Abstract
Artificial lighting allows humans to be active at night, but has many unintended consequences, including interference with ecological processes, disruption of circadian rhythms and increased exposure to insect vectors of diseases. Although ultraviolet and blue light are usually most attractive to arthropods, degree of attraction varies among orders. With a focus on future indoor lighting applications, we manipulated the spectrum of white lamps to investigate the influence of spectral composition on number of arthropods attracted. We compared numbers of arthropods captured at three customizable light-emitting diode (LED) lamps (3510, 2704 and 2728 K), two commercial LED lamps (2700 K), two commercial compact fluorescent lamps (CFLs; 2700 K) and a control. We configured the three custom LEDs to minimize invertebrate attraction based on published attraction curves for honeybees and moths. Lamps were placed with pan traps at an urban and two rural study sites in Los Angeles, California. For all invertebrate orders combined, our custom LED configurations were less attractive than the commercial LED lamps or CFLs of similar colour temperatures. Thus, adjusting spectral composition of white light to minimize attracting nocturnal arthropods is feasible; not all lights with the same colour temperature are equally attractive to arthropods.
Keywords: light emitting diodes, arthropods, phototaxis, indoor lighting, vector-borne disease
1. Introduction
Artificial night lighting is a major convenience in modern society, because an illuminated realm during the night provides more time for humans to safely stay active. Despite its practical application, contemporary night lighting poses risks to human and ecological health [1,2]. Circadian rhythms are biological cycles that run on a daily cycle and can be easily disrupted with exposure to certain wavelengths of light at night [3–5]. Night-time illumination allows humans to be more active at night, while simultaneously drawing vectors closer to humans [6]. Arthropods in particular are strongly affected by light at night, and numbers of phototactic species increase near the light sources throughout the night [7–10], which provides the basis for light traps used widely in entomology [11]. Attraction of insects to artificial lighting is also implicated in alterations in insect species distributions [12] and is a suspected but largely uninvestigated factor in declines of nocturnal species [13].
Spectral composition of light influences degree of positive phototaxis for insects [14–17]. Differences in wavelength, colour saturation and brightness of light are the most important characteristics that influence insect attraction to lights [18]. Similarities in positive phototaxis exist within orders [19]: for instance, mosquitoes and midges (Diptera) are attracted to ultraviolet (UV), blue and green light [20–23]. House flies (Glossina morsitans morsitans and Musca domestica; Diptera) show slight variation, exhibiting positive phototaxis to green and red lights in addition to UV [24]. Lepidoptera are strongly attracted to UV and blue [25–29], with a peak around 400 nm [3]. Kissing bugs (Hemiptera) are attracted to blue light and are guided by low intensity white lights [30], whereas honeybees (Hymenoptera) have positive phototaxis peaks in the UV and blue range of light [31].
The current trend in lighting technology is to replace older lamp types with energy-efficient light-emitting diode (LED) lamps for both indoor and outdoor lighting. The earliest LED lamps meant for area lighting consisted of a blue LED that was coated with a phosphor to create a full-spectrum white light that had very high emissions in the blue portion of the spectrum (i.e. a high colour temperature >5000 K; high colour temperatures appear ‘cold’, whereas low colour temperatures appear ‘warm’). Subsequent developments have led to LEDs with a range of colour temperatures for outdoor and indoor use (2700–5000 K), but all of these lamp types have more blue light emissions to which flying insects are generally attracted than do some older technologies (e.g. high-pressure sodium and low-pressure sodium lamps) [32]. Experimental investigations into insect attraction to LEDs have been mixed, with one study showing lower attraction of insects to LEDs of a range of colour temperatures than to high-pressure sodium and other lamp types [9] and another study showing greater attraction to a 4000 K LEDs than to high-pressure sodium lamps and no significant differences in attraction of insects to LEDs of different colour temperatures [33]. Furthermore, because of the full-spectrum nature of high-efficiency outdoor lights like LEDs, scientists have warned against increasing ecological effects resulting from greater blue light emissions, pointing to the effects on bats [34], insects [33], circadian rhythms across species [35,36] and ecological interactions [37–39].
Despite concern about the effects of the spectral composition of night lighting on wildlife posed by newer lighting technologies, few tools are available to predict attractiveness of any given source of light to insects. Most studies test representatives of different insect orders against specific wavelengths of light scattered across the spectrum or compare attraction for off-the-shelf lamps of various types and colour temperatures [7,9,15,33]. According to van Grunsven et al. [14], only two studies contain continuous attraction spectra that provide relative attractiveness of each wavelength for an insect group (i.e. [40] for moths and [31] for honeybees). With these curves, the attractiveness of any given light source can be calculated by multiplying the relative output of a given lamp at each wavelength by the reaction strength given for that wavelength in the model and then summing the resulting products [14]. In their recent work, van Grunsven et al. [14] found that these attractiveness curves did not perform well when lamps with high UV emissions (i.e. those measured to be highly attractive in both models) were not included in the evaluation.
In this study, we concentrate on indoor lighting that must be full spectrum to allow colour rendering. We take advantage of LEDs that create a full-spectrum light through use of multiple colours of diodes (red, blue, green; RBG) in conjunction with white diodes such that they can be adjusted in ways intended to minimize insect attraction. We use indices based on existing insect attraction curves to develop custom LED configurations and compare them with commercial LEDs and compact fluorescent bulbs of similar colour temperatures intended for indoor use. The results should also apply outdoors where full spectrum light is needed and LED technologies allow tuning of the spectrum. We note at the outset, however, that outdoor installations usually do not require full-spectrum lighting, and even lower colour temperatures and filters to avoid sensitive wavelengths would be environmentally preferable [36,41].
2. Material and methods
(a). Experimental design
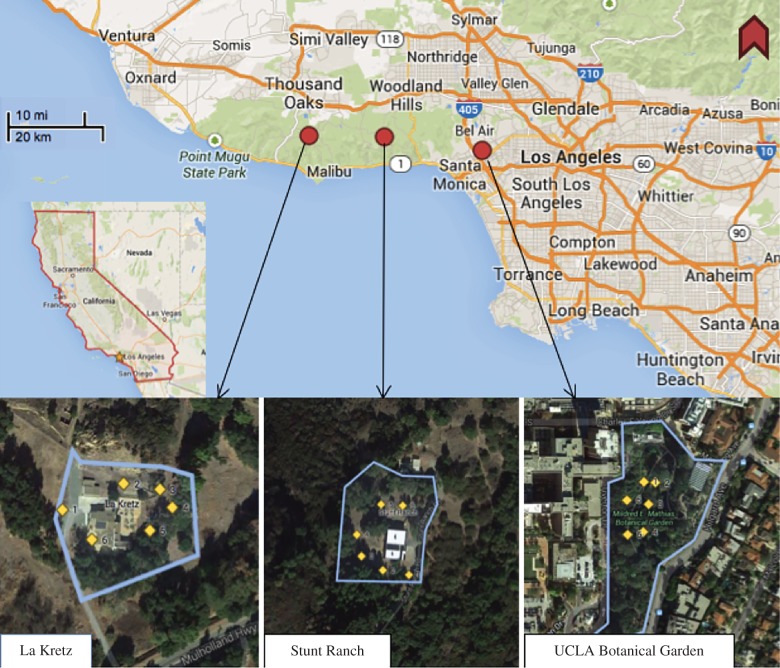
We captured arthropods in light traps at night between 17 February 2014 and 14 May 2014 at three sites in Los Angeles County, California (figure 1). An urban site was located in the UCLA Mildred E. Mathias Botanical Garden (34.066°N 118.441°W, Los Angeles, Los Angeles County). Two rural sites were UCLA La Kretz Center Field Station (34.097°N, 118.816°W, Malibu, Los Angeles County) and UCLA Stunt Ranch Santa Monica Mountains Reserve (34.093°N, 118.657°W, Calabasas, Los Angeles County). We erected six traps on each site each night, with a minimum of 11 m between traps. Each trap contained a separate light source—three tuneable LED lights consisting of RGB and W diodes produced by Philips Research Laboratories in Eindhoven, The Netherlands, one of two commercial LEDs with only W diodes, one of two commercial compact fluorescent lamps (CFLs), and an empty light housing as a ‘no light’ control (table 1).
Figure 1.
Site locations of insect traps and their placement at each field site for collecting in March–May 2014 in Los Angeles County, California. Two sites (La Kretz and Stunt Ranch) are in rural environments and the third (Mildred E. Mathias Botanical Garden) is in an urban environment. (Online version in colour.)
Table 1.
Characteristics of lamps used for light traps. A, B and C are customizable LEDs. LED1 and LED2 are two commercial LEDs, and CFL1 and CFL2 are compact fluorescent lamps. Predicted attractiveness to moths [40] and honeybees [31] is listed (see §2c).
| parameter | A | B | C | LED1 | LED2 | CFL1 | CFL2 |
|---|---|---|---|---|---|---|---|
| brand | Philips | Philips | Philips | Philips | Feit | Philips | SiaLite |
| colour temp. (K) | 3510 | 2704 | 2728 | 2700 | 2700 | 2700 | 2700 |
| CRI | 95 | 58 | 60 | 82 | 93 | 82 | 82 |
| output (lm) | 827 | 793 | 795 | 830 | 1065 | 790 | 1200 |
| pan illumination (lux) | 1675 | 1145 | 1830 | 275 | 656 | 253 | 525 |
| moth attractiveness | 0.00170 | 0.001494 | 0.001503 | 0.001676 | 0.001632 | 0.001820 | 0.001913 |
| honeybee attractiveness | 0.00100 | 0.00089 | 0.00089 | 0.001115 | 0.001070 | 0.001328 | 0.001409 |
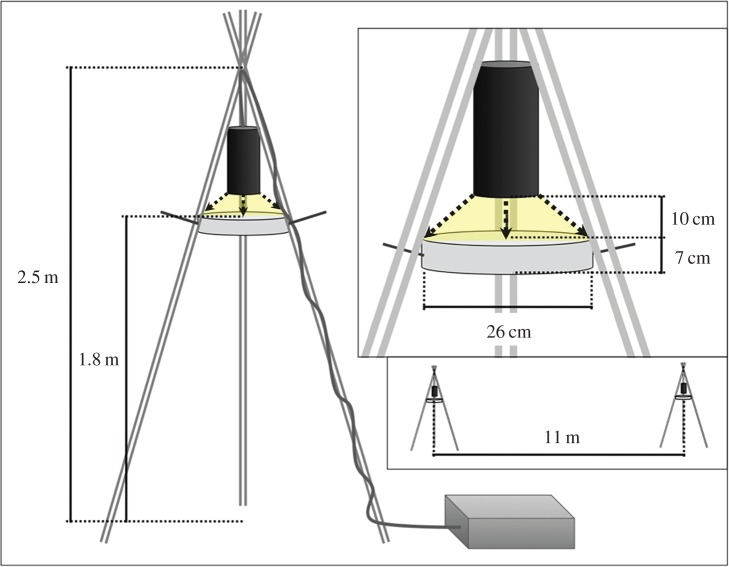
Light traps were PVC pipe tripods from which light sources were suspended above a collection receptacle (figure 2). The lights were fully shielded and directed downward at the pan trap. We constructed the pan trap from the bottom of a white plastic bucket (26 × 7 cm) that we suspended with 2-mm thick wire 10 cm below the light source and filled with soapy water to trap flying arthropods. The non-lighted control trap was an identical collection tray with the same housing but no lamp (figure 2).
Figure 2.
Schematic of light trap design, showing total height (2.5 m), collection dish height (1.8 m), distance of light source to collection dish (10 cm), height of collection dish (7 cm), diameter of collection dish (26 cm) and minimum distance between two light traps (11 m). Traps were deployed March–May 2014 in Los Angeles County, California. (Online version in colour.)
After 19 collections, we confirmed with measurements that our commercial LED lamps and CFLs were not producing the illumination specified and we replaced those two bulbs with models with greater output. Illumination produced by each lamp was measured at surface of the pan trap and at 2 m horizontally from the trap with an ExTech light meter (model no. 401027). Light from each of the lamps was directed downward at the pan trap in the same manner.
We collected samples during 32 nights: 16 at the urban site and eight each at the two rural sites. At least one night was skipped between consecutive collections at a field site to minimize any effects from the previous collection and to avoid depleting the number of arthropods.
On each night of collecting, we recorded temperature, humidity and wind speed at the beginning and end of each period of collection. Additionally, we recorded moon phase and position of traps within each site to account for potential environmental effects that could affect the number of arthropods collected in each light. We recorded ambient temperature (±0.1°C) with a digital thermometer and wind speed (±0.1 m s−1) with a handheld anemometer.
We turned lights on at sunset and off at sunrise to collect during this period; sunset times varied from 17 : 39 to 19 : 47 and sunrise times varied from 06 : 13 to 07 : 06 during the study. We rotated the location of traps each treatment clockwise at each site on successive collection nights to provide at least one night at each position. All specimens collected were immediately removed from traps after one elapsed night of collecting and taken to the laboratory for sorting.
(b). Sorting procedures
We rinsed each sample through a 255 µm mesh and preserved contents in 95% ethanol. We then transferred specimens to a Petri dish filled with 95% ethanol and observed them under a dissecting microscope. We sorted to taxonomic order with guidance from reference figures and descriptions [42,43]. Each order from each night of collecting was then prepared separately and deposited at the Natural History Museum of Los Angeles County.
(c). Light spectrum selection
Lamps with lower colour temperatures are known to attract fewer insects [8,9], so we used two LED lamp configurations that had a colour temperature of approximately 2700 K (2704 and 2728 K) and controls that had a 2700 K colour temperature. For comparison, we included one custom LED configuration with a colour temperature of 3510 K.
The three customizable LED lamps could be tuned to one of six configurations, each with emissions curves provided by the manufacturer. Modifying slightly the approach of van Grunsven et al. [14], we calculated relative attractiveness of each configuration by multiplying percentage total output at each wavelength by percentage total attractiveness for each wavelength for moths [40] and honeybees [31]. In this manner, a lamp that followed each response curve exactly would have an attractiveness of unity, whereas one that avoided those wavelengths entirely would have an attractiveness of zero. Both the insect attraction curves [31,40] were obtained in digital form from the International Commission on Illumination. We then chose the configurations of the lamps that minimized attractiveness for both curves at 3510 K colour temperature (one lamp, designated A), and at approximately 2700 K colour temperature (two lamps, B and C).
For the commercial CFLs and LEDs (both 2700 K), we used a USB650 red tide spectrometer (Ocean Optics) to measure the spectral profile and then calculated the attractiveness index for each emission spectrum.
(d). Statistical methods
We used a generalized linear model (GLM) with a Poisson distribution and log link function to analyse number of arthropods captured. We tested for and adjusted for overdispersion of the counts by estimating the overdispersion factor and adjusting the likelihood functions and confidence intervals accordingly in JMP Pro v. 11.2 (SAS Inc., Cary, NC). Environmental variables, location, pan illumination and lamp type were used to predict the number of specimens trapped for each order that contained a sufficient sample size; the remaining orders were pooled together. Illumination measured horizontally from the trap correlated highly with vertical illumination at the pan (Pearson's r = 0.97) and is not included in any models. We standardized environmental variables by their mean and range, so that all parameters ranged from zero to unity and thus their coefficients in the model could be compared. We evaluated alternative models with Akaike's information criterion scores corrected for sample size (AICc) [44].
We also performed, with a Bayesian probabilistic model, pairwise comparisons of the effect of lamp type on number of arthropods captured. A Bayesian approach reduces the risk of falsely finding a significant difference when making multiple pairwise comparisons. We computed the posterior probability distribution of the effect of lamp type conditioned to the data observed via Markov chain Monte Carlo simulations with JAGS v. 3.3.0 and R v. 3.0.1 software programs. We extracted samples from five chains, and a 5000 iteration burn-in period was used to dilute the influence of initial values in the results. Samples were thinned at five steps to reduce the time correlation between them. The correlation between successive samples was inspected with autocorrelation plots. We standardized environmental variables by their mean and standard deviation to improve the mixing properties of the chain. We also transformed the predicted variable by adding 1 to each value of captured arthropods to avoid taking the log of zero in the model.
We also calculated at the correlation (Pearson's r) between the GLM coefficients for each lamp type and the attractiveness values calculated for the lamps and did the same for the Bayesian model coefficients.
The dataset supporting this article is provided as the electronic supplementary material.
3. Results
(a). Characterization of lamps
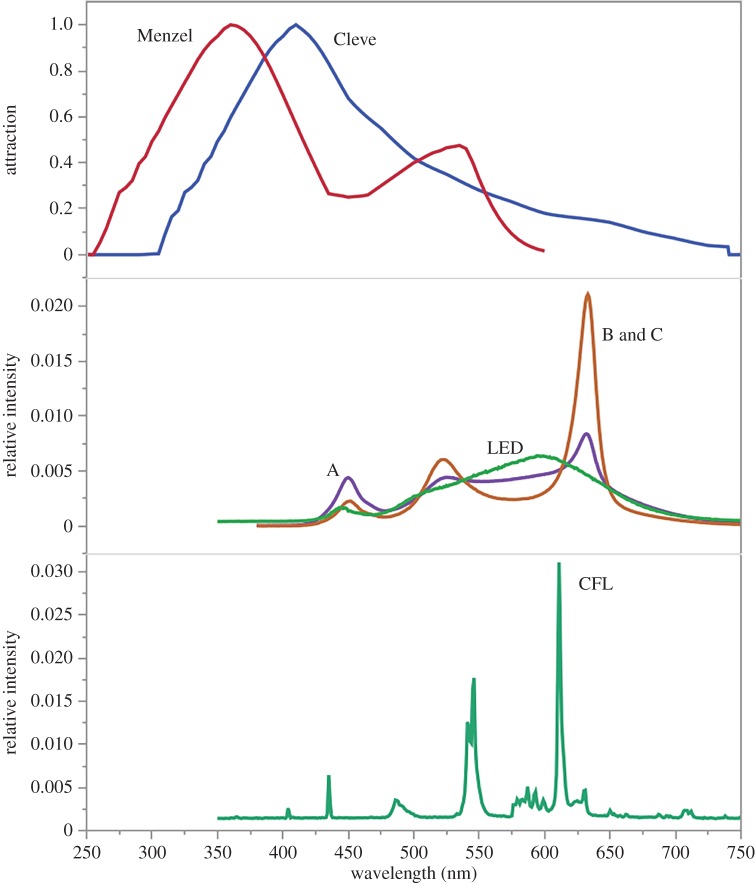
The spectral output of the 2700 K tuneable LEDs (B and C) differed substantially from that of the commercial LEDs (figure 3). The tuneable 2700 K LEDs are characterized by peaks of emission at 450 and 525 nm with the greatest output at 675 nm, whereas the commercial LEDs had a higher output across the shorter wavelengths. The 3500 K LED had emission peaks similar to the 2700 K lamps, but also had greater output at all wavelengths between those peaks. The two commercial LEDs and two CFLs used as controls were so similar in output that they were considered to be the same treatment for the remainder of the calculations (figure 3). Lamps B and C were very similar except that C had slightly more emissions in the red portion of the spectrum.
Figure 3.
Insect attraction and lamp output curves by wavelength. Top: response curves for moths (Cleve) [40] and honeybees (Menzel) [31]. Middle: output from 3500 K custom LED (A), 2700 K custom LEDs (B,C, with differences so minor they are not visible at this scale) and commercial 2700 K LEDs (LED; representing two lamps with minor differences). Bottom: output from commercial 2700 K compact fluorescent lamp (CFL; representing two lamps with minor differences). (Online version in colour.)
(b). Insect collections
We collected 5579 arthropods over 32 nights. Mean numbers of arthropods varied greatly by order and lamp type (figure 4). Diptera made up 67.5% of the specimens, Lepidoptera accounted for 12.0% and all remaining orders for the remaining 20% with Collembola (7.5%) and Hymenoptera (4.4%) making the greatest proportion.
Figure 4.
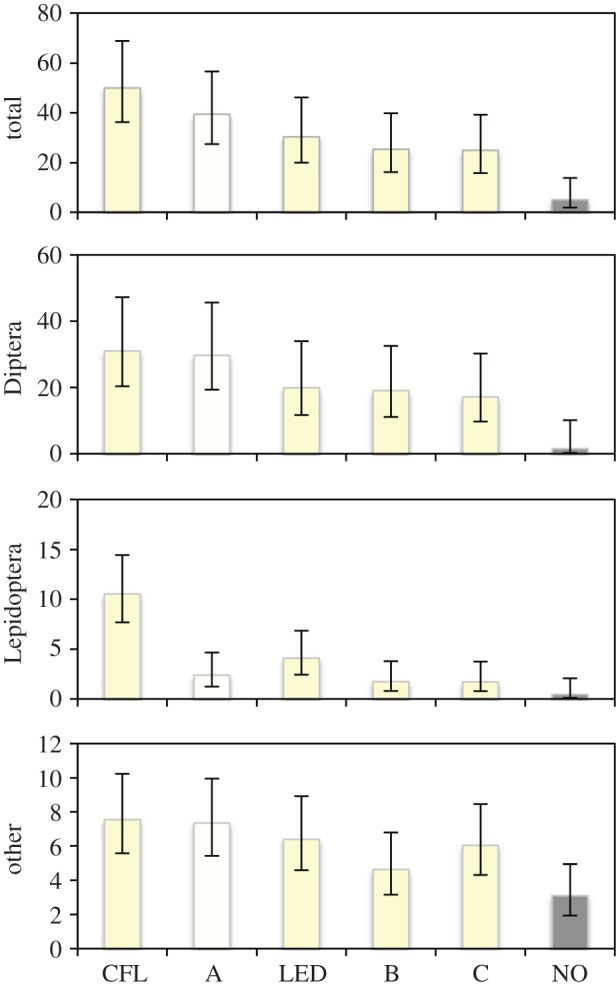
Mean of the number of specimens caught per night for all specimens, Diptera, Lepidoptera and other orders combined for each lamp type (n = 32 nights) for collections March–May 2014 in Los Angeles County, California. 95% confidence intervals calculated from generalized linear model for each grouping (see §2d). A, 3510 K custom LED; B, 2704 K custom LED; C, 2728 K custom LED; CFL, 2700 K compact fluorescent lamps; LED, commercial 2700 K LEDs; NO, control. (Online version in colour.)
(c). Environmental conditions
Overnight temperature ranged from 9.65°C to 23.25°C, humidity ranged from 19 to 89%, and winds ranged from calm to 1.6 m s−1. Temperature, wind speed and humidity were correlated, but did not exceed an absolute value of 0.55 and so were included in the multivariate analyses. Percentage moon visible ranged from 9.9 to 99.8, but we did not include it in the models because it correlated with weather variables such that results would be spurious: the high degree of light pollution in Los Angeles County would certainly confound levels of light from different moon phases, especially under cloud cover [45], and the time of moonrise may or may not occur during periods of peak invertebrate flights.
(d). Generalized linear models
With a GLM, we compared all lamp types to investigate the effect on number of total specimens, Diptera, Lepidoptera and other orders combined. Candidate models included mean temperature, relative humidity, maximum wind speed, lamp type, lamp placement in the field and illumination from the lamp as explanatory variables.
The best models (lowest AICc) for each group only included lamp type (table 2). For the Lepidoptera, the coefficient for the 2700 K CFL was greatest, followed by the commercial LED, the 3500 K LED, then the two 2700 K LEDs (table 3). For the Diptera and other orders model, the 3500 K LED was more attractive than the commercial LED, but CFL was most attractive and the tuneable 2700 K LEDs were least attractive.
Table 2.
Generalized linear models assessing contribution of lamp type, illumination, location and environmental conditions on number of Diptera, Lepidoptera, and other orders of invertebrates captured per night at two rural (n = 8 each) and one urban (n = 16) site in Los Angeles County, California, March–May, 2014.
| model variables | chi-squared | d.f. | p < chi-squared | AICc | ΔAICc |
|---|---|---|---|---|---|
| all specimens | |||||
| lamp | 35.16 | 5 | <0.0001 | 169.98 | |
| lamp, site | 114.80 | 22 | <0.0001 | 234.64 | −64.66 |
| lamp, site, humidity | 457.89 | 23 | <0.0001 | 294.41 | −124.43 |
| lamp, site, temperature, humidity | 542.70 | 24 | <0.0001 | 308.53 | −138.55 |
| lamp, site, temperature, humidity, illumination | 564.64 | 25 | <0.0001 | 312.51 | −142.53 |
| lamp, site, temperature, wind, humidity, illumination | 579.05 | 26 | <0.0001 | 318.01 | −148.03 |
| Diptera | |||||
| lamp | 27.13 | 5 | <0.0001 | 153.16 | |
| lamp, site | 123.49 | 22 | <0.0001 | 226.65 | −73.49 |
| lamp, site, humidity | 537.47 | 23 | <0.0001 | 288.21 | −135.05 |
| lamp, site, temperature, humidity | 632.91 | 24 | <0.0001 | 290.43 | −137.27 |
| lamp, site, temperature, wind, humidity | 636.64 | 25 | <0.0001 | 292.96 | −139.80 |
| lamp, site, temperature, wind, humidity, illumination | 632.52 | 26 | <0.0001 | 293.46 | −140.30 |
| Lepidoptera | |||||
| lamp | 58.76 | 5 | <0.0001 | 182.32 | |
| lamp, site | 217.15 | 22 | <0.0001 | 275.84 | −93.53 |
| lamp, site, temperature | 384.10 | 23 | <0.0001 | 343.30 | −160.98 |
| lamp, site, temperature, illumination | 495.83 | 24 | <0.0001 | 374.46 | −192.14 |
| lamp, site, temperature, wind, Illumination | 572.33 | 25 | <0.0001 | 397.24 | −214.92 |
| lamp, site, temperature, wind, humidity, illumination | 597.89 | 26 | <0.0001 | 407.30 | −224.98 |
| other orders | |||||
| lamp | 15.17 | 5 | <0.0001 | 277.85 | |
| lamp, site | 64.24 | 22 | <0.0001 | 337.48 | −59.63 |
| lamp, site, temperature, wind | 102.87 | 24 | <0.0001 | 354.33 | −76.48 |
| lamp, site, temperature | 98.88 | 23 | <0.0001 | 360.25 | −82.40 |
| lamp, site, wind, temperature, illumination | 102.39 | 25 | <0.0001 | 360.43 | −82.58 |
| lamp, site, wind, temperature, humidity, illumination | 105.18 | 26 | <0.0001 | 365.45 | −87.60 |
Table 3.
Coefficients for best generalized linear model explaining attraction of all specimens, Diptera, Lepidoptera, and other orders (mean; 95% confidence interval and chi-squared p-values). Data collected over 32 total nights at two rural and one urban site in Los Angeles County, California, March–May 2014.
| term | all specimens | Diptera | Lepidoptera | other orders |
|---|---|---|---|---|
| intercept | 3.18; 2.93–3.39; p < 0.0001 | 2.67; 2.19–2.98; p < 0.0001 | 0.83; 0.42–1.14; p = 0.0005 | 1.72; 1.57–1.86; p < 0.001 |
| lamp type [A] | 0.49; 0.12–0.86; p < 0.02 | 0.71; 0.22–1.28; p = 0.0048 | 0.04; −0.63–0.66; n.s. | 0.27; −0.02–0.55; n.s. |
| lamp type [B] | 0.05; −0.39–0.47; p = 0.80 | 0.27; −0.31–0.88; n.s. | −0.27; −1.06–0.40; n.s. | −0.18; −0.55–−0.14; n.s. |
| lamp type [C] | 0.03; −0.41–0.45; p = 0.86 | 0.17; −0.43–0.79; n.s. | −0.28; −1.09–0.39; n.s. | 0.07; −0.24–0.28; n.s. |
| lamp type [CFL] | 0.73; 0.38–1.08; p < 0.0001 | 0.76; 0.27–1.33 p = 0.0024 | 1.52; 1.11–1.98; p < 0.0001 | 0.30; 0.001–0.58; p = 0.049 |
| lamp type [LED] | 0.23; −0.18 – 0.63; p = 0.26 | 0.32; −0.25–0.92 n.s. | 0.57; 0.02–1.12; p = 0.041 | 0.13; −0.18–0.43; n.s. |
(e). Bayesian comparisons of attractiveness
Using a Bayesian log linear Poisson model, we assessed statistical significance of the difference in effects caused by lamp type on number of arthropods captured. Variables included in the model were mean temperature, mean relative humidity, lamp type, lamp placement in the field and maximum wind speed. From this statistical model, we computed a set of posterior probability distributions of the random variables representing the effect difference of two types of lamps on the number of arthropods captured. A positive value for this difference indicates that the trap illuminated by the first lamp is expected to capture more arthropods than the trap with the second lamp. The reverse is valid if the difference is negative. The difference is considered statistically significant if the 95% highest density interval (HDI) of its posterior distribution data does not contain the value zero. The mean and 95% HDI for a set of pairwise comparisons between lamp types (table 4) notably shows that the custom LEDs attracted significantly fewer insects than a commercial LED of the same colour temperature.
Table 4.
Difference of effect of different lamp types on the number of invertebrates captured in traps. Data collected over 32 total nights split among two rural (eight nights each) and one urban site (16 nights) in Los Angeles County, California, March–May 2014.
| pairwise comparison | mean (*p < 0.05) | 95% HDI | expected relative difference in insect capture (%) |
|---|---|---|---|
| all specimens | |||
| A–no | 1.97* | 1.8 : 2.13 | 717 |
| B–no | 1.66* | 1.48 : 1.82 | 526 |
| C–no | 1.65* | 1.47 : 1.82 | 521 |
| C–A | −0.32* | −0.41:−0.22 | −27 |
| C–B | 0.0019 | −0.105 : 0.098 | 0.2 |
| LED–A | −0.13* | −0.22:−0.038 | −12 |
| LED–B | 0.18* | 0.084 : 0.28 | 20 |
| LED–C | 0.19* | 0.089 : 0.28 | 21 |
| LED–CFL | −0.37* | −0.47:−0.29 | −31 |
| Diptera | |||
| A–no | 2.83* | 2.55 : 3.13 | 1695 |
| B–no | 2.61* | 2.31 : 2.9 | 1360 |
| C–no | 2.51* | 2.22 : 2.81 | 1230 |
| C–A | −0.32* | −0.44:−0.21 | −27 |
| C–B | −0.096 | −0.22 : 0.027 | −9 |
| LED–A | −0.2* | −0.32:−0.087 | −18 |
| LED–B | 0.022 | −0.1 : 0.15 | 2 |
| LED–C | 0.12 | −0.0042 : 0.24 | 12 |
| LED–CFL | −0.27* | −0.39:−0.16 | −24 |
| Lepidoptera | |||
| A–no | 1.75* | 1.2 : 2.3 | 574 |
| B–no | 1.38* | 0.81 : 1.95 | 397 |
| C–no | 1.32* | 0.76 : 1.9 | 374 |
| C–A | −0.43* | −0.78:−0.066 | −35 |
| C–B | −0.058 | −0.44 : 0.32 | −6 |
| LED–A | 0.49* | 0.18 : 0.79 | 163 |
| LED–B | 0.86* | 0.52 : 1.2 | 236 |
| LED–C | 0.92* | 0.59 : 1.2 | 251 |
| LED–CFL | −0.88* | −1.11:−0.65 | −58 |
| Hemiptera | |||
| A–no | 0.61 | −0.37 : 1.62 | 84 |
| B–no | 0.21 | −0.74 : 1.23 | 23 |
| C–no | 0.4 | −0.56 : 1.41 | 49 |
| C–A | −0.21 | −1.08 : 0.65 | −19 |
| C–B | 0.19 | −0.7 : 1.09 | 21 |
| LED–A | 0.85* | 0.098 : 1.62 | 234 |
| LED–B | 1.25* | 0.41 : 2.14 | 349 |
| LED–C | 1.06* | 0.26 : 1.86 | 288 |
| LED–CFL | 0.45 | −0.24 : 1.15 | 57 |
(f). Performance of attractiveness indices
The modelled attractiveness index for bees correlated significantly with the GLM model coefficients for Lepidoptera (r = 0.99; p = 0.0003). The modelled attractiveness index for moths correlated with GLM coefficients for Diptera (r = 0.89, p = 0.04), Lepidoptera (r = 0.91; p = 0.02). When data from the CFL were excluded (as suggested by van Grunsven et al. [14]), the modelled bee attractiveness index correlated poorly with Diptera attraction (n.s.), but extremely well with Lepidoptera attraction (r = 0.98; p = 0.015), and weakly with attraction for other orders (n.s.). Similar results were found for correlations with coefficients from the Bayesian analysis.
4. Discussion
All lamp types attracted more arthropods than the no-light control; thus, it is likely that in this regard, reducing arthropods attracted to light with currently available technology will always be a matter of mitigating the effects, which is true for many of the adverse effects of artificial night lighting [1,46,47]. Inasmuch as all light attracts arthropods, our finding that LEDs generally attract substantially fewer moths and other arthropods than a CFL with the same colour temperature is consistent with previous research [9,14,15,26,27]. It contradicts the broad claim by Pawson & Bader [33] that LEDs always worsen ecological light pollution, which was derived from comparisons of 4000 K LEDs to high-pressure sodium vapour lights (which have a lower colour temperature). Colour temperature mattered in our results, again differing from Pawson & Bader [33], with our 3500 K tuneable LED generally being more attractive to arthropod groups than the commercial 2700 K LEDs. The 3500 K LED, however, was as attractive to Diptera as the 2700 K CFL. The difference in the response of Diptera may reflect a different response spectrum for flies compared with moths and other insects; flies exhibit attraction to green and red light as well as to shorter wavelengths [24] and the 3500 K LED had emissions spread through the green and into the red.
We found that our two tuneable 2700 K LEDs were 20% and 21% less attractive to all orders combined than the commercial 2700 K LED in the Bayesian models. Because of slight differences in the housings for the lamps, the amount of light delivered on the pan traps was higher for the custom LEDs than for the commercial LED, so this result does suggest that spectrum was the dominant variable in the differences observed—a more intense custom spectrum was less attractive to arthropods than the corresponding commercially available spectrum at the same colour temperature. Notwithstanding recent results [33], previous research has shown lower colour temperature LEDs attract fewer arthropods than higher colour temperatures [9]. Our results show for the first time, to the best of our knowledge, that even at the same colour temperature, adjustment of spectral composition can influence insect attraction. For example, the two 2700 K custom LEDs attracted around three times fewer Hemiptera than the commercial 2700 K LEDs (table 4).
It may surprise some that illumination was not found to be important in predicting the attraction of invertebrates to the different light sources. The numbers of insects captured at light traps, however, does not increase linearly with illumination, but rather it increases with the square root of the ratio of the illumination from the lamp to the background illumination [48] or as a function of the logarithm of the luminance as suggested by Stevens' power law [49] and its application to sensory phenomena in insects [50]. That is, a doubling of light intensity does not result in a doubling of insects captured at light traps, meaning the influence of the intensity of our lamps on insect attraction can be expected to be smaller than the absolute differences in luminance or illumination would suggest. In our results, therefore, the spectral composition of the lamps was significantly more important than the range in illumination produced (275–1830 lux).
The experiment was designed to focus on spectrum and not on light intensity because effect of intensity will not be the same across lamp types—every lamp of a particular spectral composition will have its own curve relating light intensity to insect attraction. For example, if the spectrum of a certain lamp attracts no insects, then insect attraction and intensity are independent variables and the slope of the curve will be zero. Conversely, this slope is expected to be positive for a different lamp emission spectrum that does attract insects. Thus, any model of insect attraction that incorporates light intensity must also incorporate the interaction spectral emissions and light intensity, and these models likely would differ for taxonomic groups. Our experimental design did not include sampling at different light intensities that would have been necessary to build such a model (the lamps A, B, C were tested at only one intensity each, and commercial CFL and LEDs at only two intensities each).
Our results should encourage continued research into the usefulness of insect spectral response curves to predict the number of arthropods attracted to lights. In previous research, when lamps with UV emissions were excluded, the attractiveness curves did not explain the number of insects captured at remaining light traps [14]. In contrast, we found that for some orders the attractiveness indices correlated well with arthropods captured for four LED lamps. Several issues arise with these results. First, despite the strong correlation between the bee attractiveness spectrum [31] and model coefficients indicating Lepidoptera attraction, both with and without CFLs, these correlations are with four or five values only and many more lamps should be compared. This line of enquiry is an important direction for future research. Second, we lack an explanation or mechanism to account for the superior performance of the bee attraction curve [31] compared with the moth attraction curve [40] in predicting moth attraction. Third, partial light response curves for a range of insects suggest that outside of the general patterns (i.e. most insects are attracted to blue and UV, but some orders are also attracted to red and green), species groups may each have distinctive patterns of attraction to light and it may be unwise to seek one response curve to guide development of lamps to minimize attraction of all insect groups. Beyond minimizing blue and eliminating UV, human exposure to insect vectors may require directed experiments with individual vector species.
The pattern of attraction of individuals by Order was more similar to that previously recorded in temperate zones than in the tropics. The most common Order collected in this study was Diptera, similar to results comparing different lamp types including LEDs in an agricultural setting in The Netherlands [14], along a river in Germany [9] and between a coniferous forest and coastal grassland in New Zealand [33], whereas a similar study in an urban tropical habitat in Brazil was dominated by Isoptera [15]. The relative contributions of different orders varied by lamp type, which supports order-specific analysis of attraction. Differences between orders is likely to be important relative to managing insect vectors, where attraction of Hemiptera and Diptera are exceedingly more important than attraction of Lepidoptera. Removing wavelengths that are attractive to moths may be insufficient to minimize risk of attracting vectors, and indeed, in this study, a custom 3500 K LED was almost as attractive to Diptera as was a 2700 K CFL, whereas the same custom LED was substantially less attractive to Lepidoptera than the CFL.
Overall, our results suggest that indoor lighting sources with full spectrum light can be designed to reduce insect attraction. Some trade-off in colour rendering index and lamp efficiency is probably necessary to minimize insect attraction, but values of our prototype examples were acceptable for indoor use. Our LEDs are tuneable through use of RGB and W diodes so the efficiency penalty normally associated with RGB lamps is offset by also using W to achieve the desired colour temperature. Use of a white diode along with RGB improves the colour rendering index, because colour rendering is related to continuity of the spectrum.
Although we did not identify to species and therefore did not document individual disease vectors, the results represent progress towards development of energy-efficient indoor lighting that has promise to reduce insect-borne disease while simultaneously providing high-quality light. Potential harm to humans arises from disease-vectoring insects that transmit life-threating diseases that are documented worldwide, especially in tropical environments where protection against insects is scarce and lights at night are necessary for habitation [51]. Malaria, leishmaniasis and Chagas disease are major diseases vectored by species of Diptera and Hemiptera that can be influenced by artificial lights [52]. Transmissions of these diseases vary with species of insects involved as well as intensity and spectrum of light [6]. Fly species (Phlebotomus spp.) responsible for transmitting leishmaniasis are attracted to lights, lending evidence that exterior lighting should be considered a risk factor [53], and the hemipteran vectors of Chagas disease (Triatoma spp., Paratiatoma spp.; Reduviidae) are positively phototactic, so the importance of lighting as a continued research topic for vector borne disease is well established [6,52]. The connection between malaria and night lighting is still not fully understood, perhaps because light traps with a passive collection technique (e.g. pan traps) do not often capture mosquitos. Mosquitos do exhibit positive phototaxis, however, and are captured at lights with suction traps [6,54,55]. Disease transmission by mosquitos may, therefore, increase with artificial lighting [6], but this aspect has not yet been fully investigated. Further research into the relationship between spectral output of lamps and insect vectors is necessary to realize the potential of reducing exposure through better indoor (and outdoor) lighting.
The implications of night-time lighting for attraction of disease vectors [6], when combined with the expanding research on the effects of light on circadian rhythms and ecosystem functions [38,56–58], may persuade lighting engineers to follow a new standard that extends beyond display, price and durability, to include improved environmental and human health outcomes as well. Spectral characteristics that minimize insect attraction probably also reduce impacts on circadian rhythms, with its peak response to blue light [2]. We have demonstrated a proof-of-concept approach to minimize some of the ecological effects of both indoor and outdoor lighting installations by customizing lights to avoid sensitive portions of the visible spectrum, as has been suggested for ecological and chronobiological reasons [36,41,46]. Outdoor lighting, however, may need to be further restricted to avoid full spectrum lighting altogether to avoid adverse effects on human health, astronomical observation and ecosystems [36,41].
Supplementary Material
Acknowledgements
Willem van Hoof of Philips Research Europe produced the prototype LED lamps. We thank the UCLA La Kretz Center for California Conservation Science (especially Mario Colon), the Stunt Ranch Santa Monica Mountains Reserve, the National Park Service (permit no. SAMO-2014-SCI-002) and the UCLA Botanical Garden for access to and permission to sample at research sites. We thank F. Hölker and the anonymous reviewers for constructive and insightful comments.
Author's contributions
A.B. conceived the research topic with T.L. H.A., J.E., S.F., L.F., E.H.-Y., L.P., W.Y. and T.L. designed the experiment. H.A., J.E., S.F., L.F., E.H-.Y., L.P. and W.Y. collected and sorted the specimens, and all authors undertook data analysis and prepared the manuscript. All authors gave approval for publication.
Funding statement
The research was undertaken as part of an undergraduate practicum in environmental science at the University of California, Los Angeles. Three prototype LED lamps were loaned to UCLA by Philips Research Europe for the duration of the experiment. All other research expenses were paid by the UCLA Institute of the Environment and Sustainability. T.L. was an employee of UCLA during the experimental design and fieldwork. H.A., J.E., S.F., L.F., E.H.-Y., L.P. and W.Y. were UCLA students and received course credit for participation in the project. A.B. is an employee of Philips Research Europe.
Competing interests
A.B. is an employee of Philips, which produced the custom LEDs tested in the research.
References
- 1.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 2.Fonken LK, Nelson RJ. 2011. Illuminating the deleterious effects of light at night. F1000 Rep. Med. 3, 18 ( 10.3410/M3-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan T, Gries G. 2009. Ultraviolet and violet light: attractive orientation cues for the Indian meal moth, Plodia interpunctella. Entomol. Exp. Appl. 131, 148–158. ( 10.1111/j.1570-7458.2009.00838.x) [DOI] [Google Scholar]
- 4.Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, Orgül S, Wirz-Justice A. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab 90, 1131–1316. ( 10.1210/jc.2004-0957) [DOI] [PubMed] [Google Scholar]
- 5.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III 1995. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 332, 6–11. ( 10.1056/NEJM199501053320102) [DOI] [PubMed] [Google Scholar]
- 6.Barghini A, de Medeiros BAS. 2010. Artificial lighting as a vector attractant and cause of disease diffusion. Environ. Health Perspect. 118, 1503–1506. ( 10.1289/ehp.1002115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenbeis G, Hassel F. 2000. Zur Anziehung nachtaktiver Insekten durch Straßenlaternen—eine Studie kommunaler Beleuchtungseinrichtungen in der Agrarlandschaft Rheinhessens [Attraction of nocturnal insects to street lights – a study of municipal lighting systems in a rural area of Rheinhessin (Germany)]. Nat. Landsch. 75, 145–156. [Google Scholar]
- 8.Eisenbeis G. 2006. Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T.), pp. 281–304. Washington, DC: Island Press. [Google Scholar]
- 9.Eisenbeis G, Eick K. 2011. Studie zur Anziehung nachtaktiver Insekten an die Straßenbeleuchtung unter Einbeziehung von LEDs [Attraction of nocturnal insects to street lights: a study of lighting systems, with consideration of LEDs]. Nat. Landschaft. 86, 298–306. [Google Scholar]
- 10.Frank KD. 2006. Effects of artificial night lighting on moths. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T.), pp. 305–344. Washington, DC: Island Press. [Google Scholar]
- 11.Robinson HS. 1952. On the behaviour of night-flying insects in the neighbourhood of a bright light source. Proc. R. Entomol. Soc. Lond. A 27, 13–21. ( 10.1111/j.1365-3032.1952.tb00139.x) [DOI] [Google Scholar]
- 12.Meyer LA, Sullivan SMP. 2013. Bright lights, big city: influences of ecological light pollution on reciprocal stream–riparian invertebrate fluxes. Ecol. Appl. 23, 1322–1330. ( 10.1890/12-2007.1) [DOI] [PubMed] [Google Scholar]
- 13.Fox R. 2013. The decline of moths in Great Britain: a review of possible causes. Insect Conserv. Diver. 6, 5–19. ( 10.1111/j.1752-4598.2012.00186.x) [DOI] [Google Scholar]
- 14.van Grunsven RHA, Donners MAH, Boekee K, Tichelaar I, van Geffen KG, Groenendijk D, Berendse F, Veenendaal EM. 2014. Spectral composition of light sources and insect phototaxis, with an evaluation of existing spectral response models. J. Insect Conserv. 18, 225–231. ( 10.1007/s10841-014-9633-9) [DOI] [Google Scholar]
- 15.Poiani S, Dietrich C, Barroso A, Costa-Leonardo AM. 2014. Effects of residential energy saving lamps on the attraction of nocturnal insects. Light Res. Technol. ( 10.1177/1477153514526880) [DOI] [Google Scholar]
- 16.Barrett JR, Huber RT, Harwood FW. 1973. Selection of lamps for minimal insect attraction. Trans. ASAE 17, 710–711. ( 10.13031/2013.37607) [DOI] [Google Scholar]
- 17.Barrett JR, Killough RA, Hartsock JG. 1974. Reducing insect problems in lighted areas. Trans. ASAE 18, 329–330. ( 10.13031/2013.36852) [DOI] [Google Scholar]
- 18.Antignus Y. 2000. Manipulation of wavelength-dependent behaviour of insects: an IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res. 71, 213–220. ( 10.1016/S0168-1702(00)00199-4) [DOI] [PubMed] [Google Scholar]
- 19.Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510. ( 10.1146/annurev.ento.46.1.471) [DOI] [PubMed] [Google Scholar]
- 20.Chadee DD, Martinez R. 2000. Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indes. J. Vector Ecol. 25, 158–163. [PubMed] [Google Scholar]
- 21.Burkett DA, Butler JF. 2005. Laboratory evaluation of colored light as an attractant for femal Aedes aegypti, Aedes albopictus, Anopheles quadrimaculatus, and Culex nigripalpus. Fla Entomol. 88, 383–389. ( 10.2987/08-5815.1) [DOI] [Google Scholar]
- 22.Burkett DA. 1998. Light color attraction and dietary sugar composition for several mosquito (Diptera: Culicidae) species found in north central Florida. Dissertation, University of Florida, Gainesville, FL. [Google Scholar]
- 23.Bishop AL, Worrall R, Spohr LJ, McKenzie HJ, Barchia IM. 2004. Response of Culicoides spp. (Diptera: Ceratopogonidae) to light-emitting diodes. Aust. J. Entomol. 43, 184–188. ( 10.1111/j.1440-6055.2003.00391.x) [DOI] [Google Scholar]
- 24.Green CH. 1985. A comparison of phototactic responses to red and green light in Glossina morsitans morsitans and Musca domestica. Physiol. Entomol. 10, 165–172. ( 10.1111/j.1365-3032.1985.tb00031.x) [DOI] [Google Scholar]
- 25.Solano Lamphar HA, Kocifaj M. 2013. Light pollution in ultraviolet and visible spectrum: effect on different visual perceptions. PLoS ONE 8, e56563 ( 10.1371/journal.pone.0056563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Langevelde F, Ettema JA, Donners M, WallisDeVries MF, Groenendijk D. 2011. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 144, 2274–2281. ( 10.1016/j.biocon.2011.06.004) [DOI] [Google Scholar]
- 27.Barghini A, de Medeiros BAS. 2012. UV radiation as an attractor for insects. Leukos 9, 47–56. ( 10.1582/LEUKOS.2012.09.01.003) [DOI] [Google Scholar]
- 28.Bates AJ, et al. 2013. Assessing the value of the Garden Moth Scheme citizen science dataset: how does light trap type affect catch? Entomol. Exp. Appl. 146, 386–397. ( 10.1111/eea.12038) [DOI] [Google Scholar]
- 29.Somers-Yeates R, Hodgson D, McGregor PK, Spalding A, ffrench-Constant RH. 2013. Shedding light on moths: shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 9, 20130376 ( 10.1098/rsbl.2013.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacheco-Tucuch FS, Ramierez-Sierra MJ, Gourbière S, Dumonteil E. 2012. Public street lights increase house infestation by the Chagas disease vector Triatoma dimidiata. PLoS ONE 7, e36207 ( 10.1371/journal.pone.0036207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzel R, Greggers U. 1985. Natural phototaxis and its relationship to colour vision in honeybees. J. Comp. Physiol. A 157, 311–321. ( 10.1007/BF00618121) [DOI] [Google Scholar]
- 32.Elvidge CD, Keith DM, Tuttle BT, Baugh KE. 2010. Spectral identification of lighting type and character. Sensors 10, 3961–3988. ( 10.3390/s100403961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawson S, Bader M-F. 2014. LED lighting increases the ecological impact of light pollution irrespective of color temperature. Ecol. Appl. 24, 1561–1568. ( 10.1890/14-0468.1) [DOI] [PubMed] [Google Scholar]
- 34.Stone EL, Jones G, Harris S. 2012. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Change Biol. 18, 2458–2465. ( 10.1111/j.1365-2486.2012.02705.x) [DOI] [Google Scholar]
- 35.Pauley SM. 2004. Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med. Hypotheses 63, 588–596. ( 10.1016/j.mehy.2004.03.020) [DOI] [PubMed] [Google Scholar]
- 36.Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. 2011. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage. 92, 2714–2722. ( 10.1016/j.jenvman.2011.06.029) [DOI] [PubMed] [Google Scholar]
- 37.Gaston KJ. 2013. A green light for efficiency. Nature 497, 560–561. ( 10.1038/497560a) [DOI] [PubMed] [Google Scholar]
- 38.Gaston KJ, Bennie J, Davies TW, Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 88, 912–927. ( 10.1111/brv.12036) [DOI] [PubMed] [Google Scholar]
- 39.Davies TW, Bennie J, Inger R, de Ibarra NH, Gaston KJ. 2013. Artificial light pollution: are shifting spectral signatures changing the balance of species interactions? Glob. Change Biol. 19, 1417–1423. ( 10.1111/gcb.12166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleve K. 1964. Der Anflug der Schmetterlinge an künstliche Lichtquellen. Mitt. Dtsch. Entomol. Ges. 23, 66–76. [Google Scholar]
- 41.Zabiliūtė A, Vaicekauskas R, Vitta P, Žukauskas A. 2014. Phosphor-converted LEDs with low circadian action for outdoor lighting. Opt. Lett. 39, 563–566. ( 10.1364/OL.39.000563) [DOI] [PubMed] [Google Scholar]
- 42.Borror DJ, Triplehorn CA, Johnson NF. 1989. An introduction to the study of insects, 6th edn New York, NY: Saunders College Publishing. [Google Scholar]
- 43.Arnett RH., Jr 1993. American insects. Gainesville, FL: Sandhill Crane Press. [Google Scholar]
- 44.Burnham KP, Anderson DR. 2002. Model selection and inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 45.Kyba CCM, Ruhtz T, Fischer J, Hölker F. 2011. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLos ONE 6, e17307 ( 10.1371/journal.pone.0017307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaston KJ, Davies TW, Bennie J, Hopkins J. 2012. Reducing the ecological consequences of night-time light pollution: options and developments. J. Appl. Ecol. 49, 1256–1266. ( 10.1111/j.1365-2664.2012.02212.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rich C, Longcore T. 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press. [Google Scholar]
- 48.Bowden J. 1982. An analysis of factors affecting catches of insects in light-traps. Bull. Entomol. Res. 72, 535–556. ( 10.1017/S0007485300008579) [DOI] [Google Scholar]
- 49.Stevens SS. 1961. To honor Fechner and repeal his law. Science 133, 80–86. ( 10.1126/science.133.3446.80) [DOI] [PubMed] [Google Scholar]
- 50.Ruchty M, Roces F, Kleineidam J. 2010. Detection of minute temperature transients by thermosensitive neurons in ants. J. Neurophysiol. 104, 1249–1256. ( 10.1152/jn.00390.2010) [DOI] [PubMed] [Google Scholar]
- 51.Noor AM, Alegana VA, Gething PW, Tatem AJ, Snow RW. 2008. Using remotely sensed night-time light as a proxy for poverty in Africa. Popul. Health Metrics 6, 5 ( 10.1186/1478-7954-6-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remme JHF, et al. 2006. Tropical diseases targeted for elimination: Chagas disease, lymphatic filariasis, onchocerciasis, and leprosy. In Disease control priorities in developing countries (eds Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove A.), pp. 147–163. New York, NY: Oxford University Press. [PubMed] [Google Scholar]
- 53.dos Santos TG, de Mello Gaia MC, Brazil RP. 2003. Attraction of sand flies (Diptera: Psychodidae) to light traps in rural areas of Minas Gerais State, Brazil. J. Am. Mosq. Control Assoc. 19, 74–78. [PubMed] [Google Scholar]
- 54.Lee HI, Seo BY, Shin E-H, Burkett DA, Lee J-K, Shin YH. 2009. Efficiancy evaluation of Nozawa-style black light trap for control of Anopheline mosquitoes. Korean J. Parasitol. 47, 159–165. ( 10.3347/kjp.2009.47.2.159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suárez-Mutis MC, Fé NF, Alecrim W, Coura JR. 2009. Night and crepuscular mosquitoes and risk of vector-borne diseases in areas of piassaba extraction in the middle Negro River basin, state of Amazonas, Brazil. Mem. Inst. Oswaldo Cruz 104, 11–17. ( 10.1590/S0074-02762009000100002) [DOI] [PubMed] [Google Scholar]
- 56.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764–767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Geffen KG, van Grunsven RHA, van Ruijven J, Berendse F, Veenendaal EM. 2014. Artificial light at night causes diapause inhibition and sex-specific life history changes in a moth. Ecol. Evol. 4, 2082–2089. ( 10.1002/ece3.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley WD, Davison PI, Maxwell DL, Bendall B. 2013. Street lighting delays and disrupts the dispersal of Atlantic salmon (Salmo salar) fry. Biol. Conserv. 158, 140–146. ( 10.1016/j.biocon.2012.09.022) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.