Abstract
Objectives: Strategies to effectively identify and refer children with severe acute malnutrition (SAM) to Nutritional Rehabilitation units (NRU) can reduce morbidity and mortality.
Methods: From December 2011 to May 2012, we conducted a prospective study task-shifting inpatient malnutrition screening of Malawian children 6–60 months to lay-screeners and evaluated World Health Organization (WHO) criteria vs. the National Center for Health Statistics (NCHS) guidelines for SAM.
Results: Lay-screeners evaluated 3116 children, identifying 368 (11.8%) with SAM by WHO criteria, including 210 (6.7%) who met NCHS criteria initially missed by standard clinician NRU referrals. Overall case finding increased by 56.7%. Mid-upper arm circumference (MUAC) and bipedal edema captured 86% (181/210) NCHS/NRU-eligible children and 89% of those who died (17/19) meeting WHO criteria. Mortality of NCHS/NRU-eligible children was 10 times greater than those without SAM (odds ratio 10.5, 95% confidence interval 5.4–20.6).
Conclusions: Ward-based lay-screeners and WHO guidelines identified high-risk children with SAM missed by standard NRU referral. MUAC and edema detected the majority of NRU-eligible children.
Keywords: malnutrition screening, task-shifting, WHO growth standard, National Center for Health Statistics (NCHS) growth reference, mid-upper arm circumference (MUAC), Malawi
INTRODUCTION
Among children aged <5 years in developing countries, ∼19 million are severely malnourished, and deaths from severe malnutrition exceed 450 000 annually [1]. Identifying children with severe acute malnutrition (SAM) allows access to life-saving interventions, including referral to nutritional rehabilitation units (NRU), and may decrease mortality and future morbidity [2].
In 2006, the World Health Organization (WHO) published growth standards for SAM intended to replace the National Center for Health Statistics (NCHS) reference [3]. NCHS used anthropometric measurements from mainly formula-fed US infants, while the WHO standard uses measurements from a primarily breastfed multinational cohort [4, 5]. Both NCHS and WHO use bipedal edema and weight-for-height (WFH) <−3 standard deviations (SD) below the mean (∼70% of the NCHS median and 80% of the WHO median) as defining characteristics of SAM, but use different cutoffs for mid-upper arm circumference (MUAC) (Fig. 1). The NCHS cutoff for MUAC is <110 mm, while the WHO is ≤115 mm. All children with SAM by NCHS meet WHO criteria for severe malnutrition; some children meeting WHO criteria are only moderately malnourished per NCHS. Operationally, many inpatient NRUs throughout sub-Saharan Africa still use WFH <70% of the NCHS median as admission criteria, likely due to the input of resources needed to implement the newer WHO guidelines [6, 7].
Fig. 1.
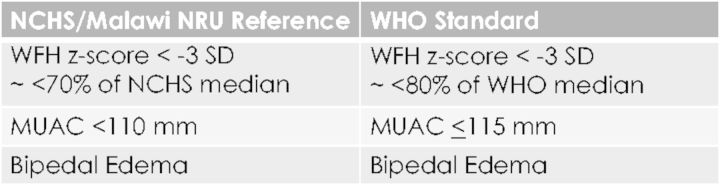
Measures for severe malnutrition in children: NCHS/NRU Reference vs. WHO Standard criteria. Abbreviations: NCHS—National Center for Health Statistics, NRU—Nutrition Rehabilitation Unit, WHO—World Health Organization, ∼—approximately, WFH—weight-for-height, MUAC—mid-upper arm circumference, mm—millimeters, SD—standard deviation.
Malawi is a sub-Saharan African country of 15.9 million people with one of the highest under-5 mortality rates globally (71/1000) [8, 9]. Thirteen percent of children <5 years are moderately to severely underweight, with 4% moderately to severely malnourished [10]. Although Malawi has implemented community-based therapeutic care to identify and treat children in the outpatient setting, mortality among hospitalized children with SAM is extremely high with estimates between 14 and 49% [11–13].
At Kamuzu Central Hospital (KCH) in Lilongwe, Malawi, formal malnutrition screening is not routinely performed on admission by clinicians. Clinicians admit children to the hospital and refer those suspected of being malnourished by gross visual inspection (either obvious edema or wasting) to the NRU for formal anthropometric and edema evaluation by a trained nurse, as well as symptom screen for diarrhea and appetite. NRU admission is based on the 2006 Malawi Ministry of Health guidelines, which consist of modified NCHS criteria: WFH <70% of the NCHS median, presence of edema, or MUAC <110 mm [14]. The WHO guidelines for severe malnutrition have not rolled-out at KCH, though they have begun to be introduced in some inpatient settings in Malawi [15]. NRU-eligible children receive daily weights, therapeutic feedings, empiric antibiotics and vitamins.
Like many sub-Saharan African hospitals, the KCH pediatrics ward suffers from staff shortages including a patient-to-nurse ratio often exceeding 100:1 [16]. The WHO recommends that task-shifting (redistribution of tasks to workers with less training) can efficiently use available workforce resources to improve clinical care [17]. Lay health-workers have been successfully used in both community- and facility-based care for a variety of conditions including HIV [18], malaria [19] and common childhood illnesses [20] in sub-Saharan Africa. Task-shifting has been used at KCH to increase HIV testing by lay-counselors and pediatric patient triage by lay-vital sign assistants [16, 21–23]. Trained volunteers screen children in a number of countries, including Malawi, as part of community-based management of acute malnutrition programs [24]; however, there is little literature describing the use of lay-screeners to identify severely malnourished hospitalized children to improve referral to inpatient NRUs.
METHODS
Study design
This was a prospective observational study with two objectives: (i) to the evaluation of the use of lay-screeners to identify children with SAM on the inpatient wards missed by the standard NRU referral; and (ii)assessment of the implications of using WHO vs. NCHS criteria on inpatient SAM prevalence. Characteristics of severely malnourished children identified by lay-screeners on the wards (meeting NCHS or WHO criteria) were compared to those who were admitted to the NRU by the standard of care referral, including case fatality rate. We assessed the distribution of anthropometric measures used to identify children with SAM and among those who died.
Study location
KCH is a tertiary care referral center serving the central region of Malawi. The pediatric wards have ∼215 beds, and admissions exceed capacity with over 13 000 annually [21].
Lay malnutrition screener training
Potential lay-screeners were recruited by asking KCH pediatric wards and NRU staff for referrals. Three people were interviewed and two were hired. Both were literate, had graduated from secondary school and were bilingual in Chichewa (local language) and English. Neither had previous health care setting experience. Lay-screener training consisted of 10 days of instruction and supervision. Training included malnutrition signs and symptoms, how to accurately measure a height and a length with height and length boards, reading a digital scale, measuring a child's weight with or without the caretaker as necessary, including use of a calculator to subtract adult weight from the total weight of child and caretaker, using laminated NCHS reference and WHO growth standard cards, performing a MUAC, measuring and documenting edema, and completing the study form. During the training period, measurements were intensively supervised for 1 week by FMC (advanced practice nurse) until competency was assured and then intermittently (about once a day for one child) for several months to ensure maintenance of quality measurements and interpretation. The use and interpretation of the NCHS- and WHO-laminated cards were also observed during supervision. Lay-screener data forms were assessed for missing data and accuracy for several weeks following training.
Study procedures
Lay-malnutrition screeners conducted bedside anthropometric measurements on children aged 6–60 months admitted to the pediatric wards between 6 December 2011 and 31 May 2012. Height (or length for children too young or ill to stand) was measured to the nearest 0.5 cm using a wooden measurement board. Weight was measured to the nearest 0.1 kg using a digital scale. Children unable to stand on the scale (due to age or illness) were measured by subtracting the caretaker’s weight from the combined weight of child and caretaker. Height and weight were used to determine WFH NCHS percent of median and WHO z-score using laminated cards. MUACs were measured using UNICEF pre-marked tapes. Bipedal edema was assessed by applying thumb pressure to the dorsum of the feet and lower extremities. Screeners recorded demographic data including sex and date of birth (or age when birth date not available) from patient health passbooks. Children screened on the wards who met either WHO or NCHS criteria for SAM were referred to the NRU for further evaluation. All children referred to the NRU received confirmatory anthropometric evaluation by NRU staff. Children not meeting NRU service requirements, but with NCHS WFH <80% of the median, were offered enrollment into outpatient therapeutic feeding programs. Demographic and anthropometric data for standard of care NRU admissions were collected from the NRU register. Mortality data were collected from the pediatric death register.
Ethical consideration
The Malawi National Health Sciences Research Committee and University of North Carolina institutional review board approved this study.
Statistical methods
Patients with complete data (age and anthropometric measures) were analyzed. Categorical data were summarized as proportions and non-normally distributed continuous variables by median and interquartile range. Associations between categorical variables were examined using chi-square tests, and between categorical and non-normally distributed continuous values with Mann–Whitney U-tests. Z-scores were compared with t-tests. Unadjusted odds ratios (OR) for death were calculated using 95% confidence intervals (CI). All tests were two-sided using a pre-determined alpha of 0.05. Emergency Nutrition Assessment for Standardized Monitoring and Assessment of Relief and Transitions software through EpiInfoTM (CDC, version 2007, Atlanta, GA, USA) was used to calculate WFH z-scores and percent of the medians for analysis. STATA (StataCorp, version 12, College Station, TX, USA) was used for all other statistical analysis.
RESULTS
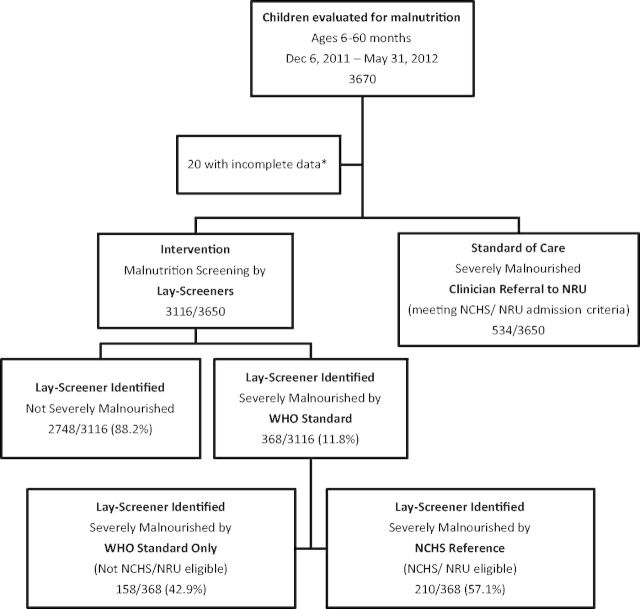
Between 6 December 2011 and 31 May 2012, a total of 3670 children aged 6–60 months were evaluated for malnutrition, of which 3650 had complete demographic and anthropometric data (Fig. 2). Lay-screeners measured 3116 children on the ward; there were 534 standard of care NRU admissions. Lay-screeners identified an additional 28.2% (210/744) of all NRU-eligible patients, accounting for 6.7% (210/3116) of all children screened on the ward. Nearly 12% (368/3116) of children on the wards evaluated by lay-screeners met WHO SAM criteria, increasing case finding of severely malnourished children by 56.7% (210/3116, 6.7% vs. 368/3116, 11.8%, p < 0.001). Children meeting WHO criteria alone accounted for 42.9% (158/368) of severely malnourished children identified on the ward by lay-screeners.
Fig. 2.
Study Flow. Severely malnourished children identified by lay-screeners on the wards meeting WHO criteria, as well as NRU eligible children initially missed by standard of care clinician referral vs. standard of care NRU admissions. *Eleven with missing ages, one with missing MUAC, two with missing height, one missing edema, five unable to calculate WFH % or z-score due to inaccurate height/weight measurement. Abbreviations: NRU—Nutrition Rehabilitation Unit, NCHS—National Center for Health Statistics, WHO—World Health Organization.
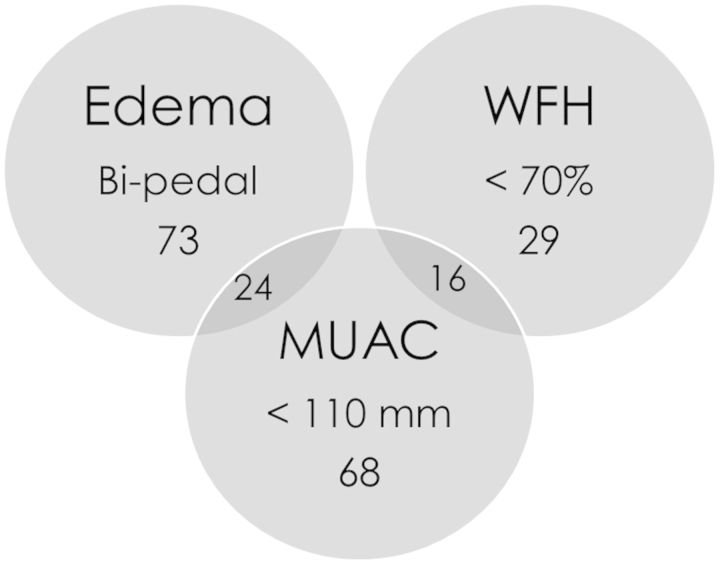
Lay-screener identified children meeting NCHS/NRU criteria were younger (median age 12.7 vs. 16.1 months, p < 0.001), with larger MUACs (median 105 vs. 100 mm, p = 0.004) compared to standard of care NRU admissions (Table 1). More standard of care NRU admissions had edema (79.8% vs. 46.2%, p = 0.001). Median NCHS WFH percentiles (74.1 vs. 74.6, p = 0.593) and WHO z-scores (−3.3, p = 0.928) were similar. MUAC and edema captured 86% (181/210) of children meeting NCHS/NRU criteria on the wards identified by lay-screeners (Fig. 3).
Table 1.
Comparison of children identified by lay-screeners on the ward meeting NCHS criteria for SAM initially missed by standard of care clinician referral vs. standard of care NRU admissions
| Standard of care Clinician Referral to NRU by NCHS criteria N = 534 N(%) | Intervention Lay-Screener Referral to NRU by NCHS criteria N = 210 N(%) | p | ||
|---|---|---|---|---|
| Age in months | <0.001 | |||
| 6–11.9 | 79 (14.8) | 65 (31.0) | ||
| 12–23.9 | 222 (41.6) | 85 (40.5) | ||
| 24–35.9 | 142 (26.6) | 35 (16.7) | ||
| 36–60 | 91 (17.0) | 25 (11.9) | ||
| Median age (IQR) | 16.1 (11.7–24.0) | 12.7 (9.5–21.6) | <0.001 | |
| Gender | ||||
| Male | 271 (50.7) | 103 (49.0) | 0.676 | |
| Female | 263 (49.3) | 107 (51.0) | ||
| Edema | ||||
| Yes | 426 (79.8) | 97 (46.2) | <0.001 | |
| No | 108 (20.2) | 113 (53.8) | ||
| MUAC < 110mm | ||||
| Yes | 196 (36.7) | 108 (51.4) | <0.001 | |
| No | 338 (63.3) | 102 (48.6) | ||
| Median MUAC (IQR) | 100 (90–110) | 105 (100–110) | 0.004 | |
| Weight for Height (WFH), n/Na | ||||
| NCHS WFH < 70%b | 31/108 (28.7) | 45/113 (39.8) | 0.082 | |
| Median NCHS WFH % (IQR) | 74.6 (68.7–82.3) | 74.1 (66.9–82.7) | 0.593 | |
| Mean WHO WFH z-score (SD) | −3.3 (1.6) | −3.3 (2.1) | 0.928 | |
| Diedc | 38 (7.1) | 15 (7.1) | 0.990 |
SAM, severe acute malnutrition; NCHS, National Center for Health Statistics; NRU, nutritional rehabilitation unit; IQR, interquartile range; MUAC, mid-upper arm circumference; mm, millimeters; SD, standard deviation.
aDenominator excludes children with edema.
b% of NCHS median.
cDied during hospitalization.
Fig. 3.
Presence of bipedal edema and/or MUAC <110 mm MUAC identified 86% (181/210) of children with severe malnutrition by NCHS criteria on the wards initially missed by NRU standard of care clinician referral. Abbreviations: WFH—weight-for-height, MUAC—mid-upper arm circumference, mm—millimeters.
Children meeting WHO SAM criteria only were younger (median age 12.8 vs. 16.2 months, p = 0.006), had larger MUACs (median 115 vs. 108 mm, p < 0.001), with higher NCHS WFH percent of the median (median 80.4 vs. 74.1%, p < 0.001) and WHO WFH z-scores (mean −2.3 vs. −3.3, p < 0.001) compared to those on the ward meeting NCHS criteria (Table 2).
Table 2.
Comparison of children with SAM initially missed by standard of care NRU clinician referral identified by lay-screeners meeting WHO standard only vs. NCHS reference on the wards
| Lay-screener identified by WHO standard only N = 158 N(%) | Lay-screener identified by NCHS reference N = 210 N(%) | p | |
|---|---|---|---|
| Age (months) | 0.027 | ||
| 6–11.9 | 70 (44.3) | 65 (31.0) | |
| 12–23.9 | 60 (38.0) | 85 (40.5) | |
| 24–35.9 | 18 (11.4) | 35 (16.7) | |
| 36–60 | 10 (6.3) | 25 (11.9) | |
| Median Age (IQR) | 12.8 (9.6–21.6) | 16.2 (11.1–24.6) | 0.006 |
| Gender | 0.253 | ||
| Male | 87 (55.1) | 103 (49.0) | |
| Female | 71 (44.9) | 107 (51.0) | |
| Edema | |||
| Yes | N/A | 97 (46.2) | |
| No | 158 (100.0) | 113 (53.8) | |
| MUAC in mm | |||
| <110 | N/A | 108 (51.4) | |
| 110 to <115 | 121 (76.7) | 31 (14.8) | <0.001 |
| ≤115 | 121 (76.7) | 139 (66.2) | 0.030 |
| >115 | 37 (23.3) | 71 (33.8) | |
| Median MUAC (IQR) | 115 (110–115) | 108 (100–125) | <0.001 |
| Weight for Height (WFH), n/Na | |||
| NCHS WFH < 70 %b | N/A | 45/113 (39.8) | |
| Median NCHS WFH % (IQR) | 80.4 (75.6–86.6) | 74.1 (66.9–82.7) | <0.001 |
| WHO WFH z-score <−3 SD | 52/158 (32.9) | 71/113 (62.8) | <0.001 |
| Mean WHO WFH z-score (SD) | −2.3 (1.3) | −3.3 (2.1) | <0.001 |
| Diedc | 4 (2.5) | 15 (7.1) | 0.048 |
SAM, severe acute malnutrition; NRU, nutritional rehabilitation unit; WHO, World Health Organization; NCHS, National Center for Health Statistics; IQR, interquartile range; MUAC, mid-upper arm circumference; mm, – millimeters; SD, standard deviation.
aDenominator excludes children with edema.
b% of NCHS median.
cDied during hospitalization.
Children identified on the wards by lay-screeners meeting NCHS criteria were more than 10 times as likely to die, and those meeting WHO criteria were seven times as likely to die, compared to those without SAM (15/210, 7.1% vs. 20/2748, 0.7%, OR 10.5, 95% CI 5.4–20.6, p < 0.0001 and 19/368, 5.1% vs. 20/2748, 0.7%, OR 7.0, 95% CI 3.8–13.2, p < 0.001, respectively). Fewer children meeting WHO criteria only, compared to NCHS criteria, died (4/158, 2.5% vs. 15/210, 7.1%, OR 0.34, 95% CI 0.12–0.99, p = 0.05). Mortality was similarly high among standard of care NRU admissions and children identified by lay-screeners meeting NCHS criteria on the wards (38/534, 7.1% vs. 15/210, 7.1% OR 1.0, 95% CI 0.55–1.84, p = 1.00) (Table 1).
Of children identified by lay-screeners, the highest case fatality rates were among those with both MUAC <110 mm and bipedal edema (5/24, 20.8%). MUAC and/or edema identified 86.7% (13/15) of children who died meeting NCHS and 90.0% (18/20) of those meeting WHO criteria (Table 3).
Table 3.
Distribution of deaths of children with SAM identified by lay-screeners by NCHS and WHO anthropometric criteria
| Deaths | |
|---|---|
| Children meeting NCHS criteria N = 210 | N = 15 (%) |
| MUAC < 110 mm only | 4 (26.7) |
| WFH < 70%a only | 2 (13.3) |
| WFH < 70% and MUAC < 110 | 1 (6.6) |
| MUAC < 110 and Edema | 5 (33.3) |
| Edema only | 3 (20.0) |
| Children meeting WHO criteria N = 368 | N = 20 (%) |
| MUAC ≤ 115 only | 2 (10.0) |
| WFH <−3SD only | 2 (10.0) |
| WFH <−3SD and MUAC ≤ 115 | 7 (35.0) |
| MUAC ≤ 115 and Edema | 6 (30.0) |
| Edema only | 3 (15.0) |
SAM, severe acute malnutrition; NCHS, National Center for Health Statistics; WHO, World Health Organization; MUAC, mid-upper arm circumference; mm, millimeters; WFH, weight for height.
a% of NCHS median.
DISCUSSION
Identifying SAM among children is the first step to reducing mortality related to this highly lethal condition. Our study highlights two successful strategies to increase case detection of SAM in a busy central hospital. First, the use of WHO standards, instead of the NCHS reference resulted in a 56.7% increase in the identification of children with SAM on the wards, all of whom are at high risk for mortality. Second, in a setting with limited resources, we demonstrated that hospital-based malnutrition screening can successfully be task-shifted from clinicians and nurses to lay-personnel. Additionally, the use of two relatively simple measurements, MUAC and edema, captured the majority of children with SAM as well as those at highest risk of death.
The prevalence of SAM on the wards using WHO criteria, compared to NCHS, increased by 1.8 times in our study. This increase is within the range of the 1.5–8 times increase reported previously, as is the increased identification of younger children [25–27]. Our ward prevalence of 12% is similar to inpatient hospital-based studies from Kenya but higher than the <5% prevalence reported by other studies using outpatient cross-sectional surveys and in refugee camps [25, 27–29]. We demonstrated that assessing MUAC and edema can capture most children with SAM in settings where measurement of height and weight are not feasible. MUAC is also less likely to be affected by hydration status in acutely ill children [30]. Relying on clinicians in busy inpatient settings to assess nutritional status for referral to NRUs often leads to assessments based on visual appearance only, which grossly underestimates the burden of malnutrition, as evidenced by the high proportion of children in our study and in others missed by this type of evaluation [31]. Formal anthropometric screening also is more likely to identify less severe cases, which can initiate earlier interventions and decrease mortality.
Mortality of children identified through WHO or NCHS guidelines was high. Although children in our study meeting WHO criteria only had lower mortality compared to those meeting NCHS guidelines, the overall case fatality rate of children meeting WHO criteria was seven times that of their non-severely malnourished ward counterparts. This is similar to other studies, which report that children with WHO WFH z-scores <−3 SD are nine times more likely to die than children with mean WFH z-scores, with similarly high mortality rates among children with MUAC ≤ 115 mm [1, 28, 32].
Limitations to our study include a lack of data on admitting diagnosis for all individual children (including HIV). Among a subset of 300 children who were severely malnourished by WHO guidelines, 17.3% were HIV-infected [33]. HIV infection is known to significantly contribute to the morbidity and mortality of hospitalized children with SAM [12, 13, 34]. Our lack of post-discharge follow-up and use of the death register for mortality data likely underestimated mortality. A recently published study in southern Malawi reported a sobering 42% case-fatality rate among children with SAM initially treated in the inpatient setting, with a quarter of children dying <90 days after discharge [12]. Underreporting of death in children meeting the WHO standard only (not currently NRU eligible) may have occurred due to shorter duration of hospitalization, as deaths among NRU-admitted children are more likely to be captured during their typically longer hospitalizations. Additionally, we may have underestimated the case finding of the standard of care referral system, as ward-based malnutrition screeners may have identified children that would have eventually been referred by a clinician. However, we only screened children after they had been evaluated by admitting staff; therefore, this is unlikely to have had a large effect. Although there is a lack of data on the accuracy and reliability of lay-screener performed malnutrition screening, our program included regular supportive supervision with immediate remediation of any identified gaps such that measurements were consistent and reliable. In addition, the use of similarly skilled individuals have been successfully used to accurately measure vital signs and perform basic triage on the pediatric wards at KCH [16].
Our study adds important information regarding the prevalence of SAM and associated mortality in hospitalized children in sub-Saharan Africa, as well as the effect of using different guidelines with differing anthropometric cutoffs. Although there has been a move to identify malnourished children in the outpatient setting, our study shows that many children with SAM are still hospitalized [35]. Hospitalized malnourished children may have different needs than their lesser or non-malnourished counterparts, including fluid resuscitation methods and need for empiric antibiotics [36, 37]. More intensive screening for malnutrition in the hospital setting may identify children at highest risk for death.
SAM remains an important cause of mortality in hospitalized children in sub-Saharan Africa. Using lay-screeners and WHO guidelines will identify more children that could access potentially life-saving interventions, including NRU resources. Widespread use of ready-to-use therapeutic food, increased antiretroviral therapy coverage and administration of empiric antibiotics are examples of encouraging developments in the management of SAM. Further research is needed to determine whether existing resources in Malawi are sufficient to adopt the WHO guidelines and if identifying more children for malnutrition interventions leads to improved morbidity and mortality outcomes.
ACKNOWLEDGEMENTS
The authors would like to thank malnutrition screeners Manna Itimu and Violet Mwambazi, and NRU nurse Monica Bottoman and the faculty and staff of the Department of Pediatrics at Kamuzu Central Hospital for their valuable contributions to this program. Elizabeth Dawson-Hawn MD (Department of Pediatrics, University of Washington) provided critical review and editorial advice. We also thank the Kizazi working group [University of Washington Global Center for Integrated Health of Women, Adolescents and Children (Global WACh)] for their support during the preparation of this article.
FUNDING
The authors have no declared conflicts of interest. This work was supported by the National Institutes of Health [Fogarty International Clinical Research Scholars and Fellows Program (R24 TW007988) and STD/AIDS Research Training Grant (T32 AI007140) to S.M.L, Heart Lung and Blood Institute (T32 HL072748-11) to E.D.M.] and Health Empowering Humanity [a 501(c)(3) non-profit organization, Houston, TX to S.M.L].
Preliminary data from this study was presented at the Section on International Child Health, American Association of Pediatrics National Conference and Exhibition, New Orleans, October 20–23, 2012.
REFERENCES
- 1.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 2.Collins S. Treating severe acute malnutrition seriously. Arch DisChild 2007;92:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, United Nations Children’s Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 4.de Onis M, Yip R. The WHO growth chart: historical considerations and current scientific issues. Bibl Nutr Dieta 1996;53:74–89. [DOI] [PubMed] [Google Scholar]
- 5.de Onis M, Garza C, Victora CG, et al. The WHO multicentre growth reference study: planning, study design, and methodology. Food Nutr Bull 2004;25(Suppl 1):S15–26. [DOI] [PubMed] [Google Scholar]
- 6.de Onis M, Wijnhoven TM, Onyango AW. Worldwide practices in child growth monitoring. J Pediat 2004;144:461–5. [DOI] [PubMed] [Google Scholar]
- 7.Kerac M, Egan R, Mayer S, et al. New WHO growth standards: roll-out needs more resources. Lancet 2009;374:100–2. [DOI] [PubMed] [Google Scholar]
- 8.United Nations Children’s Fundation. Malawi Nutrition Statistics. New York: United Nations Children’s Fundation, 2013. [Google Scholar]
- 9.UN Inter-agency Group for Child Mortality Estimation. Levels and Trends in Child Mortality. New York: United Nations Children’s Fund, 2013. [Google Scholar]
- 10.World Health Organization. World Health Organization. Global Database on Child Growth and Malnutrition. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 11.Chinkhumba J, Tomkins A, Banda T, et al. The impact of HIV on mortality during in-patient rehabilitation of severely malnourished children in Malawi. Trans R Soc Trop Med Hyg 2008;102:639–44. [DOI] [PubMed] [Google Scholar]
- 12.Kerac M, Bunn J, Chagaluka G, et al. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM Study): a prospective cohort study. PloS One 2014;9:e96030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preidis GA, McCollum ED, Mwansambo C, et al. Pneumonia and malnutrition are highly predictive of mortality among African children hospitalized with Human Immunodeficiency Virus infection or exposure in the era of antiretroviral therapy. J Pediatr 2011;159:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Government of Malawi Ministry of Health. Guidelines for the Managment of Severe Acute Malnutrition. Lilonge, Malawi: Government of Malawi, 2006. [Google Scholar]
- 15.Ahmad UN, Yiwombe M, Chisepo P, et al. Interpretation of World Health Organization growth charts for assessing infant malnutrition: a randomised controlled trial. J Paediatr Child Health 2014;50:32–9. [DOI] [PubMed] [Google Scholar]
- 16.Olson D, Preidis GA, Milazi R, et al. Task shifting an inpatient triage, assessment and treatment programme improves the quality of care for hospitalised Malawian children. Trop Med Int Health 2013;18:879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. The Global Recommendations and Guidelines on Task Shifting. Geneva: World Health Organization, 2008. [Google Scholar]
- 18.Mwai GW, Mburu G, Torpey K, et al. Role and outcomes of community health workers in HIV care in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2013;16:18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith Paintain L, Willey B, Kedenge S, et al. Community health workers and stand-alone or integrated case management of malaria: a systematic literature review. Am J Trop Med Hyg 2014;91:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huicho L, Scherpbier RW, Nkowane AM, et al. How much does quality of child care vary between health workers with differing durations of training? An observational multicountry study. Lancet 2008;372:910–6. [DOI] [PubMed] [Google Scholar]
- 21.McCollum ED, Preidis GA, Kabue MM, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PloS One 2010;5:e9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haac BE, Charles AG, Matoga M, et al. HIV testing and epidemiology in a hospital-based surgical cohort in Malawi. World J Surg 2013;37:2122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaCourse SM, Chester FM, Matoga M, et al. Counselor-based routine opt-out HIV testing increases case finding on the adult medical ward at Kamuzu central hospital in Lilongwe, Malawi [Abstract ]. In: Presented at XIX International AIDS Conference, Washington, DC, 2012. [Google Scholar]
- 24.Maleta K, Amadi B. Community-based management of acute malnutrition (CMAM) in sub-Saharan Africa: case studies from Ghana, Malawi, and Zambia. Food Nutr Bull 2014;35(Suppl 2):S34–8. [DOI] [PubMed] [Google Scholar]
- 25.de Onis M, Onyango AW, Borghi E, et al. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr 2006;9:942–7. [DOI] [PubMed] [Google Scholar]
- 26.Isanaka S, Villamor E, Shepherd S, et al. Assessing the impact of the introduction of the World Health Organization growth standards and weight-for-height z-score criterion on the response to treatment of severe acute malnutrition in children: secondary data analysis. Pediatrics 2009;123:e54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seal A, Kerac M. Operational implications of using 2006 World Health Organization growth standards in nutrition programmes: secondary data analysis. BMJ 2007;334:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkley J, Mwangi I, Griffiths K, et al. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. JAMA 2005;294:591–7. [DOI] [PubMed] [Google Scholar]
- 29.Maitland K, Berkley JA, Shebbe M, et al. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS Med 2006;3:e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mwangome MK, Fegan G, Prentice AM, et al. Are diagnostic criteria for acute malnutrition affected by hydration status in hospitalized children? A repeated measures study. Nutr J 2011;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mogeni P, Twahir H, Bandika V, et al. Diagnostic performance of visible severe wasting for identifying severe acute malnutrition in children admitted to hospital in Kenya. Bull World Health Organ 2011;89:900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull 2006;27:S7–23. [DOI] [PubMed] [Google Scholar]
- 33.LaCourse SM, Chester FM, Preidis G, et al. Use of Xpert for the diagnosis of pulmonary tuberculosis in severely malnourished hospitalized Malawian children. Pediatr J Infect Dis J 2014;33:1200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Maayer T, Saloojee H. Clinical outcomes of severe malnutrition in a high tuberculosis and HIV setting. Arch Dis Child 2011;96:560–4. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Community-based management of severe acute malnutrition. A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund. Geneva: World Health Organization, 2007. [Google Scholar]
- 36.Picot J, Hartwell D, Harris P, et al. The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review. Health Technol Assess 2012;16:1–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013;368:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]