Background: Animal microRNAs silence their target mRNAs by promoting mRNA degradation and inhibiting translation via GW182/TNRC6.
Results: TNRC6 induces silencing effects in S. cerevisiae via CCR4-NOT complex and Dhh1-Pat1 when tethered to reporter mRNAs.
Conclusion: TNRC6 utilizes the conserved mRNA fate modulators for gene silencing in yeast.
Significance: Yeast genetic tools are now available to study intricate actions of TNRC6.
Keywords: Gene Silencing, mRNA Decay, Translation Regulation, Yeast, Zebrafish, miRNA Mechanism
Abstract
The CCR4-NOT complex, the major deadenylase in eukaryotes, plays crucial roles in gene expression at the levels of transcription, mRNA decay, and protein degradation. GW182/TNRC6 proteins, which are core components of the microRNA-induced silencing complex in animals, stimulate deadenylation and repress translation via recruitment of the CCR4-NOT complex. Here we report a heterologous experimental system that recapitulates the recruitment of CCR4-NOT complex by TNRC6 in S. cerevisiae. Using this system, we characterize conserved functions of the CCR4-NOT complex. The complex stimulates degradation of mRNA from the 5′ end by Xrn1, in a manner independent of both translation and deadenylation. This degradation pathway is probably conserved in miRNA-mediated gene silencing in zebrafish. Furthermore, the mRNA fate modulators Dhh1 and Pat1 redundantly stimulate mRNA decay, but both factors are required for poly(A) tail-independent translation repression by tethered TNRC6A. Our tethering-based reconstitution system reveals that the conserved architecture of Not1/CNOT1 provides a binding surface for TNRC6, thereby connecting microRNA-induced silencing complex to the decapping machinery as well as the translation apparatus.
Introduction
The CCR4-NOT complex is a multisubunit complex involved in many aspects of mRNA metabolism (1–3). Its conserved functions include deadenylation catalyzed by the two deadenylase subunits CAF1/POP2 (CNOT7/8 in vertebrates) and CCR4 (CNOT6 in vertebrates). These two enzymes are incorporated into the complex via a direct interaction between CAF1 and the scaffold protein CNOT1 (4, 5). Thus, recruitment of the CCR4-NOT complex to mRNAs promotes deadenylation, which is usually followed by decapping and 5′-to-3′ degradation by Xrn1 (6, 7). In addition, recent studies have shown that the CCR4-NOT complex provides a link to the decapping machinery. For example, in Saccharomyces cerevisiae, the CCR4-NOT complex associates with Dhh1, a decapping activator (8). Similarly, in Drosophila and mammals, the CCR4-NOT complex interacts with the Dhh1 homolog Me31B/DDX6/RCK1/p54 and the Pat1 homolog HPat/PatL1 (9–12). HPat/PatL1 in turn associates with the decapping enzyme Dcp2 and its activators Dcp1 and Edc3, thereby organizing assembly of the decapping machinery (9, 13, 14). Moreover, Dhh1 and Pat1 also function in translation repression. In S. cerevisiae, Dhh1 or Pat1 is required for translation repression under glucose deprivation, and both Dhh1 and Pat1 repress translation initiation in vitro (15). Drosophila Me31B and vertebrate DDX6 also act as translation repressors (11, 12, 16–19). These observations imply that the CCR4-NOT complex coordinates multiple processes of mRNA degradation and translation repression rather than merely promoting deadenylation.
MicroRNAs (miRNAs)4 are small non-coding RNAs that negatively regulate gene expression by inducing translational repression, mRNA degradation, and deadenylation (20–29). miRNAs regulate their target mRNAs by associating with specific protein factors to form the miRNA-induced silencing complex (miRISC). Argonaute (Ago), a core component of miRISC, directly incorporates miRNAs (30). Drosophila GW182 and its vertebrate ortholog TNRC6A-C (trinucleotide repeat-containing 6 A-C) interact with Ago via their N-terminal glycine and tryptophan (GW) repeats, whereas their C-terminal silencing domains provide a platform for interactions with RNA regulatory factors, including poly(A)-binding protein (PABP), PAN3 of the PAN2-3 deadenylase complex, and CNOT1 (25, 31–36). The CCR4-NOT complex, which is recruited to mRNAs by miRISC, promotes deadenylation via the activities of CAF1 and CCR4 (25, 37). miRISC might further accelerate mRNA decay by recruiting decapping factors in a manner that is independent of their effects on deadenylation (38). In addition, the CCR4-NOT complex may play a role in miRNA-mediated translation repression (34, 35). miRNA can induce translation repression independent of deadenylation (39–41), and the CCR4-NOT complex does so in tethering experiments (34, 42). The interaction of a MIF4G domain of human CNOT1 with DDX6, through a structural arrangement that is analogous to the MIF4G domain of eIF4G and eIF4AI, contributes to the miRNA-mediated silencing (11, 12, 43). These observations indicate that miRISC achieves post-transcriptional silencing via conserved but intricate functions of the CCR4-NOT complex.
S. cerevisiae lacks the small RNA-producing enzyme Dicer and Ago and therefore does not produce canonical siRNAs and miRNAs. However, the basic machinery for controlling mRNA stability and translation, including the CCR4-NOT complex, decapping factors, and translation initiation factors, is highly conserved (6, 7). Notably, the yeast Pumilio-like protein Puf5/Mpt5 binds to the CCR4-NOT complex to silence and deadenylate specific mRNAs (44, 45), suggesting that the CCR4-NOT complex is involved in sequence-specific post-transcriptional regulation independent of the emergence of miRNAs. Previously, the Bartel and Roth laboratories (46, 47) showed that gene silencing by siRNA could be reconstituted in S. cerevisiae by expressing either Saccharomyces castellii Ago1 and Dicer1 or human Ago2, Dicer, and TRBP.
In this study, we successfully recapitulated two hallmarks of animal miRISC-mediated silencing in S. cerevisiae by tethering the middle domain of zebrafish TNRC6A to reporter mRNAs. Using mutant yeast strains, we showed that zebrafish TNRC6A directly stimulates decapping and 5′-to-3′ mRNA decay in a Not1-dependent but poly(A) tail- and translation-independent manner. In addition, we showed that the Dhh1 and Pat1 play crucial roles not only in stimulation of mRNA decapping but also in translational repression. These results indicate that the conserved architecture of Not1/CNOT1 provides a binding surface for TNRC6, thereby connecting miRISC to the decapping machinery and translation apparatus. Furthermore, miR-430-mediated mRNA decay was differentially susceptible to inhibition of deadenylation in zebrafish embryos. This tethering-based reconstitution system in yeast will complement miRNA studies in animal cells, in which genetic approaches are sometimes not applicable.
EXPERIMENTAL PROCEDURES
Strains and Other Methods
Yeast strains and plasmids are listed in Table 1. Information about the oligonucleotides used for poly(A) tail analysis and qRT-PCR are listed in Table 2. Polysome analysis was performed as described (48).
TABLE 1.
Yeast strains and plasmids used in this study
| Strain/Plasmids | Genotype/plasmid | Source |
|---|---|---|
| Strains | ||
| W303-1a | MATa ade2 his3 leu2 trp1 ura3 can1 | Laboratory stock |
| YIT2007 | W303-1a xrn1Δ::kanMX | Ref. 49 |
| YIT2013 | W303-1a ski2Δ::kanMX | Ref. 67 |
| YIT2030 | W303-1a pat1Δ::natMX | This study |
| YIT2031 | W303-1a dhh1Δ::kanMX | This study |
| YIT2032 | W303-1a pat1Δ::natMX4, dhh1Δ::kanMX4 | This study |
| YIT2033 | W303-1a ccr4Δ::kanMX | This study |
| YIT2034 | W303-1a caf1Δ::kanMX | This study |
| Plasmids | ||
| pIT2068 | pGAL1p-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR) URA3 CEN | Ref. 49 |
| pIT2069 | pGAL1p-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR)-MS2 URA3 CEN | Ref. 49 |
| pIT2070 | pGAL1p-No-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR) URA3 CEN | Ref. 49 |
| pIT2071 | pGAL1p-No-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR)-MS2 URA3 CEN | Ref. 49 |
| pIT2082 | pGAL1p-No-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR)-Rz URA3 CEN | Ref. 49 |
| pIT2083 | pGAL1p-No-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR)-MS2-Rz URA3 CEN | Ref. 49 |
| pIT2139 | pGAL1p-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR)-MS2-Rz URA3 CEN | This study |
| pIT2140 | pGAL1p-AUG-FLAG-MPT4ΔN-PGK1 (3′UTR)-Rz URA3 CEN | This study |
| pIT2141 | pTEF1p-FLAG-MS2 LEU2 CEN | This study |
| pIT2142 | pTEF1p-FLAG-MS2-zTNRC6AMid LEU2 CEN | This study |
| pIT2143 | pGPD1p-Dhh1-HA URA3 CEN | This study |
| pIT2144 | pGPD1p-Pat1-HA URA3 CEN | This study |
| pIT2145 | pGPD1p-Not1-HA URA3 CEN | This study |
| pIT2146 | pTEF1p-FLAG-MS2-dmGW182Mid LEU2 CEN | This study |
| pIT2147 | pGPD1p-Dhh1-FLAG LEU2 CEN | This study |
| pIT2148 | pGPD1p-Dhh1 LEU2 CEN | This study |
| pIT2149 | pGAL1p-GFP-MS2 URA3 CEN | This study |
| pIT2150 | pGAL1p-GFP-MS2-Rz URA3 CEN | This study |
| pIT2151 | pGAL11p-GFP URA3 CEN | This study |
| pIT2152 | pGAL1p-GFP-Rz URA3 CEN | This study |
| pIT2155 | pTEF1p-FLAG-MS2-zTNRC6AMid mutants QSR and W LEU2 CEN | This study |
| M44 | pCS2 + HA-λN-zTNRC6A-Mid-globin3′UTR | Ref. 40 |
| M277 | pCS2 + HA-λN-MS2-globin 3′UTR | This study |
| M338 | pCS2 + MT-CNOT7-D40A,E42E,C67E,L71E-sv40 | This study |
| M342 | pCS2 + MT-DCP2-E147A,E148A-sv40 | This study |
TABLE 2.
The oligonucleotides used for poly(A) tail analysis and qRT-PCR
| Name/Description | Sequence |
|---|---|
| PAT reverse | 5′-GGTAATACGACTCACTATAGCGAGACCCCCCCCCCTT-3′ |
| eif4ebp2 PAT forward | 5′-CAAACTATCAGAAATGCATTTAGTTCTG-3′ |
| gstm PAT forward | 5′-TCAGGTGAGTGTTGCATTTTG-3′ |
| acadl PAT forward | 5′-ATCATATGGTCTACGCCGAGAG-3′ |
| gfp forward | 5′-AAGGGCATCGACTTCAAGGAG-3′ |
| gfp reverse | 5′-GATGCCGTTCTTCTGCTTGTC-3′ |
| fluc qPCR forward | 5′-AGAACTGCCTGCGTGAGATTC-3′ |
| fluc qPCR reverse | 5′-ACCGTGATGGAATGGAACAAC-3′ |
| act1b qPCR forward | 5′-TTGTTGGACGACCCAGACATC-3′ |
| act1b qPCR reverse | 5′-ATGGGGTATTTGAGGGTCAGG-3′ |
| eif4ebp2 qPCR forward | 5′-ACGACTCAACGCAGCTACCTC-3′ |
| eif4ebp2 qPCR reverse | 5′-CGGTCCAACAGGAACTTACGA-3′ |
| gstm qPCR forward | 5′-GCTCCTGCACCTCATTTTGAAG-3′ |
| gstm qPCR reverse | 5′-ACCGGTGTATTCCAACAGCAG-3′ |
| acadl qPCR forward | 5′-CTATGTGATGCAGCGGAAGG-3′ |
| acadl qPCR reverse | 5′-TGAATGCCCGACCTACACAG-3′ |
Determination of mRNA Stability
Yeast cells were grown in minimal medium containing 2% galactose. Cells were grown to A600 = 0.6 and harvested and resuspended in medium containing 2% glucose to inhibit transcription from the GAL1 promoter. At the times indicated, the cells were harvested to prepare RNA samples using hot phenol. The mRNA levels of reporter genes were determined by Northern blotting using digoxigenin (DIG) reagents. Non-radioactive probes were prepared by PCR-based nucleic acid labeling using commercial kits. Hybridization probes were detected according to the procedure specified by the manufacturer (Roche Applied Science). The DIG-labeled probes were prepared with the following oligonucleotides: GFP (5′- GCTCTAGAATGAGTAAAGGAGAAGAACTTTTCAC-3′ and 5′- GGACTAGTTTTGTATAGTTCATCCATGCCA-3′) and 3-phosphoglycerate kinase 1 (PGK1) 3′-UTR (5′- GGGAATTTAAATTGAATTGAATTGAAATCGATAG-3′ and 5′-GGGAATTCCGATTGACCAATATATGTCTCTGAATGCC-3′). The intensity of the bands on the blots was quantified using the LAS4000 and Multi-Gauge version 3.0 (Fuji Film). Relative RNA levels were determined by comparison with a standard curve prepared with a series of dilutions of time 0 samples (just before the addition of glucose).
Western Blotting
Yeast cells were grown in minimal medium containing 2% galactose. When the culture reached an A600 of 0.6, the cells were harvested. The protein products of FLAG-tagged reporter genes were detected by Western blotting using an anti-FLAG antibody (F1804, Sigma) and a horseradish peroxidase-conjugated secondary antibody (GE Healthcare). The intensity of the bands on the blots was quantified using a LAS4000 imager and Multi-Gauge version 3.0 (Fuji Film). The relative level of the protein product was determined by comparison with a standard curve prepared with a series of dilutions. Western blotting with zebrafish embryos was performed as described previously (40).
Immunoprecipitation
Yeast cells were grown in minimal medium containing 2% glucose. When the culture reached an A600 of 0.8, the cells were harvested. Cell extracts were prepared with lysis buffer (20 mm Tris-HCl, pH 7.4, 100 mm KCl, 2 mm MgCl2, 2 mm DTT, 0.5 mm PMSF) by inversion with beads. Cell extracts were incubated with anti-FLAG M2 resin in IXA-150 buffer (50 mm Tris-HCl, pH 7.5, 150 mm KCl, 12 mm Mg(OAc)2, 1 mm DTT, 1 mm PMSF) and then washed four times and eluted with 0.5 mg/ml FLAG peptide.
Measuring the Presence of mRNA Cap Structure
20 μg of RNA samples were incubated in the absence or presence of 10 units of tobacco acid pyrophosphatase (Epicenter) at 37 °C for 90 min. After phenol extraction and ethanol precipitation, RNA samples were divided into two groups and incubated in the absence or presence of 1 unit of Terminator 5′-phosphate dependent exonuclease (Epicenter) at 30 °C for 45 min. 100 ng of in vitro-transcribed 5′-triphosphate GFP RNA was added to the reaction to confirm substrate specificity of Terminator exonuclease.
Pulse-labeling Experiments
Yeast cells were grown exponentially at 30 °C in minimal media lacking methionine and cysteine. A 10-ml aliquot of yeast cells was labeled with 100 μCi of [35S]methionine and cysteine (NEG072, PerkinElmer Life Sciences) for 10 min. This was followed by the addition of cold amino acids, to a final concentration of 40 mg/ml. The cells were then collected, and cell extracts were prepared using Y-PER (yeast protein extraction reagent) (Thermo Scientific). Cell extracts were incubated with a GFP antibody (Santa Cruz Biotechnology, Inc.) and protein G-agarose (Roche Applied Science) in IXA-100 buffer (48). The antibody-bound agarose was then washed three times. Immunoprecipitated samples were separated by SDS-polyacrylamide gel electrophoresis. The radioactivity of the precipitated proteins was measured using a Typhoon FLA 9000 imager (GE Healthcare).
Plasmid Construction
A reporter plasmid, pGAL1p-FLAG-MPT4ΔN-PGK1 (3′-UTR) was described previously (49). To construct pIT2149–2152, MPT4ΔN was replaced by GFP ORF using XbaI and BamHI sites in pGAL1p-FLAG-MPT4ΔN-PGK1 (3′-UTR).
To construct pIT2141 (pTEF1p-FLAG-MS2), a DNA fragment encoding FLAG-MS2 coat protein was amplified by PCR from pTEF1p-FLAG-MS2 (49) using the primers OIT1822 (5′-TAGATGTCTAGAATGGACTACAAGGACGACGATGACAAGGCT-3′) and OIT1948 (5′-TTGAACGGATCCGTAGATGCCGGAGTTTGCTGCG-3′) and inserted between XbaI and BamHI sites in pTEF1p (50). To construct pIT2142 (pTEF1p-FLAG-MS2-zebrafish TNRC6A Mid), a DNA fragment encoding the middle domain (residues 1310–1567) of zebrafish TNRC6A was amplified by PCR from pCS2-HA-lambdaN-zebrafish TNRC6A (40) using the primers OIT1824 (5′-TAGATGGGATCCGGAGTTCCCTGAGCTCGTTCAGTAATTTCCCT-3′) and OIT1825 (5′-TTGAACCTCGAGTCAAGAGCTGCCCCAGCCTGCCAGT-3′) and inserted between BamHI and XhoI sites in pTEF1p-FLAG-MS2. To construct pIT2155 (pTEF1p-FLAG-MS2-zebrafish TNRC6A Mid mutants QSR and W, which contains mutations that disrupt the interaction between CNOT1 and GW182/TNRC6 in human and Drosophila cells (33, 34), a DNA fragment encoding the zebrafish TNRC6A Mid domain in which Gln-Ser-Arg and Trp residues were substituted to alanines (Gln-1343, Ser-1344, Arg-1345, Trp-1349, Trp-1404, Trp-1419, Trp-1429, Trp-1484, Trp-1512, Trp-1527, Trp-1555, Trp-1565) was chemically synthesized and cloned between BamHI and XhoI sites in pTEF1p-FLAG-MS2. To construct pIT2146 (pTEF1p-FLAG-MS2-dmGW182 Mid), a DNA fragment encoding the middle domain (residues 861–1116) of dmGW182 was amplified by PCR from pAc5.1B-lambdaN-HA-DmGW182 (51) using the primers OIT2469 (5′-TAGATGCCCGGGCAAGGAGCGTCCAATCAACAATCCCGATTA-3′) and OIT2443 (5′-TTGAACGTCGACTCAGGAGCCCCAAGTGGTGTTACCACCTGTCCA-3′) and inserted between SmaI and XhoI (compatible end of SalI) sites in pTEF1p-FLAG-MS2 in which an MS2 tag was inserted between XbaI and SmaI sites. To construct pIT2143 (pGPD1-DHH1-HA), pIT2147 (pGPDp-DHH1-FLAG), and pIT2148 (pGPDp-DHH1), DHH1 ORF was amplified from the S. cerevisiae genome using the forward primer OIT2398 (5′-TAGATGGGATCCATGGGTTCCATCAATAATAACTTCAACA-3′), and reverse primers are as follows: DHH1-HA, OIT2399 (5′- TTGAACGTCGACTTACGCGTAGTCTGGGACGTCGTATGGGTAATACTGGGGTTGTGACTGACCAGGTGGC-3′); DHH1-FLAG, OIT2659 (5′-TTGAACGTCGACTTACTTGTCGTCGTCGTCCTTGTAGTCATACTGGGGTTGTGACTGACCAGGTGGC-3′); DHH1-nontag, OIT1952 (5′- TTGAACGTCGACTTAATACTGGGGTTGTGACTGACCAGGTGG-3′). Subsequently, these DNA fragments were inserted between BamHI and SalI sites in pGPD1p and pGPDp. To construct pIT2144 (pGPDp-PAT1-HA), the PAT1 gene was amplified from S. cerevisiae genome using the primers OIT2393 (5′-TAGATGGGATCCATGTCCTTCTTTGGGTTAGAAAATAGCGG-3′) and OIT2394 (5′-TTGAACGTCGACTTACGCGTAGTCTGGGACGTCGTATGGGTACTTTAGTTCTGATATTTCACCATCGCGA-3′) and inserted between BamHI and SalI sites in pGPD1p. To construct pIT2145 (pGPDp-NOT1-HA), the NOT1 gene was amplified from S. cerevisiae genome using the primers OIT2448 (5′- TAGATGGAATTCATGCTATCGGCCACATACCGTGATTTGAAC-3′) and OIT2392 (5′- TTGAACGTCGACTTACGCGTAGTCTGGGACGTCGTATGGGTATGCGTTGGATTGTAGAGGGGTTTGCCT-3′) and inserted between EcoRI and SalI sites in pGPD1p.
Tethering Assay in Zebrafish Embryos
Tethering assay in zebrafish embryos was performed as described previously (40) with some modifications. mRNAs were transcribed using an mMessage mMachine SP6 kit (Ambion). A-capped mRNA was synthesized in the presence of an ApppG cap analogue (New England Biolabs) instead of an m7GpppG cap analogue. To block polyadenylation and translation of the GFP reporter mRNA in zebrafish embryos, we injected MOs that bind to the 3′-end of the GFP reporter mRNA (PB MO; GGTACCGGGCCCAATGCATTGGCGC) and translation block MO that binds to the start codon (ACAGCTCCTCGCCCTTGCTCACCAT). MOs were purchased from Gene Tools. For mRNA injections, GFP mRNA and Fluc mRNA were diluted to a final concentration of 50 ng/μl each. HA-λN-Mid mRNA was diluted to a final concentration of 100 ng/μl. Approximately 1,000 pl of solution containing reporter mRNAs and effector mRNAs was injected into one-cell stage zebrafish embryos. A total of 10 embryos were collected at 10 h after injection, and total RNA was extracted with Isogen (NipponGene).
Poly(A) Tail Analysis
To analyze poly(A) tail length of the endogenous mRNAs, we followed the protocol of Hire-PAT (52) with the following modifications. Purified total RNA was treated with yeast PAP (Affymetrix) in the presence of 0.375 mm GTP and 0.125 mm ITP at 37 °C for 1 h. cDNA was synthesized with Perfect RT gEraser (TAKARA) according to the manufacturer's protocol except for the primer used (PAT reverse primer) and incubation temperature (44 °C). PCR was performed using GoTaq (Promega) and the primers listed below for 30–32 cycles. PCR products were separated on 6% polyacrylamide gel with 0.5× TBE, stained with GelRed (WAKO), and visualized with a LAS3000 imager (GE Healthcare). PCR products were cloned into pCRII TOPO (Invitrogen), and sequences were confirmed.
Overexpression of Dominant Negative CNOT7 and DCP2 and Inhibition of miR-430
ORFs of zebrafish cnot7 (ENSDART00000092953) and dcp2 (ENSDART00000056951) were amplified by RT-PCR and cloned into pCS2+MT vector. The following mutations were introduced to make a dominant-negative form of each protein: CNOT7, D40A/E42A (catalytic core) and C67E/L71E (CNOT6 interaction surface); DCP2, E147A/E148A (catalytic core). mRNAs were transcribed using a mMessage mMachine SP6 kit (Ambion) and diluted to a final concentration of 250 ng/μl. Approximately 1,000 pl of solution was injected into one-cell stage zebrafish embryos. To inhibit zebrafish miR-430, a mixture of morpholinos that targets miR-430a (final concentration 0.286 mm), miR-430b (143 mm), and miR-430c (71 mm) was injected at the one-cell stage. miR-430 MO sequences are as follows: miR-430a MO, ACTACCCCAACAAATAGCACTTACC; miR-430b MO, TCTACCCCAACTTGATAGCACTTTC; miR-430c MO, ACTACCCCAAAGAGAAGCACTTATG.
qRT-PCR
qRT-PCR was performed as described previously (40) using the primers in Table 2. Specific amplification was confirmed by analyzing the dissociation curve and sequencing. For the analysis of endogenous miR-430 targets, values in each injection experiment were normalized by the value of actb1. The normalized value in the miR-430 MO injection experiment was set to 1. Experiments were repeated three times.
Circularized Rapid Amplification of cDNA Ends (cRACE)
cRACE was performed essentially as described previously (53) with some modifications. Briefly, 4–5 μg of zebrafish total RNA was treated with 10 units of calf intestine alkaline phosphatase (TAKARA) for 1 h at 37 °C and extracted with phenol-chloroform. RNA was then decapped with 10 units of tobacco acid pyrophosphatase (Epicenter Biotechnologies) for 1 h at 37 °C and extracted with phenol-chloroform. We then incubated decapped RNA or an equivalent amount of naive RNA with 20 units of T4 RNA ligase (New England Biolabs) in a 120-μl reaction at 20 °C for 16 h. RNA samples were extracted with phenol-chloroform, and one-forth of the ligation products were used to make cDNA with Superscript III (Invitrogen) using eif4ebp-specific primer: 5′-CAGGGCTCACAGGTTAACTTTC-3′. First and second PCR were performed with the following primers for 22 cycles each: first primer, 5′-GCTGAGCAGCTGTGTCAAATAC-3′ (forward) and 5′-CAGGGCTCACAGGTTAACTTTC-3′ (reverse); second primer, 5′-CCGCTCGAGTTCAAGTGGAGAACAAGATCAAG-3′ (forward) and 5′-CCGGAATTCGGTATCTACAAGCCAAGCCACT-3′ (reverse). PCR products with expected size (0.8–1.2 kb) were excised and cloned into pBluescript II (SK+) with EcoRI and XhoI. Clones were analyzed by sequencing from both ends of amplicons. Clones containing the expected 3′ ends followed by a poly(A) tail and the expected 5′ sequence in this order were considered as intramolecularly ligated products and included in the analysis.
RESULTS
Tethered TNRC6A Recapitulates Hallmarks of miRNA-mediated Gene Silencing in S. cerevisiae
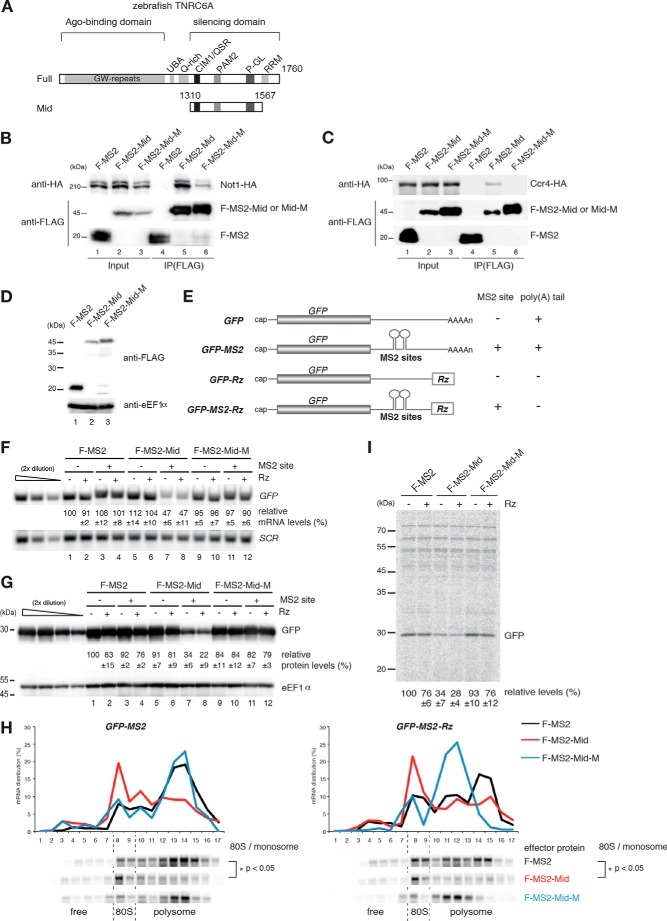
To elucidate the roles of the CCR4-NOT complex, which is recruited by GW182/TNRC6 in both mRNA decay and translation repression, we attempted to recapitulate the gene silencing activities of GW182/TNRC6 in yeast. First, we examined the interaction of the carboxyl-terminal region of zebrafish TNRC6A (Mid domain; amino acids 1310–1567) (40), which was sufficient for translation repression and deadenylation in zebrafish embryos, with the yeast CCR4-NOT complex (Fig. 1, A and B). HA-tagged Not1 and Ccr4 proteins, subunits of the yeast CCR4-NOT complex, co-immunoprecipitated with FLAG-tagged MS2 protein fused to the Mid domain (F-MS2-Mid) (Fig. 1, B and C, lanes 4 and 5). Introduction of alanine mutations in the CIM1 and W-motifs that disrupted the interaction between CNOT1 and GW182/TNRC6 in human and Drosophila cells (33, 34) (F-MS2-Mid-M) weakened the interaction of the zebrafish Mid domain with Not1 and Ccr4 (Fig. 1, B and C, lanes 5 and 6), although the expression levels of these proteins were comparable (Fig. 1D). We next examined the effects of F-MS2-Mid on the levels of mRNA and protein expressed from GFP reporter genes containing MS2 binding sites. To determine whether a poly(A) tail was required, we inserted the sequence of a hammerhead ribozyme (Rz) downstream of the MS2 binding sites (Fig. 1E) (49). Tethering of F-MS2-Mid reduced the levels of GFP-MS2 and GFP-MS2-Rz reporter mRNAs (Fig. 1F, lanes 3 and 4 and lanes 7 and 8), whereas tethering of F-MS2-Mid-M did not (Fig. 1F, lanes 7 and 8 and lanes 11 and 12). Likewise, tethering of F-MS2-Mid reduced the protein levels derived from GFP-MS2 or GFP-MS2-Rz reporters, whereas tethering of F-MS2-Mid-M did not (Fig. 1G, lanes 7 and 8 and lanes 11 and 12). Polysome analysis using sucrose density gradients revealed that levels of GFP-MS2 and GFP-MS2-Rz mRNAs reduced in the polysome fraction but increased in the 80S monosome and free fractions in cells expressing the F-MS2-Mid protein relative to cells expressing the control MS2 protein (Fig. 1H, Student's t test, p < 0.05). To directly examine translation repression by the tethered Mid domain of zebrafish TNRC6, we performed pulse-labeling experiments. Levels of synthesized GFP derived from reporter mRNAs were significantly decreased by tethered TNRC6A (Fig. 1I). The corresponding region of Drosophila GW182 also induced gene silencing in yeast (data not shown). These results indicate that tethered TNRC6 protein fragments reduced both translation efficiency and mRNA levels by recruiting the CCR4-NOT complex in S. cerevisiae in a poly(A) tail-independent manner.
FIGURE 1.
Tethered TNRC6A recapitulates hallmarks of miRNA-mediated gene silencing in S. cerevisiae. A, schematic structures of zebrafish TNRC6A and its Mid domain. B and C, interaction of FLAG-MS2-TNRC6A Mid with yeast Not1-HA and Ccr4-HA. Cell lysates of wild type cells transformed with the indicated plasmids were immunoprecipitated using anti-FLAG antibody. Total extracts (Input) and immunoprecipitates (IP) were analyzed by Western blotting using anti-HA or anti-FLAG antibody. D, the expression of the Mid domain of zebrafish TNRC6A in yeast. FLAG-tagged effector proteins were analyzed by Western blotting using anti-FLAG antibody. F-MS2, FLAG-MS2 protein; F-MS2-Mid, FLAG-MS2-TNRC6Amid fusion protein; F-MS2-Mid-M, FLAG-MS2-TNRC6Amid QSR and W mutant fusion protein. This mutant contains the mutations that disrupt the interaction of Not1 with TNRC6. E, schematic drawing of reporter genes used in Fig. 1. The filled box indicates the open reading frame. All reporter genes contain the 3′-UTR region of PGK1, in which two tandem MS2 binding sites were inserted. Rz, a hammerhead ribozyme that generates a 3′ end with no poly(A) tail. F, tethering of TNRC6A reduces mRNA levels independently of a poly(A) tail. RNA samples from wild type cells transformed with the indicated plasmids were analyzed by Northern blotting using GFP and SCR probes. The data represent the means of three independent experiments, with S.D. values. G, tethering of TNRC6Amid reduces protein levels independently of a poly(A) tail. Wild type cells harboring the indicated plasmids were grown, and protein samples were analyzed by Western blotting using anti-GFP antibody. eEF1α served as a loading control. The data represent the means of three independent experiments with S.D. values. H, tethering of TNRC6A induces translation repression, dependent on interaction with Not1 but independent of a poly(A) tail. Cell extracts were prepared from wild type cells transformed with the indicated plasmids, and polysome analysis was performed. Top, ratio (%) of mRNA distribution in each fraction. Bottom, reporter mRNA was detected by Northern blotting using a PGK1 3′UTR probe. The p value was calculated by using Student's t test. I, levels of synthesized GFP derived from reporter mRNAs were significantly reduced by tethered TNRC6 independently of a poly(A) tail. Pulse-labeling experiments were performed using the same cells as in F. GFP-MS2 and GFP-MS2-Rz protein levels are shown as the mean values of three independent experiments with S.D. values.
Tethered TNRC6 Stimulates the Degradation of mRNA from the 5′ End, Independent of Translation and a Poly(A) Tail
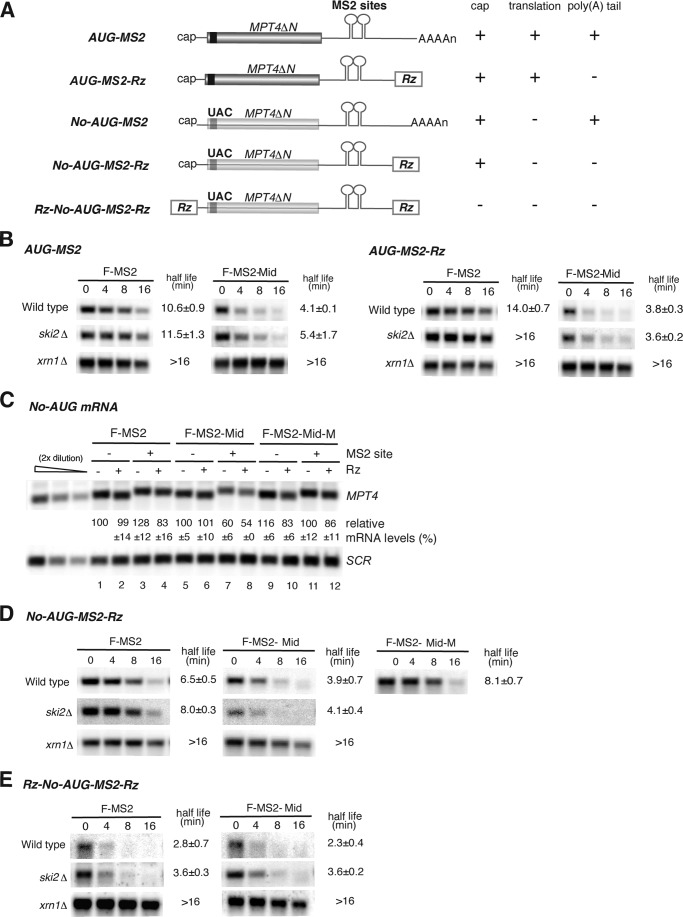
We next examined the effects of F-MS2-Mid on mRNA stability, using reporter genes containing an ORF of N-terminally truncated MPT4 (473 nucleotides) and MS2 binding sites, as described previously (Fig. 2A) (49). Consistent with the results obtained using GFP reporter mRNAs (Fig. 1F), half-life analysis revealed that tethered Mid domain dramatically stimulated mRNA degradation in a poly(A) tail-independent manner (Fig. 2B, wild type). Degradation was dependent on the 5′–3′ exonuclease Xrn1 but not the 3′–5′ exosome component Ski2, indicating that degradation occurred from the 5′ end (Fig. 2B). To dissect the effects of the tethered Mid domain on translation, deadenylation, and mRNA decay, we utilized previously constructed reporters containing MS2 binding sites. These constructs were of two types: the No-AUG-MS2 reporters are not translated because all AUG codons in the ORF of N-terminally truncated MPT4 were replaced with UAC codons (Fig. 2A), and the -Rz reporters lack a poly(A) tail (Fig. 2A) (49). The tethering of F-MS2-Mid reduced the levels of both No-AUG-MS2 and No-AUG-MS2-Rz reporter mRNAs, and this down-regulation was mediated by the CCR4-NOT complex (Fig. 2C). Half-life analysis revealed that tethering of the Mid domain accelerated the decay rate of No-AUG-MS2-Rz mRNA via an interaction with the CCR4-NOT complex (Fig. 2D, t½ = 3.9 min, F-MS2-Mid versus t½ = 8.1 min, F-MS2-Mid-M). Destabilization of No-AUG-MS2-Rz mRNA by F-MS2-Mid was significantly suppressed in xrn1Δ mutant cells (Fig. 2D, t½ > 16 min) but not in ski2Δ mutant cells (Fig. 2D, t½ = 4.1 min), further confirming that the Mid domain stimulates 5′-to-3′ mRNA decay. The Rz-No-AUG-MS2-Rz mRNA, which lacks a cap structure and is therefore intrinsically unstable, was not further destabilized by the tethering of F-MS2-Mid (Fig. 2E). Overall, these experiments suggest that in yeast, tethering of the Mid domain of zebrafish TNRC6A stimulates 5′-to-3′ degradation of the reporter mRNA independent of translation and a poly(A) tail.
FIGURE 2.
Tethered TNRC6A stimulates decapping and the degradation of mRNA from the 5′ end, independent of translation and a poly(A) tail. A, schematic drawing of reporter genes used in Fig. 2. The line represents non-translated regions. All reporter genes contain the 3′-UTR region of PGK1, in which two tandem MS2 binding sites were inserted. B, tethering of TNRC6A stimulates 5′-to-3′ mRNA decay independent of a poly(A) tail. Wild type, ski2Δ, and xrn1Δ cells containing the indicated reporter gene and plasmids were grown, RNA samples were purified at each time point, and the reporter mRNA was analyzed by Northern blotting using a DIG-labeled PGK1 3′-UTR probe. C, TNRC6A reduces mRNA levels independently of translation and a poly(A) tail. Wild type cells harboring the indicated No-AUG reporter genes were grown, and RNA samples were analyzed by Northern blotting using a DIG-labeled MPT4 probe. The data represent the means of three independent experiments with S.D. values. D and E, tethering of the Mid domain of TNRC6A promotes 5′-to-3′ mRNA decay independent of translation and a poly(A) tail in a cap-dependent manner. Wild type, ski2Δ, and xrn1Δ cells harboring No-AUG-MS2-Rz (D) and Rz-No-AUG-MS2-Rz (E) reporter gene and plasmids were grown, and RNA samples were analyzed by Northern blotting using a DIG-labeled PGK1 3′-UTR probe. The half-lives of mRNA in the indicated cells are shown as the mean values of three independent experiments with S.D. values.
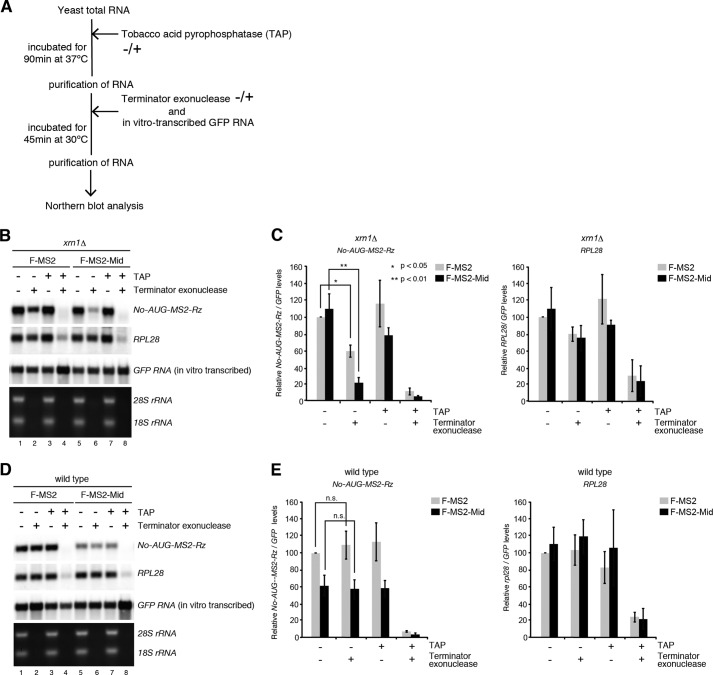
To investigate whether the decapping reaction would be accelerated by the Mid domain of zebrafish TNRC6A, we measured the levels of the decapped reporter mRNA (Fig. 3A). To this end, we utilized xrn1Δ mutant cells in which decapped mRNAs are stabilized and consequently accumulate. By treating purified RNA with Terminator exonuclease, which specifically degrades 5′-monophosphorylated RNA, the levels of decapped mRNAs could be estimated (54). In the control tethering experiment with F-MS2, the level of No-AUG-MS2-Rz mRNA was reduced to 59% by Terminator treatment, revealing that the decapped fraction was 41% (Fig. 3B, lanes 1 and 2; see also Fig. 3C for quantitation, Student's t test, p < 0.05). By contrast, Terminator exonuclease reduced the reporter mRNA tethered by F-MS2-Mid to 21%, indicating that ∼80% of the reporter mRNA was decapped by the Mid domain (Fig. 3B, lanes 5 and 6; see also Fig. 3C for quantitation, Student's t test, p < 0.01). The levels of endogenous RPL28 mRNA were reduced equally by Terminator exonuclease regardless of the tethering construct (Fig. 3B, lanes 1 and 2 and lanes 5 and 6; see also Fig. 3D). After the removal of the cap structure by treatment with tobacco acid pyrophosphatase, RPL28 and No-AUG-MS2-Rz mRNA were almost completely degraded by Terminator exonuclease (Fig. 3, B–E). A spiked 5′-triphosphorylated GFP RNA produced by in vitro transcription was not degraded by Terminator nuclease, confirming the enzyme's substrate specificity (Fig. 3, B and D). No difference was observed between F-MS2 and F-MS2-Mid in wild type cells, in which decapped mRNAs do not accumulate (Fig. 3, D and E). These results indicate that tethering of F-MS2-Mid stimulates the decapping reaction, followed by rapid degradation of the reporter mRNA from 5′ end by Xrn1, independent of translation and a poly(A) tail.
FIGURE 3.
Tethered TNRC6A stimulates the decapping reaction independent of translation and a poly(A) tail. A, schematic of the experimental procedure. B and D, TNRC6A promotes the decapping reaction, independent of translation and a poly(A) tail. RNA samples were prepared from wild type and xrn1Δ cells harboring the No-AUG-MS2-Rz reporter gene and then incubated in the absence or presence of Terminator exonuclease and tobacco acid pyrophosphatase (TAP). In vitro transcribed 5′-triphosphate GFP RNA was added after tobacco acid pyrophosphatase treatment. RNA samples were analyzed by Northern blotting. C and E, quantitation of the levels of No-AUG-MS2-Rz reporter (left) and rpl28 mRNAs (right). The levels of reporter and rpl28 mRNAs were normalized to those of the control GFP RNA as described in B and D. Error bars, S.D. The p value was calculated by using Student's t test.
Zebrafish miR-430 Stimulates mRNA Decay When the Deadenylase Activity of the CCR4-NOT Complex Is Inhibited
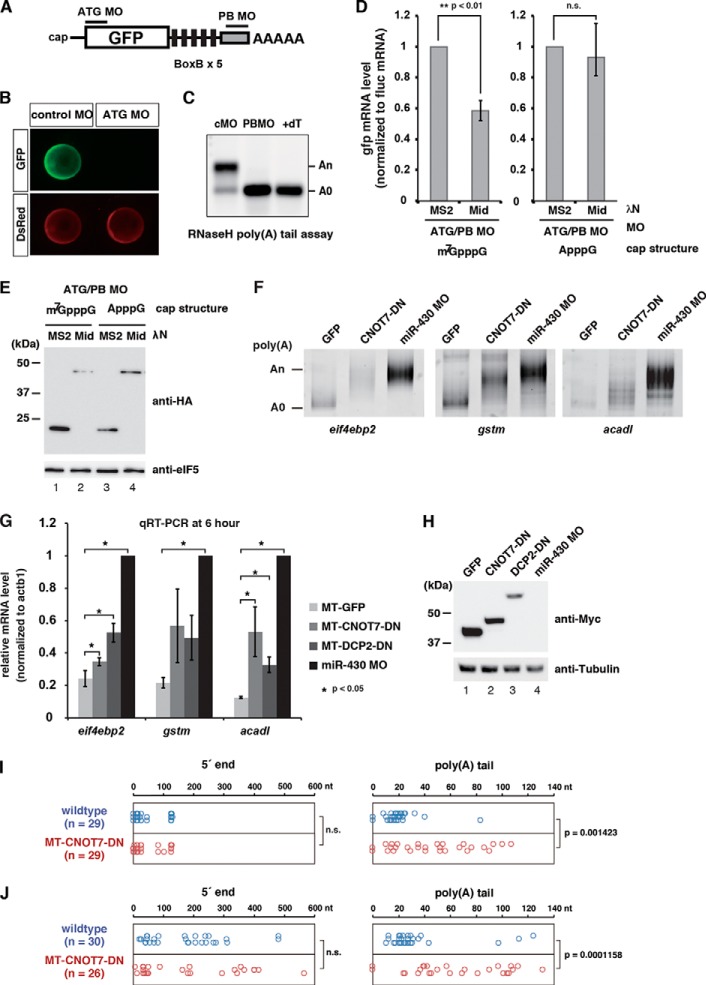
The results described above imply that TNRC6 can stimulate mRNA decay in a decapping-dependent but a poly(A) tail- and translation-independent manner. A similar observation regarding activation of decapping by miRISC has been reported in Drosophila S2 cells (25, 26, 28), supporting our findings in the hybrid assay system. Therefore, we performed three experiments in zebrafish embryos to assess the validity of our findings in yeast. First, we asked whether TNRC6A would destabilize bound mRNAs in zebrafish embryos independently of translation and a poly(A) tail. To address this question, we injected GFP reporter mRNA into zebrafish embryos and then blocked its translation using a morpholino oligomer (ATG MO) that specifically masks the start codon of the GFP ORF (Fig. 4A). In addition, we used a poly(A) tail-blocking MO (PB MO) that binds to the 3′ end of GFP mRNA to inhibit cytoplasmic polyadenylation during zebrafish embryogenesis (Fig. 4, B and C) (55). We then asked whether tethering of the Mid domain fused with the λN peptide to a GFP mRNA containing the BoxB sequence promotes mRNA decay independent of translation and a poly(A) tail. qRT-PCR analysis of reporter mRNA 10 h after injection revealed that, relative to the control tethering experiment using λN-MS2, tethering of the Mid domain promoted mRNA decay of an m7G-capped reporter mRNA whose translation and a poly(A) tail were blocked (Student's t test, p < 0.01; Fig. 4, D (left) and E). To determine whether this was due to the stimulation of decapping, we replaced the m7G cap with an A cap, because the decapping enzyme Dcp2 removes non-methylated cap structures less efficiently in vitro (56). The stability of the A-capped reporter mRNA was not significantly changed by the Mid domain (Student's t test, p = 0.53; Fig. 4, D (right) and E). These results show that the Mid domain of TNRC6A promotes mRNA decay by decapping in a translation- and poly(A) tail-independent manner in zebrafish embryos.
FIGURE 4.
Validation of the deadenylation-independent mRNA decay pathway in zebrafish embryos. A, schematic representations of the GFP reporter mRNA with BoxB sites and MOs used to analyze translation- and a poly(A) tail-independent mRNA decay by the TNRC6A Mid domain in zebrafish embryos. The translation-blocking MO (ATG MO) specifically masks the start codon of the GFP ORF. The polyadenylation-blocking MO (PB MO) binds to the end of the GFP mRNA and inhibits cytoplasmic polyadenylation during zebrafish embryogenesis. B, confirmation of the effect of ATG MO in zebrafish embryos. GFP and DsRed mRNAs were co-injected with MOs at the one-cell stage. GFP expression from the GFP reporter mRNA was completely blocked by ATG MO (green). Translation of co-injected DsRed mRNA was unaffected (red). Embryos are shown 10 h postfertilization. C, confirmation of the effect of PB MO in zebrafish embryos. GFP mRNA without a poly(A) tail was co-injected with MOs at the one-cell stage. The poly(A) tail length of the GFP mRNA was analyzed 4 h postfertilization by RNase H digestion and Northern blotting. Injected mRNA was polyadenylated after injection in the presence of control MO. PB MO completely blocked polyadenylation of the GFP mRNA. +dT, RNase H-treated sample in the presence of oligo(dT) (15), representing the completely deadenylated A(0) fragment. D, stability of the injected GFP reporter mRNA was determined by qRT-PCR 10 h after injection. Left, qRT-PCR analysis of the stability of reporter mRNA containing the 5′ m7G-cap. Right, qRT-PCR analysis of the stability of reporter mRNA containing the 5′ A-cap. GFP mRNA values were normalized against Fluc mRNA values. Normalized values of the experiments using HA-λN MS2 were set to 1. Graphs represent the means of three independent experiments. Error bars, S.D. The p value was calculated by using Student's t test. E, HA- and λN-tagged effector proteins used in D were detected by Western blotting using anti-HA antibody. eIF5 was served as loading control. F, poly(A) tail analysis of the endogenous miR-430 target mRNAs at 6 h postfertilization. mRNA encoding GFP or dominant-negative CNOT7 or MO against miR-430 was injected at the one-cell stage as indicated. The putative position of the A(0) product is shown on the left. G, qRT-PCR analysis of the miR-430 target mRNAs at 6 h postfertilization in the presence of GFP, dominant negative CNOT7, dominant negative DCP2, or miR-430 MO. mRNA levels were normalized against actb1. Normalized values of the experiments using miR-430 MO were set to 1. Graphs represent the means of three independent experiments. Error bars, S.D. The p value was calculated by using Student's t test. H, Myc-tagged effector proteins used in F and G were detected by Western blotting using anti-Myc antibody. Tubulin was served as loading control. I and J, cRACE analysis of eif4ebp2 mRNA at 5 h postfertilization in wild type (blue) and dominant negative CNOT7-injected embryos (red). Left panels, nucleotide position of 5′ end ligation junctions relative to the annotated eif4ebp2 transcript (ENSDART00000040926). Right panels, poly(A) tail length between the annotated 3′-UTR and ligated 5′ end. I, results of cRACE capturing capped mRNA; J, results of cRACE capturing decapped mRNA. The p value was calculated by using the Wilcoxon-Mann-Whitney test. n.s., not significant.
Second, we asked whether stimulation of target mRNA decapping contributes to the miRNA-mediated mRNA decay in vivo. We reasoned that if miRISC directly activates decapping, deadenylation should not be prerequisite for mRNA decay. To test this idea, we blocked miRNA-mediated deadenylation by overexpressing a mutant CNOT7 protein that lacks deadenylase activity (5) and cannot recruit the other deadenylase subunit CCR4/CNOT6 to CNOT1 (4, 5, 42). To monitor miRNA-mediated mRNA degradation, we measured the levels of three miR-430 target mRNAs (eif4ebp2, gstm, and acadl) at 6 h postfertilization, when miR-430 target mRNAs are degraded (22, 52). Overexpression of the CNOT7 mutant indeed inhibited deadenylation by miR-430, albeit not as efficiently as miR-430 antisense MO (Fig. 4F). qRT-PCR analysis revealed that inhibition of deadenylation caused accumulation of the three mRNAs to different extents (Fig. 4, G and H); in particular, although statistically significant (Student's t test, p < 0.05), eif4ebp2 appeared less sensitive to the deadenylation inhibition. Inhibition of decapping by overexpression of catalytically inactive DCP2 (56) stabilized eif4ebp2 and acadl mRNAs (Student's t test, p < 0.05) but again to different extents (Fig. 4, F and G). These results suggest that mRNAs such as eif4ebp2 are degraded by miR-430 in a manner that is less dependent on deadenylation but still dependent on decapping.
Third, we simultaneously captured both 5′ and 3′ ends of eif4ebp2 mRNA in wild type and CNOT7 DN expressed embryos at 5 h postfertilization by cRACE (53) to directly ask whether miR-430 produced decapped but polyadenylated decay intermediates. When cRACE was performed with RNA samples pretreated with calf intestine alkaline phosphatase followed by tobacco acid pyrophosphatase, capped 5′ ends that were intramolecularly ligated to 3′-OH ends could be captured (53). Indeed, amplicons from calf intestine alkaline phosphatase/tobacco acid pyrophosphatase-treated RNA samples contained 3′-to-5′ ligation junctions that converged at two 5′ sites of eif4ebp2 mRNA, presumably representing two alternative transcriptional start sites (Fig. 4I, left). Analysis of these amplicons revealed that 3′ ends of capped eif4ebp2 mRNA were mostly deadenylated (<30-nucleotide poly(A) tail) in wild type (Fig. 4I, right). On the other hand, longer poly(A) tail was retained in CNOT7 DN expressed embryos (Wilcoxon-Mann-Whitney test, p < 0.01), consistent with the poly(A) tail analysis in Fig. 4F. We then performed cRACE with naive RNA samples to capture 5′-monophosphate ends that were ligated to 3′-OH ends. Under this experimental condition, ligation junctions from both wild type and CNOT7 DN expressed embryos were located downstream of major transcriptional start sites and were broadly distributed (Fig. 4J, left), representing decapped RNA molecules being degraded in the 5′-to-3′ direction (53). Notably, the poly(A) tail length of decapped eif4ebp2 mRNA was significantly longer in CNOT7 DN expressed embryos compared with wild type (Fig. 4J, right, p < 0.01), as observed with capped eif4ebp2 mRNA. Indeed, >50% of decapped eif4ebp2 mRNA still retained a >50-nucleotide poly(A) tail in CNOT7 DN expressed embryos. This result further supported the idea that eif4ebp2 mRNA was degraded from the 5′ end by miR-430-mediated decapping even if efficient deadenylation was not ensured. Overall, these experiments confirmed that miR-430 is capable of inducing decapping and 5′-to-3′ decay independent of deadenylation in zebrafish embryos.
mRNA Decapping Factors Exert Partially Redundant Functions in Rapid mRNA Decay from the 5′ End by Tethered TNRC6
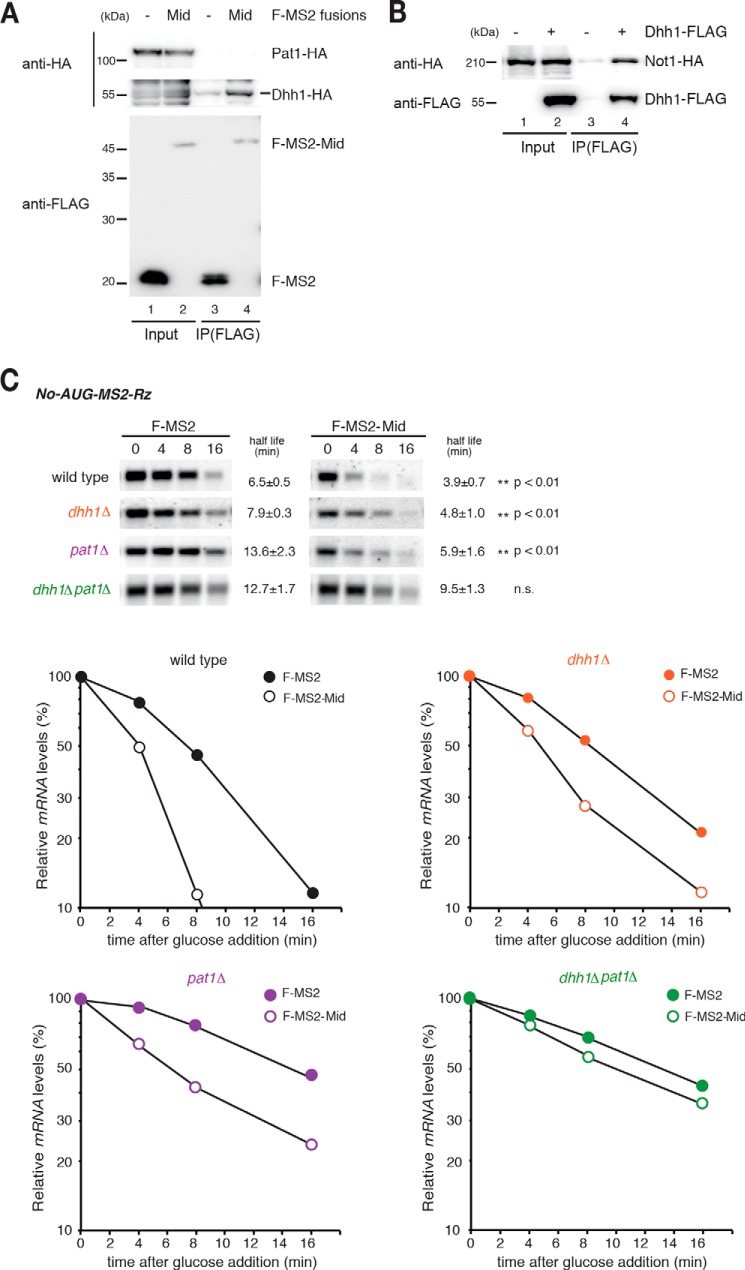
Based on the results described above and those of previous studies (25, 28, 38, 57, 58), we hypothesized that the tethered Mid domain recruits yeast CCR4-NOT complex by interacting with mRNA fate modulators, such as Dhh1 and Pat1, thereby stimulating the decapping reaction. To address this possibility, we first confirmed the interaction between FLAG-tagged F-MS2-Mid and HA-tagged Dhh1 and Pat1. Dhh1-HA protein co-immunoprecipitated with F-MS2-Mid, whereas HA-tagged Pat1 did not (Fig. 5A, lanes 3 and 4). Consistent with previous reports, HA-tagged Not1 co-immunoprecipitated with FLAG-tagged Dhh1 (Fig. 5B) (8). We then measured the stability of No-AUG-MS2-Rz mRNA in decapping activator mutants. No-AUG-MS2-Rz mRNA was dramatically stabilized in the dhh1Δpat1Δ double mutant (Fig. 5C, Student's t test, n.s.) but not in the dhh1Δ or pat1Δ single mutants (Fig. 5C, Student's t test, p < 0.01). These results provided genetic evidence that Dhh1 and Pat1 play overlapping roles in destabilization of the reporter mRNA by the CCR4-NOT complex recruited by TNRC6A.
FIGURE 5.
mRNA decapping factors exert partially redundant functions in rapid mRNA decay from the 5′ end by tethered TNRC6A. A, interaction of FLAG-MS2-Mid with yeast Dhh1-HA. Cell lysates of wild type cells transformed with the indicated plasmids were immunoprecipitated using anti-FLAG antibody. Total extract (Input) and immunoprecipitate (IP) were analyzed by Western blotting using anti-HA or anti-FLAG antibody. B, interaction of FLAG-Dhh1 with Not1-HA. Immunoprecipitation was performed as described in A. C, mRNA fate modulators Dhh1 and Pat1 have partially redundant functions in TNRC6AMid-mediated 5′-to-3′ mRNA decay independent of translation and a poly(A) tail. Top, Northern blotting analysis of No-AUG-MS2-Rz mRNA in the indicated cells. The half-lives of mRNA in the indicated cells are shown as the mean values of three independent experiments, with S.D. values. Bottom, graphs of the half-life analysis. The p value was calculated by using Student's t test.
Distinct Roles of mRNA Decapping Factors in Translation Repression and Rapid mRNA Decay from the 5′ End by Tethered TNRC6
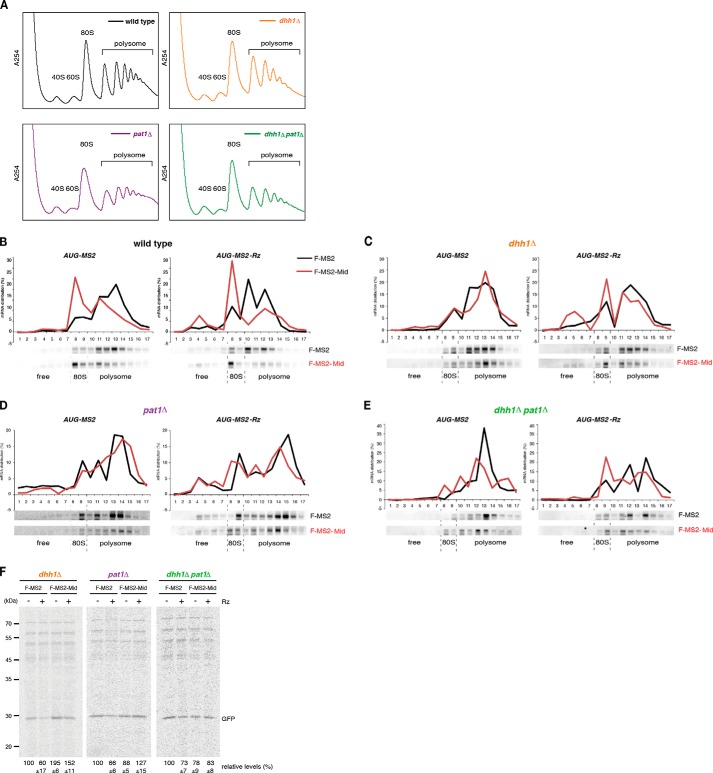
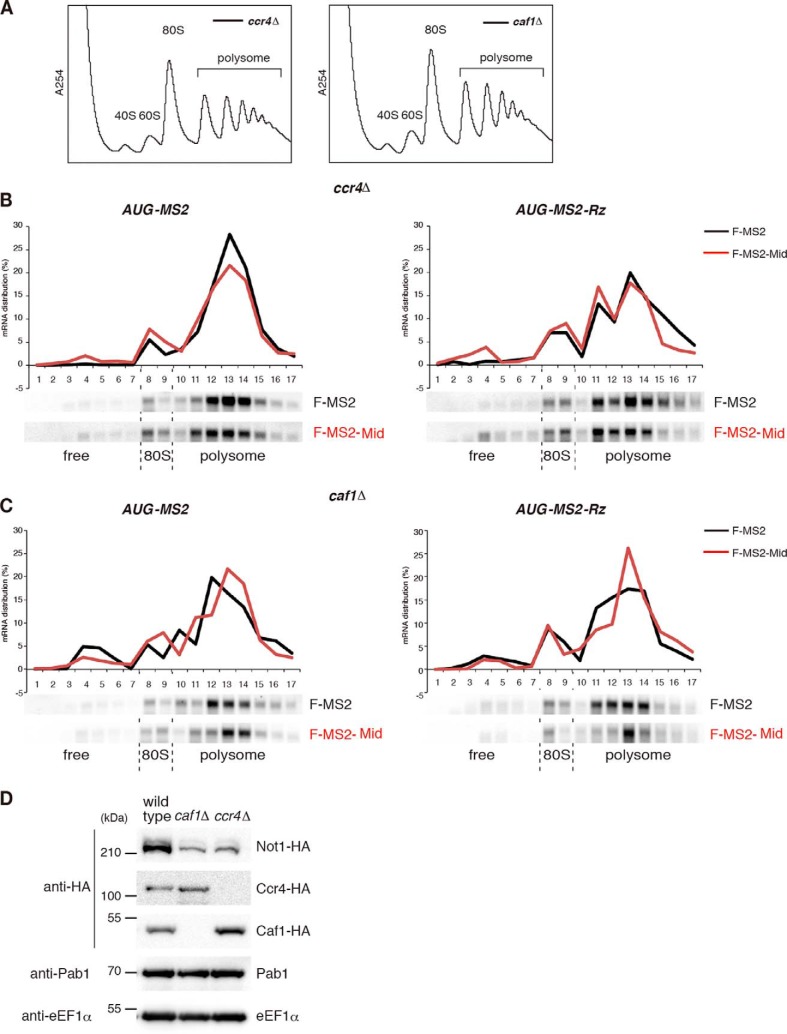
The CCR4-NOT complex has been suggested to play a role in miRNA-mediated translation repression (34, 35), and the mRNA fate modulators Dhh1 and Pat1 are also translational repressors (15–17). Furthermore, involvement of DDX6 in miRNA-mediated translation repression has been reported (11, 12, 43, 59). Therefore, we examined the roles of CCR4-NOT and decapping components in translation repression by the Mid domain. First, we performed polysome analysis to confirm that CCR4-NOT components are required for translation repression by tethered TNRC6A in yeast. Translation repression of AUG-MS2 and AUG-MS2-Rz mRNA by tethered F-MS2-Mid was almost abolished in ccr4Δ and caf1Δ mutant cells in comparison with wild type cells, independent of a poly(A) tail (Fig. 7B versus Fig. 6, A–C). Because the level of Not1 was reduced in ccr4Δ and caf1Δ mutant cells (Fig. 6D), it is likely that translation repression by tethered TNRC6 requires the integrity of CCR4-NOT complex. Next, we performed polysome analysis in dhh1Δ or pat1Δ mutant cells. Translation repression of AUG-MS2 or AUG-MS2-Rz mRNAs by the Mid domain was abrogated in dhh1Δ or pat1Δ single mutants, as well as in the dhh1Δpat1Δ double mutant (Fig. 7, A–E). These results indicate that both mRNA fate modulators are required for inhibition of translation by the tethered Mid domain of zebrafish TNRC6. To confirm the result of the polysome analysis, we performed pulse-labeling experiments to investigate whether Dhh1 and Pat1 are required for translation repression. Because N-terminally truncated MPT4 derived from AUG-MS2 or AUG-MS2-Rz mRNAs contains no methionine (other than the initiator methionine) or cysteine, we used GFP-MS2 and GFP-MS2-Rz mRNAs for the pulse-labeling experiments. The levels of synthesized GFP derived from reporter mRNAs were significantly reduced by tethered TNRC6A in the wild type (Fig. 1I), but translation repression was impaired in the dhh1Δ or pat1Δ single mutants and the dhh1Δpat1Δ double mutant cells (Fig. 7F). These results demonstrate that the two mRNA fate modulators, Dhh1 and Pat1, not only facilitate mRNA decay from the 5′ end independently of translation and a poly(A) tail but also repress translation when the CCR4-NOT complex is recruited to mRNAs by the miRISC component TNRC6A.
FIGURE 7.
The roles of decapping factors in TNRC6A-mediated translation repression in yeast. A–E, cell extracts were prepared from wild type and the indicated mutant cells transformed with the indicated plasmids, and polysome analysis was performed. Distributions of the reporter RNAs were analyzed by Northern blotting. F, pulse-labeling experiments were performed using dhh1Δ, pat1Δ, and dhh1Δpat1Δ mutant cells. GFP-MS2 and GFP-MS2-Rz protein levels reflect the mean values of three independent experiments, with S.D. values.
FIGURE 6.
The roles of CCR4-NOT complex in TNRC6A-mediated translation repression in yeast. A–C, translation repression by tethered TNRC6A was almost diminished in ccr4Δ and caf1Δ mutant cells independent of a poly(A) tail. Cell extracts were prepared from indicated cells harboring the indicated plasmid, and polysome analysis was performed. RNA samples were analyzed by Northern blotting using a DIG-labeled PGK1 3′-UTR probe. D, the expression level of Not1 reduced in caf1Δ and ccr4Δ mutant cells. Endogenous NOT1, CAF1, and CCR4 were tagged by 3× hemagglutinin (HA) in wild type, caf1Δ, and ccr4Δ mutant cells. Protein samples were analyzed by Western blotting using anti-HA, anti-Pab1 and anti-eEF1α antibodies.
DISCUSSION
In this study, we established a heterologous experimental system by tethering animal TNRC6 proteins to mRNAs in yeast. Polysome analysis, pulse-labeling experiments, and measurement of mRNA half-lives revealed that the tethered Mid domain fragment of zebrafish TNRC6A induces mRNA degradation and translation repression in yeast. This result strongly suggests that miRISC induces both translation repression and mRNA degradation via interactions with fundamental factors that are conserved across a wide range of eukaryotes. Indeed, the highly conserved proteins Dhh1 and Pat1 mediate these two functions through the CCR4-NOT complex, which is recruited by the Mid domain (Figs. 5 and 7). These two factors are involved in miRNA-mediated silencing in animals (11, 12, 25, 29, 33–35, 59), supporting the idea that TNRC6 can function in S. cerevisiae and suggesting that our system can be used to characterize the biologically relevant activities of miRISC. Although the data obtained in yeast with a truncated fragment of TNRC6 require careful validation in animal cells, the recapitulation of TNRC6-mediated silencing in yeast described here raises the possibility that genetic resources in yeast can be used to study basic principles of the miRNA system.
The Mid domain of TNRC6A interacted with the yeast CCR4-NOT complex via CIM1 and W-motifs within the Mid domain that mediate the same interactions in animals (Fig. 1B) (33, 34). This result, together with recent structural studies (11, 12), indicates that the conserved architecture of the CCR4-NOT complex provides a binding surface for GW182/TNRC6 proteins, thereby connecting miRISC to the mRNA fate modulators. Further biochemical and structural analysis in yeast will shed light on how this interaction evolved.
The interactions of GW182/TNRC6 proteins with PABP and CCR4-NOT deadenylase play crucial roles in both translational repression and degradation of miRNA targets (33–35, 37). mRNA degradation by miRNAs and GW182/TNRC6 requires both deadenylase and the DCP1-DCP2 decapping complexes (25, 60). In addition, GW182 recruits decapping enzymes to target mRNAs independently of deadenylation (38). In this study, we demonstrated that the tethered TNRC6A fragment promoted decapping and 5′-to-3′ mRNA decay in a Not1-dependent but a poly(A) tail- and translation-independent manner (Figs. 2 and 3). The levels of decapped No-AUG-MS2-Rz mRNA were significantly increased in xrn1Δ cells expressing F-MS2-Mid (Fig. 3, B and C), indicating that the tethered Mid domain of TNRC6A recruits CCR4-NOT and the decapping complex and facilitates the decapping reaction independently of a poly(A) tail and translation. Together, these results suggest that mRNA decay caused by the Mid domain can mostly be attributed to the function of the yeast CCR4-NOT complex.
Our results indicate that GW182/TNRC6 stimulates mRNA decay in yeast in a decapping-dependent but poly(A) tail- and translation-independent manner. We also found that tethering of the TNRC6A Mid domain degraded m7G-capped mRNA but not A-capped mRNA in zebrafish embryos (Fig. 4D). Moreover, miR-430 stimulated decapping and 5′-to-3′ degradation of eif4ebp2 mRNA in zebrafish embryos even when the deadenylase activity of the CCR4-NOT complex was not fully ensured (Fig. 4, F, G, and J). These results imply that, at least for some mRNAs like eif4ebp2, miRISC promotes mRNA decay from the 5′ end by directly stimulating decapping before (or in parallel to) deadenylation. Although more comprehensive study with multiple transcripts in diverse animals is prerequisite to generalize this degradation mode of miRNAs, it is noteworthy that some mRNAs that are degraded in an Ago1-dependent manner are degraded when CAF1 is knocked down in Drosophila S2 cells (25). Conversely, not all Ago1-RISC target mRNAs strictly require mRNA fate modulators for their decay (29). These observations suggest that each mRNA is differentially susceptible to decapping and deadenylation during the process of miRNA-mediated degradation.
We found that rapid decay of No-AUG-MS2-Rz mRNA by the tethered TNRC6A Mid domain was abrogated in the dhh1Δpat1Δ double mutant but not in the dhh1Δ or pat1Δ single mutants (Fig. 5). We propose that Dhh1 and Pat1 contribute to decapping via distinct pathways in the absence of translation and a poly(A) tail. In addition to inhibiting translation, Dhh1 and Pat1 may have redundant functions in the formation of the decapping complex. Consequently, in the dhh1Δpat1Δ double mutant, the decapping complex may not be able to form, resulting in a very strong defect in decapping.
A prevailing view, based on in vitro experiments, is that miRNAs inhibit translation at the initiation step by an as yet unknown mechanism (39, 61–63). A recent study has suggested that miRNAs repress translation initiation in a manner dependent upon eIF4AII, which interacts with CNOT7 of the CCR4-NOT complex in mammalian cells (64). However, because eIF4AII is present neither in Drosophila nor in unicellular eukaryotes, such as S. cerevisiae, it remains to be determined how this factor affects translation initiation in general. In this study, we showed that the tethered Mid domain of TNRC6A repressed translation in yeast (Fig. 1, G and H; see also Fig. 7B). Hence, the repressive activity of TNRC6A recapitulated in yeast is independent of eIF4AII. In addition, others also observed that eIF4AII does not interact directly with CNOT1 MIF4G domain (11, 12, 43). Furthermore, recent in vitro studies showed that miRNAs trigger dissociation of eIF4A in Drosophila and both eIF4AI and eIF4AII in humans (65, 66). Apparently, more experiments will be necessary to determine the roles of eIF4AII in translation repression by miRNAs.
In cells expressing F-MS2-Mid protein, the levels of GFP-MS2 or AUG-MS2 mRNAs were reduced in the polysome fraction, but increased in the 80 S monosome and free fractions, relative to cells expressing the control MS2 protein (Figs. 1G and 7B). This effect was observed in a reporter mRNA lacking a poly(A) tail (Figs. 1G and 7B). These results suggest that the tethered Mid domain of TNRC6A may block the formation of 48 S preinitiation complex or inhibit the steps after 80 S formation by CCR4-NOT complex independently of a poly(A) tail.
How, then, does the CCR4-NOT complex inhibit translation? Polysome analysis and pulse-labeling experiments showed that translation repression by tethering of the Mid domain of TNRC6A was abrogated in dhh1Δ or pat1Δ single mutants as well as in the dhh1Δpat1Δ double mutant (Fig. 7). Translationally repressed mRNAs in wild type cells contained a cap structure (Fig. 3, D and E). Moreover, stimulation of mRNA decay from the 5′ end was impaired only in the dhh1Δpat1Δ double mutant, whereas translation repression by the tethered TNRC6A was suppressed in the dhh1Δ and pat1Δ single mutants (Fig. 7). These results clearly indicate that translation repression is not a consequence of decapping. The mRNA fate modulators Dhh1 and Pat1 independently repress translation in vivo, and they repress translation initiation in vitro by limiting the formation of a stable 48 S preinitiation complex (15). Notably, a human homolog of Dhh1, DDX6/RCK1/p54, interacts with miRISC and contributes to translation repression in cultured cells (11, 12, 43, 59), and GW182 associates with HPat in Drosophila cells (57). Our results in yeast therefore support the model in which the CCR4-NOT complex mediates translation repression, at least in part, via recruitment of mRNA fate modulators through a direct interaction with Dhh1. Further experiments will be necessary to determine the conserved roles of decapping factors Dhh1/DDX6/RCK1/p54 and Pat1/HPat/PatL1 in translation repression by GW182/TNRC6 as well as other CCR4-NOT-interacting proteins.
Acknowledgments
We thank Dr. Yukihide Tomari for the kind gift of a reagent and for helpful discussions. We also thank members of our laboratories for discussion and critical comments on the manuscript.
This study was supported by Grant-in-aid for Scientific Research on Innovative Areas “RNA regulation” (20112006) and “Nascent chain biology” (26116003) (to T. I.) and “non-coding RNA” (24115711) (to Y. M.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by Research Grants in the Natural Sciences, Mitsubishi Foundation (to T. I.).
- miRNA
- microRNA
- DIG
- digoxigenin
- qRT-PCR and qPCR
- quantitative RT-PCR and PCR, respectively
- MO
- morpholino oligomer
- miRISC
- miRNA-induced silencing complex
- PABP
- poly(A)-binding protein
- cRACE
- circularized rapid amplification of cDNA ends
- DN
- dominant negative.
REFERENCES
- 1. Wahle E., Winkler G. S. (2013) RNA decay machines: deadenylation by the Ccr4-not and Pan2-Pan3 complexes. Biochim. Biophys. Acta. 1829, 561–570 [DOI] [PubMed] [Google Scholar]
- 2. Collart M. A., Panasenko O. O. (2012) The Ccr4-Not complex. Gene 492, 42–53 [DOI] [PubMed] [Google Scholar]
- 3. Miller J. E., Reese J. C. (2012) Ccr4-Not complex: the control freak of eukaryotic cells. Crit. Rev. Biochem. Mol. Biol. 47, 315–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basquin J., Roudko V. V., Rode M., Basquin C., Séraphin B., Conti E. (2012) Architecture of the nuclease module of the yeast Ccr4-not complex: the Not1-Caf1-Ccr4 interaction. Mol. Cell 48, 207–218 [DOI] [PubMed] [Google Scholar]
- 5. Petit A. P., Wohlbold L., Bawankar P., Huntzinger E., Schmidt S., Izaurralde E., Weichenrieder O. (2012) The structural basis for the interaction between the CAF1 nuclease and the NOT1 scaffold of the human CCR4-NOT deadenylase complex. Nucleic Acids Res. 40, 11058–11072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker R. (2012) RNA degradation in Saccharomyces cerevisae. Genetics 191, 671–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coller J., Parker R. (2004) Eukaryotic mRNA decapping. Annu. Rev. Biochem. 73, 861–890 [DOI] [PubMed] [Google Scholar]
- 8. Maillet L., Collart M. A. (2002) Interaction between Not1p, a component of the Ccr4-not complex, a global regulator of transcription, and Dhh1p, a putative RNA helicase. J. Biol. Chem. 277, 2835–2842 [DOI] [PubMed] [Google Scholar]
- 9. Ozgur S., Chekulaeva M., Stoecklin G. (2010) Human Pat1b connects deadenylation with mRNA decapping and controls the assembly of processing bodies. Mol. Cell Biol. 30, 4308–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haas G., Braun J. E., Igreja C., Tritschler F., Nishihara T., Izaurralde E. (2010) HPat provides a link between deadenylation and decapping in metazoa. J. Cell Biol. 189, 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathys H., Basquin J., Ozgur S., Czarnocki-Cieciura M., Bonneau F., Aartse A., Dziembowski A., Nowotny M., Conti E., Filipowicz W. (2014) Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Mol. Cell 54, 751–765 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y., Boland A., Kuzuoğlu-Öztürk D., Bawankar P., Loh B., Chang C. T., Weichenrieder O., Izaurralde E. (2014) A DDX6-CNOT1 complex and W-binding pockets in CNOT9 reveal direct links between miRNA target recognition and silencing. Mol. Cell 54, 737–750 [DOI] [PubMed] [Google Scholar]
- 13. Tritschler F., Braun J. E., Motz C., Igreja C., Haas G., Truffault V., Izaurralde E., Weichenrieder O. (2009) DCP1 forms asymmetric trimers to assemble into active mRNA decapping complexes in metazoa. Proc. Natl. Acad. Sci. U.S.A. 106, 21591–21596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tritschler F., Eulalio A., Helms S., Schmidt S., Coles M., Weichenrieder O., Izaurralde E., Truffault V. (2008) Similar modes of interaction enable Trailer Hitch and EDC3 to associate with DCP1 and Me31B in distinct protein complexes. Mol. Cell Biol. 28, 6695–6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coller J., Parker R. (2005) General translational repression by activators of mRNA decapping. Cell 122, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minshall N., Thom G., Standart N. (2001) A conserved role of a DEAD box helicase in mRNA masking. RNA 7, 1728–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura A., Amikura R., Hanyu K., Kobayashi S. (2001) Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development 128, 3233–3242 [DOI] [PubMed] [Google Scholar]
- 18. Anderson J. S., Parker R. (1996) RNA turnover: the helicase story unwinds. Curr. Biol. 6, 780–782 [DOI] [PubMed] [Google Scholar]
- 19. Minshall N., Kress M., Weil D., Standart N. (2009) Role of p54 RNA helicase activity and its C-terminal domain in translational repression, P-body localization and assembly. Mol. Biol. Cell 20, 2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fabian M. R., Sonenberg N., Filipowicz W. (2010) Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 [DOI] [PubMed] [Google Scholar]
- 21. Lim L. P., Lau N. C., Garrett-Engele P., Grimson A., Schelter J. M., Castle J., Bartel D. P., Linsley P. S., Johnson J. M. (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 [DOI] [PubMed] [Google Scholar]
- 22. Giraldez A. J., Mishima Y., Rihel J., Grocock R. J., Van Dongen S., Inoue K., Enright A. J., Schier A. F. (2006) Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79 [DOI] [PubMed] [Google Scholar]
- 23. Mishima Y., Giraldez A. J., Takeda Y., Fujiwara T., Sakamoto H., Schier A. F., Inoue K. (2006) Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Curr. Biol. 16, 2135–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu L., Fan J., Belasco J. G. (2006) MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. U.S.A. 103, 4034–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. (2006) mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20, 1885–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eulalio A., Huntzinger E., Nishihara T., Rehwinkel J., Fauser M., Izaurralde E. (2009) Deadenylation is a widespread effect of miRNA regulation. RNA 15, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huntzinger E., Izaurralde E. (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12, 99–110 [DOI] [PubMed] [Google Scholar]
- 28. Rehwinkel J., Behm-Ansmant I., Gatfield D., Izaurralde E. (2005) A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11, 1640–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eulalio A., Rehwinkel J., Stricker M., Huntzinger E., Yang S. F., Doerks T., Dorner S., Bork P., Boutros M., Izaurralde E. (2007) Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev. 21, 2558–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meister G. (2013) Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet 14, 447–459 [DOI] [PubMed] [Google Scholar]
- 31. Eulalio A., Helms S., Fritzsch C., Fauser M., Izaurralde E. (2009) A C-terminal silencing domain in GW182 is essential for miRNA function. RNA 15, 1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Till S., Lejeune E., Thermann R., Bortfeld M., Hothorn M., Enderle D., Heinrich C., Hentze M. W., Ladurner A. G. (2007) A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat. Struct. Mol. Biol. 14, 897–903 [DOI] [PubMed] [Google Scholar]
- 33. Fabian M. R., Cieplak M. K., Frank F., Morita M., Green J., Srikumar T., Nagar B., Yamamoto T., Raught B., Duchaine T. F., Sonenberg N. (2011) miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 18, 1211–1217 [DOI] [PubMed] [Google Scholar]
- 34. Chekulaeva M., Mathys H., Zipprich J. T., Attig J., Colic M., Parker R., Filipowicz W. (2011) miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 18, 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braun J. E., Huntzinger E., Fauser M., Izaurralde E. (2011) GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 44, 120–133 [DOI] [PubMed] [Google Scholar]
- 36. Christie M., Boland A., Huntzinger E., Weichenrieder O., Izaurralde E. (2013) Structure of the PAN3 pseudokinase reveals the basis for interactions with the PAN2 deadenylase and the GW182 proteins. Mol. Cell 51, 360–373 [DOI] [PubMed] [Google Scholar]
- 37. Fabian M. R., Mathonnet G., Sundermeier T., Mathys H., Zipprich J. T., Svitkin Y. V., Rivas F., Jinek M., Wohlschlegel J., Doudna J. A., Chen C. Y., Shyu A. B., Yates J. R., 3rd, Hannon G. J., Filipowicz W., Duchaine T. F., Sonenberg N. (2009) Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol. Cell 35, 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishihara T., Zekri L., Braun J. E., Izaurralde E. (2013) miRISC recruits decapping factors to miRNA targets to enhance their degradation. Nucleic Acids Res. 41, 8692–8705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fukaya T., Tomari Y. (2012) MicroRNAs mediate gene silencing via multiple different pathways in drosophila. Mol. Cell 48, 825–836 [DOI] [PubMed] [Google Scholar]
- 40. Mishima Y., Fukao A., Kishimoto T., Sakamoto H., Fujiwara T., Inoue K. (2012) Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 109, 1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukaya T., Tomari Y. (2011) PABP is not essential for microRNA-mediated translational repression and deadenylation in vitro. EMBO. J. 30, 4998–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bawankar P., Loh B., Wohlbold L., Schmidt S., Izaurralde E. (2013) NOT10 and C2orf29/NOT11 form a conserved module of the CCR4-NOT complex that docks onto the NOT1 N-terminal domain. RNA. Biol. 10, 228–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rouya C., Siddiqui N., Morita M., Duchaine T. F., Fabian M. R., Sonenberg N. (2014) Human DDX6 effects miRNA-mediated gene silencing via direct binding to CNOT1. RNA 20, 1398–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldstrohm A. C., Seay D. J., Hook B. A., Wickens M. (2007) PUF protein-mediated deadenylation is catalyzed by Ccr4p. J. Biol. Chem. 282, 109–114 [DOI] [PubMed] [Google Scholar]
- 45. Goldstrohm A. C., Hook B. A., Seay D. J., Wickens M. (2006) PUF proteins bind Pop2p to regulate messenger RNAs. Nat. Struct. Mol. Biol. 13, 533–539 [DOI] [PubMed] [Google Scholar]
- 46. Drinnenberg I. A., Weinberg D. E., Xie K. T., Mower J. P., Wolfe K. H., Fink G. R., Bartel D. P. (2009) RNAi in budding yeast. Science 326, 544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suk K., Choi J., Suzuki Y., Ozturk S. B., Mellor J. C., Wong K. H., MacKay J. L., Gregory R. I., Roth F. P. (2011) Reconstitution of human RNA interference in budding yeast. Nucleic Acids Res. 39, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inada T., Aiba H. (2005) Translation of aberrant mRNAs lacking a termination codon or with a shortened 3′-UTR is repressed after initiation in yeast. EMBO J. 24, 1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsuboi T., Inada T. (2010) Tethering of poly(A)-binding protein interferes with non-translated mRNA decay from the 5′ end in yeast. J. Biol. Chem. 285, 33589–33601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mumberg D., Müller R., Funk M. (1994) Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chekulaeva M., Filipowicz W., Parker R. (2009) Multiple independent domains of dGW182 function in miRNA-mediated repression in Drosophila. RNA 15, 794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bazzini A. A., Lee M. T., Giraldez A. J. (2012) Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336, 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rissland O. S., Norbury C. J. (2009) Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat. Struct. Mol. Biol. 16, 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Braun J. E., Truffault V., Boland A., Huntzinger E., Chang C. T., Haas G., Weichenrieder O., Coles M., Izaurralde E. (2012) A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat. Struct. Mol. Biol. 19, 1324–1331 [DOI] [PubMed] [Google Scholar]
- 55. Aanes H., Winata C. L., Lin C. H., Chen J. P., Srinivasan K. G., Lee S. G., Lim A. Y., Hajan H. S., Collas P., Bourque G., Gong Z., Korzh V., Aleström P., Mathavan S. (2011) Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res. 21, 1328–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Dijk E., Cougot N., Meyer S., Babajko S., Wahle E., Séraphin B. (2002) Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO. J. 21, 6915–6924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jäger E., Dorner S. (2010) The decapping activator HPat a novel factor co-purifying with GW182 from Drosophila cells. RNA Biol. 7, 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barišić-Jäger E., Krecioch I., Hosiner S., Antic S., Dorner S. (2013) HPat a decapping activator interacting with the miRNA effector complex. PLoS One 8, e71860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chu C. Y., Rana T. M. (2006) Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4, e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen C. Y., Zheng D., Xia Z., Shyu A. B. (2009) Ago-TNRC6 triggers microRNA-mediated decay by promoting two deadenylation steps. Nat. Struct. Mol. Biol. 16, 1160–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zdanowicz A., Thermann R., Kowalska J., Jemielity J., Duncan K., Preiss T., Darzynkiewicz E., Hentze M. W. (2009) Drosophila miR2 primarily targets the m7GpppN cap structure for translational repression. Mol. Cell 35, 881–888 [DOI] [PubMed] [Google Scholar]
- 62. Thermann R., Hentze M. W. (2007) Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 447, 875–878 [DOI] [PubMed] [Google Scholar]
- 63. Mathonnet G., Fabian M. R., Svitkin Y. V., Parsyan A., Huck L., Murata T., Biffo S., Merrick W. C., Darzynkiewicz E., Pillai R. S., Filipowicz W., Duchaine T. F., Sonenberg N. (2007) MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 317, 1764–1767 [DOI] [PubMed] [Google Scholar]
- 64. Meijer H. A., Kong Y. W., Lu W. T., Wilczynska A., Spriggs R. V., Robinson S. W., Godfrey J. D., Willis A. E., Bushell M. (2013) Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 340, 82–85 [DOI] [PubMed] [Google Scholar]
- 65. Fukao A., Mishima Y., Takizawa N., Oka S., Imataka H., Pelletier J., Sonenberg N., Thoma C., Fujiwara T. (2014) MicroRNAs trigger dissociation of eIF4AI and eIF4AII from target mRNAs in humans. Mol. Cell 56, 79–89 [DOI] [PubMed] [Google Scholar]
- 66. Fukaya T., Iwakawa H. O., Tomari Y. (2014) MicroRNAs block assembly of eIF4F translation initiation complex in Drosophila. Mol. Cell 56, 67–78 [DOI] [PubMed] [Google Scholar]
- 67. Kuroha K., Tatematsu T., Inada T. (2009) Upf1 stimulates degradation of the product derived from aberrant messenger RNA containing a specific nonsense mutation by the proteasome. EMBO Rep. 10, 1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]