Abstract
The pathogenic fungus, Cryptococcus neoformans, is known to undergo phenotypic variation, which affects its virulence in the host. Recent investigations on C. neoformans cells in humans have validated the concept that phenotypic variation is present and relevant for the outcome of chronic cryptococcosis. The C. neoformans capsule is not the only trait that varies among strains. An emerging variant is the “old cell phenotype” generated when C. neoformans undergoes replicative aging. This phenotype, which other than larger size also exhibits a thickened cell wall, inhibits phagocytosis and killing by antifungals in vitro. In concert with the finding that old cells accumulate in vivo, this emergent trait could have significant impact on cryptococcal virulence and infection, and contribute to treatment failure.
Keywords: aging, C. neoformans, pathogen, phenotypic variation, virulence
Fungi, such as Saccharomyces cerevisiae and Schizosaccharomyces pombe have been invaluable model organisms in the study of aging [1–5], a field that has been far removed from fungal pathogenesis until recently. Recent studies in the fungal pathogens, Candida albicans and Cryptococcus neoformans, have revealed that aging increases phenotypic variation within the pathogen population as it expands in the host environment over time [6–9]. Old C. neoformans cells have been shown to be biologically advantageous in vitro compared with young cells [6,8] and could constitute an unanticipated phenotypic variant that could potentially alter the virulence of C. neoformans during infection. This interesting phenomenon could be relevant to other eukaryotic and some prokaryotic pathogens where asymmetrical aging occurs, and where phenotypic variation emerges during host pathogen interaction.
Capsule induction is a major determinant of phenotypic variation
Several studies have demonstrated that during chronic infection with C. neoformans, phenotypic variants emerge [10–14]. Such ‘microevolution’ has been documented in serial isolates [15,16] and experimental murine infection [17]. The most thoroughly investigated phenotypic trait is capsule size, which can be variable among strains and even within a cryptococcal population. Older studies have inversely correlated capsule volume and induction [18] with phagocytosis indices [19] and variable antibody binding [20]. Capsule growth in C. neoformans is tightly coordinated with cell cycle progression [21]. Accordingly, mutants of a G1-type cyclin, Cln1 [22] that exhibit a longer G1 phase also produce a larger capsule. Capsule size is regulated by several transcription factors, including Ada2, Rim101, and Gat201 [23,24]. In addition, capsule sizes can vary and depend on the microenvironment of infection. Polysaccharide capsules are more induced in C. neoformans residing in the lung compared with the yeast found in the brain environment [25,26]. Size matters as successful phagocytosis is important for the ability of C. neoformans to transmigrate across the blood–brain barrier, and capsular changes have been documented to affect dissemination to the brain [27,28]. Consistent with that view, a recent large phenotypic analysis of C. neoformans strains derived from cerebrospinal fluids (CSF) of humans with chronic cryptococcal meningitis determined that strains with high levels of fungal uptake by macrophages in vitro were associated with higher CSF fungal burden and decreased long-term patient survival. Interestingly, high-uptake strains were also hypocapsular and exhibited greater laccase activity and increased survival ex vivo in purified CSF [29].
Cell size independent of capsule size generates phenotypic variants
It must be noted that cell size variation is not only dependent on capsule induction but can also occur when the cell body size varies [6]. In fact, C. neoformans cells with a variable range of cell sizes have been observed in murine infection studies [30]. Recent investigations focused on replicative aging in cryptococcal populations have shown that size variation also occurs during the process of replicative aging and can be observed in chronic rat and human cryptococcosis [8]. The cell size increase seen during aging proportionally affects the capsule and the cell body [31], and thus is not only due to an over-induced capsule. Size increase from replicative aging has been observed in other fungi as well [6,9]. It remains questionable whether cell size is truly predetermined and correlated with overall life span as this was concluded from investigations done with a S. cerevisiae mutant collection [32]. There it was found that the smaller the cell size at birth, the longer the replicative life span (RLS) of the mutant (greater number of replications per cell). A study done in our laboratory with 18 C. neoformans strains did not validate that finding and found no correlation with cell body size and life span (Figure 1A). Interestingly, this study indicated that cell size at death appeared to somewhat correlate with life span (Figure 1B). In Mycobacterium smegmatis, a rod-shaped bacterium that replicates asymmetrically, birth and elongation rates also did not correlate [33]. Birth size may thus not limit life span in pathogens per se and underlie different selection pressures, especially in facultative intracellular pathogens like C. neoformans [34–38].
Figure 1. Birth size of Cryptococcus neoformans cells from various clinical isolates does not strongly correlate with the cell’s replicative life span (RLS).
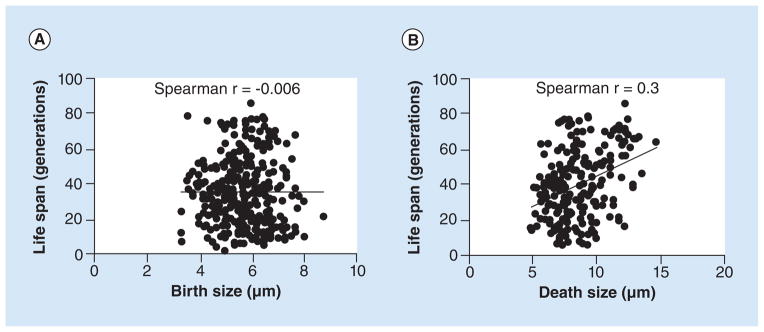
(A) The size at birth of 359 C. neoformans cells did not correlate with their respective median RLSs (Spearman’s r = −0.006). (B) Death size of 235 C. neoformans cells appeared to correlate with their respective median RLSs (Spearman’s r = 0.3).
Titan cells
Murine infection studies described yet another cell phenotype, namely large-bodied cells termed ‘titan cells’, which are much larger than old cells that emerge in the process of replicative aging [6,8,39]. Titan cells can occur within 24 h of pulmonary murine infection [40], whereas old cells occur after weeks of meningeal rat infection [8]. Titan cells grow to cells that are 5–10 times larger than the cell size of the inoculum and demonstrate larger capsules [40]. Both titan [39,40] and old cells [8] are either not at all or not easily phagocytosed by macrophages, and show increased resistance to oxidative stress. Notably, titan cells are polyploid, and it is not clear at this point whether old cells are polyploid as well. Interestingly, genome duplication can occur in fungi without cell division as observed in S. cerevisiae [41]. Regardless of the differences in these cellular morphologies, both types may be important to cryptococcal disease, depending on the time and site of infection. Titan cells likely result in phenotypic variants early on, whereas old cells require many replications and can only emerge over time.
The thickness of the cell wall can increase phenotypic variability
Cell walls of fungi are important defense barriers and also targets of antifungal medication. They contain immunologically relevant epitopes, and therefore their components are vital to immune responses, and importantly, can also contribute to altered virulence [42–46]. A less studied aspect of the C. neoformans cell wall is the generation of bud scars, which are left on the mother cell after a bud separates [47]. In aging S. cerevisiae, the cell wall becomes weakened with the accumulation of these bud scars [48]. By contrast, in aging C. neoformans, bud scars heal as the cell wall is rearranged during budding [49,50]. In fact, the cell wall has been documented to thicken with age, and its thickness can permit differentiation between old mother cells and their young daughter cells (Figure 2). A thickened cell wall in older cells could conceivably explain a lower effective fungicidal activity (EFA <0.5 log), which is observed in some cryptococcosis patients that are treated with antifungals and correlates with poor outcome [51,52]. Particularly, the thickened cell wall may keep polyenes and azoles from interacting with ergosterol in the fungal cell membrane, and echinocandins from interacting with glucans in the fungal cell wall. In fact, old C. neoformans cells show increased resistance to the polyene amphotericin B (AMB) and the azole fluconazole as demonstrated in time killing assays [6,8], which are not dependent on growth. Similar resistance was observed in old C. albicans cells when exposed to variable concentrations of the echinocandin caspofungin [6]. Already in C. neoformans cells that have undergone only 10 replications, killing assays have demonstrated enhanced resistance [6,8]. This finding in combination with the fact that AMB has poor penetration in the central nervous system warrants more studies directed toward drug resistance in C. neoformans. Here, it is noteworthy to acknowledge that phenotypic differences, such as cell wall thickness, between young and old cells would not be detected with in vitro minimum inhibitory concentration assays, which are dependent on growth. These assays are performed with young exponentially growing pathogen populations [53] and present selection pressures that are very different from fungal cells grown over weeks and months in vivo [8].
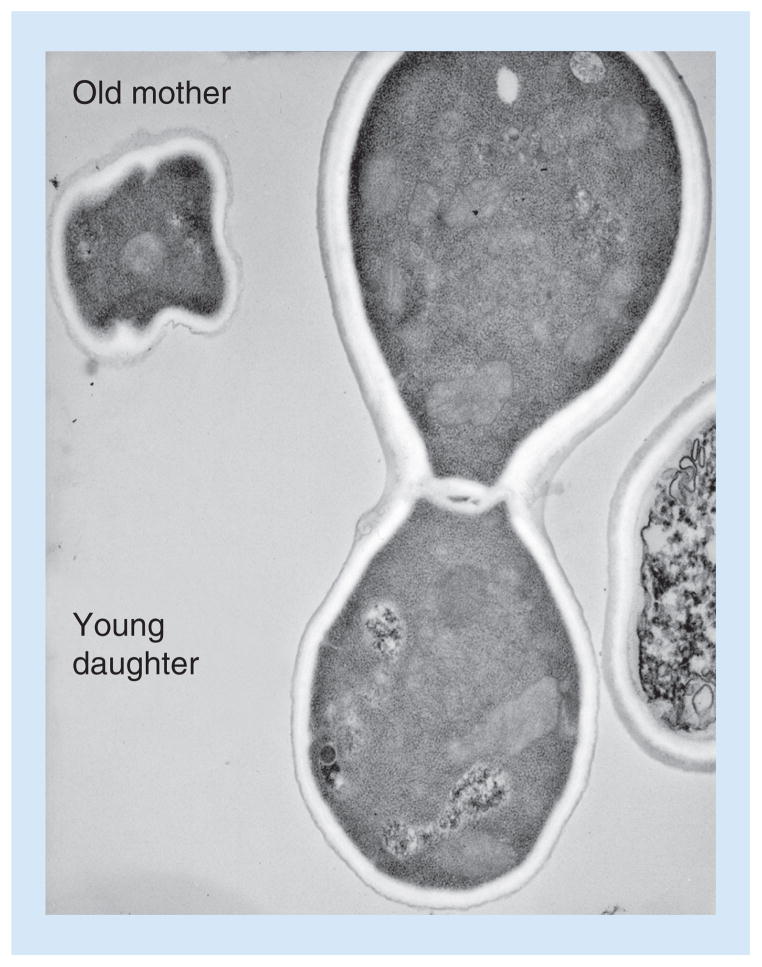
Figure 2. The thickness of the Cryptococcus neoformans cell wall increases with division.
A representative image showing that the cell wall of an old C. neoformans mother cell is relatively thicker than its young daughter cell.
Phenotypic switching elicits hypervirulent variants of C. neoformans
Phenotypic switching is defined as the spontaneous emergence of colonies that have an altered colony morphology [54], and this phenomenon is observed at a higher frequency than somatic mutation and can revert to the unaltered or parent type [53,55–57]. Therefore, phenotypic switching constitutes a controlled epigenetically driven process that allows C. neoformans to change ‘phenotypes’ without the risk of mutation. Phenotypic switch variants exhibit enhanced virulence in murine infection models and, therefore, are selected in the host environment [14,56–60]. This is not unique to C. neoformans, and in fact, phenotypic switch variants in C. glabrata also similarly exhibit enhanced virulence [61,62].
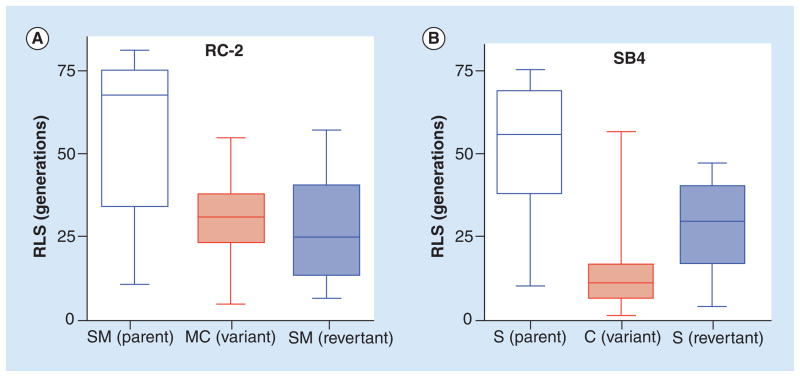
One interesting observation is that hypervirulent switch variants, which result from phenotypic switching, appear to exhibit a shortened replicative life span [8]. Specifically, this means that phenotypic switching results in a significant loss of the average number of total replications that the switch variant can undergo when compared with the parent (Figure 3). This finding underscores the fact that life span is regulated and not fixed and that phenotypic switching may be epigenetically linked to replicative aging. Also aging of yeast cells promotes an increased rate of switching to hypervirulent variants in C. neoformans [6]. Most likely, this is the result of age-induced genomic instability [63,64], which may also affect heteroresistance [65] and chromosomal loss [66–68], both of which have been shown to influence cryptococcal virulence. Therefore, hypervirulent variants are by no means the predominant mechanism of generating phenotypic variants.
Figure 3. Replicative life span appears to be regulated in phenotypic switch variants.
(A) RLS of the hypervirulent variant (MC) was significantly shortened compared with the parent type (SM) of a serotype D strain RC-2. This RLS was not recovered when MC was reverted back to SM. (B) RLS of the hypervirulent variant (C) was significantly shortened compared with the parent type (S) of a serotype A strain SB4. This RLS was not recovered when C was reverted to S.
C: Serrated; MC: Mucoid; RLS: Replicative life span; S: Smooth; SM: Smooth.
Selection of phenotypic variants & cryptococcal cells with the ‘old phenotype’ (cells of advanced replicative age)
C. neoformans switch variants, such as the hyper-virulent mucoid variant that arises from smooth parent cells, affect a small proportion of the pathogen population. However, novel phenotypes can be very stable, are thus inherited by progeny yeast cells, and selected in the setting of the host environment. Specifically, it is the significant changes in the capsular polysaccharide that affect viscosity and biophysical characteristics of the polysaccharide in these variants and confer an advantage while promoting their selection [6]. In fact, despite slower doubling times that are observed both in older cells [8] and in hypervirulent switch variants [14,59], these variants still persist and dominate the pathogen population [6,56]. These doubling time differences hint at the fact that rapid reproduction might be traded in fungi for other advantages. In S. cerevisiae, mutations that extend life span have been shown to cause defects in reproduction and fitness [69]. Unlike the switch variants, the trait of being old is not passed on to the progenies, which are young with every replication. The exception to this are long-lived S. cerevisiae mutants where the life span is inherited [69], or extremely old cells that give rise to progeny with a 30% reduced life span [70]. Therefore, the fact that old cells are observed during chronic infection suggests that immense selection pressures that kill off the predominantly young population are operative in the host environment. Data suggest that host immune cells, which include macrophages, as well as anti-fungal treatment, such as AMB, constitute some of the selection pressures in vivo [8].
Conclusion & future perspective
The ability of C. neoformans to generate phenotypic variants could help explain differences in the outcome of infection, which still has a significant mortality [71]. Recent data correlated capsular size to raised intracranial pressure and lowered inflammatory response in patient spinal fluid [72] and found that the pathogen population size was more heterogeneous in vivo than in vitro. This heterogeneity was in agreement with a pathogen population that had undergone microevolution and persisted despite various selection pressures during chronic infection. More investigations that focus on the actual in vivo evolved pathogen population have to be pursued. Now methods are available that will permit us to determine the age of individual C. neoformans cells in an in vivo specimen. Future studies are planned to test the intriguing hypothesis that aging of cells within a pathogen population is an unanticipated emergent phenotypic trait that contributes to cryptococcal virulence and resilience.
EXECUTIVE SUMMARY.
Phenotypic variation in C. neoformans has been established to be imperative to the ability of the pathogen to persist during chronic infection.
Despite extensive studies of this phenomenon, cryptococcosis remains a formidable threat for parts of the world.
In order to better understand what causes the generation of variants and promotes their selection during infection, newer approaches need to be taken.
Studies on the epigenetic regulation of capsular induction, cell size, cell wall thickness, aging and phenotypic switching may provide insight into the unique and unanticipated emergence of phenotypic variants that contribute to cryptococcal persistence, resilience and ultimately virulence.
Acknowledgments
The authors thank Emily Cook and Neena Jain for their technical expertise.
Footnotes
Financial & competing interests disclosure
BC Fries is supported by NIH awards R01 AI059681 and R21 AI114259. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Smith ED, Kennedy BK, Kaeberlein M. Genome-wide identification of conserved longevity genes in yeast and worms. Mech Ageing Dev. 2007;128(1):106–111. doi: 10.1016/j.mad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy BK, Guarente L. Genetic analysis of aging in Saccharomyces cerevisiae. Trends Genet. 1996;12(9):355–359. [PubMed] [Google Scholar]
- 3.Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464(7288):513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roux AE, Quissac A, Chartrand P, Ferbeyre G, Rokeach LA. Regulation of chronological aging in Schizosaccharomyces pombe by the protein kinases Pka1 and Sck2. Aging Cell. 2006;5(4):345–357. doi: 10.1111/j.1474-9726.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- 5.Roux AE, Chartrand P, Ferbeyre G, Rokeach LA. Fission yeast and other yeasts as emergent models to unravel cellular aging in eukaryotes. J Gerontol A Biol Sci Med Sci. 2010;65(1):1–8. doi: 10.1093/gerona/glp152. [DOI] [PubMed] [Google Scholar]
- 6••.Jain N, Cook E, Xess I, Hasan F, Fries D, Fries BC. Isolation and characterization of senescent Cryptococcus neoformans and implications for phenotypic switching and pathogenesis in chronic cryptococcosis. Eukaryot Cell. 2009;8(6):858–866. doi: 10.1128/EC.00017-09. This is the first major work to link replicative aging to a fungal pathogen and highlights the importance of studying this emerging variant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Cordero RJ, Pontes B, Guimaraes AJ, et al. Chronological aging is associated with biophysical and chemical changes in the capsule of Cryptococcus neoformans. Infect Immun. 2011;79(12):4990–5000. doi: 10.1128/IAI.05789-11. This is the first major work to study chronological aging in C. neoformans and showcases the importance of aging to generate variation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Bouklas T, Pechuan X, Goldman DL, Edelman B, Bergman A, Fries BC. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. MBio. 2013;4(4) doi: 10.1128/mBio.00455-13. This is the first study to show that old C. neoformans cells display a potentially advantageous phenotype in vitro and can accumulate in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu XH, Meng FL, Hu Y, Zhou JQ. Candida albicans, a distinctive fungal model for cellular aging study. Aging cell. 2008;7(5):746–757. doi: 10.1111/j.1474-9726.2008.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta G, Fries BC. Variability of phenotypic traits in Cryptococcus varieties and species and the resulting implications for pathogenesis. Future Microbiol. 2010;5(5):775–787. doi: 10.2217/fmb.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietrella D, Fries B, Lupo P, Bistoni F, Casadevall A, Vecchiarelli A. Phenotypic switching of Cryptococcus neoformans can influence the outcome of the human immune response. Cell Microbiol. 2003;5(8):513–522. doi: 10.1046/j.1462-5822.2003.00297.x. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero A, Fries BC. Phenotypic switching in Cryptococcus neoformans contributes to virulence by changing the immunological host response. Infect Immun. 2008;76(9):4322–4331. doi: 10.1128/IAI.00529-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcfadden DC, Fries BC, Wang F, Casadevall A. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot Cell. 2007;6(8):1464–1473. doi: 10.1128/EC.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain N, Guerrero A, Fries BC. Phenotypic switching and its implications for the pathogenesis of Cryptococcus neoformans. FEMS Yeast Res. 2006;6(4):480–488. doi: 10.1111/j.1567-1364.2006.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasi E, Brozzetti A, Francisci D, et al. Evidence of microevolution in a clinical case of recurrent Cryptococcus neoformans meningoencephalitis. Eur J Clin Microbiol Infect Dis. 2001;20(8):535–543. doi: 10.1007/s100960100549. [DOI] [PubMed] [Google Scholar]
- 16.Ormerod KL, Morrow CA, Chow EW, et al. Comparative genomics of serial isolates of Cryptococcus neoformans reveals gene associated with carbon utilization and virulence. G3 (Bethesda) 2013 doi: 10.1534/g3.113.005660. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries BC, Casadevall A. Serial isolates of Cryptococcus neoformans from patients with AIDS differ in virulence for mice. J Infect Dis. 1998;178(6):1761–1766. doi: 10.1086/314521. [DOI] [PubMed] [Google Scholar]
- 18.Cherniak R, Morris LC, Belay T, Spitzer ED, Casadevall A. Variation in the structure of glucuronoxylomannan in isolates from patients with recurrent Cryptococcal meningitis. Infect Immun. 1995;63(5):1899–1905. doi: 10.1128/iai.63.5.1899-1905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. 2003;33(7):1957–1967. doi: 10.1002/eji.200323848. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Hermoso D, Dromer F, Janbon G. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect Immun. 2004;72(6):3359–3365. doi: 10.1128/IAI.72.6.3359-3365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Rodas R, Cordero RJ, Trevijano-Contador N, et al. Capsule growth in Cryptococcus neoformans is coordinated with cell cycle progression. MBio. 2014;5(3):e00945–e00914. doi: 10.1128/mBio.00945-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martín JP, Di Pietro A. Morphogenesis and Pathogenicity in Fungi. Springer; NY, USA: 2012. [Google Scholar]
- 23.O’meara TR, Norton D, Price MS, et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6(2):e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135(1):174–188. doi: 10.1016/j.cell.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun. 1998;66(10):5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis. 1993;167(1):186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- 27.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi M, Li SS, Zheng C, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120(5):1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabiiti W, Robertson E, Beale MA, et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest. 2014;124(5):2000–2008. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147(Pt 8):2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- 31.Cordero RJ, Bergman A, Casadevall A. Temporal behavior of capsule enlargement by Cryptococcus neoformans. Eukaryot Cell. 2013;12(10):1383–1388. doi: 10.1128/EC.00163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Dungrawala H, Hua H, et al. Cell size and growth rate are major determinants of replicative lifespan. Cell Cycle. 2011;10(1):144–155. doi: 10.4161/cc.10.1.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Aldridge BB, Fernandez-Suarez M, Heller D, et al. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science. 2012;335(6064):100–104. doi: 10.1126/science.1216166. This study links aging to prokaryotic pathogen and is important in showcasing the universality of this trait in generating variation in pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68(7):4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman DL, Lee SC, Mednick AJ, Montella L, Casadevall A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun. 2000;68(2):832–838. doi: 10.1128/iai.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci USA. 2001;98(26):15245–15250. doi: 10.1073/pnas.261418798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmesser M, Tucker S, Casadevall A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001;9(6):273–278. doi: 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 38.Coelho C, Bocca AL, Casadevall A. The intracellular life of Cryptococcus neoformans. Annu Rev Pathol. 2014;9:219–238. doi: 10.1146/annurev-pathol-012513-104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Zaragoza O, Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol. 2013;16(4):409–413. doi: 10.1016/j.mib.2013.03.006. This study highlights cell size as important to generating variants that can have significant consequences for cryptococcal virulence and infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okagaki LH, Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot Cell. 2012;11(6):820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondorosi E, Roudier F, Gendreau E. Plant cell-size control: growing by ploidy? Curr Opin Plant Biol. 2000;3(6):488–492. doi: 10.1016/s1369-5266(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 42.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168(6):2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 43.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006;6(4):513–524. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 44.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog. 2010;6(4):e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker LG, Specht CA, Donlin MJ, Lodge JK. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell. 2007;6(5):855–867. doi: 10.1128/EC.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker LG, Specht CA, Lodge JK. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryot Cell. 2011;10(9):1264–1268. doi: 10.1128/EC.05138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinclair D, Mills K, Guarente L. Aging in Saccharomyces cerevisiae. Annu Rev Microbiol. 1998;52:533–560. doi: 10.1146/annurev.micro.52.1.533. [DOI] [PubMed] [Google Scholar]
- 48.Powell CD, Quain DE, Smart KA. Chitin scar breaks in aged Saccharomyces cerevisiae. Microbiology. 2003;149(Pt 11):3129–3137. doi: 10.1099/mic.0.25940-0. [DOI] [PubMed] [Google Scholar]
- 49.Zaragoza O, Telzak A, Bryan RA, Dadachova E, Casadevall A. The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol Microbiol. 2006;59(1):67–83. doi: 10.1111/j.1365-2958.2005.04928.x. [DOI] [PubMed] [Google Scholar]
- 50.Mcfadden D, Zaragoza O, Casadevall A. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol. 2006;14(11):497–505. doi: 10.1016/j.tim.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45(1):76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 52•.Bicanic T, Brouwer AE, Meintjes G, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS. 2009;23(6):701–706. doi: 10.1097/QAD.0b013e32832605fe. This study made use of serial isolates to demonstrate treatment failure as established by a measure of ‘effective fungicidal activity.’. [DOI] [PubMed] [Google Scholar]
- 53.Jain N, Wickes BL, Keller SM, et al. Molecular epidemiology of clinical Cryptococcus neoformans strains from India. J Clin Microbiol. 2005;43(11):5733–5742. doi: 10.1128/JCM.43.11.5733-5742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slutsky B, Buffo J, Soll DR. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- 55.Fries BC, Goldman DL, Cherniak R, Ju R, Casadevall A. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect Immun. 1999;67(11):6076–6083. doi: 10.1128/iai.67.11.6076-6083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fries BC, Taborda CP, Serfass E, Casadevall A. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J Clin Invest. 2001;108(11):1639–1648. doi: 10.1172/JCI13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldman DL, Fries BC, Franzot SP, Montella L, Casadevall A. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc Natl Acad Sci USA. 1998;95(25):14967–14972. doi: 10.1073/pnas.95.25.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain N, Li L, Mcfadden DC, et al. Phenotypic switching in a Cryptococcus neoformans variety gattii strain is associated with changes in virulence and promotes dissemination to the central nervous system. Infect Immun. 2006;74(2):896–903. doi: 10.1128/IAI.74.2.896-903.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain N, Li L, Hsueh YP, et al. Loss of allergen 1 confers a hypervirulent phenotype that resembles mucoid switch variants of Cryptococcus neoformans. Infect Immun. 2009;77(1):128–140. doi: 10.1128/IAI.01079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guerrero A, Jain N, Wang X, Fries BC. Cryptococcus neoformans variants generated by phenotypic switching differ in virulence through effects on macrophage activation. Infect Immun. 2010;78(3):1049–1057. doi: 10.1128/IAI.01049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srikantha T, Zhao R, Daniels K, Radke J, Soll DR. Phenotypic switching in Candida glabrata accompanied by changes in expression of genes with deduced functions in copper detoxification and stress. Eukaryot Cell. 2005;4(8):1434–1445. doi: 10.1128/EC.4.8.1434-1445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lachke SA, Joly S, Daniels K, Soll DR. Phenotypic switching and filamentation in Candida glabrata. Microbiology. 2002;148(Pt 9):2661–2674. doi: 10.1099/00221287-148-9-2661. [DOI] [PubMed] [Google Scholar]
- 63.Gravel S, Jackson SP. Increased genome instability in aging yeast. Cell. 2003;115(1):1–2. doi: 10.1016/s0092-8674(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 64.Mcmurray MA, Gottschling DE. An age-induced switch to a hyper-recombinational state. Science. 2003;301(5641):1908–1911. doi: 10.1126/science.1087706. [DOI] [PubMed] [Google Scholar]
- 65.Sionov E, Lee H, Chang YC, Kwon-Chung KJ. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 2010;6(4):e1000848. doi: 10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ormerod KL, Fraser JA. Balancing stability and flexibility within the genome of the pathogen Cryptococcus neoformans. PLoS Pathog. 2013;9(12):e1003764. doi: 10.1371/journal.ppat.1003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu G, Wang J, Choi J, et al. Variation in chromosome copy number influences the virulence of Cryptococcus neoformans and occurs in isolates from AIDS patients. BMC Genomics. 2011;12:526. doi: 10.1186/1471-2164-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Averette AF, Desnos-Ollivier M, Ni M, Dromer F, Heitman J. Genetic diversity and genomic plasticity of Cryptococcus neoformans AD hybrid strains. G3 (Bethesda) 2012;2(1):83–97. doi: 10.1534/g3.111.001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delaney JR, Murakami CJ, Olsen B, Kennedy BK, Kaeberlein M. Quantitative evidence for early life fitness defects from 32 longevity-associated alleles in yeast. Cell Cycle. 2011;10(1):156–165. doi: 10.4161/cc.10.1.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy BK, Austriaco NR, Jr, Guarente L. Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span. J Cell Biol. 1994;127(6 Pt 2):1985–1993. doi: 10.1083/jcb.127.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 72••.Robertson EJ, Najjuka G, Rolfes MA, et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J Infect Dis. 2014;209(1):74–82. doi: 10.1093/infdis/jit435. This study demonstrated that the pathogen population in vivo is more heterogeneous in capsule size, which invariably affects intracranial pressure and inflammatory response in the patient. [DOI] [PMC free article] [PubMed] [Google Scholar]