1. The challenge
Daily, lunar and seasonal cycles of natural light have been key forms of environmental variation across the Earth's surface since the first emergence of life. They have driven the development of biological phenomena from the molecule to the ecosystem, including metabolic and physiological pathways, the behaviour of individuals, geographical patterns of adaptation and species richness, and ecosystem cycles (e.g. [1–4]). Indeed, biological systems are arguably organized foremost by light [5–7].
The natural patterns of light have over the last 100 years come to be greatly disrupted through the introduction of artificial light into the night-time environment: artificial light at night (ALAN). This derives from a diversity of sources, including street lighting, advertising lighting, architectural lighting, security lighting, domestic lighting and vehicle lighting. ALAN disrupts natural patterns of light both via direct effects of illumination from these sources as well as via skyglow (the scattering by atmospheric molecules or aerosols in the atmosphere of ALAN that is emitted or reflected upwards; [8–10]).
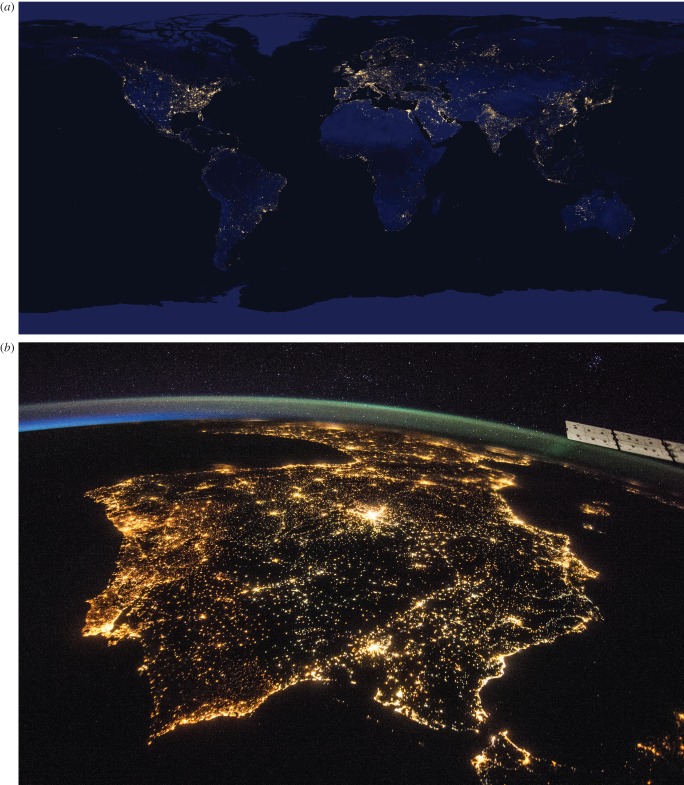
On the ground this disruption of natural patterns of light takes two principal forms [11]. First, light has been introduced in places, times and at intensities at which it does not naturally occur. This has been firmly fixed in the public imagination through the creation from satellite and astronaut acquired night-time imagery of pictures of the Earth which illustrate the extent of ALAN, of urbanization and of major centres of human population (figure 1; e.g. [12,13]). Given the nature of such images, it is challenging to use these to make some categorical quantification of the extent of ALAN, although it is plainly much more widespread than urban infrastructure alone, mainly because of the skyglow effect. One estimate that accounted explicitly for the effects of skyglow was that 18.7% of the global land area experienced ALAN [12], another based more directly on satellite imagery that 11.4% of terrestrial and 0.2% of marine areas of the globe experienced ALAN [11], and another that ALAN is increasing at around 6% per annum with huge geographical variation (0–20%; [14]).
Figure 1.
Remote imagery of artificial light at night. (a) Monthly composite satellite image of night-time light from the Visible Infrared Imaging Radiometer Suite (VIIRS) day/night band (DNB). NASA Earth Observatory image, using Suomi NPP VIIRS data provided by NOAA National Geophysical Data Center. (b) Photograph of the Iberian Peninsula at night from the International Space Station showing Spain and Portugal. Image courtesy of the Earth Science and Remote Sensing Unit, NASA Johnson Space Center (http://eol.jsc.nasa.gov).
Second, ALAN is introducing light with a spectrum that is different from those of sunlight, moonlight or starlight [11]. The spectrum of ALAN depends fundamentally on the kind of lighting device that is being used, ranging from narrow (e.g. low pressure sodium) to broad bandwidths (e.g. high intensity discharge and light-emitting diode—LED, [15]). The dominant technology tends to vary geographically, but can be locally quite heterogeneous. However, there is a general trend towards the use of ‘whiter lighting′ sources, often with a strong component in the blue portion of the spectrum (especially using LEDs; e.g. [16]).
Unlike many other anthropogenic changes that have been wrought on the environment (e.g. in CO2, temperature, habitat change), those resulting from ALAN are entirely unprecedented. There have been no natural analogues, at any time scale, to the nature, extent, distribution, timing or rate of spread of ALAN [11,17].
The introduction of ALAN has provided significant and substantial benefits to humankind [18,19]. However, if biological systems are fundamentally shaped by light, and ALAN has changed the patterns of light in novel and extensive ways, it seems logical to predict that ALAN will have numerous biological impacts. This is not a new argument. Concerns as to the biological impacts of ALAN have been expressed for a long time (e.g. [20–23]). Numerous studies have also been published that demonstrate such impacts (for recent examples, see [24–31]). However, understanding the genuine severity of the problem is both challenging and timely: with the large scale and rapid introduction of LED lights and the use of ‘smart illumination′ [16], we now have the opportunity to adjust ALAN to reduce any negative environmental impacts provided that there is a good understanding of the effects of both intensity and spectral composition of ALAN. This special issue is a step toward addressing that research challenge, which takes several key forms. In this introductory paper, we distinguish those associated with light, with individual organisms and with populations, communities and ecosystems.
2. Light
In the main, understanding of the patterns of ALAN has come from analyses of satellite imagery (e.g. [12,32]), aerial surveys (e.g. [33,34]) and ground-based measurements of direct illumination and skyglow ([8,35]; some produced from citizen science data, e.g. [36]). These have proven invaluable and will continue to provide significant insights. However, to improve understanding of the biological impacts of ALAN, such an approach has to be enriched in a number of ways.
First, more attention needs to be paid to differences in the nature and relative importance of the three main sources of ALAN, namely direct illumination, light scattered by cloud cover and light scattered from a clear sky [37]. While ALAN is perhaps most commonly envisaged in terms of direct illumination (e.g. typical diagrammatic representations of ALAN focus on the immediate illumination from one or more streetlights), the atmospheric scattering of light, and the resultant skyglow, is likely also to be very important. This is particularly so because while direct illumination may extend metres to hundreds of metres and is readily blocked by obstacles, skyglow may extend kilometres to hundreds of kilometres [17] and is little influenced even by terrain blocking [37]. Indeed, much more needs to be known about the nature of skyglow, exploiting the computational tools that are now available [37].
Second, more attention needs to be paid to the spectra of ALAN. ALAN tends to be mapped in terms of the intensity of illumination, commonly with respect to human vision. However, biological processes (e.g. photosynthesis, circadian clocks, vision) vary markedly in the components of spectra to which they are most sensitive. Of concern here is not simply the spectrum of direct illumination but also of skyglow, and how the two interact. For example, in urban areas clouds have a much bigger impact on the proportion of red than blue light redirected towards the surface [38]. Relative to white or blue light sources, reddened sources are believed to reduce skyglow owing to the stronger Rayleigh scattering at short wavelengths [10,39].
Third, and perhaps most critically, too little is presently known about what ALAN is actually experienced by organisms, and how this varies. In the absence of better information, it has commonly to be assumed that the average levels of ALAN in an area are those experienced by the organisms that occur there. However, particularly given the spatial resolutions at which it is mapped, animals can often behaviourally avoid the typically more heterogeneous patterns of ALAN through the spatial and temporal habitat and movement choices that they make. In one of the few examples we are aware of to date, Dominoni & Partecke [40] show that urban blackbirds do actually experience longer subjective day lengths as a consequence of ALAN.
3. Individual organisms
The vast majority of studies of the biological impacts of ALAN concern the effects on individual organisms. These span studies of gene expression (e.g. [41,42]), physiology (e.g. [43–46]), foraging [24,47–50], daily movements [51–55], migratory behaviour (e.g. [56,57]), reproductive behaviour (e.g. [58–62]) and mortality (e.g. [63,64]). There is almost a complete lack of published examples in which no effect was documented (but see [35]), suggesting either that biological impacts are quite pervasive or the potential for a severe ‘file drawer′ problem (see [65]) in the literature. Although a file drawer problem of some degree would not be surprising, the truth most probably lies somewhere in between.
What is lacking at this point is a well-developed understanding of how the biological impacts of ALAN change with variation among individual organisms, life stages, spatial-temporal contexts and with the form of the ALAN. With respect to the organisms, key challenges are to determine: (i) how intraspecific responses to ALAN vary among and within classes of individuals (e.g. sex, age, body size); (ii) how responses to ALAN vary among a wide array of different species—much reliance is presently placed on studies of birds and mammals [66], with almost no knowledge about effects on microorganisms (but see [67]) and plants (but see [68]), and little known about invertebrates (with the exception of moths; but see [69] for a review, and [68,70,71]); (iii) how responses to ALAN by laboratory organisms or humans extrapolate to organisms in the wild—particularly notable is the evidence of significant stress and disease impacts in laboratory or domestic situations [72], and the limited studies of these effects in wild organisms; and (iv) how metabolic, physiological and behavioural responses to ALAN influence organismal fitness—studies are beginning to uncover such fitness consequences [62].
With respect to the ALAN itself, the challenges are to determine: (i) the form of dose (intensity)-response relationships for a range of biological impacts of ALAN—almost exclusively, studies to date have contrasted predominantly two ALAN treatments (ALAN versus no ALAN), preventing determination of thresholds and the overall shapes of dose-response functions; and (ii) the form of spectral-response relationships for a range of biological impacts of ALAN—again, as with dose-response relationships, the state-of-the-art experiments are employing just a few spectral treatments [68,71,73] or typical light sources for outdoor lighting with different colour spectra (see [74,75]). Understanding of both dose-response and spectral-response relationships, and their interaction, will be critical to providing the best advice on how to limit the negative biological impacts of ALAN.
4. Populations, communities and ecosystems
While ALAN has been widely documented to have effects on the physiology and behaviour of individual organisms, the extent to which this translates into impacts on populations, communities and ecosystems remains poorly understood. The principal problem here is simply that the number of studies that have been conducted is extremely small (see [27,67,68,71,76,77]).
A challenge in determining the influence of ALAN on populations is that while it can potentially influence each of the key demographic parameters (births, deaths, immigration, emigration), it is difficult to study each of these effects for a single study species [78]. Those species for which births and deaths are relatively easy to measure are commonly those for which immigration and emigration are hard to determine, and vice versa.
Although there are documented examples of the impacts of ALAN on prey influencing their predators, and of impacts on predators influencing their prey (e.g. [50,79,80]), the manner in which such influences ramify through communities remains poorly understood [11]. Bennie et al. [68] report experimental evidence of bottom-up, but not top-down, effects in simple plant–herbivore–predator communities.
To predict the ecological consequences of ALAN in natural systems reliably, it is critical to have a better understanding of longer term processes that moderate the susceptibility of populations, communities and ecosystems to an illuminated environment. Much of the available knowledge is based on short-term experiments within one generation time (often days to weeks) that do not allow the consideration of response mechanisms, such as acclimation, adaptation, physiological, behavioural and even evolutionary compensatory mechanisms linked to environmental context and seasonal timing. For example, an illumination period of more than 1 year was necessary to cause a clear change in an ALAN-naive freshwater microbial community [67].
Although largely unknown, it has to be expected that effects on ecosystem functions and services do occur [7,11]. In the tropics, for example, nocturnal seed dispersers such as bats are crucial for ecosystem functioning. It was found that natural forest succession and connectivity of forest patches may suffer owing to ALAN through a reduction in nocturnal seed disperser activity in illuminated areas [29]. Another example is microbial communities living in aquatic sediments. These are highly diverse and play an important role in the global carbon cycle. Hölker et al. [67] report ALAN-induced changes in the species composition of such sediment communities. This has implications for ecosystem functions (here carbon mineralization) and could even shift the system from negative to positive net ecosystem production at night.
To determine the effects of ALAN on populations, communities and ecosystems most effectively, it is necessary to establish replicated field experiments. The first such experiments report findings in this special issue—the ECOLIGHT experiment [68], the ‘Verlust der Nacht′ experiment [67] and the LightOnNature experiment [71]. Early evidence is suggesting that there may be marked between-year variation in the influences of ALAN, emphasizing the importance of developing or maintaining long-term experiments including several generations of key species.
5. Conclusion
Over just the last few years there has been an explosion of research interest in the biological impacts of ALAN (albeit the topic has deep historical roots; for reviews see [69,81–83]). This has been fuelled by (i) several policy reports that have highlighted its likely importance among the plethora of anthropogenic influences on the environment (e.g. [84–86]); (ii) the need to cut energy costs by altering public lighting systems and the associated potential for environmental gains [87,88]; (iii) the wide-scale change to LED lighting and calls for the design of eco-friendly spectral composition of lamps [14,66,89] and arguably, (iv) the serendipitous contemporaneous emergence both of major independent funded research programs experimentally addressing ecological impacts of ALAN [67,68,71] and of the interdisciplinary ‘Loss of the Night Network′ funded by the EU COST program. The resultant body of research work has mapped out the potential breadth of biological impacts of ALAN, has highlighted many important targets for future work and has begun to identify ways in which practical steps can be taken to reduce environmental concerns. This special issue contributes further to that trajectory.
Acknowledgements
We are grateful to the authors who kindly agreed to contribute to this special issue, to the reviewers who provided valuable comments on the manuscripts, and to H. Eaton and other members of the editorial and production staff of Philosophical Transactions of the Royal Society B who have as ever been a delight to work with. We thank J. Bennie and S. Gaston for comments on the manuscript.
Funding statement
The research leading to this paper has received funding from the European Research Council under the European Union′s Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 268504 to K.J.G.; from the Verlust der Nacht project, funded by the Federal Ministry of Education and Research, Germany to F.H. (BMBF-033L038A); and from the Dutch Technology Foundation STW, which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs, to M.E.V.
References
- 1.Arendt J. 1998. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev. Reprod. 3, 13–22. ( 10.1530/ror.0.0030013) [DOI] [PubMed] [Google Scholar]
- 2.Hays GC. 2003. A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503, 163–170. ( 10.1023/B:HYDR.0000008476.23617.b0) [DOI] [Google Scholar]
- 3.Urbanski J, Mogi M, O'Donnell D, DeCotiis M, Toma T, Armbruster P. 2012. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 179, 490–500. ( 10.1086/664709) [DOI] [PubMed] [Google Scholar]
- 4.Bennie J, Duffy JP, Inger R, Gaston KJ. 2014. The biogeography of time partitioning in mammals. Proc. Natl Acad. Sci. USA 111, 13 727–13 732. ( 10.1073/pnas.1216063110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronfeld-Schor N, Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181. ( 10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 6.Bradshaw WE, Holzapfel CM. 2010. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72, 147–166. ( 10.1146/annurev-physiol-021909-135837) [DOI] [PubMed] [Google Scholar]
- 7.Hölker F, Wolter C, Perkin EK, Tockner K. 2010. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25, 681–682. ( 10.1016/j.tree.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 8.Kyba CCM, Ruhtz T, Fischer J, Hölker F. 2011. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS ONE 6, e17307 ( 10.1371/journal.pone.0017307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies TW, Bennie J, Inger R, Gaston KJ. 2013. Artificial light alters natural regimes of night-time sky brightness. Sci. Rep. 3, 1722 ( 10.1038/srep01722) [DOI] [Google Scholar]
- 10.Luginbuhl CB, Boley PA, Davis DR. 2014. The impact of light source spectral power distribution on sky glow. J. Quant. Spectrosc. Radiat. Transf. 139, 21–26. ( 10.1016/j.jqsrt.2013.12.004) [DOI] [Google Scholar]
- 11.Gaston KJ, Duffy JP, Gaston S, Bennie J, Davies TW. 2014. Human alteration of natural light cycles: causes and ecological consequences. Oecologia 176, 917–931. ( 10.1007/s00442-014-3088-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinzano P, Falchi F, Elvidge CD. 2001. The first World Atlas of the artificial night sky brightness. Mon. Notes R. Astron. Soc. 328, 689–707. ( 10.1046/j.1365-8711.2001.04882.x) [DOI] [Google Scholar]
- 13.Davies TW, Duffy JP, Bennie J, Gaston KJ. 2014. The nature, extent and ecological implications of marine light pollution. Front. Ecol. Environ. 12, 347–355. ( 10.1890/130281) [DOI] [Google Scholar]
- 14.Hölker F, et al. 2010. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc. 15, 13. [Google Scholar]
- 15.Elvidge CD, Keith DM, Tuttle BT, Baugh KE. 2010. Spectral identification of lighting type and character. Sensors 10, 3961–3988. ( 10.3390/s100403961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haitz R, Tsao JY. 2011. Solid-state lighting—why it will succeed, and why it won't be overtaken. Opt. Photonics 6, 26–30. ( 10.1002/opph.201190325) [DOI] [Google Scholar]
- 17.Kyba CCM, Hölker F. 2013. Do artificially illuminated skies affect biodiversity in nocturnal landscapes? Landscape Ecol. 28, 1637–1640. ( 10.1007/s10980-013-9936-3) [DOI] [Google Scholar]
- 18.Gaston KJ, Gaston S, Bennie J, Hopkins J. In press. Environmental benefits and costs of artificial nighttime lighting. Environ. Rev. [Google Scholar]
- 19.Meier J, Hasenöhrl U, Krause K, Pottharst M. (ed). 2015. Urban lighting, light pollution and society. New York, NY: Routledge. [Google Scholar]
- 20.Squires WA, Hanson HE. 1918. The destruction of birds at the lighthouses on the coast of California. Condor 20, 6–10. ( 10.2307/1362354) [DOI] [Google Scholar]
- 21.Verheijen FJ. 1960. The mechanisms of the trapping effect of artificial light sources upon animals. Arch. Néerland. Zool. 13, 1–107. ( 10.1163/036551660X00017) [DOI] [Google Scholar]
- 22.Frank K. 1988. Impact of outdoor lighting on moths: an assessment. J. Lepidopterists Soc. 42, 63–93. [Google Scholar]
- 23.Kerenyi NA, Pandula E, Feuer G. 1990. Why the incidence of cancer is increasing—the role of ‘light pollution’. Med. Hypotheses 33, 75–78. ( 10.1016/0306-9877(90)90182-E) [DOI] [PubMed] [Google Scholar]
- 24.Dwyer RG, Bearhop S, Campbell HA, Bryant DM. 2012. Shedding light on light: benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 82, 478–485. ( 10.1111/1365-2656.12012) [DOI] [PubMed] [Google Scholar]
- 25.Le Tallec T, Perret M, Théry M. 2013. Light pollution modifies the expression of daily rhythms and behavior patterns in a nocturnal primate. PLoS ONE 8, e79250 ( 10.1371/journal.pone.0079250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazor T, Levin N, Possingham HP, Levy Y, Rocchini D, Richardson AJ, Kark S. 2013. Can satellite-based night lights be used for conservation? The case of nesting sea turtles in the Mediterranean. Biol. Conserv. 159, 63–72. ( 10.1016/j.biocon.2012.11.004) [DOI] [Google Scholar]
- 27.Meyer LA, Sullivan SMP. 2013. Bright lights, big city: influences of ecological light pollution on reciprocal stream-riparian invertebrate fluxes. Ecol. Appl. 23, 1322–1330. ( 10.1890/12-2007.1) [DOI] [PubMed] [Google Scholar]
- 28.Picchi MS, Avolio L, Azzani L, Brombin O, Camerini G. 2013. Fireflies and land use in an urban landscape: the case of Luciola italica L. (Coleoptera: Lampyridae) in the city of Turin. J. Insect Conserv. 17, 797–805. ( 10.1007/s10841-013-9562-z) [DOI] [Google Scholar]
- 29.Lewanzik D, Voigt CC. 2014. Artificial light puts ecosystem services of frugivorous bats at risk. J. Appl. Ecol. 52, 388–394. ( 10.1111/1365-2664.12206) [DOI] [Google Scholar]
- 30.Pawson SM, Bader MK-F. 2014. LED lighting increases the ecological impact of light pollution irrespective of color temperature. Ecol. Appl. 24, 1561–1568. ( 10.1890/14-0468.1) [DOI] [PubMed] [Google Scholar]
- 31.van Geffen KG, van Grunsven RHA, van Ruijven J, Berendse F, Veenendaal EM. 2014. Artificial light at night causes diapause inhibition and sex-specific life history changes in a moth. Ecol. Evol. 4, 2082–2089. ( 10.1002/ece3.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennie J, Davies T, Duffy J, Inger R, Gaston KJ. 2014. Contrasting trends in light pollution across Europe. Sci. Rep. 4, 3789 ( 10.1038/srep03789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuechly HU, Kyba CCM, Ruhtz T, Lindemann C, Wolter C, Fischer J, Hölker F. 2012. Aerial survey of light pollution in Berlin, Germany, and spatial analysis of sources. Remote Sens. Environ. 126, 39–50. ( 10.1016/j.rse.2012.08.008) [DOI] [Google Scholar]
- 34.Hale JD, Davies G, Fairbrass AJ, Matthews TJ, Rogers CD, Sadler JP. 2013. Mapping lightscapes: spatial patterning of artificial lighting in an urban landscape. PLoS ONE 8, e61460 ( 10.1371/journal.pone.0061460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkin EK, Hölker F, Heller S, Berghahn R. 2014. Artificial light and nocturnal activity in gammarids. PeerJ 2, e279 ( 10.7717/peerj.279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyba CCM, Wagner JM, Kuechly HU, Walker CE, Elvidge CD, Falchi F, Ruhtz T, Fischer J, Hölker F. 2013. Citizen science provides valuable data for monitoring global night sky luminance. Sci. Rep. 3, 1835 ( 10.1038/srep01835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubé M. 2015. Physical behaviour of anthropogenic light propagation into the nocturnal environment. Phil. Trans. R. Soc. B 370, 20140117 ( 10.1098/rstb.2014.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyba CCM, Ruhtz T, Fischer J, Hölker F. 2012. Red is the new black: how the colour of urban skyglow varies with cloud cover. Mon. Notes R. Astron. Soc. 425, 701–708. ( 10.1111/j.1365-2966.2012.21559.x) [DOI] [Google Scholar]
- 39.Aubé M, Roby J, Kocifaj M. 2013. Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. PLoS ONE 8, e67798 ( 10.1371/journal.pone.0067798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominoni DM, Partecke J. 2015. Does light pollution alter daylength? A test using light loggers on free-ranging European blackbirds (Turdus merula). Phil. Trans. R. Soc. B 370, 20140118 ( 10.1098/rstb.2014.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashkenazi L, Haim A. 2012. Light interference as a possible stressor altering HSP70 and its gene expression levels in brain and hepatic tissues of golden spiny mice. J. Exp. Biol. 215, 4034–4040. ( 10.1242/jeb.073429) [DOI] [PubMed] [Google Scholar]
- 42.Haim A, Zubidat AE. 2015. Artificial light at night: melatonin as a mediator between the environment and epigenome. Phil. Trans. R. Soc. B 370, 20140121 ( 10.1098/rstb.2014.0121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brainard GC, Richardson BA, Hurlbut EC, Steinlechner S, Matthews SA, Reiter RJ. 1984. The influence of various irradiances of artificial light, twilight, and moonlight on the suppression of pineal melatonin content in the Syrian hamster. J. Pineal Res. 1, 105–119. ( 10.1111/j.1600-079X.1984.tb00202.x) [DOI] [PubMed] [Google Scholar]
- 44.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. 2011. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 7, 468–471. ( 10.1098/rsbl.2010.1108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulin C, Bruyant F, Laprise M-H, Cockshutt AM, Vandenhecke JMR, Huot Y. 2013. The impact of light pollution on diel changes in the photophysiology of Microcystis aeruginosa. J. Plankton Res. 36, 286–291. ( 10.1093/plankt/fbt088) [DOI] [Google Scholar]
- 47.Bakken LE, Bakken GS. 1977. American redstart feeding by artificial light. Auk 94, 373–374. [Google Scholar]
- 48.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435–1439. ( 10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 49.Santos CD, Miranda AC, Granadeiro JP, Lourenco PM, Saraiva S, Palmeirim JM. 2010. Effects of artificial illumination on the nocturnal foraging of waders. Acta Oecol. 36, 166–172. ( 10.1016/j.actao.2009.11.008) [DOI] [Google Scholar]
- 50.Becker A, Whitfield AK, Cowley PD, Järnegren J, Næsje TF. 2013. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. Appl. Ecol. 50, 43–50. ( 10.1111/1365-2664.12024) [DOI] [Google Scholar]
- 51.Moore MV, Pierce SM, Walsh HM, Kvalvik SK, Lim JD. 2000. Urban light pollution alters the diel vertical migration of Daphnia. Verh. Intern. Ver. Limnol. 27, 779–782. [Google Scholar]
- 52.Kuijper DPJ, Schut J, van Dullemen D, Toorman H, Goossens N, Ouwehand J, Limpens HJGA. 2008. Experimental evidence of light disturbance along the commuting routes of pond bats (Myotis dasycneme). Lutra 51, 37–49. [Google Scholar]
- 53.Stone EL, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127. ( 10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 54.Riley WD, Bendall B, Ives MJ, Edmonds NJ, Maxwell DL. 2012. Street lighting disrupts the diel migratory pattern of wild Atlantic salmon, Salmo salar L., smolts leaving their natal stream. Aquaculture 330–333, 74–81. ( 10.1016/j.aquaculture.2011.12.009) [DOI] [Google Scholar]
- 55.Mathews F, Roche N, Aughney T, Jones N, Day J, Baker J, Langton S. 2015. Barriers and benefits: implications of artificial night-lighting for the distribution of common bats in Britain and Ireland. Phil. Trans. R. Soc. B 370, 20140124 ( 10.1098/rstb.2014.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans WR, Akashi Y, Altman NS, Manville AM., II 2007. Response of night-migrating songbirds in cloud to colored and flashing light. N. Am. Birds 60, 476–488. [Google Scholar]
- 57.Poot H, Ens BJ, Vries HDe, Donners MAH, Wernand MR, Marquenie JM. 2008. Green light for nocturnally migrating birds. Ecol. Soc. 13, 47. [Google Scholar]
- 58.Miller MW. 2006. Apparent effects of light pollution on singing behavior of American robins. Condor 108, 130–139. ( 10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2) [DOI] [Google Scholar]
- 59.Kempenaers B, Borgström P, Löes P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 60.Titulaer M, Spoelstra K, Lange CYMJG, Visser ME. 2012. Activity patterns during food provisioning are affected by artificial light in free living great tits (Parus major). PLoS ONE 7, e37377 ( 10.1371/journal.pone.0037377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Da Silva A, Valcu M, Kempenaers B. 2015. Light pollution alters the phenology of dawn and dusk singing in common European songbirds. Phil. Trans. R. Soc. B 370, 20140126 ( 10.1098/rstb.2014.0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Jong M, Ouyang JQ, Da Silva A, van Grunsven RHA, Kempenaers B, Visser ME, Spoelstra K. 2015. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Phil. Trans. R. Soc. B 370, 20140128 ( 10.1098/rstb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones J, Francis CM. 2003. The effects of light characteristics on avian mortality at lighthouses. J. Avian Biol. 34, 328–333. ( 10.1111/j.0908-8857.2003.03183.x) [DOI] [Google Scholar]
- 64.Rodríguez A, Rodríguez B, Curbelo ÁJ, Pérez A, Marrero S, Negro JJ. 2012. Factors affecting mortality of shearwaters stranded by light pollution. Anim. Conserv. 15, 519–526. ( 10.1111/j.1469-1795.2012.00544.x) [DOI] [Google Scholar]
- 65.Rosenthal R. 1979. The ‘file drawer problem’ and tolerance for null results. Psychol. Bull. 86, 638–641. ( 10.1037/0033-2909.86.3.638) [DOI] [Google Scholar]
- 66.Spoelstra K, Visser ME. 2013. The impact of artificial light on avian ecology. In Avian urban ecology (eds Gil D, Brumm H.), pp. 21–28. Oxford, UK: OUP. [Google Scholar]
- 67.Hölker F, Wurzbacher C, Weißenborn C, Monaghan MT, Holzhauer SIJ, Premke K. 2015. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Phil. Trans. R. Soc. B 370, 20140130 ( 10.1098/rstb.2014.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennie J, Davies TW, Cruse D, Inger R, Gaston KJ. 2015. Cascading effects of artificial light at night: resource-mediated control of herbivores in a grassland ecosystem. Phil. Trans. R. Soc. B 370, 20140131 ( 10.1098/rstb.2014.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaston KJ, Bennie J, Davies TW, Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 88, 912–927. ( 10.1111/brv.12036) [DOI] [PubMed] [Google Scholar]
- 70.Jones TM, Durrant J, Michaelides EB, Green MP. 2015. Melatonin: a possible link between the presence of artificial light at night and reductions in biological fitness. Phil. Trans. R. Soc. B 370, 20140122 ( 10.1098/rstb.2014.0122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spoelstra K, van Grunsven RHA, Donners M, Gienapp P, Huigens ME, Slaterus R, Berendse F, Visser ME, Veenendaal E. 2015. Experimental illumination of natural habitat—an experimental set-up to assess the direct and indirect ecological consequences of artificial light of different spectral composition. Phil. Trans. R. Soc. B 370, 20140129 ( 10.1098/rstb.2014.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stevens RG, Zhu Y. 2015. Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Phil. Trans. R. Soc. B 370, 20140120 ( 10.1098/rstb.2014.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone EL, Wakefield A, Harris S, Jones G. 2015. The impacts of new street light technologies: experimentally testing the effects on bats of changing from low-pressure sodium to white metal halide. Phil. Trans. R. Soc. B 370, 20140127 ( 10.1098/rstb.2014.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eisenbeis G. 2006. Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T.), pp. 281–304. Washington, DC: Island Press. [Google Scholar]
- 75.Longcore T, Aldern HL, Eggers JF, Flores S, Franco L, Hirshfield-Yamanishi E, Petrinec LN, Yan WA, Barroso AM. 2015. Tuning the white light spectrum of light emitting diode lamps to reduce attraction of nocturnal arthropods. Phil. Trans. R. Soc. B 370, 20140125 ( 10.1098/rstb.2014.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764–767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perkin EK, Hölker F, Tockner K, Richardson JS. 2014. Artificial light as a disturbance to light-naïve streams. Freshwater Biol. 59, 2235–2244. ( 10.1111/fwb.12426) [DOI] [Google Scholar]
- 78.Gaston KJ, Bennie J. 2014. Demographic effects of artificial nighttime lighting on animal populations. Environ. Rev. 22, 1–8. ( 10.1139/er-2014-0041) [DOI] [Google Scholar]
- 79.Buchanan BW. 1993. Effects of enhanced lighting on the behaviour of nocturnal frogs. Anim. Behav. 45, 893–899. ( 10.1006/anbe.1993.1109) [DOI] [Google Scholar]
- 80.Heiling AM. 1999. Why do nocturnal orb-web spiders (Araneidae) search for light? Behav. Ecol. Sociobiol. 46, 43–49. ( 10.1007/s002650050590) [DOI] [Google Scholar]
- 81.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 82.Rich C, Longcore T. (eds). 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press. [Google Scholar]
- 83.Perkin EK, Hölker F, Richardson JS, Sadler JP, Wolter C, Tockner K. 2011. The influence of artificial light on stream and riparian ecosystems: questions, challenges, and perspectives. Ecosphere 2, 122 ( 10.1890/ES11-00241.1) [DOI] [Google Scholar]
- 84.Health Council of the Netherlands. 2000. Impact of outdoor lighting on man and nature. Publication No. 2000/25E. The Hague, The Netherlands: Health Council of the Netherlands. [Google Scholar]
- 85.The Royal Commission on Environmental Pollution. 2009. Artificial light in the environment. London, UK: TSO. [Google Scholar]
- 86.US Department of Energy. 2012. Light at night: the latest science See http://apps1.eere.energy.gov/buildings/publications/pdfs/ssl/sslwhitepaper_nov2010.pdf.
- 87.Gaston KJ. 2013. A green light for efficiency. Nature 497, 560–561. ( 10.1038/497560a) [DOI] [PubMed] [Google Scholar]
- 88.Kyba CCM, Hänel A, Hölker F. 2014. Redefining efficiency for outdoor lighting. Energy Environ. Sci. 7, 1806–1809. ( 10.1039/c4ee00566j) [DOI] [Google Scholar]
- 89.Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. 2011. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage. 92, 2714–2722. ( 10.1016/j.jenvman.2011.06.029) [DOI] [PubMed] [Google Scholar]