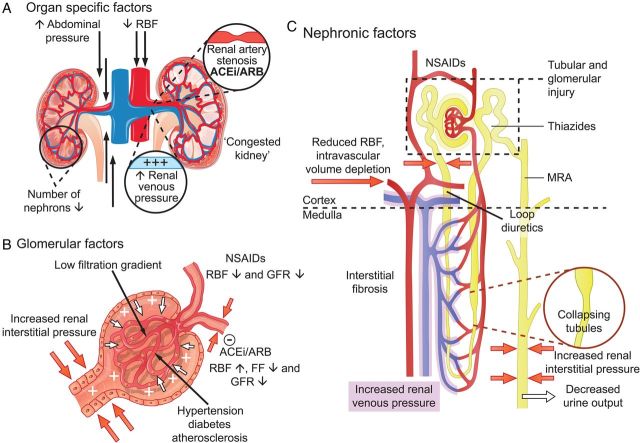
Renal impairment is one of the most powerful predictors of a poor clinical outcome in heart failure (HF). The risk of death in patients with reduced glomerular filtration rate (GFR) is more than double that of patients without renal impairment. In addition, a decline in eGFR (irrespective of cause) is associated with a 60–80% higher mortality.1
Although this change in eGFR is termed ‘worsening renal function’ (WRF) in most studies, there is great variation in the definition of what is meant by WRF across individual studies. Not only does the marker of interest (serum creatinine, cystatin C, or estimated GFR) vary, but so does the magnitude of change that is considered significant. Recently, the term ‘acute kidney injury’ (AKI), adopted from the nephrology literature, has been used to refer to increases in creatinine (or reductions in eGFR), although in many cases we believe this description has been used incorrectly. To complicate matters further, there are three different sets of criteria for AKI, namely RIFLE, AKIN, and KDIGO. To add to this confusion, both impairment in renal function at baseline and deterioration of renal function have been described as the so-called ‘cardiorenal syndrome’, even though there is no evidence to suggest that this is a pathophysiologically plausible entity.2 This cardiorenal interplay includes haemodynamic derangement, where decreased cardiac output and increased venous congestion lead to decreased renal perfusion pressure (reflecting arterial inflow and venous efflux) and decreased GFR. Increased renal venous pressure can also lead to renal interstitial hypertension, and possibly tubular hypertrophy, fibrosis, and tubular injury.2,3 Other factors that may influence cardiorenal interaction include the use of renin–angiotensin–aldosterone system (RAAS) inhibitors, which can inhibit the autoregulatory response to reduced perfusion pressure and renal blood flow, leading to decreased GFR, while at the same time improving clinical status and longer-term outcomes in HF. Finally, concomitant diseases, such as long-standing atherosclerosis, hypertension, and diabetes complicate matters further. Figure 1 provides a (limited) overview of known pathophysiological associations at the organ, glomerular, and nephron level. It highlights the complexity and the numerous factors that are involved in the pathophysiology of deterioration in renal function and the influence of various factors on the association with a clinical outcome. To evaluate clinical significant changes in renal function in the light of these aspects is therefore not as easy as it seems at the first glance.
Figure 1.
Factors involved in the cause and association with an outcome of changes in renal function in heart failure. (A) Organ-specific factors. The main determinants of decreased glomerular filtration rate are a decrease in renal blood flow and an increase in central and renal venous pressure. The latter can be caused by intravascular congestion, but also by an increase in intra-abdominal pressure. Owing to increased renal venous pressure, renal interstitial pressure rises, which results a ‘congested kidney’ since the kidney is encapsulated (B and C). Renal artery stenosis is present in ∼25% of heart failure patients, which can further compromise renal blood flow, especially in the presence of renin–angiotensin–aldosterone system inhibitors. (B) Glomerular factors. Decreased renal blood flow and low blood pressure trigger renal autoregulation, preserving glomerular filtration rate by increasing filtration fraction by increased efferent vasoconstriction. The use of renin–angiotensin–aldosterone system-inhibitors inhibits this process, which increases renal blood flow, but leads (in some patients) to a reduction in glomerular filtration rate (pseudo-worsening renal function). Non-steroidal anti-inflammatory drugs inhibit prostaglandin synthesis, thereby impairing prostaglandin associated increase/dependent renal blood flow. Increased interstitial pressure causes increased pressure in Bowman's capsule, which directly opposes filtration, in a glomerulus where the filtration gradient is already low due to a decreased renal blood flow and increased renal venous pressure. Concomitant diseases have direct, but differential effect on glomerular filtration, glomerular integrity and podocyte function, as well as autoregulation. (C) Nephronic factors. Different therapies have different renal effects and exert their action at specific sites as indicated in this diagram. Intravascular volume depletion (in the presence or absence of congestion) can lead to impaired renal perfusion and decreased glomerular filtration rate. The combination of increased interstitial pressure, reduced arterial perfusion, concomitant disease and therapies can cause tubular and glomerular injury. Increased renal venous pressure causes increased renal interstitial pressure, resulting in collapsing of renal tubules, which decreases glomerular filtration rate, and eventually leads to decreased urine output, sodium retention, and congestion. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; FF, filtration fraction; GFR, glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; NSAIDs, non-steroidal anti-inflammatory drugs; RAAS, renin–angiotensin–aldosterone system; RBF, renal blood flow.
What is the Cardiologist to make all of this? Each of the nephrology community's guidelines on AKI describe three levels of AKI, termed ‘Risk’, ‘Injury’, and ‘Failure’ in the RIFLE classification, and stages 1–3 in the AKIN and KDIGO classifications (Table 1). As with definitions of WRF, all AKI definitions consider changes in serum creatinine, with larger increases corresponding to greater degrees of AKI. However, these classifications require additional and crucial criteria which are usually neglected in studies in HF. Specifically, the AKIN criteria stipulate that the changes in creatinine should occur within 48 h, while RIFLE requires the change to occur within 1–7 days and to be sustained >24 h; the KDIGO guidelines require one, the other or both of these additional criteria.4 Not only this, but the nephrology definitions of AKI also stipulate measurement of low urine output as an important criterion.
Table 1.
Characteristics of definitions of acute kidney injury and worsening renal function
| KDIGO |
HF literature |
Suggested definitiona |
||||
|---|---|---|---|---|---|---|
| Acute kidney injury (AKI) |
Worsening renal function (WRF) |
WRF in chronic HF/AKI in acute HF |
||||
| Stage | Serum creatinine | Urine output | Definitions used |
Serum creatinine/eGFR | Additional criteria | |
| 1 | Increase 1.5–1.9 times baseline within 1–7 days OR ≥ 26.5 µmol/L increase within 48 h | <0.5 mL/kg/h for 6–12 h | Definitions based on creatinine | >/≥26.5 µmol/L increase ≥26.5 µmol/L and ≥25% increase >/≥44 µmol/L increase ≥ 1.5 times baseline ≥ 25% increase and above 176 µmol/L |
Chronic HF (WRF)a | |
| ≥26.5 µmol/L and ≥ 25% increase in sCrb OR ≥ 20% decrease in eGFR over 1–26 weeks | Deterioration in HF status but not leading to hospitalization | |||||
| Acute HF (AKI)a | ||||||
| 2 | Increase 2.0–2.9 times baseline | <0.5 mL/kg/h for ≥12 h | Definitions based on cystatin C | > 0.3 mg/L increase in cystatin C | Increase 1.5–1.9 times baseline sCr within 1–7 days before or during hospitalization OR ≥26.5 µmol/L increase in sCrb within 48 h OR Urine output <0.5 mL/kg/h for 6–12 h | Deterioration in HF status or failure to improve OR Need for inotropes, ultrafiltration or renal replacement therapy |
| 3 | Increase ≥3.0 times baseline OR Increase >354 µmol/L OR Initiation of renal replacement therapy | < 0.3 mL/kg/h for ≥24 h OR Anuria ≥12 h |
Definitions based on eGFR | ≥ 20% decrease ≥ 25% decrease > 5 mL/min/year decrease |
||
For conversion from µmol/L to mg/dL divide by 88.4.
aAny deterioration in renal function that does not meet these criteria should be regarded as pseudo-WRF or pseudo-AKI where there is no evidence of associated harm with the exception of very large increases in serum creatinine (doubling or > 88.4 µmol/L increase) which should always be a reason to refer for further investigation. Consider alternative reasons for increases in creatinine/cystatin C or decrease in eGFR other than WRF/AKI such as intravascular depletion, dehydration, excessive diuresis, medication that alters tubular handling of creatinine, and RAAS inhibitors.
bOr ≥ 0.3 mg/L increase in cystatin C.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; HF, heart failure; KDIGO, Kidney Disease: Improving Global Outcomes; MRA, mineralocorticoid receptor antagonist; sCr, serum creatinine; WRF, worsening renal function.
In studies in chronic HF, we generally do not have repeated measures of creatinine over such short periods of time (or measures of urine output) and therefore the prevalence of true AKI in chronic HF is unknown. On the other hand, WRF over longer periods of time have been reported, but it should be acknowledged that next to a rise in creatinine, the definition of WRF should also include deterioration in HF status to reflect the overall worse clinical condition.
In acute HF, definitions based on short-term changes in creatinine make more sense. However, even in acute HF, few studies have provided detailed data on urine output and even fewer on how urine output relates to changes in creatinine or eGFR. Moreover, the value of measuring urine output may be questionable when the main treatment given is a diuretic. Therefore, even the most commonly used definition of WRF (≥ 26.5 µmol/L increase in creatinine) can only be regarded as approximating to stage 1 AKI or ‘Risk’ using the RIFLE criteria. It is only associated with substantially higher mortality rates in the absence of relief of congestion and improvement in HF status. More severe forms of AKI (stage 2 or 3) are rare in acute HF but if these do occur, prognosis is poor and intensive care management, including renal replacement therapy is often required.
So, where do we go from here? Specifically, we caution the extension of RIFLE/AKIN/KDIGO AKI definitions to include patients experiencing a rise in serum creatinine during successful treatment of acute decompensated HF, at least in the absence of subjective evidence of significant renal injury or failure. In many ways, cardiologists and nephrologists may be treating two distinct but interacting conditions that are simplistically defined by the same biomarker changes. We should also acknowledge that changes in creatinine that occur in HF often have a different cause than AKI described in nephrology guidelines. Acute kidney injury in patients with renal disease is the direct consequence of the disease, while in HF the cause of changes in creatinine is more often indirect. Even more importantly, we now realize that not all increases in creatinine in HF are a bad sign. For instance, in the same way decongestion may lead to haemoconcentration, which is associated with an improved clinical outcome,5,6 a reduction in intravascular volume may lead to increased serum creatinine concentrations, although the extent of this phenomenon is currently unknown. Increases in creatinine during the initiation of evidence-based renin–angiotensin–aldosterone system-blocking therapies in chronic HF may even be associated with better outcomes.7 As a general concept, increases in serum creatinine that parallel improvement in symptoms, signs and weight loss in acute HF are not associated with a poor outcome.5 It seems unreasonable to describe these patients as having AKI or WRF, terms that imply the patient is likely to experience a worse outcome than had creatinine not risen. Additionally, we must acknowledge that in most circumstances AKI and WRF are two distinct entities; AKI indicates that renal injury has occurred, which might or might not be reversible, while WRF represents a functional decline in GFR which may be present without the occurrence of renal injury (AKI), and may or may not be associated with a worsening clinical outcome depending on circumstances.
A more intuitive approach would be to combine a clinical response (using clinical signs of congestion and changes in weight, urine output, haematocrit, natriuretic peptides, and potentially other biomarkers in the future) with changes in renal function measures to distinguish between true AKI (which might be termed Cardiorenal syndrome), and something which might be better described as pseudo-AKI (or pseudo-WRF when only a functional decrease in eGFR is observed) (Table 1). The first is worrisome; the second can be thought of as part of an appropriate response to therapy. As such, it is probably not only the change in creatinine per se that should determine the definition of WRF and poor outcome, but rather the clinical context in which the change in creatinine occurs. An example of the combined assessment of a clinical response and changes in serum creatinine was recently published by Valente et al.8 who showed that a good diuretic response (quantified as weight loss per 40 mg furosemide) was associated with improved clinical outcome. However, the incidence of ‘WRF’ was the highest in the group of patients with the best and worst diuretic response. According to our definition, patients with an increase in creatinine and a good diuretic response had pseudo-AKI, whereas those with an increase in creatinine and a poor diuretic response had true AKI. Additionally, we should realize that a stable serum creatinine is a far better predictor of a good outcome compared with the prognostic information any change in serum creatinine carries. As our proposed definitions are new, no existing study has examined the ability of potential biomarkers of renal injury to differentiate between true and pseudo-AKI.
In the end, it all comes down to the fact that the rise in creatinine itself is not as important as a determinant of outcome, but rather its cause and the clinical context during which these changes develop. We hope these definitions will re-focus thinking about changes in creatinine on the patient's clinical status, treatments used and the patient's response to these, bearing in mind that not every increase in creatinine is an ominous prognostic sign. We also hope that recognition of pseudo-AKI and WRF will prevent withdrawal of potentially life-saving therapy.
Conflict of interest: K.D. is supported by the Netherlands Heart Institute (ICIN) and an ESC Heart Failure Association Research Grant.
References
- 1.Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–446. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. The cardiorenal syndrome in heart failure. Prog Cardiovasc Dis. 2011;54:14–53. doi: 10.1016/j.pcad.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 5.Van der Meer P, Postmus D, Ponikowski P, Cleland JG, O'Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013;61:1973–1981. doi: 10.1016/j.jacc.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 6.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–524. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study) J Am Coll Cardiol. 2012;60:2082–2089. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 8.Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–1293. doi: 10.1093/eurheartj/ehu065. [DOI] [PubMed] [Google Scholar]