Abstract
Background
Screening mammography utilization in Vermont has declined since 2009 during a time of changing screening guidelines and increased interest in personalized screening regimens. This study evaluates whether the breast cancer risk distribution of the state’s screened population changed during the observed decline.
Methods
We examined the breast cancer risk distribution among screened women between 2001 and 2012 using data from the Vermont Breast Cancer Surveillance System. We estimated each screened woman’s 5-year risk of breast cancer using the Breast Cancer Surveillance Consortium risk calculator. Annual screening counts by risk group were normalized and age-adjusted to the Vermont female population by direct standardization.
Results
The normalized rate of low-risk (5-year breast cancer risk of <1%) women screened increased 8.3% per year (95% confidence interval [CI] = 4.8 to 11.9) between 2003 and 2008 and then declined by −5.4% per year (95% CI = −8.1 to −2.6) until 2012. When stratified by age group, the rate of low-risk women screened declined −4.4% per year (95% CI = −8.8 to 0.1; not statistically significant) for ages 40 to 49 years and declined a statistically significant −7.1% per year (95% CI = −12.1 to −2.0) for ages 50 to 74 years during 2008 to 2012. These declines represented the bulk of overall decreases in screening after 2008, with rates for women categorized in higher risk levels generally exhibiting small annual changes.
Conclusions
The observed decline in women screened in Vermont in recent years is largely attributable to reductions in screening visits by women who are at low risk of developing breast cancer.
Screening mammography reduces mortality from breast cancer through early detection (1); however, there is disagreement about optimal screening regimens (2,3). This is in part because of new evidence concerning the harms and benefits of mammography (4), including concerns regarding overdiagnosis (5). Especially for women aged less than 50 years (6), these concerns have led to an emphasis on individualized decision-making approaches to screening participation and interval (7,8).
Risk-based approaches to breast cancer screening have been increasingly emphasized in recent years (7,9–12). This trend has paralleled the 2009 US Preventive Services Task Force (USPSTF) recommendation that the decision to screen before age 50 years should be based on individual factors and patient context (13), and the American Cancer Society’s 2007 recommendation that mammography should be supplemented with magnetic resonance imaging for high-risk women of any age (14). Health-care providers use a wide variety of risk assessment strategies (15), with family history used as the predominant clinical discriminant (16–18). Additionally, both patients and providers can quantitatively assess the individual risk of developing breast cancer within 5 years using online calculators that implement the Breast Cancer Risk Assessment Tool model and the more recent Breast Cancer Surveillance Consortium (BCSC) model, which also includes mammographic breast density (19–22).
Patient utilization of screening mammography is known to vary by personal breast cancer risk (23–25), as well as by a number of factors, including educational attainment, health-care access, and community socioeconomic status (26–31). However, it is not clear how the risk profile of women using screening mammography has changed during the recent period of changing screening guidelines, scientific debate, and media controversy.
We previously reported a decline in breast cancer screening rates in Vermont after the 2009 USPSTF recommendations using a statewide mammography registry (32). The purpose of this study was to evaluate whether the breast cancer risk distribution of the screened population in Vermont has changed during the observed decline in utilization. Using a cross-sectional analysis of statewide registry data, we examined the distribution of breast cancer risk among the screened population in Vermont between 2001 and 2012.
Methods
Data
The Vermont Breast Cancer Surveillance System has collected longitudinal patient data regarding mammography screening and breast cancer outcomes in Vermont since 1994. It is part of both the National Cancer Institute’s BCSC (33) and the recently formed Population-based Research Optimizing Screening through Personalized Regimens program. At each screening mammography visit to any breast imaging facility in Vermont, the patient completes a standardized questionnaire that includes health history and demographic information. Radiologists and mammography technologists provide information on the clinical mammography findings and the reason for the visit. This study was Health Insurance Portability and Accountability Act compliant and was approved by the University of Vermont Institutional Review Board with a waiver of informed consent. Approximately 5% of the women in the Vermont Breast Cancer Surveillance System indicated that they did not wish their data to be used in research through an opt-out mechanism and were thus excluded from the study.
Study Population
Our sample consists of women aged 40 to 74 years who had screening mammography examinations in Vermont between 2001 and 2012. Data from one imaging facility (representing approximately 11% of the mammograms performed in Vermont) was excluded because of incomplete data during the study period. Mammography examinations of women reporting a personal history of breast cancer (n = 28610), breast augmentation (n = 4954), or race/ethnicity of Native American (n = 1295) were excluded from the study because the BCSC risk model is not validated for those groups (34). In instances of women who received multiple screening examinations within a calendar year, the first mammogram was retained and subsequent examinations excluded (n = 2234) so that the sample is restricted to one screening mammography visit per woman per year. The total sample for all years included 588429 screening mammography examinations among 121473 individual women.
Measuring Individual Breast Cancer Risk
We used the BCSC risk model in SAS software version 9.3 (SAS Institute, Cary, NC) to assess a woman’s 5-year risk for breast cancer at the time of each recorded mammogram (21,34). The model calculates an individual’s risk of developing breast cancer based on the following variables: age, breast density as defined by the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) (35), first-degree family history of breast cancer, history of biopsy, and race/ethnicity. The resultant risk scores were grouped according to the following risk categories: low (<1%), average (1%–1.66%), intermediate (1.67%–2.49%), high (2.5%–3.99%), and very high (≥4%) (36).
Statistical Analyses
Our analyses were based on cross-sectional comparisons of screened women across calendar years. A number of our variables of interest had missing data (Table 1). We created 10 imputed datasets using multivariable imputation by chained equations in Stata software version 13 (StataCorp, College Station, TX). This method generates imputed values based on a set of individual imputation models respective to each variable with missing observations (37).
Table 1.
Characteristics of women undergoing screening mammography, Vermont 2001 to 2012
| Variable | Observed No. (%) |
|---|---|
| Total screening mammograms | 588429 (100) |
| Age, y | |
| 40–49 | 188183 (32.0) |
| 50–59 | 212611 (36.1) |
| 60–69 | 141994 (24.1) |
| 70–74 | 45641 (7.8) |
| Missing | 0 (0.0) |
| Mammographic breast density | |
| Almost entirely fat | 71649 (12.2) |
| Scattered fibroglandular densities | 228364 (38.8) |
| Heterogeneously dense | 164604 (28.0) |
| Extremely dense | 25483 (4.3) |
| Missing | 98329 (16.7) |
| First-degree family history | |
| No | 449556 (76.4) |
| Yes | 92161 (15.7) |
| Missing | 46712 (7.9) |
| Biopsy history | |
| No | 425461 (72.3) |
| Yes | 123182 (20.9) |
| Missing | 39786 (6.8) |
| Race/ethnicity | |
| White, non-Hispanic | 563025 (95.7) |
| Black, non-Hispanic | 1535 (0.3) |
| Asian | 3736 (0.6) |
| Hispanic | 9337 (1.6) |
| Missing | 10796 (1.8) |
The imputation model included all variables in Table 1, in addition to height, weight, age at menarche, age at first birth, and year of screening (37). Missing data was similarly distributed across risk categories within each of the BCSC risk model input variables, with the exception of a modestly elevated percentage of missing density data for women categorized as high risk (25% missing vs 13%–18% missing for other risk groups). The model used augmented regression to manage cases of perfect prediction for the density variable, a common occurrence with categorical variables (38). The screening frequencies and standard errors of imputed variables, as well as the average annual BCSC risk scores, were combined across imputed datasets obtained using Rubin’s rules (39,40).
Annual population denominators of Vermont women by age were obtained from the US Census intercensal estimates for 2001 to 2009 and the postcensal estimates for 2010 to 2012 (41). The health service area (42) for the excluded imaging facility consists of a single county; thus we excluded that county from the statewide population counts. We used the Census data to normalize and age-adjust the count of annual mammography screenings in each risk group by direct standardization to account for the state’s growing representation of older ages over time. The annual age-specific counts of screened women in each risk group were divided by the age-specific Vermont female population in that year, and the resultant rates were combined within risk groups by age-adjustment to the 2001 to 2012 Vermont female population.
Joinpoint regression analyses were performed using software available from the National Cancer Institute (43). The models used weighted least squares regression, with calendar year as the independent variable and the natural logarithm of the age-adjusted normalized utilization rate as the dependent variable. The analyses identified the lines of best fit using a sequence of permutation tests, and we used a maximum of two joinpoints, which identified statistically significant changes in trend at the P less than .05 level. The model also estimated annual percentage change (APC) between points. The directional sign (+/−) and 95% confidence interval of each APC is reported and, using a two-sided test, the APC is considered statistically significant if the confidence interval does not include zero (44).
Results
As presented in Table 2, the age-adjusted annual breast cancer screening utilization rate of women aged 40 to 74 years climbed from 38.7% (95% confidence interval [CI] = 38.4 to 39.3) in 2001 to 42.5% (95% CI = 42.2 to 42.7) in 2008 and then declined to 37.5% (95% CI = 37.3 to 37.8) in 2012. For women aged 40 to 49 years, the utilization rate peaked in 2008 at 38.5% (95% CI = 38.0 to 38.9) and then declined to a 12-year low of 33.0% (95% CI = 32.5 to 33.5) in 2012. For women aged 50 to 74 years, the utilization rate also peaked in 2008 at 44.7% (95% CI = 44.4 to 45.1), before declining to 40.1% (95% CI = 39.7 to 45.4) in 2012.
Table 2.
Average Breast Cancer Surveillance Consortium risk scores by year and age group*
| Year | Aged 40 to 74 y | Aged 40 to 49 y | Aged 50 to 74 y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible population in Vermont | Women screened in Vermont | Age-adjusted screening utilization % (95% CI) | Average BCSC risk score (95% CI) | Eligible population in Vermont | Women screened in Vermont | Age-adjusted screening utilization % (95% CI) | Average BCSC risk score (95% CI) | Eligible population in Vermont | Women screened in Vermont | Age-adjusted screening utilization % (95% CI) | Average BCSC risk score (95% CI) | |
| 2001 | 113892 | 43512 | 38.7 (38.4 to 39.3) | 1.56 (1.55 to 1.57) | 47405 | 15922 | 33.6 (33.2 to 34.0) | 0.90 (0.89 to 0.91) | 66487 | 27590 | 41.6 (41.2 to 42.5) | 1.94 (1.93 to 1.95) |
| 2002 | 116159 | 44015 | 38.3 (38.0 to 38.6) | 1.59 (1.58 to 1.60) | 47521 | 15956 | 33.6 (33.1 to 34.0) | 0.92 (0.91 to 0.93) | 68638 | 28059 | 40.9 (40.6 to 41.3) | 1.97 (1.95 to 1.98) |
| 2003 | 118658 | 45087 | 38.3 (38.0 to 38.6) | 1.61 (1.60 to 1.62) | 47657 | 16112 | 33.8 (33.4 to 34.2) | 0.93 (0.92 to 0.94) | 71001 | 28975 | 40.9 (40.5 to 41.2) | 2.00 (1.98 to 2.01) |
| 2004 | 120821 | 46821 | 39.0 (38.7 to 39.3) | 1.60 (1.59 to 1.61) | 47476 | 16273 | 34.3 (33.8 to 34.7) | 0.92 (0.91 to 0.92) | 73345 | 30548 | 41.7 (41.3 to 42.3) | 1.96 (1.95 to 1.97) |
| 2005 | 122487 | 46092 | 37.8 (37.5 to 38.1) | 1.51 (1.50 to 1.52) | 46936 | 15690 | 33.4 (33.0 to 33.9) | 0.87 (0.86 to 0.88) | 75551 | 30402 | 40.3 (39.9 to 44.6) | 1.84 (1.82 to 1.85) |
| 2006 | 124117 | 49794 | 40.2 (39.9 to 43.5) | 1.47 (1.46 to 1.48) | 46338 | 16868 | 36.4 (36.0 to 36.8) | 0.84 (0.84 to 0.85) | 77779 | 32926 | 42.4 (42.0 to 42.7) | 1.79 (1.78 to 1.80) |
| 2007 | 125536 | 50696 | 40.4 (40.1 to 40.7) | 1.47 (1.46 to 1.48) | 45149 | 16231 | 35.9 (35.5 to 36.4) | 0.84 (0.83 to 0.85) | 80387 | 34465 | 42.9 (42.5 to 43.2) | 1.76 (1.75 to 1.77) |
| 2008 | 127132 | 54109 | 42.5 (42.2 to 42.7) | 1.44 (1.44 to 1.45) | 44090 | 16961 | 38.5 (38.0 to 38.9) | 0.82 (0.81 to 0.83) | 83042 | 37148 | 44.7 (44.4 to 45.1) | 1.73 (1.72 to 1.74) |
| 2009 | 128582 | 54415 | 42.1 (41.9 to 42.4) | 1.48 (1.47 to 1.49) | 43055 | 16438 | 38.2 (37.7 to 38.6) | 0.82 (0.81 to 0.83) | 85527 | 37977 | 44.4 (44.0 to 44.7) | 1.77 (1.76 to 1.78) |
| 2010 | 130055 | 51882 | 39.6 (39.3 to 39.8) | 1.51 (1.50 to 1.52) | 42252 | 14604 | 34.6 (34.1 to 35.0) | 0.83 (0.82 to 0.84) | 87803 | 37278 | 42.4 (42.0 to 42.7) | 1.77 (1.76 to 1.78) |
| 2011 | 131258 | 51848 | 39.1 (38.8 to 39.3) | 1.54 (1.53 to 1.55) | 41067 | 14027 | 34.2 (33.7 to 34.6) | 0.83 (0.82 to 0.84) | 90191 | 37821 | 41.8 (41.5 to 42.2) | 1.81 (1.80 to 1.82) |
| 2012 | 131878 | 50158 | 37.5 (37.3 to 37.8) | 1.58 (1.57 to 1.59) | 39678 | 13101 | 33.0 (32.5 to 33.5) | 0.84 (0.83 to 0.85) | 92200 | 37057 | 40.1 (39.7 to 45.4) | 1.84 (1.83 to 1.85) |
* Table reflects counts of women screened in Vermont who fit our study inclusion criteria (aged 40–74 years; no history of breast augmentation; non-Native American race/ethnicity; and those who did not screen at one excluded imaging facility) and women who did not opt out of research. Eligible population by age group is based on US intercensal and postcensal estimates provided by the US Census (41). BCSC = Breast Cancer Surveillance Consortium; CI = confidence interval.
Among women aged 40 to 74 years obtaining a screening mammogram, the individual 5-year risk of developing breast cancer varied between an average of 1.61% in 2003 (95% CI = 1.60 to 1.62) and 1.44% in 2008 (95% CI = 1.44 to 1.45). After a period of decline in risk scores between 2003 and 2008, average risk increased to 1.58% (95% CI = 1.57 to 1.59) in 2012. As seen in Table 2, there were similar trends in the average risk for women in each age group.
Figure 1A shows normalized utilization rates by BCSC risk category among women aged 40 to 74 years between 2001 and 2012. The normalized rate of women categorized as low risk (<1% 5-year risk) climbed at an APC of 8.3 (95% CI = 4.8 to 11.9) between 2003 and 2008, then declined at an APC of −5.4 (95% CI = −8.1 to −2.6) until 2012. Rates for women categorized as average risk (1%–1.66% 5-year risk) saw no changes in trend, with a level APC of 0.2 (95% CI = −0.4 to 0.7) between 2001 and 2012. For women who were categorized as intermediate risk (1.67%–2.49% 5-year risk) and high risk (2.5%–3.99% 5-year risk), small yet steady and statistically significant declines in rates were observed throughout the study period, with the former exhibiting an APC of −1.5 (95% CI = −2.0 to −0.9) and the latter exhibiting an APC of −2.1 (95% CI = −3.0 to −1.2). There were no statistically significant APCs among normalized rates for very high–risk women (≥4% 5-year risk).
Figure 1.
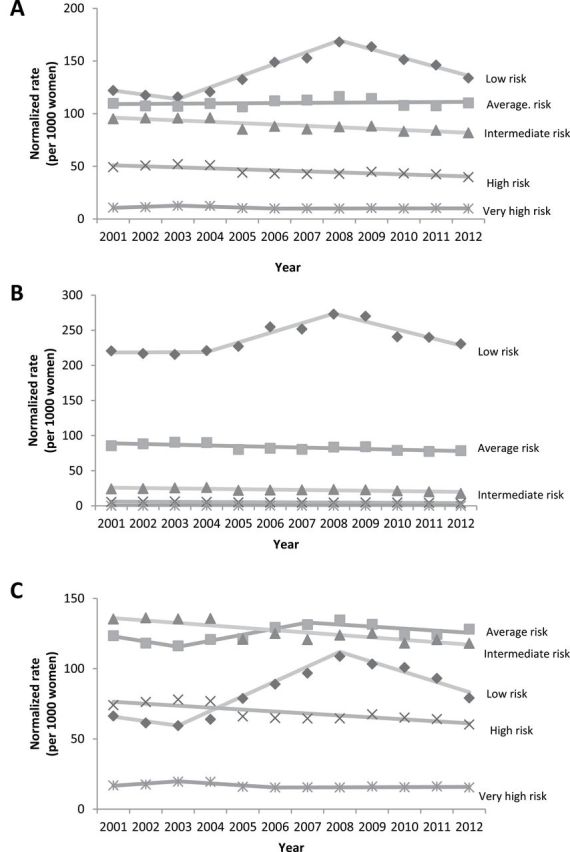
Normalized rates of screened women in each 5-year Breast Cancer Surveillance Consortium (BCSC) risk category, 2001 to 2012. A) Ages 40 to 74 years. B) Ages 40 to 49 years. C) Ages 50 to 74 years. To find the normalized rate, the annual age-specific counts of screened women in each risk group were divided by the age-specific Vermont female population in that year, and the resultant rates were combined within risk groups by age-adjustment to the 2001 to 2012 Vermont female population. Five-year BCSC risk score categories: low (<1%), average (1%–1.66%), intermediate (1.67%–2.49%), high (2.5%–3.99%), and very high (≥4%).
The results of analyses stratified by age group (40–49 and 50–74 years) are shown in Figure 1, B and C. Throughout the study period, the majority of women screened in the group aged 40 to 49 years were categorized as low risk (Figure 1B). From 2004 to 2008, the normalized rate of women identified each year as low risk climbed, with an APC of 5.8 (95% CI = −1.8 to 13.9), and then declined between 2008 and 2012 with a non-statistically significant APC of −4.4 (95% CI = −8.8 to 0.1). Moderate declines in the normalized rates of women identified as average (APC = −1.2; 95% CI = −1.9 to −0.5) and intermediate risk (APC = −2.3; 95% CI = −3.5 to −1.1) occurred over the course of the full study period. Women categorized as high or very high risk in the group aged 40−49 years represent a very small proportion (<1%) of each annual sample, and they exhibited no statistically significant changes in utilization trends throughout the study period.
Most women screened in the group aged 50 to 74 years were identified each year as average or intermediate risk (Figure 1C). Women categorized as average risk exhibited a non-statistically significant increase in their normalized rate between 2003 and 2007 (APC = 3.5, 95% CI = −2.4 to 9.9) and then a modest decline until 2012 (APC = −1.1; 95% CI = −3.4 to 1.2). The rates of women screened at intermediate (APC = −1.3; 95% CI = −1.9 to −0.8) and high risk (APC = −2.0; 95% CI = −2.9 to −1.1) declined modestly across the study period. Women identified as low risk saw the most variation in their annual rates of screening. The low-risk category climbed at an APC of 13.7 (95% CI = 6.4 to 21.5) from 2003 to 2008 and then subsequently declined at a statistically significant APC of −7.1 (95% CI = −12.1 to −2.0) between 2008 and 2012.
Discussion
The decline in screening utilization in Vermont is largely attributable to decreases in screening examinations for low-risk women. Since 2008, the normalized utilization rate declined annually by −5.4% (95% CI = −8.1 to −2.6) among low-risk women, whereas average- and higher-risk groups experienced relatively little change.
Although age is a strong determinant of breast cancer risk (15,45), our results indicate that the decline in utilization among low-risk women after 2008 is not solely explained by decreases in screening participation by younger women or the underlying population changes in Vermont’s age structure. In analyses stratified by age group, we observed that a decline in the normalized rate of screened, low-risk women occurred in both the group aged 40 to 49 years and the group aged 50 to 74 years, accounting for a majority of the overall reduction in screening utilization in each. Notably, this decline in screening for women at low risk was greater in magnitude in the group aged 50 to 74 years. For the group aged 40 to 49 years, women categorized as average or intermediate risk exhibited steady declines in normalized screening levels over the course of the study period, whereas women at low risk exhibited a statistically significant change in trend in 2008.
There are a number of recent studies that characterize breast cancer risk among screened women (6,21,45–49); however there has been little examination of trends in risk over time. Strengths of our study include both the availability of long-term data and a reliance on medical record dates, which provide a more accurate representation of screening attendance than self-reported behavior (50). Additionally, the incorporation of multiple breast cancer risk factors into one index allows for a more comprehensive quantification of risk than relying on any single factor (21) and represents a readily available measurement of risk as opposed to a patient’s genetic predisposition.
Nevertheless, certain limitations to this study should be considered. Although we have observed a decline in the normalized levels of low-risk women receiving mammography screening, we cannot assess the decision-making processes that have influenced this trend. We are also unable to assess in detail economic influences related to the 2007 to 2009 recession. Within Vermont, unemployment rose from 4.1% in January 2008 to a peak of 7.2% in mid-2009, then declined to 4.6% by the end of 2012 (51). Health insurance coverage for Vermont women aged 40 to 74 years remained relatively high throughout the period studied, fluctuating between 93% and 95% (52). Thus it does not appear that the economic recession or changes in health insurance coverage can explain the observed trends in screening after 2009. It is also not possible to know whether the breast cancer risk distribution of the state population has changed over time; however, it seems unlikely that clinical risk factors would have substantially done so in the past 5 years. In addition, the observed trends in risk could have been impacted by changes in assessment of breast density, which is a risk factor in the BCSC risk model. The distribution of breast density has historically fluctuated in Vermont, in part because of changes in BI-RADS density assessment practices (35), as well as the subjectivity of practitioner interpretation (53). Between 2003 and 2008, there was an upward trend of women with almost entirely fatty breasts screened in the state, reflecting changes in reporting that were responsive to the American College of Radiology’s fourth edition BI-RADS Breast Imaging Atlas (35). Across the wider BCSC, density reporting has remained generally unchanged since 2003 (54). Because most of the associated variation in breast density reporting in Vermont occurred before 2008, outside influences on density reporting do not appear to explain the declining mammography utilization rates for women categorized as low risk between 2008 and 2012. Furthermore, although there may be temporal variation in breast density measurement trends, its use as a clinical factor in assessing risk has gained traction in recent years (55), as evidenced by the national advocacy movement for legally requiring providers to report density findings (56). Given the dependence of risk assessment on a factor that can be subject to changes in reporting, some caution is needed when analyzing changes in the distribution of risk over time, especially at subnational scales. This will remain important in the coming years as the density distribution may adjust to the parameters of the newly released fifth edition of the BI-RADS Atlas (54). Finally, the use of the BCSC risk model does not account for all contributors to breast cancer risk, such as paternal inheritance or other genetic factors.
Our findings raise a number of questions and considerations regarding a patient’s decision to screen. Although any individual’s motivation for seeking screening is complex and not addressed directly here, the wide variation in screening exams for women at low risk between 2003 and 2012 may be an indicator that low-risk patients of any age are more responsive to the public discourse and guidance for breast cancer screening. The climb in utilization observed before the new USPSTF guidelines may in part reflect their 2002 guidelines that were inclusive of a large age range, as well as earlier public health efforts to maximize screening. Utilization rates among low-risk women showed the greatest magnitude increases during that time. Meanwhile, demand for mammography screening may be less variable among women who perceive themselves at higher risk. There is some evidence that self-perceived risk is associated with guideline adherence and more frequent mammography screening (47,57,58), and yet women may tend to not have accurate perceptions about their personal risk (57). To complicate matters, confusion among the public about the newest screening recommendations is widespread (59). Finally, the views of primary care providers about mammography screening vary widely (60–62), and recommendation by a provider for screening is one of the strongest predictors of women undergoing screening mammography (31). These considerations highlight the need for further study of patient- and provider-level considerations of risk in the decision to screen.
In conclusion, we find that declines in screening examinations for women at low risk of breast cancer account for a majority of the recent declines in mammography screening utilization in Vermont. The potential health implications of these changing patterns in screening utilization are unclear. In a period of overall declines in screening utilization, it is reassuring that utilization has declined most in low-risk women rather than among higher-risk women. Nevertheless, optimal screening strategies based on risk require further evidence to guide clinical practice. The long-term outcomes for breast cancer incidence and mortality associated with these observed changes are unknown, providing an important area of future research and breast cancer monitoring.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (U54 CA163303 to BLS; P01 CA154292 to KK; U01 CA070013 to BMG).
The authors are solely responsible for the study design, data generation, analysis and interpretation of the data, writing the manuscript, and decision to submit the manuscript for publication.
We would like to thank Mark Bowman, Rachael Chicoine, Cindy Groseclose, Kathleen Howe, Dawn Pelkey, and Tiffany Pelkey for their dedicated efforts in data collection and administrative support. We also thank the mammography facilities, radiologists, and women of Vermont for providing data for this study.
References
- 1. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–W242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marmot MG. Sorting through the arguments on breast screening. JAMA. 2013;309(24):2553–2554. [DOI] [PubMed] [Google Scholar]
- 3. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–1692. [DOI] [PubMed] [Google Scholar]
- 4. Marmot MG, Altman DG, Cameron DA, et al. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. [DOI] [PubMed] [Google Scholar]
- 6. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years a systematic review and meta-analysis. Ann Intern Med. 2012;156(9):635–U79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerlikowske K. Evidence-based breast cancer prevention: the importance of individual risk. Ann Intern Med. 2009;151(10):750–752. [DOI] [PubMed] [Google Scholar]
- 8. Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–U50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years a comparative modeling study of risk. Ann Intern Med. 2012;156(9):609–U39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tice JA, O’Meara ES, Weaver DL, et al. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105(14):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qaseem A, Snow V, Sherif K, et al. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;146(7):511–515. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong K, Eisen A, Weber B. Assessing the risk of breast cancer. N Engl J Med. 2000;342(8):564–571. [DOI] [PubMed] [Google Scholar]
- 13. US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–727. [DOI] [PubMed] [Google Scholar]
- 14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 15. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–691. [DOI] [PubMed] [Google Scholar]
- 16. Sabatino SA, McCarthy EP, Phillips RS, et al. Breast cancer risk assessment and management in primary care: provider attitudes, practices, and barriers. Cancer Detect Prev. 2007;31(5):375–383. [DOI] [PubMed] [Google Scholar]
- 17. Burke W, Culver J, Pinsky L, et al. Genetic assessment of breast cancer risk in primary care practice. Am J Med Genet. 2009;149A(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haas JS, Kaplan CP, Gregorich SE, et al. Do physicians tailor their recommendations for breast cancer risk reduction based on patient’s risk? J Gen Intern Med. 2004;19(4):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Breast Cancer Surveillance Consortium. Breast Cancer Surveillance Consortium Risk Calculator https://tools.bcsc-scc.org/BC5yearRisk/ Accessed February 12, 2014.
- 20. National Cancer Institute. National Cancer Institute Breast Cancer Risk Assessment Tool http://www.cancer.gov/bcrisktool/ Accessed Feburary 12, 2014.
- 21. Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–W75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast-cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. [DOI] [PubMed] [Google Scholar]
- 23. McCaul KD, Branstetter AD, Schroeder DM, et al. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15(6):423–429. [DOI] [PubMed] [Google Scholar]
- 24. Walker MJ, Chiarelli AM, Knight JA, et al. Perceived risk and adherence to breast cancer screening guidelines among women with a familial history of breast cancer: a review of the literature. Breast. 2013;22(4):395–404. [DOI] [PubMed] [Google Scholar]
- 25. Gross CP, Filardo G, Singh HS, et al. The relation between projected breast cancer risk, perceived cancer risk, and mammography use—results from the National Health Interview Survey. J Gen Intern Med. 2006;21(2):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dailey AB, Kasl SV, Holford TR, et al. Neighborhood-level socioeconomic predictors of nonadherence to mammography screening guidelines. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2293–2303. [DOI] [PubMed] [Google Scholar]
- 27. Carney PA, Goodrich ME, MacKenzie T, et al. Utilization of screening mammography in New Hampshire—a population-based assessment. Cancer. 2005;104(8):1726–1732. [DOI] [PubMed] [Google Scholar]
- 28. Rahman SMM, Dignan MB, Shelton BJ. Factors influencing adherence to guidelines for screening mammography among women aged 40 years and older. Ethn Dis. 2003;13(4):477–484. [PMC free article] [PubMed] [Google Scholar]
- 29. Carney PA, O’Malley J, Buckley DI, et al. Influence of health insurance coverage on breast, cervical, and colorectal cancer screening in rural primary care settings. Cancer. 2012;118(24):6217–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith ML, Hochhalter AK, Ahn S, et al. Utilization of screening mammography among middle-aged and older women. J Womens Health. 2011;20(11):1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schueler KM, Chu PW, Smith-Bindman R. Factors associated with mammography utilization: a systematic quantitative review of the literature. J Womens Health. 2008;17(9):1477–1498. [DOI] [PubMed] [Google Scholar]
- 32. Sprague BL, Bolton KC, Mace JL, et al. Registry-based study of trends in breast cancer screening mammography before and after the 2009U.S. Preventive Services Task Force recommendations. Radiology. 2014;270(2):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast cancer surveillance consortium: a national mammography screening and outcomes database. Am J Roentgenol. 1997;169(4):1001–1008. [DOI] [PubMed] [Google Scholar]
- 34. Balch S. bcscrisk.sas v1.0. NCI-funded Breast Cancer Surveillance Consortium (HHSN261201100031C). https://tools.bcsc-scc.org/BC5yearRisk Accessed October 23, 2013.
- 35. D’Orsi CJ, Bassett LW, Berg WA, et al. Mammography, 4th edition. In: D’Orsi CJ, Mendelson EB, Ikeda DM, et al. , eds. Breast Imaging Reporting and Data System: ACR BI-RADS—Breast Imaging Atlas. Reston, VA: American College of Radiology; 2003;179–189. [Google Scholar]
- 36. Shepherd JA, Kerlikowske K, Ma L, et al. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 38. White IR, Daniel R, Royston P. Avoiding bias due to perfect prediction in multiple imputation of incomplete categorical variables. Comp Stat Data Analysis. 2010;54(10):2267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 40. UCLA: Statistical Computing Group. Introduction to Stata http://www.ats.ucla.edu/stat/stata/faq/ologit_mi_marginsplot.htm Accessed February 12, 2014.
- 41. U.S. Census Bureau. Population Estimates https://www.census.gov/popest/methodology/ Accessed January 24, 2014.
- 42. Makuc DM, Haglund B, Ingram D, et al. Health service areas for the United States. Vital Health Stat. 1991;2(112):1–102. [PubMed] [Google Scholar]
- 43. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Joinpoint Regression Program, Version 4.0.1—January 2013. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 44. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351; correction: 2001;20(4):655. [DOI] [PubMed] [Google Scholar]
- 45. Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010 http://seer.cancer.gov/csr/1975_2010/ Accessed May 20, 2014.
- 46. Weisstock CR, Rajapakshe R, Bitgood C, et al. Assessing the breast cancer risk distribution for women undergoing screening in British Columbia. Cancer Prev Res. 2013;6(10):1084–1092. [DOI] [PubMed] [Google Scholar]
- 47. Walker MJ, Chiarelli AM, Knight JA, et al. Perceived risk and adherence to breast cancer screening guidelines among women with a familial history of breast cancer: a review of the literature. Breast. 2013;22(4):395–404. [DOI] [PubMed] [Google Scholar]
- 48. Brinton JT, Barke LD, Freivogel ME, et al. Breast cancer risk assessment in 64,659 women at a single high-volume mammography clinic. Acad Radiol. 2012;19(1):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wernli KJ, DeMartini WB, Ichikawa L, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med. 2014;174(1):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cronin KA, Miglioretti DL, Krapcho M, et al. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. US Department of Labor Bureau of Labor Statistics. Local Area Unemployment Statistics http://www.bls.gov/lau/ Accessed March 14, 2014.
- 52. King M, Ruggles S, Alexander JT, et al. Integrated Public Use Microdata Series, Current Population Survey (CPS): Version 3.0. [Machine-readable database]. Minneapolis: University of Minnesota. Data based on the US Census Bureau’s Annual March CPS. http://cps.ipums.org/cps/index.shtml Accessed March 12, 2014.
- 53. Spayne MC, Gard CC, Skelly J, et al. Reproducibility of BI-RADS breast density measures among community radiologists: a prospective cohort study. Breast J; 2012;18(4):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013;123–. [Google Scholar]
- 55. Boyd NF, Martin LJ, Yaffe M, et al. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dehkordy SF, Carlos RC. Dense breast legislation in the United States: state of the states. J Am Coll Radiol. 2013;10(12):899–902. [DOI] [PubMed] [Google Scholar]
- 57. Katapodi MC, Lee KA, Facione NC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38(4):388–402. [DOI] [PubMed] [Google Scholar]
- 58. Murabito JM, Evans JC, Larson MG, et al. Family breast cancer history and mammography—Framingham Offspring Study. Am J Epidemiol. 2001;154(10):916–923. [DOI] [PubMed] [Google Scholar]
- 59. Squiers LB, Holden DJ, Dolina SE, et al. The public’s response to the US Preventive Services Task Force’s 2009 recommendations on mammography screening. Am J Prev Med. 2011;40(5):497–504. [DOI] [PubMed] [Google Scholar]
- 60. Yasmeen S, Romano PS, Tancredi DJ, et al. Screening mammography beliefs and recommendations: a web-based survey of primary care physicians. BMC Health Serv Res. 2012;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hinz EK, Kudesia R, Rolston R, et al. Physician knowledge of and adherence to the revised breast cancer screening guidelines by the United States Preventive Services Task Force. Am J Obstet Gynecol. 2011;205(3):e1–e5. [DOI] [PubMed] [Google Scholar]
- 62. Meissner HI, Klabunde CN, Han PK, et al. Breast cancer screening beliefs, recommendations, and practices. Cancer. 2011;117(14):3101–3111. [DOI] [PubMed] [Google Scholar]


