Background: Astrocyte pH regulation is essential for brain function.
Results: Bicarbonate-independent acid load recovery in whole-mount astrocytes was achieved primarily via V-ATPase, which also held astrocytes at a basic resting pH.
Conclusion: V-ATPase is the major bicarbonate-independent H+ extruder in a whole-mount astrocyte population.
Significance: High levels of V-ATPase expression will isolate astrocyte pH regulation from cytoplasm changes in other ion species.
Keywords: Astrocyte, pH Regulation, Proton Transport, Sodium-Proton Exchange, Vacuolar ATPase, Glia, Acid Extrusion, Optic Nerve, White Matter
Abstract
The mechanisms of HCO3−-independent intracellular pH (pHi) regulation were examined in fibrous astrocytes within isolated neonatal rat optic nerve (RON) and in cultured cortical astrocytes. In agreement with previous studies, resting pHi in cultured astrocytes was 6.82 ± 0.06 and inhibition of the V-ATPase H+ pump by Cl− removal or via the selective inhibitor bafilomycin had only a small effect upon resting pHi and recovery following an acid load. In contrast, resting pHi in RON astrocytes was 7.10 ± 0.04, significantly less acidic than that in cultured cells (p < 0.001), and responded to inhibition of V-ATPase with profound acidification to the 6.3–6.5 range. Fluorescent immuno-staining and immuno-gold labeling confirmed the presence V-ATPase in the cell membrane of RON astrocyte processes and somata. Using ammonia pulse recovery, pHi recovery in RON astrocyte was achieved largely via V-ATPase with sodium-proton exchange (NHE) playing a minor role. The findings indicate that astrocytes in a whole-mount preparation such as the optic nerve rely to a greater degree upon V-ATPase for HCO3−-independent pHi regulation than do cultured astrocytes, with important functional consequences for the regulation of pH in the CNS.
Introduction
Astrocytes are responsible for extracellular ion homeostasis in the CNS, including H+. pH can have profound effects upon excitability, neurotransmission, and injury responses, and the mechanisms of pH regulation in astrocytes have been examined in detail previously. Acute intracellular pH (pHi) balance in the CNS is determined by two factors: (i) the intrinsic buffering power of neural cells, and (ii) the activity of membrane transporters that move H+ equivalents across cell membranes, resulting in cellular acid extrusion or acid loading (1–3). Acid extruders can either directly remove H+ from cells or import HCO3−, whereas acid loaders are thought to exclusively remove HCO3−. Two HCO3−-dependent transporters, the Na-HCO3 cotransporter and the Na+-driven Cl-HCO3 exchanger, have been described in astrocytes (3, 4) in addition to an electro-neutral Cl-HCO3 exchanger (5). HCO3−-independent transporters maintain intracellular pH by transporting H+ either directly or coupled to another ionic gradient. Studies using cultured astrocytes indicate that the Na-H exchanger (NHE)2 is the principal HCO3−-independent H+ extrusion protein in the CNS, coupling H+ extrusion to the inward Na+ gradient (3, 6–9). Astrocytes also express a V-type H-ATPase that can be blocked by bafilomycin, although the involvement of this pump in pH maintenance and recovery following an acid load is limited in cultured cells (10, 11).
The majority of information available regarding H+ extrusion from astrocytes was obtained from culture preparations. These have the advantage of low background signal and ease of use but the disadvantage that the cells may behave differently from cells in situ, where they are integrated into complex neural networks. Using postnatal day 0–4 (P0–P4) rat optic nerves, we have now examined the involvement of V-ATPase and NHE in the maintenance of steady-state pHi and pHi recovery following an acid load in astrocytes. This white matter tract is pre-myelinated, allowing high-contrast imaging of ion-sensitive intracellular dyes, and contains a population of early maturing fibrous astrocytes. The nerve was transected at either end, and cells were imaged in the central region and are therefore in a native, effectively undamaged environment. We report that in contrast to cultured astrocytes, V-ATPase is the dominant HCO3−-independent H+ extrusion mechanism in this in situ astrocyte population.
EXPERIMENTAL PROCEDURES
Rat Optic Nerve Preparation
Postnatal day 0–2 (P0–P2) Lister Hooded or P0–P4 Wistar rats of both sexes were humanely killed, and the optic nerves were dissected according to regulations from the Home Office, United Kingdom, with approval from the local Animal Welfare and Ethical Review Board (AWERB) of the University of Leicester and Plymouth University. The nerves were placed in HEPES-buffered artificial cerebrospinal fluid (aCSF) (see below). The pH-sensitive fluorescent dye 2′,7′-bis-(2-carboxyethyl)-5 (and 6)-carboxyfluorescein (BCECF) (Molecular Probes Inc.) was used to measure pHi. BCECF-AM (10 μm) was dissolved in dimethyl sulfoxide, 10% Pluronic acid, and cells were loaded in HEPES-aCSF at room temperature for 90 min (15 min for cultures). After washing, the ends of the nerves were fixed to a glass coverslip with a small amount of cyanoacrylate glue and sealed into a Plexiglas perfusion chamber with silicone grease (atmosphere chamber, Warner Instruments). The chamber was mounted on an Eclipse TE2000-U inverted microscope (Nikon), continuously superfused at a rate of 2 ml/min, and maintained in an oxygenated environment (see Ref. 12) for further details). The chamber temperature was maintained at 37 °C with a flow-through feedback tubing heater (Warner Instruments) positioned immediately before the chamber and a feedback objective heater (Bioptechs) that warmed the objective to 37 °C. This combination of heating systems regulated the temperature of the bath and coverslip to 37 °C, as established periodically with a temperature probe.
Astrocyte Cultures
Astrocytes were isolated and cultured using an established protocol (13). Brains from BALB/c mice embryos (embryonic days 14–16) were collected in Hanks' balanced salt solution (Gibco) on ice, and after removal of the meninges, the tissue was digested for 2 min at 37 °C with 2 ml of 1% trypsin solution. The digestion was stopped with 10 ml of DMEM (Gibco) with 10% FCS (Gibco). After washing, the tissue was triturated in 2 ml of 0.5% DNase solution. 10 ml of DMEM, 10% FCS medium was added, and cells were centrifuged for 5 min at 200 × g. The pellet was resuspended in DMEM, 10% FCS incubation media with 50 units/ml penicillin and 50 mg/ml streptomycin (P/S) (Invitrogen), and the cells were plated in poly-l-lysine (100 μg/ml) precoated flasks (1.8–3.5 × 107 cells/flask). The medium was partially exchanged every 3 days. After 15–20 days, a mixed glial culture was achieved. The cells were separated by shaking, leaving the astrocyte layer, which was washed twice with serum-free Hanks' balanced salt solution prior to trypsinization. Glial fibrillary acidic protein (GFAP) staining of sister cultures showed 93.9% of cells being GFAP+. Astrocytes were centrifuged at 4 °C for 5 min at 200 × g and resuspended with incubation media DMEM, 10% FCS, P/S and plated on coverslips, which were incubated at 37 °C until mounted in an atmosphere chamber as described above.
Cell Imaging
Cells within the optic nerve or on coverslips were illuminated at 440 and 490 nm by a monochromator (Optoscan; Cairn Research), and images were collected every 30 s at 510 nm using an appropriate filter set (Chroma Technology) and a cooled CCD camera (CoolSNAP HQ; Roper Scientific). Cells were initially brought into focus during illumination at 490 nm, usually selecting a field containing >30 cells in optic nerves and >60 in astrocyte cultures. In the case of optic nerves, all the cells loading the dye at this age are astrocytes (12), and a typical optic nerve cell loading with BCECF is shown in Fig. 1A. The brightest 15 or 30 cells were selected for analysis to avoid data biasing between nerves or cultures, respectively. Once mounted in the microscope, nerves or cell cultures were left to equilibrate for 10–20 min in HEPES-aCSF to remove any unhydrolyzed dye before the start of each experiment. Regions of interest were drawn around individual cells, and following background subtraction, the fluorescence ratio for each was plotted against time.
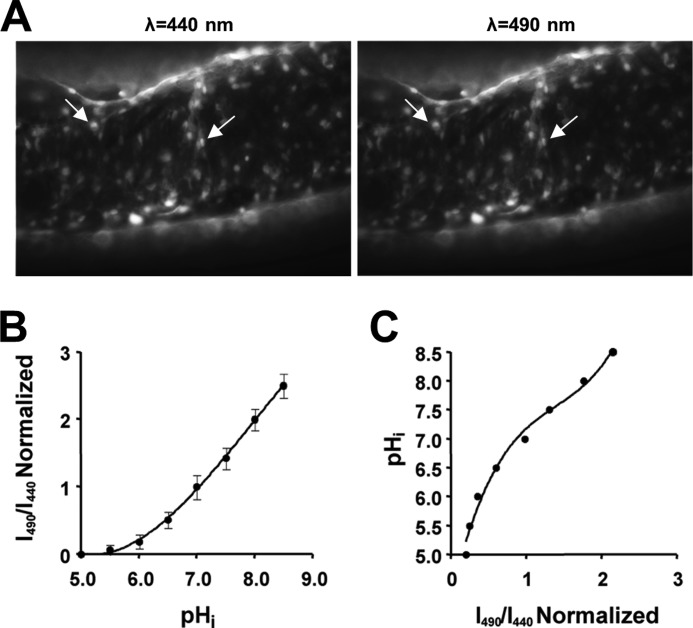
FIGURE 1.
Cell loading of BCECF-AM and pHi calibration in optic nerve astrocytes. A, images of BCECF-AM loading in rat optic nerve astrocytes. A large number of cells can be seen to have loaded the dye (e.g. arrows) during excitation at 440 and 490 nm. B, plot of the calibration curve obtained from optic nerve astrocytes. 490/440 ratios were obtained after exposing cells to high K+/nigericin calibration solutions at pH values from 5.5 to 8.5. The 490/440 ratio was normalized to the ratio at pH 7.0. Values are given as mean ± S.E. C, replotted calibration curve with the data fitted to a third order polynomial function. This is used for correlating pHi values to normalized 490/440 ratios obtained from single-point calibrations at the end of experiments.
Calibration
The 490/440 fluorescence ratio was calibrated against a lookup table giving a single point reference at the end of each experiment. The lookup table was produced by superfusing a series of optic nerves with a high K+/nigericin solution driving pHi to that of the superfusate and giving one point on a 490/440 versus pHi plot (8, 14). The high K+/nigericin solution contained (in mm): 10 Na+, 130 K+, 10 Cl−, 130 gluconic acid, 0.6 Ca2+, 0.6 MgSO4, 10 glucose, and 10 PIPES for pH ≤ 6.8 or 10 HEPES for pH ≥ 7.0. For Nigericin (10 μm), gramicidin (5 μm), and ouabain (1 mm) solutions, pH was adjusted with NaOH, giving eight different solutions with pH ranging from 5.0 to 8.5 in intervals of 0.5. 490/440 ratios were calculated to obtain the calibration curve shown in Fig. 1B, normalized to pH 7.0. As in previous studies (8, 14), the calibration curve was replotted and fitted with a third order polynomial function obtaining a best-fit value R2 of 0.98 (Fig. 1C). The 490/440 data from individual experiments was then converted to pHi values by exposing the cell to a pH 7.0 calibration solution at the end of each experiment, applying the resulting 490/440 value to the third order polynomial function and converting the whole data series using these values.
Acid Loading and H+ Buffering
Rat optic nerves or astrocyte cultures were acid-loaded using the NH4+ prepulse technique (15). Briefly, nerves or cultures were prepared as described above and exposed to HEPES-aCSF containing 20 mm (NH4)2SO4. NH3 freely permeates the cell membrane and reacts with free H+ in the cytoplasm, causing a rapid increase in pHi. Slow NH4+ influx follows, acidifying the cell, and subsequent removal of the extracellular NH3/NH4+ causes a rapid decrease in pHi to below the starting value. Optic nerve astrocytes were exposed to NH3/NH4+ for 5 min, whereas exposure time for cultured astrocytes was reduced to 2 min due to problems with cell viability. The shorter exposure time for cultured astrocytes allowed for a full recovery of pHi consistent with Ref. 1. The shorter ammonia pulse also caused a smaller acidification and thus recovery over a narrowed band of pHi values (6.6–7.0) as compared with optic nerve astrocytes.
H+-buffering power was calculated using an established protocol (1, 8, 14). The total cellular H+-buffering capacity (βT) is the product of an HCO3-dependent component (βHCO3 = 2.3 [HCO3−]i) and an intrinsic component (βi). By measuring the pHi increases produced by exposing cells to different external concentrations of NH4+ under conditions where pHi regulation is interrupted (8) and in the absence of HCO3−, βT = βi, which can be calculated as
Nerves were initially superfused with HEPES-aCSF followed by HEPES-aCSF with Na+ and Cl− substituted to block pH regulation in the astrocytes (see below). Three rat optic nerves (RONs) were exposed to [NH4+] in concentrations of 1, 2.5, 5, 10, or 20 mm. Following each pulse, pHi, was extrapolated to time 0, Δ[NH4+]i was calculated using the Henderson-Hasselbalch equation, and βT = βi was calculated and plotted against the resulting pHi (4, 8).
Rat Optic Nerve Extracellular pH
Ion-sensitive microelectrodes were manufactured as described previously (16). Electrodes were pulled from glass capillaries (1.5-mm outer diameter, 0.86-mm inner diameter, Harvard Apparatus) with a Sutter model P-97 puller (Sutter Instrument Co.). Electrodes were salinized using N,N-dimethyltrimethylsilylamine (TMSDMA), backfilled with HEPES-aCSF (pH 7.45), and front-filled with the ion-exchange resin (hydrogen ionophore I: mixture A, Sigma-Aldrich). The reference electrode was pulled as above and filled with HEPES-aCSF. Experimental solutions were kept at 37 °C and continuously aerated. The Ag-AgCl bath electrode was connected to the superfusate using a 1 m KCl agar bridge to avoid drift in the Cl−-free solution. Dissected rat optic nerves were stored in HEPES-aCSF for at least 1 h to allow axon sealing. Nerves were then fixed onto coverslips as described above. Following impalement, the nerve was left to recover for 5–10 min before the start of experiments. The recordings were performed and recorded with a high impedance differential electrometer (FD223a, World Precision Instruments), a IX-228 data acquisition unit, and LabScribe2 software (both iWorx Systems, Inc.) with sampling at 1 Hz.
Due to differences in composition between electrode fillings and the experimental Na+/Cl−-free HEPES-aCSF, we compensated for the junction potential by recording the change in junction potential in the chamber following experiments and subtracting it from the in-nerve recordings. The electrodes were calibrated using HEPES-aCSF with pH levels ± 1 unit relative to the control (6.4 and 8.4, respectively). pH sensitivity of the electrodes was found to be similar in control and Na+/Cl−-free (NMDG gluconate-substituted) HEPES-aCSF.
Antibody Staining
Nerves were transferred to 0.1 m PBS prior to fixing in 4% paraformaldehyde for 30 min at room temperature. The nerves were then cryoprotected (20–30% sucrose for 5 min), transferred to Tissue-Tek medium (Sigma), and frozen using ethanol and dry ice. 20-μm sections were cut by cryostat and mounted onto microscope slides. The freshly cut sections were then submerged in 0.1 m PBS for 5 min and blocked in 0.1 m PBS containing 10% goat serum and 0.5% Triton-X for 120 min at room temperature prior to overnight exposure to primary antibody at 4 °C in the same solution. Monoclonal GFAP (Molecular Probes, 1:200) and rabbit anti-V-ATPase F (sc-20947, Santa Cruz Biotechnology, 1:100) were used. Slides were then washed (3 × 5 min) and incubated in the appropriate Alexa Fluor-conjugated secondary antibody (Molecular Probes, 1:1000) for 60 min at room temperature. The sections were then washed sequentially in 0.5, 0.1, and 0.05 m PBS containing 10% normal serum. Images were collected using an Olympus IX70 confocal microscope (60× objective). In all cases, controls treated as above but without primary antibody were blank.
Electron Microscopy
Optic nerves were post-fixed in 3% glutaraldehyde/Sorenson's Ringer prior to post-fixing with 2% osmium tetroxide and dehydration prior to infiltration in epoxy. Sections were counterstained with uranyl acetate and lead citrate and examined with a Jeol 100CX electron microscope (see Ref. 17 for details). For post-embedding immuno-labeling, primary antibody was applied to the sections overnight, and appropriate 20-nm gold particle secondary antibodies were applied following washing (see Ref. 18 for further details).
Solutions and Chemicals
HEPES-buffered aCSF contained (in mm): 126 NaCl, 4 KCl, 2 NaH2PO4, 2 MgSO4, 25 HEPES, 10 glucose, 2 calcium gluconate, 2 sodium cyclamate, pH 7.45, adjusted with HCl. For Na+-free HEPES-buffered aCSF, NaCl was replaced by 128 mm NMDG Cl− and 2 mm KCl was replaced by 2 mm KH2PO4, omitting both sodium cyclamate and NaH2PO4. For Cl−-free aCSF, NaCl was replaced by 128 mm sodium cyclamate, and KCl was replaced by 2 mm K+ gluconate and 2 mm KH2PO4. The Na+- and Cl−-free solution contained (in mm) 2 MgSO4, 25 HEPES, 10 glucose, 2 calcium gluconate, 2 potassium gluconate, 2 KH2PO4, and 130 NMDG gluconate prepared by titrating the NMDG-containing solution with gluconate to pH 7.45. Osmolarity for all solutions was measured using a vapor pressure osmometer (Camlab) and adjusted with sucrose to 315 mosm. All chemicals were purchased from Sigma-Aldrich) unless otherwise stated.
Statistics
Static significance was tested using one-way analysis of variance with Bonferroni's post hoc test or Student's t test as appropriate (GraphPad, Prism).
RESULTS
Na+- and Cl−-dependent pHi Regulation Differs between in Situ and Cultured Astrocytes
We determined the contribution of the Cl−-dependent V-ATPase to resting pHi levels in both cultured astrocytes and in situ rat optic nerve astrocytes. Optic nerve and cultured astrocytes superfused for 15–25 min with HEPES-aCSF displayed baseline pHi of 7.10 ± 0.04 (n = 4 optic nerves, 60 cells) and 6.82 ± 0.06 (n = 4 cultures, 90 cells), respectively (p < 0.01; Fig. 2, A and C). Cl− removal did not significantly affect the pHi of cultured astrocytes, which fell to 6.71 ± 0.07 (p > 0.05; n = 60; Fig. 2, A and C), whereas pHi in optic nerve astrocytes fell significantly to 6.30 ± 0.04 (p < 0.001; n = 4 optic nerves, 60 cells; Fig. 2, A and C). Perfusion with the selective V-ATPase inhibitor bafilomycin (50 nm) reduced optic nerve astrocyte resting pHi to 6.52 ± 0.02 (p < 0.01;c Fig. 2, B and C) over a similar 20–30-min time course to the acidification produced by Cl− removal. Bafilomycin reduced pHi in cultured astrocytes by ∼0.15 pH units to 6.64 ± 0.04 (n = 4 cultures, 90 cells), which was significantly less than the ∼0.61 pH unit change found in optic nerve astrocytes. The plateau pHi levels in optic nerve and cultured astrocytes exposed to bafilomycin were not significantly different (p > 0.5), and V-ATPase activity in optic nerve cells therefore accounts for their alkaline resting pHi as compared with cultured cells. To confirm the Cl−-dependent nature of the V-ATPase activity in optic nerve astrocyte, pHi recovery following a 30-min period of 0 Cl− perfusion was shown to be blocked by bafilomycin upon Cl− restoration (Fig. 2D).
FIGURE 2.
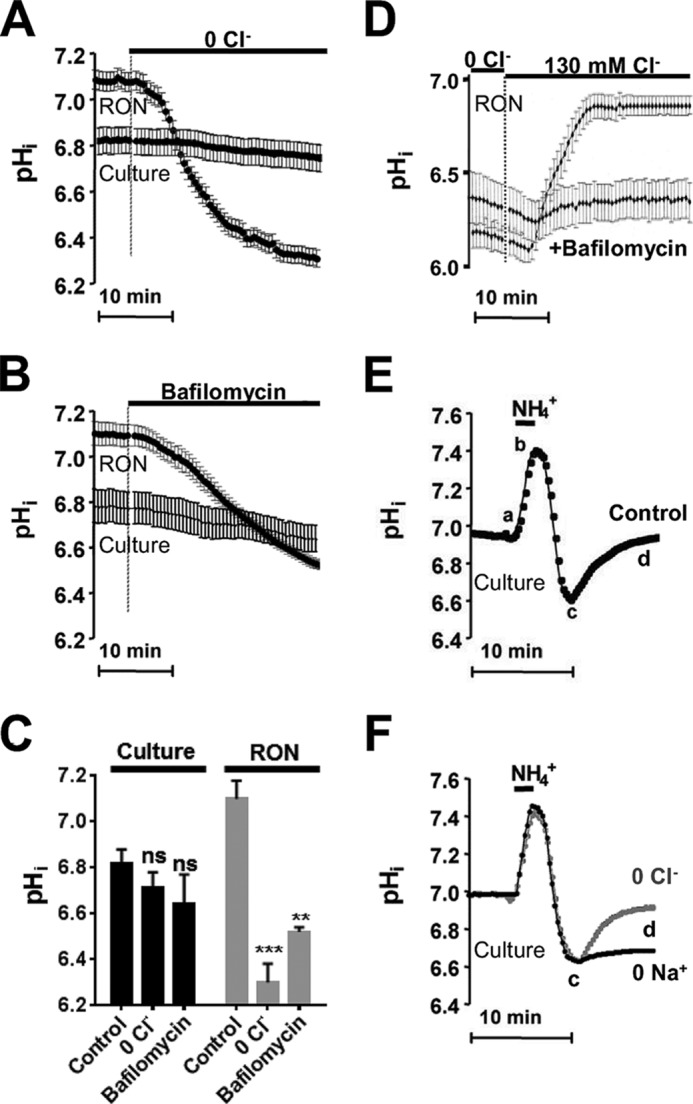
The effect of bafilomycin or ionic substitution upon HCO3−-independent pHi in rat optic nerve and cultured astrocytes. A, substitution of Cl− (0 Cl−) in HEPES-buffered, nominally HCO3−-free, aCSF evokes a large acidification in RON astrocytes, but not in cultured astrocytes (Culture). B, exposure to the V-ATP blocker bafilomycin (50 nm) evokes a large acidification in optic nerve astrocytes but not in cultured astrocytes. C, summary of data in A and B. Values are given as mean ± S.E. Significance is given relative to the respective control: **, p < 0.001, ***, p < 0.0001. ns, not significant. D, pHi of optic nerve astrocytes acidified by Cl− removal is normalized following restoration of physiological extracellular Cl− in a bafilomycin-sensitive fashion. E, recovery of pHi from an acid load in cultured astrocytes in control solution. Baseline pHi was stable prior to the onset of a 2 min 20 mm ammonia pulse. Segment a-b is the alkalinization evoked by NH4 exposure, segment b-c is the acid load generated after switching back to ammonia-free conditions, and segment c-d is the pHi recovery. F, as in E; pHi recovery in cultured astrocytes was reduced in the absence of extracellular Na+ (0 Na+) but unaffected by Cl− substitution (0 Cl−).
The differential effect of V-ATPase inhibition between cultured and in situ astrocytes suggests higher rates of H+ extrusion via this route in optic nerve astrocytes. Following an acid load (exposure to a 20 mm ammonia pulse) in cultured astrocytes, pHi fell from a baseline of 6.98 ± 0.05 to 6.60 ± 0.07 before recovering over a 7-min period (Fig. 2E, segment c-d) to 6.96 ± 0.07 (p > 0.05 versus baseline; n = 4 cultures, 90 cells). Na+ removal largely abolished the pHi recovery following an acid load, with pHi reaching 6.69 ± 0.07 (n = 4 cultures, 90 cells; Fig. 2F), which is consistent with prior studies that report largely Na+-dependent forms of HCO3−-independent acid extrusion in these cells (8, 9). In contrast, removal of Cl− from the medium had no significant effect upon pHi recovery in cultured astrocytes (Fig. 2F).
V-ATPase Is the Principal Acid Extrusion Mechanisms in Optic Nerve Astrocytes
We determined the Na+ and Cl− dependence of acid extrusion in optic nerve astrocytes, using ammonia pulse recovery. Baseline pHi was 7.14 ± 0.08 (n = 4 optic nerves, 60 cells), and following the acid load, pHi did not fully recover, returning to a mean of 6.81 ± 0.07 (p < 0.001, Fig. 3A). The recovery from an acid load in Cl−-free medium was significantly reduced in optic nerve astrocytes, reaching a plateau pHi of 6.36 ± 0.16 (p < 0.001 versus control), whereas pHi recovered to 6.78 ± 0.12 in Na+-free medium (p < 0.001 versus Cl−-free; n = 4 nerves, 60 cells, Fig. 3B) after 20 min. Following ammonia loading, perfusion with the NHE inhibitor EIPA resulted in pHi recovery to 6.76 ± 0.10 (n = 4 nerves, 60 cells; Fig. 3C), comparable with the recovery in Na+-free medium (p > 0.89). In the presence of the V-ATPase inhibitor bafilomycin, pHi recovery reached a steady value of 6.57 ± 0.13 (Fig. 3D, n = 4 optic nerves, 60 cells), not significantly different from that obtained in Cl−-free medium (p > 0.31). A combination of EIPA + Cl−-free medium (Fig. 3E) or bafilomycin + Na+-free medium (Fig. 3F) prevented pHi recovery following an acid load.
FIGURE 3.
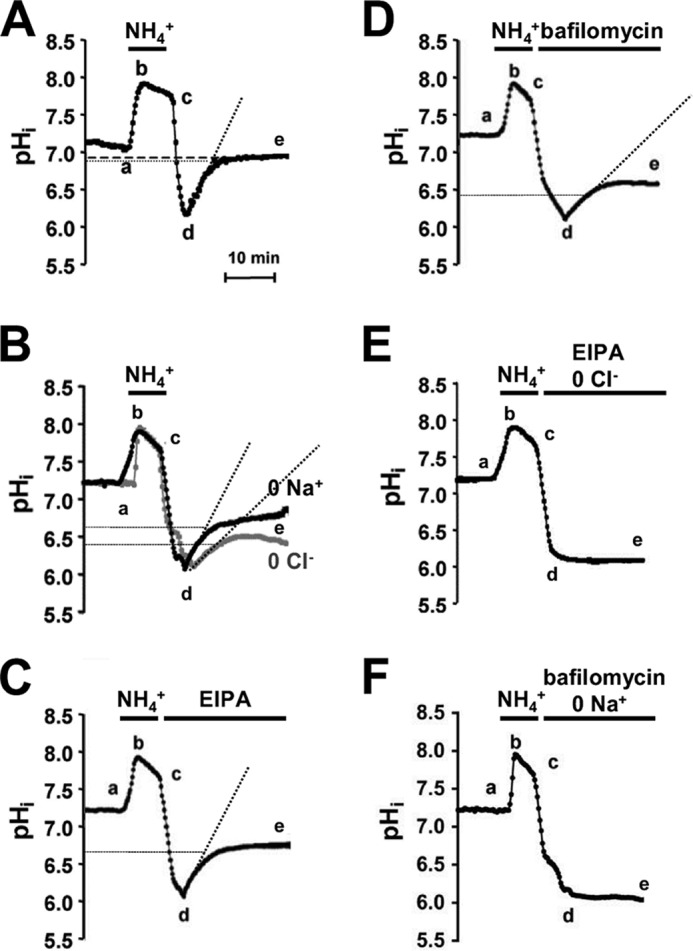
HCO3−-independent H+ extrusion in neonatal optic nerve astrocytes. A, mean pHi changes in HEPES-aCSF following a 5-min 20 mm ammonia pulse (segment a-c). pHi recovered after return to ammonia-free conditions (segment d-e), but not completely (note thick dashed line). B, as in A; the recovery was reduced by removal of extracellular Na+ (0 Na+) and Cl− (0 Cl−). C and D, mean pHi recovery following acid load in the presence of the Na-H exchange inhibitor EIPA or the V-ATPase inhibitor bafilomycin. Both inhibitors reduced the recovery, although bafilomycin reduced it to the greatest extent. E and F, pHi recovery was prevented by the following combinations: NHE inhibitor EIPA and Cl− removal or V-ATPase inhibitor bafilomycin combined with Na+ removal. Dotted lines on A–D indicate recovery rates within the first 5 min following removal of NH4+. All values are given as mean ± S.E.
pHi recovery rates calculated for the 5-min period following the removal of ammonia confirm the significance of V-ATP in optic nerve astrocytes. The rate in control HCO3−-free conditions was 0.15 pH units/min, whereas recovery in the absence of NHE (0 Na+ or EIPA) or V-ATPase activity (0 Cl− or bafilomycin) was 0.12 and 0.07 pH units/min, respectively. These results demonstrate that V-ATPase, in addition to maintaining resting pHi, is the major contributor to acid extrusion following an acidic challenge in optic nerve astrocytes.
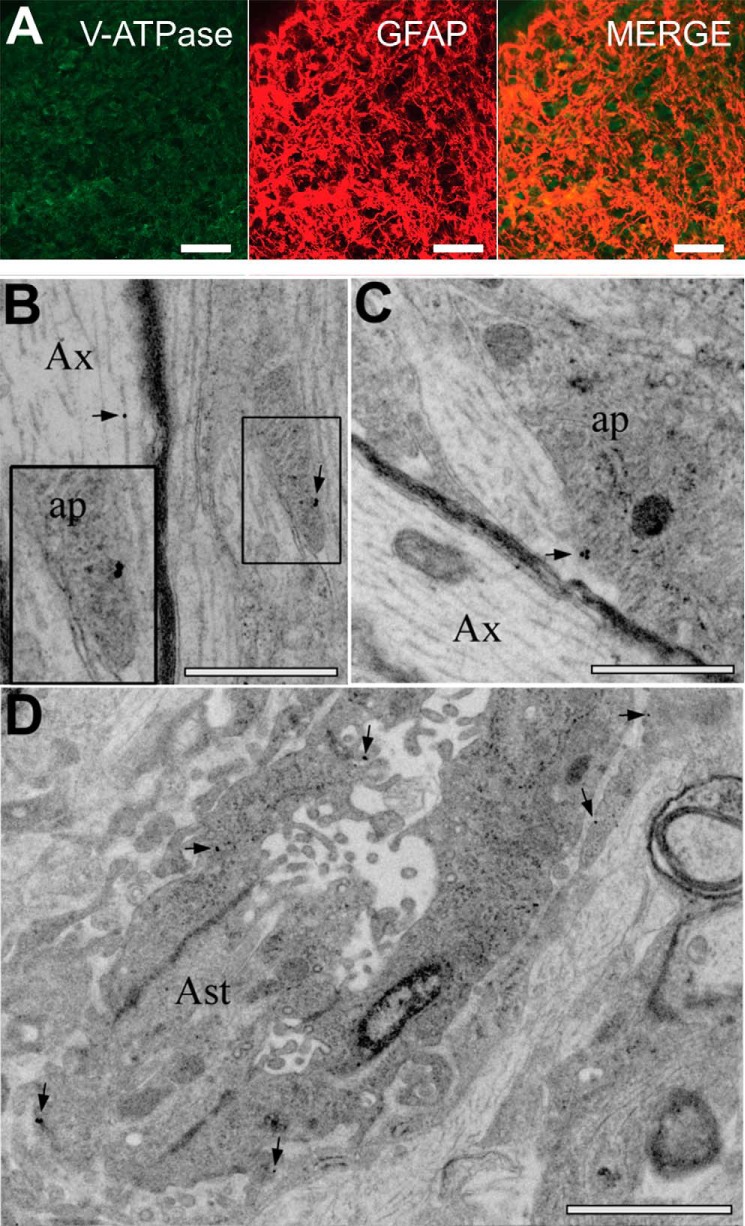
Immuno-staining showed co-expression of V-ATPase and the astrocyte marker GFAP, confirming the presence of significant V-ATPase expression in neonatal optic nerve astrocytes (Fig. 4A). At the ultrastructural level, immuno-gold labeling of V-ATPase revealed that the protein was present in the cell membrane of astrocyte processes neighboring axons (Fig. 4, B and C) and in astrocyte somata (Fig. 4D), in addition to the previously documented staining of axonal vesicular tubular complex (18). The contribution of V-ATPase to pHi regulation in these cells was therefore not a result of H+ loading into glial synaptic-type vesicles.
FIGURE 4.
V-ATPase expression in P2 rat optic nerve. A, V-ATPase (green, left), and GFAP (middle, red) expression in the same section, overlaid on the right. Note the clear astrocytic expression of V-ATPase, in addition to expression between astrocytes that may correspond to axonal protein. B–D, immuno-gold labeling of electron micrographs for V-ATPase reactivity in the long section, showing gold particles (arrows) localized to the membrane of astrocyte processes (ap, B and C), and somata (Ast, D). Note that axons (Ax) also contain particles. Note the presence of glial filaments in the astrocyte processes and the wide-bore endoplasmic reticulum, dark bodies, and glycogen particles in the somata (see Ref. 17). The box in B is shown at higher magnification. Scale bars = 10 μm in A; 500 nm in B and C; and 1 μm in D.
Buffering Capacity and Extracellular pH
The buffering capacity of astrocyte in situ has never been assessed, and calculation of the buffering capacity requires displacement of pHi by known acid loads in the absence of active pH regulation. This is generally achieved by exposure to various NH4+ concentrations, and the calculation hereof requires knowledge of both pHi and extracellular pH (pHo). pHi regulation was eliminated in optic nerve astrocytes by removal of Na+ and Cl−, producing a decrease in pHi from the resting point of 7.15 to 6.07 ± 0.02 (p < 0.001; n = 4 nerves, 60 cells, Fig. 5A). pHo in neonatal rat optic nerve superfused with HEPES-buffered aCSF (pH 7.45) deviated from that of the bath due to the presence of diffusion barriers between the two compartments and was recorded as 7.28 ± 0.02 (n = 4 nerves) using ion-sensitive microelectrodes (Fig. 5B). Perfusion with 0 Na+/0 Cl− produced a small alkaline increase in the nerve extracellular space to pH 7.36 ± 0.02 (n = 4, p < 0.05, Fig. 5B). Following interruption of pHi regulation by the ionic substitution, optic nerve astrocytes were acid-loaded with NH4+ at various concentrations (8) (Fig. 5, A and C), and the intrinsic buffering capacity (βi) was calculated (see “Experimental Procedures”). There was a non-linear relation between βi and pHi with a maxima around pH 6.5.
FIGURE 5.
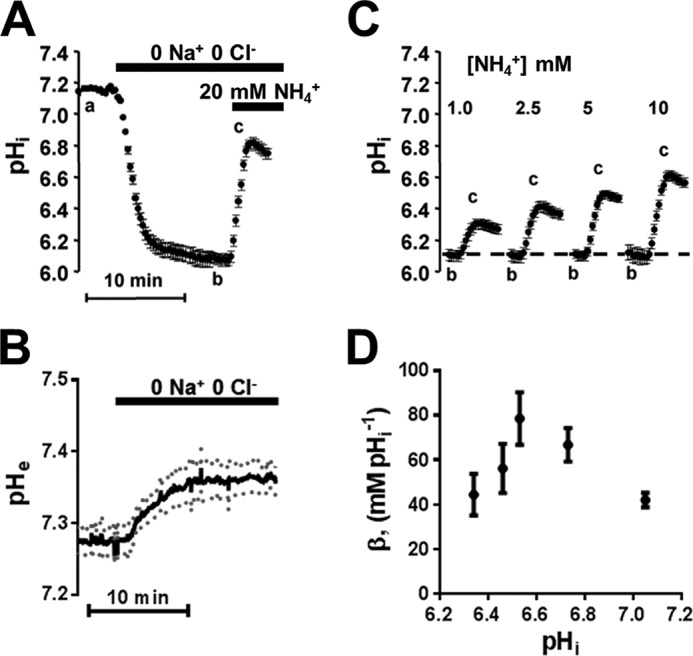
Intrinsic buffering capacity and pHo were determined following blockade of Na-H exchange and V-ATPase acid extrusion by NMDG gluconate substitution of Na+ and Cl−. A, Na+ and Cl− removal produced a 1.08 ± 0.08 pH units acidification in optic nerve astrocytes, and exposure to NH4+ (20 mm) in the absence of acid extrusion mechanisms evoked an alkalinization. B, pHo measured in optic nerve using ion-sensitive microelectrodes was increased by 0.08 pH units following Na+ and Cl− removal. C and D, exposure to NH4+ as in A (1–10 mm) resulted in increasing alkalinization. The buffering capacity (βi) for each [NH4+] concentration was calculated and plotted against the resulting pHi. Note that the pH change has been extrapolated back to time 0 for each solution. All values are given as mean ± S.E.
DISCUSSION
The ease with which mammalian astrocyte cultures can be generated and the control the preparation allows of the extracellular medium have resulted in a particular focus on the physiological properties of these cells. When it comes to ion homeostasis, cell culture studies have revealed the range of transport proteins that astrocytes can express and the physiological functions that they can perform. The list of pH-regulating membrane proteins identified in this way includes NHE, Na-HCO3 cotransport, Na+-driven Cl-HCO3 exchange, Cl-HCO3 exchange, and a V-ATPase, most of which have also been functionally identified in lower animal preparations (see Refs. 2 and 3 for reviews). The few studies of pH regulation in mammalian astrocytes in vivo or in whole-mount preparations indicate the presence of Na-HCO3 cotransport in adult retinal astrocytes (19) and of Na-HCO3 cotransport and NHE in P8–20 medullary astrocytes (20). The current investigation is the first to examine the contribution made by the transporters NHE and V-ATPase in the nominal absence of HCO3− in a whole-mount astrocyte population, revealing the predominance of V-ATPase, a finding with significant functional implications for CNS pH handling.
Neonatal White Matter Astrocytes
The white matter astrocyte population in the CNS matures early relative to other neural cell types. Arising from progenitor cells, including radial glia (21, 22), mature phenotype GFAP+ astrocytes are present in the human fetal brain from about post-conception weeks 11 to 14 (23), and populate the intermediate zone that will ultimately form cortical white matter from post-conception weeks 16 to 18 (24). Fibrous white matter astrocytes are established particularly early; for example, they appear in the cortex before protoplasmic gray matter astrocytes (24, 25). Studies of astrocyte development in central white matter are complicated by the complex morphological arrangement of axon tracts at different maturational stages. Large differences in astrocyte development have been noted, for example, between closely apposed fiber tracts in the optic radiations (24). These complications are avoided in the optic nerve, which is a uniform and structurally isolated white matter tract where astrocytes are generated from astrocyte precursor cells rather than by transformation of radial glia (26–29). By mid-gestation in humans, optic nerve astrocyte maturation is largely complete, predating the onset of myelination by ∼20 weeks (26).
In animal studies, astrocyte precursor cells populate the RON at fetal day 17, and GFAP+ astrocytes are generated continually until the end of the first week of life (27–29). At birth, >70% of cells in the RON are astrocytes with many of the features of mature cells including a radiating stellate morphology, expression of glial filaments, and organelle structures typical of the mature phenotype (such as endoplasmic reticulum, mitochondria, and Golgi). These astrocytes selectively accumulate AM-conjugated dyes (12), and the current study has taken advantage of the early maturation of fibrous astrocytes to examine pHi regulation in an effectively intact whole-mount preparation.
Astrocytes in Situ Rely Primarily on V-ATPase for HCO3−-independent pHi Regulation
V-ATPase is thought to be present in nearly every eukaryotic cell, where it acidifies intracellular organelles such as lysosomes, secretory, and synaptic vesicles. In the plasma membrane, V-ATPase functions to acidify the extracellular space, facilitating nephronal acidification, resorption of bone by osteoclasts, or reabsorption of bicarbonate in renal proximal tubules (reviewed in Refs. 32 and 33). In the absence of HCO3−, NHE1 has been identified as the principal acid extrusion mechanism in astrocytes (10, 30, 31) (see Ref. 3 for review), with V-ATPase being a minor contributor in cultured rat hippocampal astrocytes (10) and not involved in baseline pHi maintenance in cultured cortical astrocytes (30). V-ATPase is dependent upon extracellular [Cl−] (34–36), and consistent with the low activity of V-ATPase in cultures, we found only a minor change in pHi of cultured astrocytes upon Cl− removal or block by the selective inhibitor bafilomycin. In optic nerve astrocytes, these conditions resulted in significant acidification, leading to a pHi convergence in the two astrocyte populations. HCO3−-independent pH recovery in cultured astrocytes was unaffected by Cl− removal, but was highly Na+-dependent. These findings align with data on astrocyte cultures showing that H+ extrusion in HEPES-buffered medium at a pHi of 6.05 is mediated by EIPA- and amiloride-sensitive NHE (8). In comparison, pH recovery from an acid load in optic nerve astrocytes exhibited little Na+ or EIPA sensitivity, whereas Cl− removal or bafilomycin greatly reduced acid extrusion. These data are consistent with the hypothesis that HCO3−-independent pHi regulation in optic nerve astrocytes is mediated predominantly by V-ATPase with NHE playing a minor role. Consistent with this hypothesis, immuno-staining at the light and ultrastructural levels shows robust V-ATPase expression in the cell membrane of these astrocytes.
Buffering Capacity of Astrocytes in Situ
Buffering power in cultured cerebral and hippocampal astrocytes is essentially constant in the pH range 6–7 (37, 38) or linearly decreases with increasing pHi (8, 31). We found a non-linear relation between buffering power and pHi in optic nerve astrocytes. Measurements of buffering power are inherently variable, especially at low pH values (e.g. Ref. 31), and any inferred linear relationship with pH is possibly unwarranted. Buffering power distribution is the sum of the concentration and pKa of all proton-buffering molecules in the cell (39); the buffering distribution therefore depends on the cellular composition and will not necessarily follow pH linearly.
We found that the intrinsic buffering capacity of the optic nerve astrocytes was above 40 mm (pH)−1 throughout the pH range ∼6.3–7.1. This is in the 26–50 mm (pH)−1 range found in hippocampal slices, whole-brain cortical tissue homogenate, and isolated synaptosomes (37, 40–42), but substantially greater than the 10.5–14.1 mm (pH)−1 range of cultured astrocytes (8, 31, 38). It is possible that buffering capacity is generally reduced in cultured cells as compared with cells in vivo. A high intrinsic buffering capacity paired with a presumably higher bicarbonate buffering power due to the elevated resting pHi of in situ astrocytes would render the cells more resistant to pH fluctuations than their cultured counterparts.
Conclusion
Excitability in the neonatal rat optic nerve is mediated by long-duration, slow-conducting action potentials (43), and excitability-dependent changes in the extracellular space are exaggerated in this tissue. Extracellular [K+] can rise ∼20 mm during electrical stimulation of neonatal optic nerve axons (44), and neighboring astrocytes express relatively high levels of voltage-gated Na+ channels, which are down-regulated in the absence of axonal contact (45). Large activity-dependent elevations in extracellular [K+] are likely to evoke significant rises in intracellular [Na+] in these astrocytes, which would influence pHi regulation mediated via NHE. This astrocyte population may therefore require a form of pHi regulation that is independent of intracellular [Na+], such as that provided by V-ATPase. Reliance upon V-ATPase for HCO3−-independent pHi regulation, in contrast to NHE, may therefore have functional utility for neonatal white matter astrocytes. However, the assumption that CNS astrocytes in general express low levels of V-ATPase and high levels of NHE is based almost entirely upon cell culture studies, and the current findings question the validity of this assumption. Voltage-gated Na+ channels and Na+-dependent transport expression are widespread among astrocyte populations, and intracellular [Na+] has recently been implicated in the regulation of astrocyte signaling at synapses (46). Synapses are also sites where pHi regulation is an essential function of astrocyte processes; for example, activity-dependent acidification at NMDA-type glutamate receptors would have profound consequences for synaptic integration (47). Na+-independent pHi regulation may therefore be a general requirement for perisynaptic astrocyte processes. Because astrocytes in vitro do not form processes that are integrated into a neural network, they may fail to express the levels of V-ATPase found in the current study.
In general, astrocytes expressing high levels of V-ATPase will have an elevated capacity for pHi regulation, in particular at the acid pH levels that prevail under ischemic conditions in the CNS. These are conditions under which such a transporter might be required because HCO3− levels will be reduced, limiting HCO3−-dependent pHi regulation, whereas NHE is unlikely to function efficiently due to reduced extracellular pH (3). In this regard, it is interesting that astrocytes contain the only significant energy reserve in the CNS in the form of glycogen (48), and V-ATPase may therefore participate in pH maintenance during limited periods of ischemia. High levels of functional V-ATPase expression in astrocytes therefore may have important implications for both the physiology and the pathophysiology of the CNS.
This work was supported by Biotechnology and Biological Sciences Research Council (BBSRC) Grant BB/J016969/1 (to R. F.).
This article was selected as a Paper of the Week.
- NHE
- Na-H exchanger
- BCECF
- 2′,7′-bis-(2-carboxyethyl)-5 (and 6)-carboxyfluorescein
- AM
- acetoxymethyl
- aCSF
- artificial cerebrospinal fluid
- GFAP
- glial fibrillary acidic protein
- NMDG
- N-methyl-d-glucamine
- EIPA
- ethyl-isopropyl amiloride
- RON
- rat optic nerve
- P
- postnatal day(s).
REFERENCES
- 1. Roos A., Boron W. F. (1981) Intracellular pH. Physiol. Rev. 61, 296–434 [DOI] [PubMed] [Google Scholar]
- 2. Deitmer J. W., Rose C. R. (1996) pH regulation and proton signalling by glial cells. Prog. Neurobiol. 48, 73–103 [DOI] [PubMed] [Google Scholar]
- 3. Chesler M. (2003) Regulation and modulation of pH in the brain. Physiol. Rev. 83, 1183–1221 [DOI] [PubMed] [Google Scholar]
- 4. Bevensee M. O., Apkon M., Boron W. F. (1997) Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J. Gen. Physiol. 110, 467–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shrode L. D., Putnam R. W. (1994) Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia 12, 196–210 [DOI] [PubMed] [Google Scholar]
- 6. Boyarsky G., Ransom B., Schlue W. R., Davis M. B., Boron W. F. (1993) Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia 8, 241–248 [DOI] [PubMed] [Google Scholar]
- 7. Pizzonia J. H., Ransom B. R., Pappas C. A. (1996) Characterization of Na+/H+ exchange activity in cultured rat hippocampal astrocytes. J. Neurosci. Res. 44, 191–198 [DOI] [PubMed] [Google Scholar]
- 8. Bevensee M. O., Weed R. A., Boron W. F. (1997) Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role Of HCO3. J. Gen. Physiol. 110, 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cengiz P., Kintner D. B., Chanana V., Yuan H., Akture E., Kendigelen P., Begum G., Fidan E., Uluc K., Ferrazzano P., Sun D. (2014) Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLoS One 9, e84294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pappas C. A., Ransom B. R. (1993) A depolarization-stimulated, bafilomycin-inhibitable H+ pump in hippocampal astrocytes. Glia 9, 280–291 [DOI] [PubMed] [Google Scholar]
- 11. Philippe J. M., Dubois J. M., Rouzaire-Dubois B., Cartron P. F., Vallette F., Morel N. (2002) Functional expression of V-ATPases in the plasma membrane of glial cells. Glia 37, 365–373 [PubMed] [Google Scholar]
- 12. Fern R. (1998) Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J. Neurosci. 18, 7232–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCarthy K. D., de Vellis J. (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. (1988) pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3. Am. J. Physiol. 255, C844–C856 [DOI] [PubMed] [Google Scholar]
- 15. Boron W. F., De Weer P. (1976) Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J. Gen. Physiol. 67, 91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voipio J., Pasternack M., MacLeod K. T. (1994) Ion-sensitive microelectrodes. in The Plymouth Workshop Handbook (Ogden D., ed), pp. 275–316, The Company of Biologists, Cambridge, UK [Google Scholar]
- 17. Thomas R., Salter M. G., Wilke S., Husen A., Allcock N., Nivison M., Nnoli A. N., Fern R. (2004) Acute ischemic injury of astrocytes is mediated by Na-K-Cl cotransport and not Ca2+ influx at a key point in white matter development. J. Neuropathol. Exp. Neurol. 63, 856–871 [DOI] [PubMed] [Google Scholar]
- 18. Alix J. J., Dolphin A. C., Fern R. (2008) Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. J. Physiol. 586, 4069–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman E. A. (1999) Sodium-bicarbonate cotransport in retinal astrocytes and Muller cells of the rat. Glia 26, 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erlichman J. S., Cook A., Schwab M. C., Budd T. W., Leiter J. C. (2004) Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R289–R302 [DOI] [PubMed] [Google Scholar]
- 21. Freeman M. R. (2010) Specification and morphogenesis of astrocytes. Science 330, 774–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howard B. M., Zhicheng M. o., Filipovic R., Moore A. R., Antic S. D., Zecevic N. (2008) Radial glia cells in the developing human brain. Neuroscientist 14, 459–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wierzba-Bobrowicz T., Lechowicz W., Kosno-Kruszewska E. (1997) A morphometric evaluation of morphological types of microglia and astroglia in human fetal mesencephalon. Folia Neuropathol. 35, 29–35 [PubMed] [Google Scholar]
- 24. Roessmann U., Gambetti P. (1986) Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol. 70, 308–313 [DOI] [PubMed] [Google Scholar]
- 25. Takashima S., Becker L. E. (1983) Developmental changes of glial fibrillary acidic protein in cerebral white matter. Arch. Neurol. 40, 14–18 [DOI] [PubMed] [Google Scholar]
- 26. Sturrock R. R. (1975) A light and electron microscopic study of proliferation and maturation of fibrous astrocytes in the optic nerve of the human embryo. J. Anat. 119, 223–234 [PMC free article] [PubMed] [Google Scholar]
- 27. Vaughn J. E. (1969) An electron microscopic analysis of gliogenesis in rat optic nerves. Z. Zellforsch. Mikrosk. Anat. 94, 293–324 [DOI] [PubMed] [Google Scholar]
- 28. Skoff R. P., Price D. L., Stocks A. (1976) Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J. Comp. Neurol. 169, 313–334 [DOI] [PubMed] [Google Scholar]
- 29. Mi H., Barres B. A. (1999) Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J. Neurosci. 19, 1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amos B. J., Chesler M. (1998) Characterization of an intracellular alkaline shift in rat astrocytes triggered by metabotropic glutamate receptors. J. Neurophysiol. 79, 695–703 [DOI] [PubMed] [Google Scholar]
- 31. McLean L. A., Roscoe J., Jorgensen N. K., Gorin F. A., Cala P. M. (2000) Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am. J. Physiol. Cell Physiol. 278, C676–C688 [DOI] [PubMed] [Google Scholar]
- 32. Beyenbach K. W., Wieczorek H. (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577–589 [DOI] [PubMed] [Google Scholar]
- 33. Breton S., Brown D. (2013) Regulation of luminal acidification by the V-ATPase. Physiology 28, 318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaunitz J. D., Gunther R. D., Sachs G. (1985) Characterization of an electrogenic ATP and chloride-dependent proton translocating pump from rat renal medulla. J. Biol. Chem. 260, 11567–11573 [PubMed] [Google Scholar]
- 35. Fernández R., Malnic G. (1998) H+ ATPase and Cl− interaction in regulation of MDCK cell pH. J. Membr. Biol. 163, 137–145 [DOI] [PubMed] [Google Scholar]
- 36. Malnic G., Geibel J. P. (2000) Cell pH and H+ secretion by S3 segment of mammalian kidney: role of H+-ATPase and Cl−. J. Membr. Biol 178, 115–125 [DOI] [PubMed] [Google Scholar]
- 37. Katsura K., Mellergård P., Theander S., Ouyang Y. B., Siesjö B. K. (1993) Buffer capacity of rat cortical tissue as well as of cultured neurons and astrocytes. Brain Res. 618, 283–294 [DOI] [PubMed] [Google Scholar]
- 38. Pappas C. A., Ransom B. R. (1994) Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J. Neurophysiol. 72, 2816–2826 [DOI] [PubMed] [Google Scholar]
- 39. Boron W. F. (2004) Regulation of intracellular pH. Adv. Physiol. Educ. 28, 160–179 [DOI] [PubMed] [Google Scholar]
- 40. Jean T., Frelin C., Vigne P., Barbry P., Lazdunski M. (1985) Biochemical properties of the Na+/H+ exchange system in rat brain synaptosomes. Interdependence of internal and external pH control of the exchange activity. J. Biol. Chem. 260, 9678–9684 [PubMed] [Google Scholar]
- 41. Nachshen D. A., Drapeau P. (1988) The regulation of cytosolic pH in isolated presynaptic nerve terminals from rat brain. J. Gen. Physiol. 91, 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roberts E. L., Jr., Chih C. P. (1998) The pH buffering capacity of hippocampal slices from young adult and aged rats. Brain Res. 779, 271–275 [DOI] [PubMed] [Google Scholar]
- 43. Foster R. E., Connors B. W., Waxman S. G. (1982) Rat optic nerve: electrophysiological, pharmacological and anatomical studies during development. Brain Res. 255, 371–386 [DOI] [PubMed] [Google Scholar]
- 44. Ransom B. R., Yamate C. L., Connors B. W. (1985) Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J. Neurosci. 5, 532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Minturn J. E., Sontheimer H., Black J. A., Ransom B. R., Waxman S. G. (1992) Sodium channel expression in optic nerve astrocytes chronically deprived of axonal contact. Glia 6, 19–29 [DOI] [PubMed] [Google Scholar]
- 46. Kirischuk S., Parpura V., Verkhratsky A. (2012) Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 35, 497–506 [DOI] [PubMed] [Google Scholar]
- 47. Traynelis S. F., Cull-Candy S. G. (1991) Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J. Physiol. 433, 727–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ransom B. R., Fern R. (1997) Does astrocytic glycogen benefit axon function and survival in CNS white matter during glucose deprivation? Glia 21, 134–141 [PubMed] [Google Scholar]