828. ( 10.1093/jnci/95.11.825) [DOI] [PubMed] [Google Scholar]
- 23.Anisimov VN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, Semenchenko AV, Yashin AI. 2004. Effect of exposure to light-at-night on life span and spontaneous carcinogenesis in female CBA mice. Int. J. Cancer 111, 475–479. ( 10.1002/ijc.20298) [DOI] [PubMed] [Google Scholar]
- 24.Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. 2011. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36, 1062–1069. ( 10.1016/j.psyneuen.2011.01.004) [DOI] [PubMed] [Google Scholar]
- 25.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. 2009. Influence of light at night on murine anxiety- and depressive-like responses. Behav. Brain Res. 205, 349–354. ( 10.1016/j.bbr.2009.07.001) [DOI] [PubMed] [Google Scholar]
- 26.Vinogradova IA, Anisimov VN, Bukalev AV, Ilyukha VA, Khizhkin EA, Lotosh TA, Semenchenko AV, Zabezhinski MA. 2010. Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in young but not in old rats. Aging. 2, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonken LK, Haim A, Nelson RJ. 2012. Dim light at night increases immune function in Nile grass rats, a diurnal rodent. Chronobiol. Int. 29, 26–34. ( 10.3109/07420528.2011.635831) [DOI] [PubMed] [Google Scholar]
- 28.Dominoni DM, Partecke J. 2013. Long-term effects of chronic artificial night light exposure on life-history traits of songbirds. Integr. Comp. Biol. 53, E55. [Google Scholar]
- 29.Dominoni DM, Quetting M, Partecke J. 2013. Long-term effects of chronic light pollution on seasonal functions of European blackbirds (Turdus merula). PLoS ONE. 8, e85069 ( 10.1371/journal.pone.0085069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18 664–18 669. ( 10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arendt J, Skene D. 2005. Melatonin as a chronobiotic. Sleep Med. Rev. 9, 25–39. ( 10.1016/j.smrv.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 32.Zawilska JB. 1996. Melatonin as a chemical indicator of environmental light-dark cycle. Acta Neurobiol. Exp. 56, 757–767. [DOI] [PubMed] [Google Scholar]
- 33.Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ. 2010. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 85, 607–623. ( 10.1111/j.1469-185X.2009.00118.x) [DOI] [PubMed] [Google Scholar]
- 34.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. 1958. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 80, 2587 ( 10.1021/ja01543a060) [DOI] [Google Scholar]
- 35.Vivien-Roels B, Pevet P. 1993. Melatonin—presence and formation in invertebrates. Experientia. 49, 642–647. ( 10.1007/BF01923945) [DOI] [Google Scholar]
- 36.Hardeland R, Poeggeler B. 2003. Non-vertebrate melatonin. J. Pineal Res. 34, 233–241. ( 10.1034/j.1600-079X.2003.00040.x) [DOI] [PubMed] [Google Scholar]
- 37.Reiter RJ, Rosales-Corral SA, Manchester LC, Tan DX. 2013. Peripheral reproductive organ, health and melatonin: ready for prime time. Int. J. Mol. Sci. 14, 7231–7272. ( 10.3390/ijms14047231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bembenek J, Sehadova H, Ichihara N, Takeda M. 2005. Day/night fluctuations in melatonin content, arylalkylamine N-acetyltransferase activity and NAT mRNA expression in the CNS, peripheral tissues and hemolymph of the cockroach, Periplaneta americana. Comp. Biochem. Physiol. B. 140, 27–36. ( 10.1016/j.cbpc.2004.03.017) [DOI] [PubMed] [Google Scholar]
- 39.Itoh MT, Hattori A, Nomura T, Sumi Y, Suzuki T. 1995. Melatonin and arlyalklamine N-acetyltransferase activity in the silkworm, Bombyx mori. Mol. Cell Endocrinol. 115, 59–64. ( 10.1016/0303-7207(95)03670-3) [DOI] [PubMed] [Google Scholar]
- 40.Itoh MT, Shinozawa T, Sumi Y. 1999. Circadian rhythms of melatonin-synthesizing enzyme activities and melatonin levels in planarians. J. Pineal. Res. 830, 165–173. [DOI] [PubMed] [Google Scholar]
- 41.Itoh MT, Sumi Y. 1998. Melatonin and serotonin N-acetyltransferase activity in developing eggs of the cricket Gryllus bimaculatus. Brain Res. 781, 91–99. ( 10.1016/S0006-8993(97)01220-1) [DOI] [PubMed] [Google Scholar]
- 42.Dominoni DM, Goymann W, Helm B, Partecke J. 2013. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds. Front. Zool. 10, 60 ( 10.1186/1742-9994-10-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redman J, Armstrong S, Ng K. 1983. Free-running activity rhythms in the rat: entrainment by melatonin. Science 219, 1089–1091. ( 10.1126/science.6823571) [DOI] [PubMed] [Google Scholar]
- 44.Underwood H, Harless M. 1985. Entrainment of the circadian activity rhythm of a lizard to melatonin injections. Behav. Physiol. 35, 267–270. ( 10.1016/0031-9384(85)90348-8) [DOI] [PubMed] [Google Scholar]
- 45.Lu J, Cassone V. 1993. Daily melatonin administration synchronizes circadian patterns of brain metabolism and behavior in pinealectomized house sparrows, Passer domesticus. J Comp. Physiol. 173, 775–782. ( 10.1007/BF02451908) [DOI] [Google Scholar]
- 46.Heigl S, Gwinner E. 1994. Periodic melatonin in the drinking water synchronizes circadian rhythms in sparrows. Naturwissenschaften 81, 83–85. ( 10.1007/BF01138466) [DOI] [Google Scholar]
- 47.Poeggeler B. 1993. Melatonin and the light-dark zeitgeber in vertebrates, invertebrates and unicellular organisms—introduction. Experientia 49, 611–613. ( 10.1007/BF01923940) [DOI] [PubMed] [Google Scholar]
- 48.Morita M, Best JB. 1984. Effects of photoperiods and melatonin on planarian asexual reproduction. J. Exp. Zool. 231, 273–282. ( 10.1002/jez.1402310212) [DOI] [Google Scholar]
- 49.Finocchiaro L, Callebert J, Launay JM, Jallon JM. 1988. Melatonin biosynthesis in Drosophila—its nature and effects. J. Neurochem. 50, 382–387. ( 10.1111/j.1471-4159.1988.tb02923.x) [DOI] [PubMed] [Google Scholar]
- 50.Hintermann E, Jeno P, Meyer UA. 1995. Isolation and characterisation of an arylalkyamine N-acetyltransferase from Drosophila melanogaster. FEBS Lett. 375, 148–150. ( 10.1016/0014-5793(95)01198-N) [DOI] [PubMed] [Google Scholar]
- 51.Jallon JM, Callebert J, Launay JM, Finocchiaro L. 1990. Melatonin in Drosophila melanogaster heads—wild and mutant, period. In Régulation des cycles saisonniers chez les invertébrés (eds Ferron P, Missonnier J, Mauchamp B.), pp. 213–216. Paris, France: Institut National de la Recherche Agronomique. [Google Scholar]
- 52.Sainath SB, Swetha C, Reddy PS. 2013. What do we (need to) know about the melatonin in crustaceans? J. Exp. Zool. A. 319, 365–377. ( 10.1002/jez.1800) [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Qin Y, Li X, Song D, Qi M. 2007. Brain melatonin content and polyethism in adult workers of Apis mellifera and Apis cerana (Hym., Apidae). J. App. Entomol. 131, 734–739. ( 10.1111/j.1439-0418.2007.01229.x) [DOI] [Google Scholar]
- 54.Abran D, Anctil M, Ali MA. 1994. Melatonin activity rhythms in eyes and cerebral ganglia of Aplysia californica. Gen. Comp. Endocrinol. 96, 215–222. ( 10.1006/gcen.1994.1176) [DOI] [PubMed] [Google Scholar]
- 55.Itoh MT, Hattori A, Sumi Y, Suzuki T. 1995. Day-night changes in melatonin levels in different organs of the cricket (Gryllus bimaculatus). J. Pineal Res. 18, 165–169. ( 10.1111/j.1600-079X.1995.tb00156.x) [DOI] [PubMed] [Google Scholar]
- 56.Thakurdas P, Sharma S, Vanlalhriatpuia K, Sinam B, Chib M, Shivagaje A, Joshi D. 2009. Light at night alters the parameters of the eclosion rhythm in a tropical fruit fly, Drosophila jambulina. Chronobiol. Int. 26, 1575–1586. ( 10.3109/07420520903529765) [DOI] [PubMed] [Google Scholar]
- 57.Yamano H, Watari Y, Arai T, Takeda M. 2001. Melatonin in drinking water influences a circadian rhythm of locomotor activity in the house cricket, Acheta domesticus. J. Insect Physiol. 47, 943–949. ( 10.1016/S0022-1910(01)00067-1) [DOI] [Google Scholar]
- 58.Bentkowski P, Markowska M, Pijanowska J. 2010. Role of melatonin in the control of depth distribution of Daphnia magna. Hydrobiologia 643, 43–50. ( 10.1007/s10750-010-0134-x) [DOI] [Google Scholar]
- 59.Reiter R, Poeggeler B, Tan D, Chen L, Manchester L. 1993. Antioxidant capacity of melatonin: a novel action not requiring a receptor. Neuroendocrinol. Lett. 15, 103–116. [Google Scholar]
- 60.Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. 1993. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocrine J. 1, 57–60. [Google Scholar]
- 61.Garcia JJ, et al. 2014. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J. Pineal Res. 56, 225–237. ( 10.1111/jpi.12128) [DOI] [PubMed] [Google Scholar]
- 62.Skulachev VP. 2009. New data on biochemical mechanism of programmed senescence of organisms and antioxidant defense of mitochondria. Biochemistry (Mosc) 74, 1400–1403. ( 10.1134/S0006297909120165) [DOI] [PubMed] [Google Scholar]
- 63.Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. 2007. Melatonin role in the mitochondrial function. Front. Biosci. 12, 947–963. ( 10.2741/2116) [DOI] [PubMed] [Google Scholar]
- 64.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. 2003. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 50, 1129–1146. [PubMed] [Google Scholar]
- 65.Chuffa LGA, et al. 2011. Long-term melatonin treatment reduces ovarian mass and enhances tissue antioxidant defenses during ovulation in the rat. Brazil J. Med. Biol. Res. 44, 217–223. ( 10.1590/S0100-879X2011007500018) [DOI] [PubMed] [Google Scholar]
- 66.Benot S, Goberna R, Reiter RJ, Garcia-Maurino S, Osuna C, Guerrero JM. 1999. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res. 27, 59–64. ( 10.1111/j.1600-079X.1999.tb00597.x) [DOI] [PubMed] [Google Scholar]
- 67.Bonilla E, Medina-Leendertz S, Diaz S. 2002. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp. Gerontol. 37, 629–638. ( 10.1016/S0531-5565(01)00229-7) [DOI] [PubMed] [Google Scholar]
- 68.Coto-Montes A, Hardeland R. 1999. Antioxidative effects of melatonin in Drosophila melanogaster: antagonization of damage induced by the inhibition of catalase. J. Pineal Res. 27, 154–158. ( 10.1111/j.1600-079X.1999.tb00610.x) [DOI] [PubMed] [Google Scholar]
- 69.Lavara-Culebras E, Munoz-Soriano V, Gomez-Pastor R, Matallana E, Paricio N. 2010. Effects of pharmacological agents on the lifespan phenotype of Drosophila DJ-1 beta mutants. Gene 462, 26–33. ( 10.1016/j.gene.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 70.Teran R, Bonilla E, Medina-Leendertz S, Mora M, Villalobos V, Paz M, Arcaya JL. 2012. The life span of Drosophila melanogaster is affected by melatonin and thioctic acid. Invest. Clin. 53, 250–261. [PubMed] [Google Scholar]
- 71.Calvo JR, Gonzalez-Yanes C, Maldonado MD. 2013. The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 55, 103–120. ( 10.1111/jpi.12075) [DOI] [PubMed] [Google Scholar]
- 72.Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM. 2013. Melatonin: buffering the immune system. Int. J. Mol. Sci. 14, 8638–8683. ( 10.3390/ijms14048638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pohanka M. 2013. Impact of melatonin on immunity: a review. Cent. Eur. J. Med. 8, 369–376. ( 10.2478/s11536-013-0177-2) [DOI] [Google Scholar]
- 74.Beckage N. 2008. Insect immunology. San Diego: Academic Press. [Google Scholar]
- 75.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. 1980. Light supresses melatonin secretion in humans. Science 210, 1267–1269. ( 10.1126/science.7434030) [DOI] [PubMed] [Google Scholar]
- 76.Brainard GC, Richardson BA, Petterborg LJ, Reiter RJ. 1982. The effect of different light intensities on pineal melatonin content. Brain Res. 233, 75–81. ( 10.1016/0006-8993(82)90931-3) [DOI] [PubMed] [Google Scholar]
- 77.Figueiro MG, Wood B, Plitnick B, Rea MS. 2011. The impact of light from computer monitors on melatonin levels in college students. Neuroendocrinol. Lett. 32, 158–163. [PubMed] [Google Scholar]
- 78.Firth BT, Belan I, Kennaway DJ. 2006. Persistence of a plasma melatonin rhythm in constant darkness and its inhibition by constant light in the sleepy lizard, Tiliqua rugosa. J. Pineal Res. 41, 15–20. ( 10.1111/j.1600-079X.2006.00322.x) [DOI] [PubMed] [Google Scholar]
- 79.Kumar V, Van't Hof TJ, Gwinner E. 2007. Circadian behavioral and melatonin rhythms in the European starling under light-dark cycles with steadily changing periods: evidence for close mutual coupling? Hormones Behav. 52, 409–416. ( 10.1016/j.yhbeh.2007.04.011) [DOI] [PubMed] [Google Scholar]
- 80.Vera LM, Lopez-Olmeda JF, Bayarri MJ, Madrid JA, Sanchez-Vazquez FJ. 2005. Influence of light intensity on plasma melatonin and locomotor activity rhythms in tench. Chronobiol. Int. 22, 67–78. ( 10.1081/CBI-200038157) [DOI] [PubMed] [Google Scholar]
- 81.Moore CB, Siopes TD. 2000. Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gent. Comp. Endocrinol. 119, 95–104. ( 10.1006/gcen.2000.7496) [DOI] [PubMed] [Google Scholar]
- 82.Vinogradova IA, Anisimov V. 2013. Melatonin prevents the development of the metabolic syndrome in male rats exposed to different light/dark regimens. Biogerontology 14, 401–409. ( 10.1007/s10522-013-9437-4) [DOI] [PubMed] [Google Scholar]
- 83.Itoh MT, Sumi Y. 2000. Circadian clock controlling egg hatching in the cricket (Gryllus bimaculatus). J. Biol. Rhythm. 15, 241–245. ( 10.1177/074873040001500305) [DOI] [PubMed] [Google Scholar]
- 84.Drayton JM, Jennions MD. 2011. Inbreeding and measures of immune function in the cricket Teleogryllus commodus. Behav. Ecol. 22, 486–492. ( 10.1093/beheco/arr005) [DOI] [Google Scholar]
- 85.Ozkan E, et al. 2012. The measurement of plasma melatonin levels by high performance liquid chromatography. J. Exp. Int. Med. 2, 85–88. [Google Scholar]
- 86.Siva-Jothy MT, Moret Y, Rolff J. 2005. Insect immunity: an evolutionary ecology perspective. Adv. Insect. Physiol. 32, 1–48. ( 10.1016/S0065-2806(05)32001-7) [DOI] [Google Scholar]
- 87.Ribeiro C, Brehélin M. 2006. Insect haemocytes: what type of cell is that? J. Insect Physiol. 52, 417–429. ( 10.1016/j.jinsphys.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 88.Kanost MR, Gorman MJ. 2008. Phenoloxidases in insect immunity. In Insect immunology (ed. Beckage N.), pp. 69–96. San Diego, CA: Academic Press. [Google Scholar]
- 89.Cox DR. 1972. Regression models and life-tables. J. R. Stat. Soc. B. 34, 187–220. [Google Scholar]
- 90.Maestroni GJM, Conti A. 1993. Melatonin and the immune system. In Melatonin and the pineal gland: from basic science to clinical application (eds Touitou Y, Arendt J, Pevet P.), pp. 295–302. Amsterdam, The Netherlands: Excerpta Medica. [Google Scholar]
- 91.Holker F, Wolter C, Perkin EK, Tockner K. 2010. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25, 681–682. ( 10.1016/j.tree.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 92.Bailey NW. 2011. A test of the relationship between cuticular melanism and immune function in wild-caught Mormon crickets. Physiol. Entomol. 36, 155–164. ( 10.1111/j.1365-3032.2011.00782.x) [DOI] [Google Scholar]
- 93.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. 2011. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 7, 468–471. ( 10.1098/rsbl.2010.1108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borniger JC, McHenry ZD, Salloum BAA, Nelson RJ. 2014. Exposure to dim light at night during early development increases adult anxiety-like responses. Physiol. Behav. 133, 99–106. ( 10.1016/j.physbeh.2014.05.012) [DOI] [PubMed] [Google Scholar]
- 95.McNamara KB, van Lieshout E, Jones TM, Simmons LW. 2013. Age-dependent trade-offs between immunity and male, but not female, reproduction. J. Anim. Ecol. 82, 235–244. ( 10.1111/j.1365-2656.2012.02018.x) [DOI] [PubMed] [Google Scholar]
- 96.Eisner T, Smedley SR, Young DK, Eisner M, Roach B, Meinwald J. 1996. Chemical basis of courtship in a beetle (Neopyrochroa flabellata): Cantharidin as ‘nuptial gift’. Proc. Natl. Acad. Sci. USA 93, 6499–6503. ( 10.1073/pnas.93.13.6499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruning A, Holker F, Wolter C. 2011. Artificial light at night: implications for early life stages development in four temperate freshwater fish species. Aquat. Sci. 73, 143–152. ( 10.1007/s00027-010-0167-2) [DOI] [Google Scholar]
- 98.Cary GA, Cuttler AS, Duda KA, Kusema ET, Myers JA, Tilden AR. 2012. Melatonin: neuritogenesis and neuroprotective effects in crustacean x-organ cells. Comp. Biochem. Physiol. A. 161, 355–360. ( 10.1016/j.cbpa.2011.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. 2007. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42, 28–42. ( 10.1111/j.1600-079X.2006.00407.x) [DOI] [PubMed] [Google Scholar]
- 100.Gao N, Hardie J. 1997. Melatonin and the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 43, 615–620. ( 10.1016/S0022-1910(97)00015-2) [DOI] [PubMed] [Google Scholar]
- 101.Kashian DR, Dodson SI. 2004. Effects of vertebrate hormones on development and sex determination in Daphnia magna. Environ. Toxicol. Chem. 23, 1282–1288. ( 10.1897/03-372) [DOI] [PubMed] [Google Scholar]
- 102.Terron MP, Garrido M, Rodriguez AB. 2013. Beneficial effects of melatonin-rich and melatonin-enriched foods on health. Int. J. Health Nut. 4, 1–14. [Google Scholar]