Abstract
Human induced pluripotent stem cells (hiPSCs), like embryonic stem cells, are under intense investigation for novel approaches to model disease and for regenerative therapies. Here, we describe the derivation and characterization of hiPSCs from a variety of sources and show that, irrespective of origin or method of reprogramming, hiPSCs can be differentiated on OP9 stroma towards a multi-lineage haemo-endothelial progenitor that can contribute to CD144+ endothelium, CD235a+ erythrocytes (myeloid lineage) and CD19+ B lymphocytes (lymphoid lineage). Within the erythroblast lineage, we were able to demonstrate by single cell analysis (flow cytometry), that hiPSC-derived erythroblasts express alpha globin as previously described, and that a sub-population of these erythroblasts also express haemoglobin F (HbF), indicative of fetal definitive erythropoiesis. More notably however, we were able to demonstrate that a small sub-fraction of HbF positive erythroblasts co-expressed HbA in a highly heterogeneous manner, but analogous to cord blood-derived erythroblasts when cultured using similar methods. Moreover, the HbA expressing erythroblast population could be greatly enhanced (44·0 ± 6·04%) when a defined serum-free approach was employed to isolate a CD31+ CD45+ erythro-myeloid progenitor. These findings demonstrate that hiPSCs may represent a useful alternative to standard sources of erythrocytes (RBCs) for future applications in transfusion medicine.
Keywords: pluripotent stem cell, erythropoiesis, globin expression
The advent of reprogramming technologies and induced pluripotent stem cells (Park et al, 2007; Takahashi et al, 2007; Yu et al, 2007; Lowry et al, 2008) have paved the way for a host of novel applications, including disease modelling and regenerative therapies, which were previously inconceivable. We have been able to demonstrate that human induced pluripotent stem cells (hiPSCs) can mimic early developmental haemopoiesis to yield B lymphocytes (Carpenter et al, 2011) and provide cardiomyocytes for heart repair (Carpenter et al, 2012). Here, we have asked whether human iPSCs can be used to mimic human developmental erythropoiesis, including definitive erythropoiesis, and to express adult haemoglobin (HbA), which would facilitate potential applications in haematology and transfusion medicine.
Developmental haemopoiesis is thought to proceed in successive waves, each yielding haemopoietic progeny with increased multi-lineage potential. Ultimately, intra-embryonic haemopoiesis yields a stem cell that will supply the blood lineage throughout adult life (Dieterlen-Lievre et al, 1976; Cumano et al, 2001; Cumano & Godin, 2007), and this has been shown to arise in the aorta-gonad-mesonephros (AGM) and other major arteries at around embryonic day E10·5 in the mouse (de Bruijn et al, 2000) by a process of endothelial to haemopoietic transition (Eilken et al, 2009; Bertrand et al, 2010; Boisset et al, 2010; Kissa & Herbomel, 2010). Prospective haemopoietic stem cells then colonize the fetal liver, spleen and, later, the bone marrow. Prior to this, the first wave of haemopoiesis arises in the yolk sac at around E7·0–E7·5 (Palis & Yoder, 2001; Baron, 2003), yielding primitive erythrocytes (and primitive macrophages). A second wave of haemopoiesis (at around E8·25) contributes to the first definitive progeny of erythro-myeloid progenitors (EMPs) (Palis et al, 1999, 2001; England et al, 2011) and slightly later at E9·0, to the first B-1 B and T lymphocytes (Godin et al, 1993; Ghosn et al, 2011; Yoshimoto et al, 2011, 2012). Although arising in both the yolk sac and AGM, it is still unclear whether the progeny from this first definitive wave represents an independent progenitor to that of the HSC.
If hiPSC are to be considered for future applications in transfusion medicine, it is important to demonstrate that hiPSC-derived erythropoiesis is capable of producing RBCs that express adult globins, as has been described for mouse ES cells (Wiles & Keller, 1991). Distinguishing features between primitive and definitive erythropoiesis are the expression of specific globins (Wood & Stamatoyannopoulos, 1976; Stamatoyannopoulos, 2005; Sankaran et al, 2010a), specifically from the beta globin locus. ε-globin expression has been used to identify primitive erythroblasts (Fraser et al, 2007), while γ globin (both HBG1 and HBG2) is expressed in fetal definitive erythroblasts (Peschle et al, 1985; Johnson et al, 2000; Stamatoyannopoulos, 2005; Sankaran et al, 2010b). Erythrocytes from adult haemopoietic stem cells typically express adult alpha and beta globin (β or HBB in humans, or βmajor and βminor or Hbb-b1 and Hbb-b2 in mouse). Previous attempts to describe beta globin expression from hiPSCs include reverse transcription polymerase chain reaction (RT-PCR) and mass spectrometry (Lu et al, 2008; Qiu et al, 2008; Lapillonne et al, 2010; Dias et al, 2011; Kobari et al, 2012) that generally reveals a low level of beta globin expression. This approach however does not permit single cell analysis to define discrete sub-populations that would be expected from multiple waves of haemopoiesis.
Here we describe the haematopoietic differentiation from various hiPSC lines, generated in a variety of ways. We describe the formation of a multi-potent CD34+ haemato-endothelial progenitor that can undergo definitive erythropoiesis, where arising erythroblasts express fetal haemoglobin (HbF) and adult haemoglobin (HbA). Notably, expression profiles assessed by flow cytometry revealed that hiPSC-derived erythroblasts have a highly similar pattern to that observed from cultured cord blood CD34+-derived erythroblasts, where erythroblasts express HbF, with a sub-population of HbF+HbA+ cells. We also describe defined conditions that permit the isolation of a CD31+ CD45+ EMP, and show that in extended culture on OP9 stroma, that erythroblasts can exhibit enhanced co-expression of both HbF and HbA. We also present karyotypic and genetic analysis of hiPSC lines, and discuss potential safety considerations from acquired genetic abnormalities in these cells.
Materials and methods
Derivation and culture of human iPS cells
Erythroid differentiation was studied with hiPSC lines derived from a variety of sources. We used previously reported hiPSC lines derived from human adult fibroblasts, and new hiPSC lines derived from cord blood and adult peripheral blood mononuclear cells, to assess the effects of source and reprogramming strategy on erythroid development. Fibroblast sources of hiPSCs (C19) were generated using retrovirus essentially as previously reported (Takahashi et al, 2007) and described by us elsewhere (Carpenter et al, 2011). HiPSCs derived from cord blood mononuclear cells or erythroblasts were designated OC1 and CE1 respectively, from cord blood CD34+ cells were designated O3·1–O3·3, and from adult peripheral mononuclear cells designated OPM2. Cord blood and adult peripheral blood was collected anonymously, with informed written consent, following ethics approval by the Oxford and Berkshire Research Ethics Committee and with Institutional R&D approval. All blood cells were reprogrammed using OriP plasmids (pEP4EO2SCK2MEN2L and pEP4EO2SET2K) from Addgene (Cambridge, MA, USA) as previously reported (Yu et al, 2009) where the OPM2 line was generated without feeders, as described also by Yu et al (2011).
Previously we have described hiPSC characterization and routine passage (Carpenter et al, 2011) whilst we also assessed pluripotency by teratoma formation, after transplantation of 1 × 106 cells in 30% Matrigel™, under the testicular capsule of anaesthetized non-obese diabetic severe combined immunodeficiency (NOD SCID) mice. After 21 d, teratomas were recovered, fixed, sectioned and stained with haematoxylin and eosin, using standard immuno-histochemical practice, prior to identification of tissues by microscopy.
Molecular karyotyping using SNP microarrays
For each sample, 200 ng DNA was hybridized to a CytoSNP-12 v2.1 microarray following the manufacturer's recommended guidelines (Illumina Inc., San Diego, CA, USA). Data analyses were performed using GenomeStudio V2011.1 (Illumina Inc.) and Nexus v6.1 Discovery Edition (BioDiscovery, Hawthorne, CA, USA). Please see Supplemental Methods for assessment criteria.
Induction of haemopoietic differentiation of hiPSCs upon co-culture with OP9 stroma
Here, we co-cultured all hiPSC lines on OP9 stroma, as originally described for hES cells (Vodyanik et al, 2005) and hiPSCs (Carpenter et al, 2011). After 10 d of co-culture with OP9 stroma, we harvested the differentiated hiPSCs cells by 20 min digestion with collagenase Type IV (1 mg/ml) and further trypsinization (0·05% for 10 min at 37°C). Cells were further mechanically disrupted and then washed, and CD34+ cells isolated by magnetic-activated cell separation (MACS) using CD34 microbeads (Miltenyi Biotec, Bisley, Surrey, UK). Further assessment was conducted by live cell staining for CD45, CD144 and CD31, performed in 100 μl phosphate-buffered saline (PBS) with 0·5% (w/v) bovine serum albumin (BSA; Sigma-Aldrich, Dorset, UK) with 10 μl FcR block agent (Miltenyi Biotec) on ice with 10–20 μl of relevant antibody (details of all antibody clone identification in Supplemental Methods).
Assessing the haemo-endothelial and B cell lymphoid potential across hiPSC lines on MS5 stroma
Briefly we used MS5 stroma (obtained from Professor Igor Slukvin, University of Wisconsin, Madison, WI, USA) as described previously (Carpenter et al, 2011) and in Supplemental Methods. For B cell lymphopoiesis, cells were supplemented with IL7 (20 ng/ml), IL3 (10 ng/ml), SCF (50 ng/ml), and FLT3L 50 ng/ml (all from R&D Systems, Abingdon, UK). For erythropoiesis, cells were supplemented with SCF (50 ng/ml), EPO (3 u/ml), IGF1 (40 ng/ml), IL3 (10 ng/ml) and dexamethasone (1 μmol/l). Cells were then either stained by immunohistochemistry for the presence of endothelial networks using anti-CD146 antibody (AP conjugated) or cells were harvested by collagenase and trypsin digestion as above, prior to staining with the relevant conjugated antibodies, CD45-allophycocyanin (APC) and CD10-phycoerythrin (PE), CD71-PE, CD235a-APC and anti-HLA Class I antibody (all from BD Biosciences, Oxford, UK), and (CD19-peridinin chlorophyll cyanin 5·5 (PerCPCy5·5) and fluorescein isothicyanate (FITC) from eBiosciences, prior to analysis by flow cytometry (using an LSR II from BD Biosciences).
Assessing HbF and HbA haemoglobin expression profiles across hiPSC-derived erythroblasts
CD34+ multi-lineage progenitors were first isolated on OP9 stroma as described above, and then subjected to a differentiation strategy based on that described for suspension cultures (England et al, 2011). After MACS separation, CD34+ cells were introduced to either suspension culture in StemSpan (Stem Cell Technologies, Vancouver, BC, Canada) with SCF (50 ng/ml), EPO (3 u/ml), IGF1 (40 ng/ml), IL3 (10 ng/ml) and dexamethasone (1 μmol/l). Cells were harvested on day 14 and freshly stained with CD235a-APC and 4′,6-diamidino-2-phenylindole (DAPI) (1 μg/ml) and, after three washes in PBS, fixed in 4% (w/v) paraformaldehyde and permeabilized prior to staining with 2·5 μl anti-alpha globin antibody-FITC conjugate (ab19191; Abcam, Cambridge, UK), 2·5 μl anti-HbF-PE conjugate (clone 2D12; BD Biosciences), or anti-HbA (500 ng) (clone 4B8; Perkin Elmer, Cambridge, UK) co-incubated with 1 μg of anti-IgG2b-Alex488 (Invitrogen, Life Technologies Corporation, Paisley, UK). Experiments were repeated three times across four hiPSC cell lines, with mean data from HbF+/HbA− and HbF+/HbA+ populations presented. Cellular controls included fresh blood from cord blood and adult peripheral blood, and mononuclear cells cultured in parallel with hiPSC-derived CD34+ cells.
Assessment of erythroid potential of hiPSCs differentiated in defined conditions
hiPSC lines were grown to high density in mTeSR media, prior to differentiation as described previously (Carpenter et al, 2012), with additional supplementation of VEGF (10 ng/ml) from day 4–8, and SCF (100 ng/ml), FLT3L (100 ng/ml) TPO (20 ng/ml), IL3 (10 ng/ml) and IL6 (10 ng/ml) from day 8–12 (all from R & D Systems). The presence of CD31+ and CD45+ cells on day 14, designated EMPs, was confirmed by live cell immuno-histochemistry using anti-CD31-PE conjugate (50 μl/ml) (Miltenyi Biotec) and anti-CD45-488 conjugated antibodies (10 μl/ml) (Invitrogen) and fluorescence microscopy. Fluorescence-activated cell sorting (FACS) analysis was used to confirm dual positive CD31+ CD45+. CD31+ progenitors were isolated from day 12 cultures by MACS separation, and erythropoiesis was induced for a further 14 d by re-plating onto OP9 stroma in alpha-minimal essential medium with 10% fetal calf serum (FCS) and supplemented with SCF (100 ng/ml), EPO (2 u/ml), IGF1 (40 ng/ml) and dexamethasone (1 μmol/l), with half-feeds every 4 d. On day 26 of differentiation, cells were stained and FACS analysis undertaken for HbF and HbA assessment as described above.
Results
hiPSC derivation and characterization
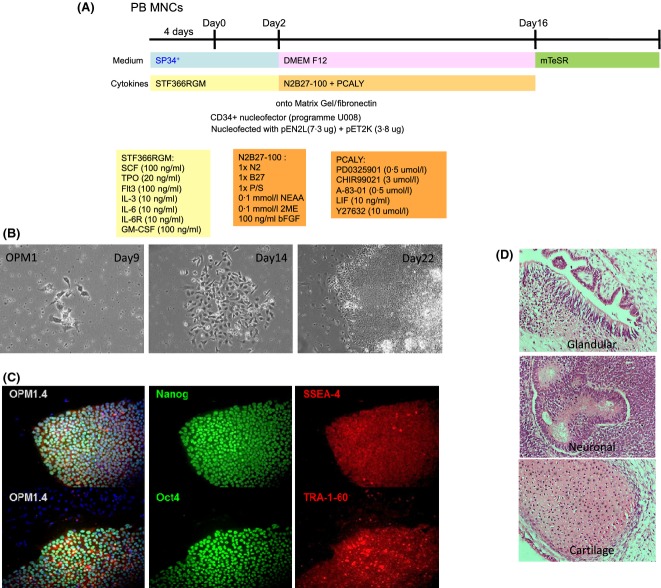
Derivation of multiple hiPSC lines was undertaken for a systematic assessment of erythropoiesis and to demonstrate expression of HbF and HbA at a single cell level. We used previously reported lines (C18 and C19) derived from human adult fibroblasts (Carpenter et al, 2011), and new lines using OriP plasmid vectors (Yu et al, 2009) to assess whether erythropoiesis was more or less efficient between approaches. New lines were derived from cord blood mononuclear cells (OC1) or cord erythroblasts (CE1) after 7 d expansion in conditions that promote erythroid growth. The reprogramming regime is included for lines generated with OriP vectors (Fig1A). We also reprogrammed adult peripheral blood mononuclear cells in the absence of feeders (OPM2). In all conditions tested, hiPSC colonies were apparent from day 9 onwards, and this was especially obvious in chemically defined conditions because adherent cells were easily identified (Fig1B). Colonies were picked from day 21 onwards and expanded as lines using mTeSR media without feeders. By passage 6, the generated lines were tested for the ES-specific antigens SSEA4, TRA-1-60, Oct4 and Nanog. The peripheral blood-derived hiPSC line OPM2, is shown as a representative example (Fig1C). All selected lines were demonstrated to be pluripotent, and form teratomas in vivo (Fig1D).
Figure 1.
Reprogramming of human hiPSC lines from various sources (fibroblasts and blood) using retrovirus and plasmids. (A) Generation of hiPSC lines using OriP episomal plasmids with a small molecule approach that is independent of mouse embryo fibroblast feeders. (B) hiPS colonies can be observed within 14 d, shown as bright field images on Day 9 and 14. A typical hiPSC/embryonic stem cell morphology (compact colonies and high nucleus-cytoplasm ratio) can be observed by day 22. (C) Once hiPSC lines were established, these were then confirmed to express stem cells markers, Nanog, Oct4, SSEA4 and TRA-1-60. (D) hiPSC lines were shown to be pluripotent, as demonstrated by teratoma formation in vivo and contribution to the three germ layers: glandular (endoderm) neuronal (neuro-ectoderm) and cartilage (mesoderm) tissues. hiPSC, human induced pluripotent stem cells; PB MNCs, peripheral blood mononuclear cells.
Molecular karyotyping and genetic analysis
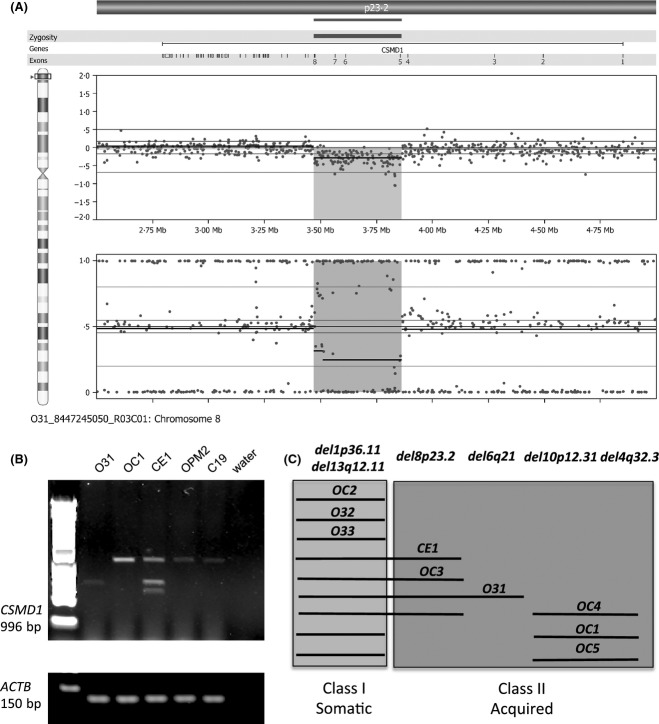
When analysed by G banding, all lines that were tested were cytogenetically normal, except for C18, which carried a balanced reciprocal translocation and was not considered further. Molecular analysis using the CytoSNP-12 v2.1 microarray (Illumina Inc), showed that all of the assessed cell lines harboured a subset of copy number variants (CNVs) that have been noted previously in the Database of Genomic Variants (http://dgv.tcag.ca/dgv/app/home). In addition, we identified genomic rearrangements in six of the nine cell lines originating from the same donor. These acquired (Class II) rearrangements ranged in size from a single exon deletion of PLXDC1 (validated by direct sequencing, in three of the nine cell lines, data not shown) to a mosaic 8·2 Mb region of copy neutral loss of heterozygosity (cnLOH) involving chromosome bands 17p13.1-13.3 (see Table S1). Of particular note were copy number (CN) losses involving the large CSMD1 gene, seen in four of the nine cell lines. Here we show a representation of the CNV within the CSMD1 gene (Fig2A) and demonstrate its effect on transcript size across hiPSC lines. O31 (not used later in erythroid differentiation studies) and CE1 were shown to have truncated transcripts as resolved by RT-PCR and agarose electrophoresis (Fig2B). OC1 and OPM2 represent controls. Figure2C shows in brief the various CNV mutations observed in lines derived from a single donor, where Class I (somatic) and Class II (acquired) mutations can be identified. A more complete list documenting CN and cnLOH events of CNVs is given in Table SI.
Figure 2.
Genomic analysis reveals common copy number variations across hiPSC-derived lines. Two hundred nanograms of DNA was hybridized to a CytoSNP-12 v2.1 and data analyses was performed using GenomeStudio V2011.1 and Nexus v6.1. A representation for the copy number variant occurring at del8p23.2 is shown in A, which was confirmed by genomic polymerase chain reaction, that revealed truncated transcripts for the CSMD genes, across several hiPSC lines (B). (C) A schematic identifying the mutations across hiPSC lines from one donor (OC, O3 and CE lines), which are common to more than one line, and indicate the presence of somatic mutations (common to all lines) and acquired or Class II mutations that arose independently during culture.
Deriving multi-lineage haemo-endothelial progenitors across hiPSC lines
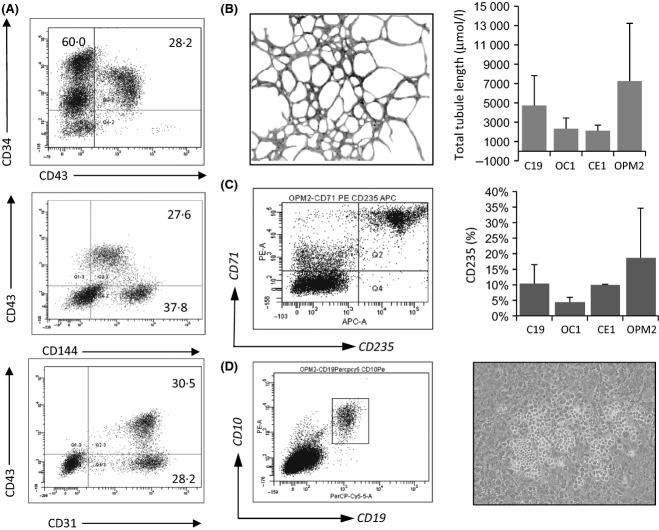
Previously we have described a system that can yield CD34+ progenitors capable of forming endothelium, erythroid cells and B lymphocytes (Carpenter et al, 2011) and so we employed this method to stringently assess haemo-endothelial potential across hiPSC lines. A haemato-endothelial sub-population that is positive for CD43, and thus committed to the haemopoietic lineage (Vodyanik et al, 2006) was identified after 10 d co-culture with OP9 stoma (Fig3A). After MACS selection of CD34+ cells, containing a CD34+CD43+ subpopulation, they were seeded onto MS5 stroma under erythroid or lymphoid conditions. All hiPSC lines tested are able to undergo multi-lineage differentiation towards endothelium (seen in all conditions) that stained positive for CD146 (Fig3B), erythroid cells that were positive for CD71 and CD235a (Fig3C) and B lymphocytes that co-expressed CD10 and CD19 (Fig3D), whilst a bright field image of B lymphocytes growing on MS5 stroma, from CE1 hiPSC, is also shown in Fig3D. Mean data for contribution to these lineages is also provided to demonstrate that all lines tested can give rise to red blood cells and endothelium. Mean data for B cell differentiation is extremely robust for hiPSC line C19 (at 3·4% SEM 0·33 n = 6), and B cell differentiation was confirmed for CE1 (at 1·4% n = 1) and OPM2 lines (at 2·5% n = 2). A brightfield image in Fig3D demonstrates typical morphology of B lymphocytes within the MS5 stroma.
Figure 3.
Multi-lineage haemopoietic differentiation of hiPSC lines upon OP9 co-culture. (A) Human induced pluripotent stem cells (hiPSC) lines from a variety of sources were assessed for their haemopoietic potential. hiPSC colonies were harvested and co-cultured on OP9 stroma for 10 d. Haemopoietic progeny arising after 10 d co-culture with OP9 were harvested by collagenase/trypsin treatment and analysed for the appearance of markers typical of haemogenic endothelium committed to the haemopoietic lineage that includes staining for CD34+ with CD43+, and CD43+ with CD144+ and CD31+. CD34+ cells were isolated by magnetic-activated cell separation, and seeded onto MS5 feeders and exposed to either (B) CD146+ networks, (C) lymphoid (SCF, Flt3L, IL7 and IL3) or (D) erythroid (SCF, EPO, IGF, IL3 and dexamethasone) conditions for a further 21 d. Here we see the appearance of either CD19+CD10+ B lymphocytes or CD71+ CD235a+ erythroblasts, as well as CD146+ endothelial networks, indicating that all hiPSC lines differentiate into a multi-lineage progenitor that is capable of haemato-endothelial differentiation. Mean data are provided in the far right panels where n = 2–3, and standard deviation is shown.
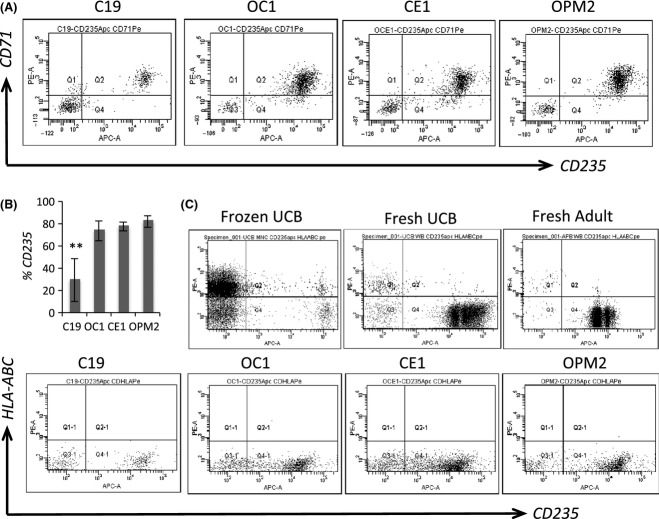
Erythropoiesis across hiPSC lines derived
To rigorously assess erythropoiesis across hiPSC lines, we first characterized general phenotypic features after 10 d differentiation on OP9 stroma and 14 d in erythroid conditions. Differentiated hiPSC lines co-expressed CD71 and CD235a (Glycophorin A) (Fig4A) and have comparable levels of purity (Fig4B), although hiPSCs from fibroblasts (C19) may underperform in this assay, contributing to a lower proportion of CD235a+CD71+ erythroblasts when compared to blood-derived hiPSCs. Most notably for diagnostics and applications in transfusion medicine, is that when compared to normal cellular counterparts, umbilical cord blood (UCB) and adult peripheral blood, Class I HLA expression was down-regulated, on iPS-derived erythroblasts, but not on CD235a− mononuclear cells, and this was seen across all lines tested (Fig4C). We also demonstrated that hiPSC-derived erythroblasts express a range of surface antigens associated with red cells, and at comparable levels to fresh UCB and adult peripheral blood (Supplemental 2 Figure S1b), except for CD71, which remained high. Erythroblasts from both CD34-derived and hiPSC-derived sources were heterogenous and remained nucleated, as revealed by Leishman's stain (Supplemental 2 Figure S1b).
Figure 4.
Systematic analysis reveals efficient erythropoiesis across hiPSC lines, and down regulation of Class I HLA. Here we describe erythropoiesis across all human induced pluripotent stem cells (hiPSC) lines, from CD34+ multi-lineage progenitors (from OP9 co-culture), differentiated in liquid culture in StemSpan medium with EPO, SCF, IGF-1, and dexamethasone. Efficient erythropoiesis was demonstrated (A and B) for OC1-, OCE1-, and OPM2-derived erythroblasts that reached nearly 80% purity. Populations from C19 had a significantly lower proportion of CD235a+ erythroblasts. When assessed for Class I HLA down-regulation (C), across lines, Class I HLA expression was low on CD235a+ erythroblasts, and in line with that seen for cellular controls, frozen umbilical cord blood (UCB), fresh UCB and fresh adult peripheral blood.
Of particular interest in this study, is whether erythroblasts from different hiPSC sources express HbF and HbA. We assessed the globin expression profiles of erythroblasts from hiPSC lines, compared to relevant controls, using the nCounter Analysis System (Nanostring Technologies, Seattle, WA, USA) where individual mRNA molecules can be visualized and counted directly. Quantitative data for human globin expression is provided in Supplemental 2, Table S2, which highlights high-level expression of ζ and ε globin (embryonic) as well as αand γ globin (fetal) for hiPSC-derived RBCs expanded in StemPro34, whilst β globin (adult) expression is low at 1–2% of UCB CD34-derived RBC expression levels.
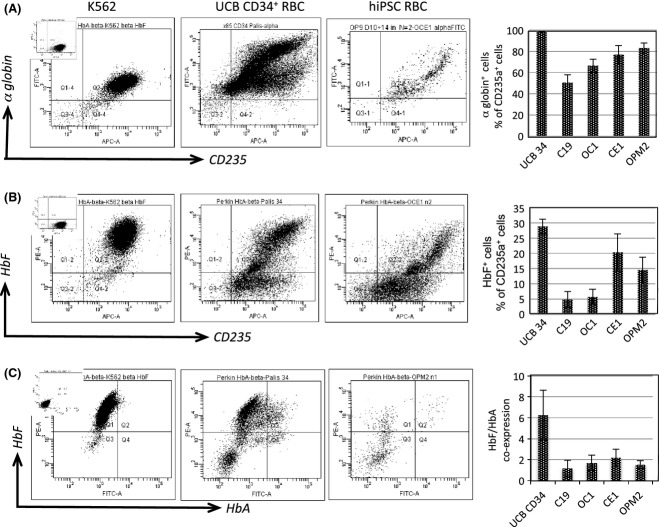
To resolve globin expression profiles at a single cell level, and more specifically that of fetal and adult haemoglobin, we firstly assessed α globin expression against CD235a, and observed that a majority of erythroblasts express high levels of α globin (50–80% depending on lines) and have a very similar pattern of staining to cultured UCB CD34+-derived erythroblasts (Fig5A). Next we wanted to determine the HbF expression profile of hiPSC-derived erythroblasts, plotted against CD235a (Glycophorin A). Here (Fig5B) we found that staining profile between cultured UCB-derived CD34+ cells and hiPSC-derived erythroblasts was similar, although there was a lower proportion of cells double positive for CD235a and HbF in the hiPSC samples (5–20%) compared with the control UCB CD34+ (29·4 ± 5·1%). Of particular note was the high level of HbF expression in UCB CD34+-derived erythroblasts, which suggests a bias towards expression of HbF, during in-vitro culture.
Figure 5.
hiPSCs give rise to erythroblasts that express alpha globin as HbF and HbA. (A) Representative plots for α globin staining against CD235a, from erythroblasts expanded in liquid culture from CD34+ multi-lineage progenitors cultured on OP9 stroma. Gating initially on live (DAPI−) cells, staining was observed in K562 cells (left panel), UCB CD34+-derived RBCs (middle panel) and hiPSC-derived RBCs (right panel). Mean data [n = 3 ± standard error of the mean (SEM)] are shown also (far right panel). We observed distinct and separate populations arising for both UCB CD34+-derived and hiPSC-derived erythroblasts. When analysed for both (A) alpha globin and (B) HbF with CD235a we saw a distinct sub-population that was strongly positive for α globin and to a lesser extent, positive for HbF+ (5–20%) across lines, but which was similar to that seen for cultured UCB CD34+-derived erythroblasts. Mean data across hiPSCs lines (far right panel) is shown to confirm that a HbF+ CD235a+ sub-population was formed in all lines tested. (C) When assessed using antibodies specific to HbF and HbA, controls had a range of expression patterns, with K562 and UCB CD34+-derived RBCs being mainly HbF+HbA− with a small sub-population being HbF+HbA+ (which typically accounted for 2–5% of cells). When hiPSC-derived erythroblasts were assessed, again there was a range of HbF and HbA expression at around 1–2%, in a highly heterogeneous manner, but which mirrors that observed for cultured UCB CD34+-derived erythroblasts. The globin expression panel (far right) shows mean data (n = 3 ± SEM) of HbF/HbA co-expression as a% of total CD235a+ DAPI− cells. hiPSC, human induced pluripotent stem cells; UCB, umbilical cord blood; RBC, red blood cells.
Additional experiments were aimed at assessing adult or HbA globin expression, as a further indicator for definitive erythropoiesis, and to identify a potentially better source of erythroblasts for therapeutic applications. To accurately monitor HbF and HbA co-expression by flow cytometry, we firstly generated a stringent gating strategy developed using appropriate cellular controls (Fig5). Here we used fresh cord blood and peripheral blood as positive controls for HbA expression, and K562 erythro-myeloid cells as a negative control. HbF however, was high in K562 cells, with a minor population evident in adult and cord blood as described previously (Prus & Fibach, 2013). When these criteria were applied to assess erythroblasts across hiPSC lines, co-expression of HbF and HbA was seen in a small sub-population of hiPSCs (1–2%), in a manner that is highly heterogeneous, but in this sub-population βglobin expression was substantially higher than negative fractions, and mirrored that observed for cord blood CD34+-derived erythroblasts. Levels of HbF+HbA− and HbF+HbA+ fractions are given across hiPSC lines from three independent experiments.
Demonstrating definitive erythropoiesis in defined conditions
Previously we have described an efficient protocol for directed differentiation of hiPSCs towards the cardiovascular lineage (Carpenter et al, 2012). Here we aimed to test whether this system could also be adapted to provide erythroblasts in a defined, serum and feeder-free approach. As VEGF is known to promote erythropoiesis from pluripotent sources (Cerdan et al, 2004) we tested whether the addition of VEGF (and subsequently with haematopoietic then erythroid cytokines, see Fig6A) to differentiating cardiovascular cultures could promote erythropoiesis and if the erythroblasts formed could express HbF and HbA. Figure6B shows a differentiating culture of hiPSCs and demonstrates the presence of cells co-expressing CD31+ and CD45+ that appeared to be weakly dual-positive. This was confirmed by MACS using anti-CD31 microbeads, and subsequent flow cytometry analysis (Fig6C) where the CD31+CD45+ population, considered the EMP (Palis et al, 1999; England et al, 2011; McGrath et al, 2011) is also positive for CD235a+. This can be induced four-fold by exposure to VEGF from day 4–8 (0·3667 ± 0·15 vs. 1·53 ± 0·43 n = 3).
Figure 6.
hiPSCs from a CD31+ CD45+ erythro-myeloid progenitor under defined conditions, are closely associated with the vasculature and become CD235+. (A) Schematic to describe the defined culture conditions used to derive the eryhtro-myeloid progenitors, and to then expand erythroblasts on OP9 stroma. (B) CD45+ (green) CD31+ (red) cells were closely associated with endothelial networks, suggestive of a haemogenic endothelial origin. (C) Flow cytometric demonstration that CD31+, can be purified by magnetic-activated cell separationusing anti-CD31 microbeads. (i) isotype controls, (ii) whole cell fraction, (iii) negative fraction and (iv) positive fraction, showing a clear enrichment for a CD31+ CD45+ progenitors (left panel). Furthermore, the CD31+ fraction expressed CD235a, but CD31 expression is lost upon erythroid maturation. Data shown is representative of four separate experiments. hiPSC, human induced pluripotent stem cells; DxM, dexamethasone.
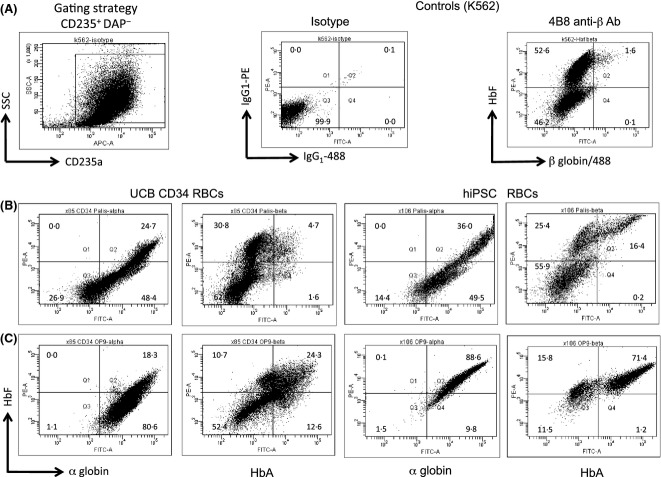
We also wanted to assess the impact of co-culture with OP9 stroma, which has been shown to induce β globin expression (Choi et al, 2012). When cells were purified by MACS using anti-CD31 beads, and co-cultured on OP9 stroma under erythroid conditions, they were shown to undergo erythropoiesis and give rise to haemoglobinized eryhthroblasts after 14 d (Fig7). When erythroblasts from defined differentiation conditions were assessed specifically for HbF and HbA expression, we observed (as for haematopoietic differentiation on OP9 stroma) that hiPSC-derived erythroblasts co-express HbF and HbA, but did so at much improved levels, whereby nearly all CD235a+ erythroblasts were HbF+ (72·9 ± 5·81%) and a significant proportion of these co-expressed HbF+ HbA+ (44·0 ± 6·04%). This was confirmed by significantly enhanced levels of HBB mRNA as determined using the Nanostring assay (Supplemental 2).
Figure 7.
hiPSC-derived erythro-myeloid progenitors can express HbF and HbA upon OP9 co-culture. (A) The gating strategy used to identify DAPI+ cells (inset) and subsequently, CD235a+ cells, for assessment of globin profile (left panel), with isotype control shown for α globin staining (middle panel) and K562 negative cellular control with HbA staining profile and gating strategy also shown (right panel). HbF and HbA expression profile by flow cytometry of CD31+ cells grown (B) in suspension culture (StemPro34 with erythroid factors) and (C) in OP9 co-culture. Compared to UCB CD34-derived RBCs, hiPSC-derived RBCs have very similar populations arising, notably that they are strongly positive for α globin expression, irrespective of culture conditions. However, there was a clear effect of co-culture on OP9 stroma (C), which promotes HbF expression over that of suspension cultures (B) particularly for hiPSC-derived RBCs to (at 72·9 ± 5·81%), Of greatest significance however is that HbA expression in hiPSC-derived RBCs is also greatly enhanced upon co-culture with OP9 (at 44·0 ± 6·04%). hiPSC, human induced pluripotent stem cells; UCB, umbilical cord blood; RBC, red blood cells; SSC, sideways scatter.
Discussion
We have demonstrated here that hiPSCs, generated from multiple different sources are capable of generating a multi-lineage haemato-endothelial progenitor, contributing towards myeloid, lymphoid and endothelial lineages. Additionally we show that hiPSCs, when analysed at a single cell level, are able to undergo definitive erythropoiesis where erythroblasts express HbF and HbA. This observation, of high expression levels of HbF and HbA in a small sub-population (1–2%) of erythroblasts from pluripotent sources, is unique in human studies. When we assessed defined conditions for definitive erythropoiesis, we observed an EMP (CD31+CD45+) that was enhanced four-fold by supplementation with VEGF, could proliferate on OP9 stroma to generate erythroblasts that co-express HbF and HbA to much higher levels (44·0 ± 6·04%) and was expressed in a manner analogous to UCB CD34-derived erythroblasts. These findings demonstrate that, irrespective of the reprogramming strategy or source of tissue (adult fibroblast versus perinatal or adult mononuclear cells), hiPSCs are suitable for multi-lineage haematopoiesis, and that definitive erythropoiesis can be reproducibly observed, using a variety of strategies and regardless of issues such as epigenetic memory (Kim et al, 2010; Stadtfeld et al, 2012). Furthermore, we show that across iPSC lines, erythroblasts lack expression of Class I HLA, which mirrors normal erythropoiesis, and has important implications both for diagnostics and transfusion medicine. Given that there is still much controversy regarding the derivation of B cells from pluripotent stem cells (Martin et al, 2008), it is of note that B cell lymphopoiesis was demonstrated from multiple hiPSCs sources.
Additionally, we have described various somatic and acquired mutations across hiPSCs lines, where some CNVs are common in hiPSC lines, as described previously (Martins-Taylor et al, 2011). Of the acquired genomic rearrangements reported here, a number of the genes involved have been associated with cancer or cell growth. For example, the 8·2 Mb region of cnLOH in a single cell line included TP53, a well known tumour suppressor gene that is mutated in the germ-line in Li-Fraumeni syndrome (Srivastava et al, 1990), and somatically in a range of other cancers, reviewed elsewhere (Hainaut & Wiman, 2009). LINC00290 lies is a region of recurrent chromosomal alterations identified in adrenocortical tumours (Letouze et al, 2012). Mutations within the CSMD1 gene investigated here have been associated with aggressive tumour formation (Scholnick & Richter, 2003; Farrell et al, 2008; Kamal et al, 2010) and it has been shown previously to have potential tumour suppressor activity (Tang et al, 2012). Although truncated in the CE line used in this study, the impact of this mutation is unclear, although it did not prevent efficient (or definitive) erythropoiesis. These findings highlight the importance of assessing hiPSC-derived tissues for genomic aberrations, however it is envisaged that efficient enucleation during terminal differentiation of erythropoiesis will help to negate any safety concerns that may arise from such somatic or acquired mutations. Of note, it has been recently shown that enucleation is possible in hiPSC-derived erythroblasts, in vivo (Kobari et al, 2012).
With regard to developmental erythropoiesis, globin expression is known to undergo changes associated with both maturational switching and lineage switching (Stamatoyannopoulos, 2005; Sankaran et al, 2010a). Whilst expression of embryonic globins (ε and ζ) and α globin predominate in primitive erythropoiesis (Fraser et al, 2007, 2010) and in pluripotent sources of erythroblasts (Lu et al, 2008; Qiu et al, 2008; Lapillonne et al, 2010; Dias et al, 2011; Kobari et al, 2012), gamma (γ) globin expression is also widely reported. Here however, γ globin is associated with a lineage switch from primitive to definitive erythropoiesis (England et al, 2011; McGrath et al, 2011). Previous reports show generally low levels of HBB transcript by RT-PCR or protein by mass spectrometry (Qiu et al, 2008; Lapillonne et al, 2010). This is unsurprising, because protocols for pluripotent stem cell differentiation generally recapitulate the primitive wave of erythropoiesis where embryonic globins are largely expressed (Lu et al, 2008). We show that by direct quantification (Nanostring Assay), that a variety of embryonic globins are expressed that include ζ and ε. However, upon assessment of single cells by FACS profiling, we were able to uniquely identify a small sub-population of erythroblasts that co-express both HbF and HbA (HbA antibody specific to β globin), therefore suggesting definitive erythropoiesis. This is entirely consistent with the first definitive wave of haemopoiesis and definitive erythropoiesis, and which results in an EMP capable of differentiating to express HbF and HbA in model systems (England et al, 2011; McGrath et al, 2011).
When we assessed defined conditions for definitive erythropoiesis, an EMP (CD31+CD45+) could be identified, which was enhanced four-fold by supplementation with VEGF. VEGF has been shown to promote endothelial expansion in such cells (Yang et al, 2008) and is also known to promote erythropoiesis (Cerdan et al, 2004). Importantly for clinical applications, this method was serum-free and feeder-free, and was highly permissive for definitive erythropoiesis, yielding CD235a+ erythroblasts that were predominantly HbF+, and that co-expressed both HbF+ and HbA+ (44·0 ± 6·04%). The observation of a CD31+CD45+ progenitor arising from the endothelial vessels within the defined culture conditions closely mirrors the formation of the EMP from yolk sac endothelium during the first definitive wave of erythropoiesis (Palis et al, 1999; England et al, 2011; McGrath et al, 2011). Improved switching of globin expression from HbF to HbA during erythroid expansion on OP9 stroma mirrors that reported by Kobari et al (2012), who demonstrated induction of HbA expression in hiPSC-derived erythroblasts in vivo. HbF expression is unlikely therefore to be a barrier to clinical use, but instead its expression may yet serve as an indicator for the definitive erythroid lineage, that may be then induced to express HbA.
In conclusion, we have demonstrated that hiPSCs lines, derived in a variety of ways, can all yield a multi-lineage haemato-endothelial progenitor that can be used to model early developmental haemopoiesis, such as B cell lymphopoiesis and erythropoiesis. We have also demonstrated formation of a EMP that can undergo definitive erythropoiesis to co-express HbF and HbA. The capacity of hiPSCs to undergo definitive erythropoiesis analogous to that observed from cord blood implies that hiPSCs could 1 d be considered for clinical use. However, there are many hurdles to be overcome before this will be widely applicable, particularly with regard to logistics of scale-up for manufacture, whilst safety concerns will also be paramount. After reprogramming of somatic cells and extensive proliferation in culture, efficient enucleation in vitro during terminal differentiation will be critical. However, as hiPSCs can mirror many aspects of early developmental haematopoiesis, they do represent a potential alternative to donor tissue for future applications in transfusion medicine.
Acknowledgments
We would like to acknowledge the support from an NHSBT project grant (LC, SMW), the OSCI (LC, SMW) and the NHSBT Trust fund (SMW, LC). AF was funded by an MRC-OSCI DPhil studentship award. The authors thank Drs Maxim Vodyanik and Igor Slukvin (University of Wisconsin, Madison, WI, USA) for the provision of OP9 cells (originally from Dr Nakano, Kyoto University, Yoshida, Japan). This article also summarizes independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programmes (Grant Reference Numbers RP-PG-0310-1001, RP-PG-0310-1003 and RP-PG-0310-1004). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. AP, SK and JT are supported by the NIHR Biomedical Research Centre, Oxford, with funding from the Department of Health's NIHR Biomedical Research Centres funding scheme and Wellcome Trust (090532/Z/09/Z).
Authorship contribution
CTY contributed towards conception and the majority of experiments. AF conducted haematopoietic differentiation and contributed B cell data. ATP, JT and SK contributed towards array experiments and data interpretation. PAG and NT contributed teratoma studies. SM conducted the Nanostring Assay. DJR, AN, SMW and LC obtained funding, reviewed and supervized the research. LC conceived the experiments, performed experiments and wrote the manuscript. All authors reviewed and approved the manuscript.
Conflicts of interest
There are no conflicts of interest to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1Extended Methods.
Table S1CNVs mapped across hiPSC lines.
Data S2Additional Methods.
Table S2. Nanostring analysis of globin mRNA.
Fig S1. Red blood cell phenotyping and Leishman's staining.
Fig S2. Negative cellular controls to indicate specific staining for alpha and gamma globin.
References
- Baron MH. Embryonic origins of mammalian hematopoiesis. Experimental Hematology. 2003;31:1160–1169. doi: 10.1016/j.exphem.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY. Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E. Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. &. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC. Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo Journal. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L, Malladi R, Yang CT, French A, Pilkington KJ, Forsey RW, Sloane-Stanley J, Silk KM, Davies TJ, Fairchild PJ, Enver T. Watt SM. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117:4008–4011. doi: 10.1182/blood-2010-08-299941. &. [DOI] [PubMed] [Google Scholar]
- Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K. Watt SM. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells and Development. 2012;21:977–986. doi: 10.1089/scd.2011.0075. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdan C, Rouleau A. Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103:2504–2512. doi: 10.1182/blood-2003-07-2563. &. [DOI] [PubMed] [Google Scholar]
- Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA. Slukvin II. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Reports. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A. Godin I. Ontogeny of the hematopoietic system. Annual Reviews of Immunology. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. &. [DOI] [PubMed] [Google Scholar]
- Cumano A, Ferraz JC, Klaine M, Di Santo JP. Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. &. [DOI] [PubMed] [Google Scholar]
- Dias J, Gumenyuk M, Kang H, Vodyanik M, Yu J, Thomson JA. Slukvin II. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells and Development. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterlen-Lievre F, Beaupain D. Martin C. Origin of erythropoietic stem cells in avian development: shift from the yolk sac to an intraembryonic site. Annals of Immunology (Paris) 1976;127:857–863. &. [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S. Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. &. [DOI] [PubMed] [Google Scholar]
- England SJ, McGrath KE, Frame JM. Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. doi: 10.1182/blood-2010-07-299743. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C, Crimm H, Meeh P, Croshaw R, Barbar T, Vandersteenhoven JJ, Butler W. Buckhaults P. Somatic mutations to CSMD1 in colorectal adenocarcinomas. Cancer Biology & Therapy. 2008;7:609–613. doi: 10.4161/cbt.7.4.5623. &. [DOI] [PubMed] [Google Scholar]
- Fraser ST, Isern J. Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ST, Isern J. Baron MH. Use of transgenic fluorescent reporter mouse lines to monitor hematopoietic and erythroid development during embryogenesis. Methods in Enzymology. 2010;476:403–427. doi: 10.1016/S0076-6879(10)76022-5. &. [DOI] [PubMed] [Google Scholar]
- Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA. Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lievre F. Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. &. [DOI] [PubMed] [Google Scholar]
- Hainaut P. Wiman KG. 30 years and a long way into p53 research. Lancet Oncology. 2009;10:913–919. doi: 10.1016/S1470-2045(09)70198-6. &. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Buck S, Chiu CH, Gage DA, Shen TL, Hendrickx AG, Gumucio DL. Goodman M. Humans and old world monkeys have similar patterns of fetal globin expression. Journal of Experimental Zoology. 2000;288:318–326. doi: 10.1002/1097-010X(20001215)288:4<318::AID-JEZ4>3.0.CO;2-0. &. [DOI] [PubMed] [Google Scholar]
- Kamal M, Shaaban AM, Zhang L, Walker C, Gray S, Thakker N, Toomes C, Speirs V. Bell SM. Loss of CSMD1 expression is associated with high tumour grade and poor survival in invasive ductal breast carcinoma. Breast Cancer Research and Treatments. 2010;121:555–563. doi: 10.1007/s10549-009-0500-4. &. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP. Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K. Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. &. [DOI] [PubMed] [Google Scholar]
- Kobari L, Yates F, Oudrhiri N, Francina A, Kiger L, Mazurier C, Rouzbeh S, El Nemer W, Hebert N, Giarratana MC, Francois S, Chapel A, Lapillonne H, Luton D, Bennaceur-Griscelli A. Douay L. Human induced pluripotent stem cells can reach complete terminal maturation: in vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica. 2012;97:1795–1803. doi: 10.3324/haematol.2011.055566. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne H, Kobari L, Mazurier C, Tropel P, Giarratana MC, Zanella-Cleon I, Kiger L, Wattenhofer-Donze M, Puccio H, Hebert N, Francina A, Andreu G, Viville S. Douay L. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letouze E, Rosati R, Komechen H, Doghman M, Marisa L, Fluck C, de Krijger RR, van Noesel MM, Mas JC, Pianovski MA, Zambetti GP, Figueiredo BC. Lalli E. SNP array profiling of childhood adrenocortical tumors reveals distinct pathways of tumorigenesis and highlights candidate driver genes. Journal of Clinical Endocrinology Metabolism. 2012;97:E1284–E1293. doi: 10.1210/jc.2012-1184. &. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT. Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SJ, Feng Q, Park JS, Vida L, Lee BS, Strausbauch M, Wettstein PJ, Honig GR. Lanza R. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Woll PS, Ni Z, Zuniga-Pflucker JC. Kaufman DS. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112:2730–2737. doi: 10.1182/blood-2008-01-133801. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Taylor K, Nisler BS, Taapken SM, Compton T, Crandall L, Montgomery KD, Lalande M. Xu RH. Recurrent copy number variations in human induced pluripotent stem cells. Nature Biotechnology. 2011;29:488–491. doi: 10.1038/nbt.1890. &. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, Bulger M. Palis J. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. doi: 10.1182/blood-2010-12-325357. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J. Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Experimental Hematology. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. &. [DOI] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C. Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. &. [DOI] [PubMed] [Google Scholar]
- Palis J, Chan RJ, Koniski A, Patel R, Starr M. Yoder MC. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4528–4533. doi: 10.1073/pnas.071002398. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW. Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2007;451:141–146. doi: 10.1038/nature06534. &. [DOI] [PubMed] [Google Scholar]
- Peschle C, Mavilio F, Care A, Migliaccio G, Migliaccio AR, Salvo G, Samoggia P, Petti S, Guerriero R, Marinucci M, Lazzaro D, Russo G. Masterroberardino G. Haemoglobin switching in human embryos: asynchrony of zeta – alpha and epsilon – gamma-globin switches in primitive and definite erythropoietic lineage. Nature. 1985;313:235–238. doi: 10.1038/313235a0. &. [DOI] [PubMed] [Google Scholar]
- Prus E. Fibach E. Heterogeneity of F cells in beta-thalassemia. Transfusion. 2013;53:499–504. doi: 10.1111/j.1537-2995.2012.03769.x. &. [DOI] [PubMed] [Google Scholar]
- Qiu C, Olivier EN, Velho M. Bouhassira EE. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Xu J. Orkin SH. Advances in the understanding of haemoglobin switching. British Journal of Haematology. 2010a;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Xu J. Orkin SH. Transcriptional silencing of fetal hemoglobin by BCL11A. Annals of the New York Academy of Sciences. 2010b;1202:64–68. doi: 10.1111/j.1749-6632.2010.05574.x. &. [DOI] [PubMed] [Google Scholar]
- Scholnick SB. Richter TM. The role of CSMD1 in head and neck carcinogenesis. Genes Chromosomes Cancer. 2003;38:281–283. doi: 10.1002/gcc.10279. &. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Zou ZQ, Pirollo K, Blattner W. Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348:747–749. doi: 10.1038/348747a0. &. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh RM, Chen T, Ooi SS, Kim SY, Bestor TH, Shioda T, Park PJ. Hochedlinger K. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nature Genetics. 2012;44:398–405. doi: 10.1038/ng.1110. &, S391-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Experimental Hematology. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. &. [DOI] [PubMed] [Google Scholar]
- Tang MR, Wang YX, Guo S, Han SY. Wang D. CSMD1 exhibits antitumor activity in A375 melanoma cells through activation of the Smad pathway. Apoptosis. 2012;17:927–937. doi: 10.1007/s10495-012-0727-0. &. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Bork JA, Thomson JA. Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. &. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Thomson JA. Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles MV. Keller G. Multiple haemopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. &. [DOI] [PubMed] [Google Scholar]
- Wood WG. Stamatoyannopoulos G. Globin synthesis during erythroid cell maturation in alpha thalassemia. Hemoglobin. 1976;1:135–151. doi: 10.3109/03630267608991676. &. [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ. Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. &. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K. Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH. Yoder MC. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. &. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II. Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chau KF, Vodyanik MA, Jiang J. Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS ONE. 2011;6:e17557. doi: 10.1371/journal.pone.0017557. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1Extended Methods.
Table S1CNVs mapped across hiPSC lines.
Data S2Additional Methods.
Table S2. Nanostring analysis of globin mRNA.
Fig S1. Red blood cell phenotyping and Leishman's staining.
Fig S2. Negative cellular controls to indicate specific staining for alpha and gamma globin.