Abstract
Methamphetamine (METH) exposure results in long-term damage to the dopamine system in both human METH abusers and animal models. One factor that has been heavily implicated in this METH-induced damage to the dopaminergic system is the activation of D1 Dopamine (DA) receptors. However, a significant caveat to the studies investigating the role of the receptor in such toxicity is that genetic and pharmacological manipulations of the D1 DA receptor also mitigate METH-induced hyperthermia. Importantly, METH-induced hyperthermia is tightly associated with the neurotoxicity, such that simply cooling animals during METH exposure protects against the neurotoxicity. Therefore, it is difficult to determine whether D1 DA receptors per se play an important role in METH-induced neurotoxicity or whether the protection observed simply resulted from a mitigation of METH-induced hyperthermia. To answer this important question, the current study infused a D1 DA receptor antagonist into striatum during METH exposure while controlling for METH-induced hyperthermia. Here we found that even when METH-induced hyperthermia is maintained, the coadministration of a D1 DA receptor antagonist protects against METH-induced neurotoxicity, strongly suggesting that D1 DA receptors play an important role in METH-induced neurotoxicity apart from the mitigation of METH-induced hyperthermia.
Keywords: dopamine receptor, dopamine, methamphetamine, neurotoxicity
Introduction
Exposure to a neurotoxic regimen of methamphetamine (METH) results in significant damage to the dopaminergic system, particularly in the caudate-putamen of human METH abusers and in the striatum of rodents [16, 19, 23, 26]. Several factors have been implicated in such toxicity, however the mechanism through which METH exposure results in damage to the central DA system has not been clearly elucidated.
One such factor that has been heavily associated with METH-induced neurotoxicity is increased extracellular dopamine (DA) and the subsequent activation of D1 DA receptors. It is well established that METH exposure results in a significant increase in extracellular DA [14, 21, 22]. And, increased extracellular DA may result in neurotoxicity via activation of D1 DA receptors. For example, D1 DA receptor antagonists systemically co-administered with METH partially protect against METH-induced neurotoxicity [6]. Similarly, intrastriatal infusion of D1 DA receptor antagonist during METH exposure has also been reported to protect against METH-induced neurotoxicity [14]; however, the protection may at least partially have resulted from mitigation of METH-induced hyperthermia, a factor also tightly associated with METH-induced neurotoxicity [1, 5]. More recently however, animals with a deletion of the D1 DA receptor gene were also reported to be protected against METH-induced neurotoxicity—an effect that appears to not solely depend upon attenuation of METH-induced hyperthermia [3]. Thus, due to the inconclusive, yet promising, results appearing in the literature the present work was conducted to further examine whether blockade of D1 DA receptors in striatum during METH exposure protects against METH-induced DA nerve terminal degeneration when METH-induced hyperthermia is maintained. To answer this question, we conducted studies in which the D1 DA receptor antagonist SCH23390 (R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol) was infused directly into the striata of animals just prior to and during administration of the binge regimen of METH while manipulating the ambient temperature in the rats' environment in order to maintain METH-induced hyperthermia.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) were housed in wire mesh cages in a temperature-controlled room on a 12:12-hr light:dark cycle with free access to food and water. All animal care and experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (8th Ed., National Research Council) and were approved by the Institutional Animal Care and Use Committee at the University of Utah.
Surgical Procedures
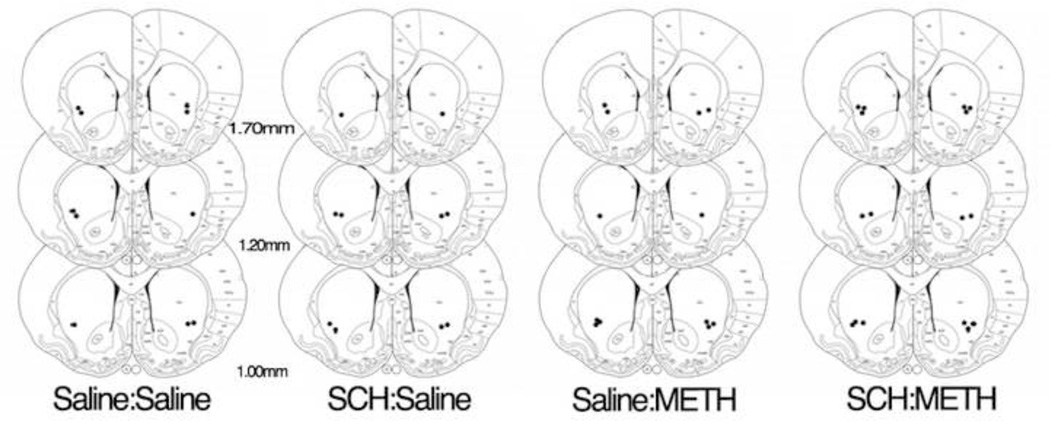
One week prior to METH or saline treatment, male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) were anesthetized with ketamine/xylazine (90/10 mg/kg, i.p.) and placed in a stereotaxic apparatus. 21-gauge guide cannulae were bilaterally implanted (Plastics One, Roanoke, VA) and were lowered to end just dorsal to the dorsal striatum (mm from bregma: AP: +0.5mm, ML: ± 3.0mm, from skull DV: −3.2mm). The guides were secured with skull screws and dental acrylic and dummy cannulae were inserted. Subsequent infusions were made through 33-gauge infusion cannulae extending 3.8 mm beyond the guides (Figure 2).
Figure 2.
Diagram indicates location of infusion sites in striatum with the black dots representing placement of infusion sites. Numbers represent mm from Bregma.
Intrastriatal Infusions and METH Administration
On the treatment day (post natal day (PND60), 30 min prior to saline or METH injections, intrastriatal infusions of either saline (0.1 µl/1 min, 0.9% saline) or the D1 receptor antagonist, R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol (SCH23390 (2 µg/µl in 0.9% saline as previously described [14, 24], at 0.1 µl/min Sigma-Aldrich, D), through the guide cannulae began. Infusions continued until 1 hr after the last injection of either saline or METH, therefore producing a total elapsed time of infusion of 7.5 hr.
METH and saline injections were conducted as previously described [11]. Briefly, on the treatment day (PND60), rats (4–8 per treatment group) were individually housed in plastic tub cages (Instech Laboratories Inc.). Animals received injections of (±)-METH-HCl (10 mg free base/kg, s.c.; kindly provided by the National Institute on Drug Abuse) or 0.9% saline (1 ml/kg, s.c.) at 2-hr intervals resulting in a total of four injections. Rectal temperatures were monitored using a digital thermometer (BAT-12, Physitemp Instruments, Clifton, NJ) to ensure the presence of METH-induced hyperthermia. Baseline temperatures for each animal were taken 30 min prior to the first injection and 1 hr after each subsequent injection. If the body temperature of an animal exceeded 40.5°C, the animal was cooled by transferring it to a cage placed over wet ice until the body temperature fell below 39°C. Conversely, cages of SCH23390 infused, METH-treated animals were placed on a heating pad with a heating lamp in order to maintain METH-induced hyperthermia (39°C–40.4°C). Approximately 18 hr after the last injection, animals were returned to their home cages in the colony room.
Tissue Preparation
Animals were sacrificed 7 days after the last METH or saline injection via exposure to CO2 for 1 min. Following decapitation, brains were rapidly removed and submerged in 4% paraformaldehyde with 0.9% NaCl for 24 hr at 4°C, then cryoprotected in 30% sucrose in phosphate buffered saline (PBS) and stored at 4°C. The brains were then sectioned at 30 µm on a freezing microtome (Microm, HM 440E). For each animal, sections of striatum just anterior to, at the site of infusion, and just posterior to the infusion were collected and stored at 4°C in 1mg/ml sodium azide.
Immunohistochemistry
DAT immunohistochemistry was performed to evaluate METH-induced DA depletions and was conducted as previously described [11]. Briefly, sections underwent heat-mediated antigen retrieval for 20 min. Sections were then washed, incubated for 10 min in 0.1M PBS containing 3% H2O2, washed again in PBS, and blocked. Tissue was then incubated overnight at 4°C in a primary antibody solution (Millipore, MAB369, 1:5000). The following day, tissue was washed, and incubated in a secondary antibody solution (Vector Labs, BA-4000, 1:200). Finally, tissue was incubated in avidin-biotinylated peroxidase complex solution (ABC Elite Kit, Vector Labs, PK-6100) and the reaction terminated by washing in PBS. The tissue sections were then incubated in nickel-enhanced diaminobenzidine tetrahydrochloride (Ni-DAB; Vector, SK-4100), washed again, mounted onto slides, dried, dehydrated and coverslipped with VectaMount (Vector Labs, H-5000).
Image Acquisition and Analysis
Images were analyzed as previously described [11] and the analysis was conducted by an experimenter blinded to treatment conditions. Briefly, images were digitized and densitometric analysis was performed using the NIH ImageJ software (http://imagej.nih.gov/ij/), yielding background-subtracted, average gray values in striatum. Three striatal sections per rat were analyzed and averaged. Average gray values were then compared across treatment groups.
Statistical Analysis
Statistical analysis was performed using a two-factor ANOVA (Infusion × Treatment) followed by post hoc analysis via Tukey’s HSD test. Statistical analysis on body temperatures was conducted using a MANOVA with repeated measures (Infusion × Treatment × Time) followed by a Student’s t-test post hoc analysis at individual time points to determine main effects of infusions and treatments.
Results
METH-induced Hyperthermia
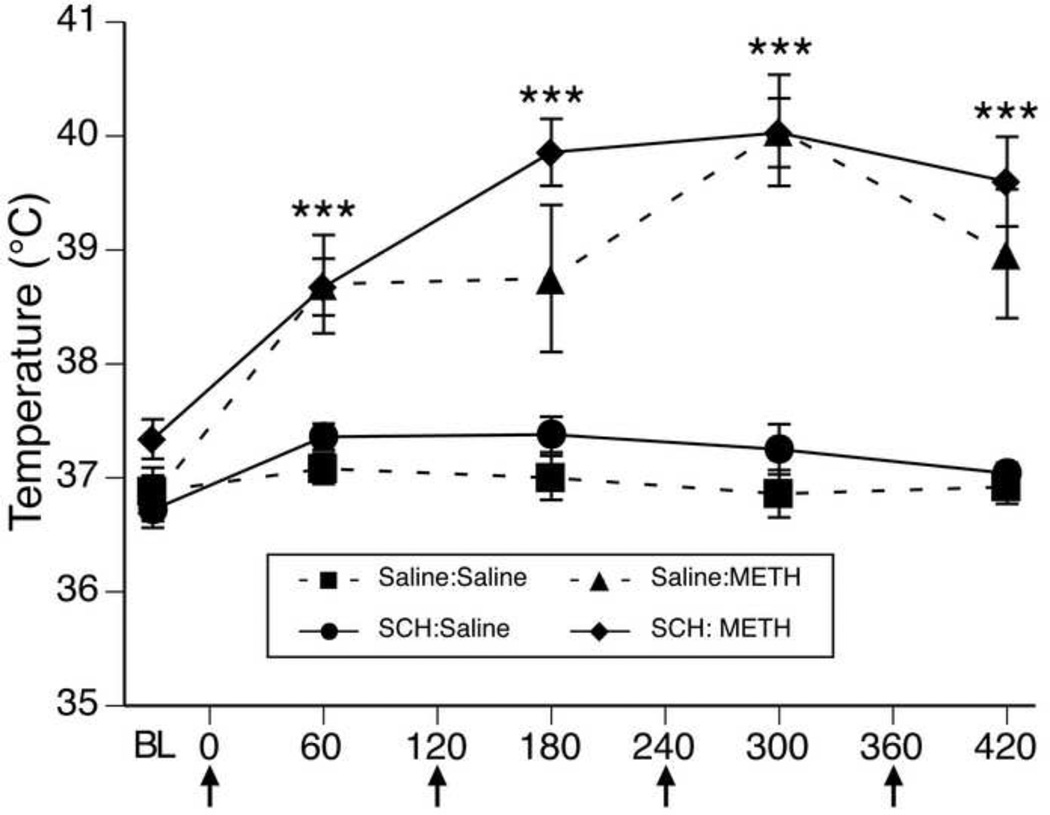
For body temperature data collected during METH or saline treatment (Figure 1), MANOVA revealed a significant main effects of infusion (F(1,20)= 8.98, p<0.01), a significant main effect of treatment (F(1,20)= 250.9, p<0.0001) and a significant effect of time (F(4,17)= 26.8, p<0.0001). Furthermore, there was also a significant treatment × time interaction (F(4,17)= 13.7, p<0.0001). Post hoc analysis revealed that the temperatures of animals receiving METH were not different from controls at baseline (0 min, t=1.29, p=0.2), but were significantly greater than those receiving saline at all four time points after the injections of METH began (60 min, t=5.03, p<0.0001; 180 min, t=5.30, p<0.0001; 300 min, t=8.52, p<0.0001; 420 min, t=5.90, p<0.0001). Importantly, there was no time × infusion interaction (F(4,17)= 0.6, p>0.7) or an infusion × treatment × time interaction (F(4,17)= 0.7, p>0.6), indicating the maintenance of METH-induced hyperthermia in the rats receiving intrastriatal infusions of SCH23390.
Figure 1.
Body temperatures (mean±SEM; n=4–8) of animals that received systemic injections of saline (4 × 1 mL/kg, s.c. at 2-hr intervals) or (±)-METH (4 × 10 mg/kg, s.c. at 2-hr intervals) and intrastriatal infusions of either saline or SCH222390. Treatment group designations indicate infusion:treatment, resulting in the four treatment groups: Saline:Saline (S:S); SCH:Saline (SCH:S); Saline:METH (S:M); and SCH:METH (SCH:M). Temperatures were obtained 30 min prior to the first injection (baseline; BL) and 1 hr after each subsequent injection. X-axis values represent minutes after the first injection and arrows represent the time of each saline or METH injection. ***p<0.0001 significant effect of METH at this time point.
METH-Induced Dopamine Depletions
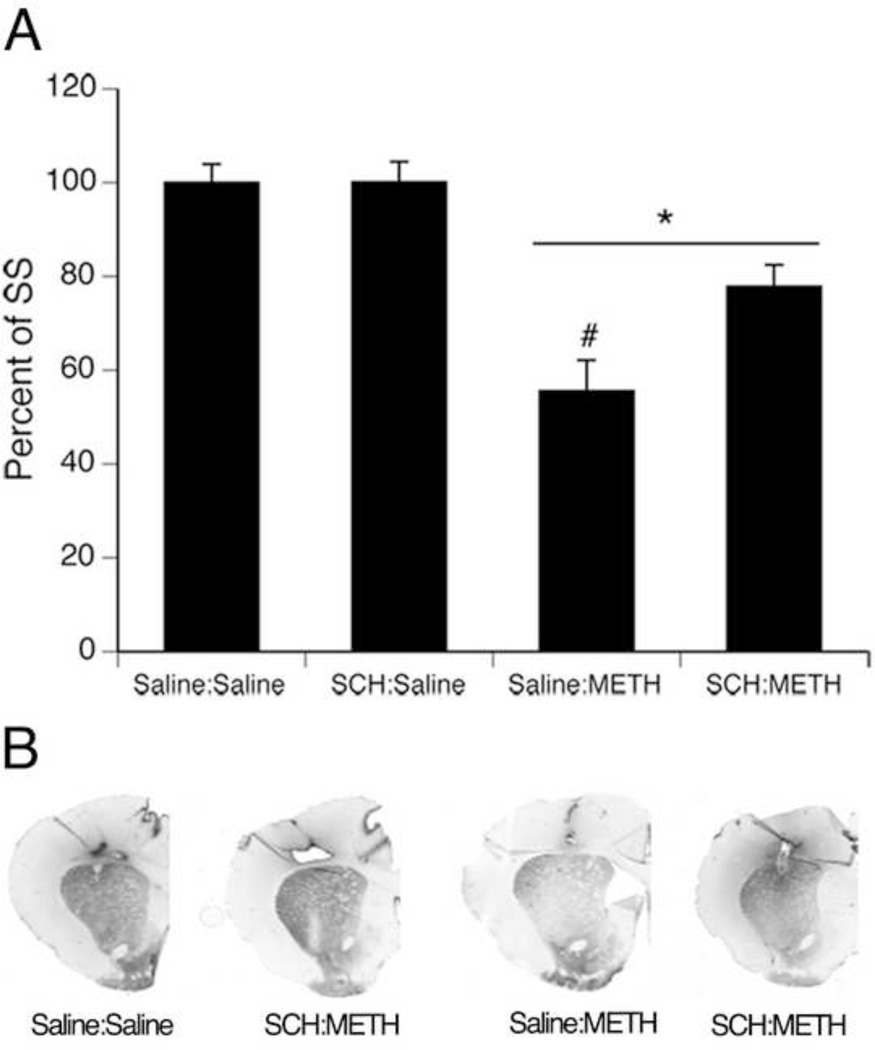
METH-treated rats showed significant decreases in DAT immunohistochemical staining compared to saline-treated controls, however SCH23390 infusions resulted in partial protection against such toxicity (Figure 3). A two-factor ANOVA of the average gray value of DAT immunohistochemical signal in striatum revealed significant main effects of infusion (F(1,20) =4.6, p<0.05) and treatment (F(1,20) =40.4, p<0.0001), and a significant infusion × treatment interaction (F(1,20) =4.5, p<0.05). Post hoc analysis of the interaction revealed that animals treated with a neurotoxic regimen of METH (saline:METH and SCH:METH) had significantly lower DAT immunohistochemical signal compared to saline-treated controls (saline:saline and SCH:saline) (Tukey’s HSD test, p values <0.05; Figure 2). Importantly, animals infused with saline and treated with METH (saline:METH) showed significantly lower DAT immunohistochemical signal in striatum compared to all other treatment groups (saline:saline, SCH:saline, and SCH:METH) (Tukey’s HSD test, p values <0.05; Figure 3).
Figure 3.
Striatal DAT immunohistochemical signal of animals that received systemic injections of saline (4 × 1 mL/kg, s.c. at 2-hr intervals) or (±)-METH (4 × 10 mg/kg, s.c. at 2-hr intervals) and intrastriatal infusions of either saline or SCH23390. Animals were sacrificed 7 days following METH or Saline treatment on PND60. Treatment group designations indicate infusion:treatment, resulting in the four treatment groups: Saline:Saline (S:S); SCH:Saline (SCH:S); Saline:METH (S:M); and SCH:METH (SCH:M). * indicates significant differences from Saline:Saline and SCH:Saline, p<0.05; # indicates significant difference from SCH:METH, p<0.05. (B) Representative images of DAT immunohistochemistry
Discussion
Methamphetamine abuse has long been associated with significant and persistent dopaminerigic system damage. Recent work has also suggest that individuals with a history of METH abuse are at a higher risk for developing Parkinson’s Disease compared to both healthy controls and individuals with a history of cocaine abuse [7, 8]. Thus work determining the cascade of events during and following METH exposure that ultimately results in these dopaminergic deficits is essential for the development of therapeutic interventions.
One such factor that has been heavily associated with METH-induced neurotoxicity is increased extracellular DA concentrations and subsequent activation of D1 DA receptors. However one problem with many of these studies is that D1 DA receptor antagonists coadministered during METH exposure also disrupt METH-induced hyperthermia. And, METH-induced hyperthermia in itself is tightly associated with METH-induced neurotoxicity, as simply cooling animals during or following METH exposure results in protection against such toxicity [2]. Thus, the field is unable to distinguish whether protection observed in these studies resulted from blockade of D1 DA receptors or from the mitigation of METH-induced hyperthermia. To clarify the role of these receptors in such toxicity, we performed intrastriatal infusions of the D1 DA receptor antagonist, SCH23390 during METH exposure, while controlling for METH-induced hyperthermia.
Baseline temperatures for animals were collected 30 minutes prior to saline or METH injections and were recorded every hour after each subsequent injection. Results of these recordings indicate that animals administered METH showed significantly elevated core body temperatures compared to controls (Figure 1). Importantly, animals that were treated with METH and that received SCH23390 infusions did not show significantly different core body temperatures compared to animals treated with METH and that received saline infusions (Figure 1), thus we were able to maintain METH-induced hyperthermia in animals treated with METH and infused with SCH23390. METH-induced DA depletions were determined using DAT immunohistochemistry as routinely performed in our laboratory [10, 11]. Here we found that animals that received bilateral intrastriatal infusions with the D1-DA receptor antagonist SCH23390, showed a significant degree of protection from METH-induced neurotoxicity compared to their counterparts treated with METH and infused with saline. Together, these data indicate that the neuroprotection observed in animals infused with SCH23390 during METH injections resulted from the blockade of D1 DA receptors in the striatum, and not from disruption of METH-induced hyperthermia.
Interestingly, D1 DA receptors are located postsynaptic to DA nerve terminals that undergo degeneration following METH exposure [17]. Therefore, although unexplored in the current study, one possible mechanism through which D1 DA receptor activation may play a causal role in damage to DA terminals is through altered basal ganglia output secondary to METH-induced DA release and activation of D1 DA receptors ultimately leading to excessive corticostriatal excitation and GLU-mediated excitotoxicity to DA nerve terminals. For instance, the striatum receives significant GLU inputs from corticostriatal projections [4, 12]. And, corticostriatal activity can be regulated by nigrothalamic and thalamocortical projections, as γ-aminobutyric acid (GABA) release from D1 DA receptor-containing, striatonigral neurons activates GABA-A receptors in the substantia nigra pars reticulata (SNpr) and decreases thalamic neuron firing [9, 20, 25]. Nigrothalamic activity can then influence glutamatergic thalamocortical and corticostriatal projections [15]. Studies have also shown that METH-induced GLU release is associated with GABA release in the SNpr and decreased GABA release in the thalamus [18] and that GABA-A receptor antagonism in the SNpr can reduce METH-induced GABA release in the thalamus, as well as GLU release and DA nerve terminal degeneration in the striatum [18]. Other work has also shown that intrastriatal infusions of D1 DA receptor antagonists prevent apomorphine-induced cortical immediate-early gene expression and sensorimotor responsiveness [24], further implicating activation of D1 DA receptors in striatum in influencing corticostriatal activity. Lastly, recent evidence has shown that NMDA-type GLU receptor antagonist applied epidurally to the cortex reduces both METH-induced c-fos gene expression and DA nerve terminal degeneration in striatum [13]. Taken together, these data suggest that METH may increase GLU release and ultimately DA neuron toxicity via the activation of D1 DA receptors on striatonigral efferent neurons.
Conclusions
Previous work had strongly suggested that D1 DA receptor antagonists play an important role in METH-induced DA terminal degeneration. However, a significant caveat of these studies is that METH-induced hyperthermia was also disrupted. Here, we coadminaistered a D1DA receptor antagonist with METH while controlling for METH-induced hyperthermia and show that blocking this receptor significantly protects against METH-induced DA terminal degeneration through a mechanism separate from the mitigation of METH-induced hyperthermia, thus strongly suggesting that D1 DA receptors play a significant role in METH-induced neurotoxicity.
Highlights.
METH-induced hyperthermia was maintained in rats receiving intrastriatal SCH23390.
Intrastriatal SCH23390 infusions protected against METH-induced DA depletions.
This protection was independent of the mitigation of METH-induced hyperthermia.
Abbreviations
- DA
dopamine
- METH
methamphetamine
- PND
post-natal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Participated in Research Design: Friend and Keefe
Conducted Experiments: Friend and Keefe
Performed Data Analysis: Friend and Keefe
Wrote or contributed to the writing of the manuscript: Friend and Keefe
Contributor Information
Danielle M. Friend, Email: da.friend@utah.edu.
Kristen A. Keefe, Email: k.keefe@utah.edu.
References
- 1.Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- 2.Ali SF, Newport RR, Holson W, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hyperthermia decrease methamphetamine neurotoxicity in mice. Ann N Y Acad Sci. 1995;765:338. doi: 10.1111/j.1749-6632.1995.tb16610.x. [DOI] [PubMed] [Google Scholar]
- 3.Ares-Santos S, Granado N, Oliva I, O'Shea E, Martin ED, Colado MI, Moratalla R. Dopamine D(1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis. 2012;45:810–820. doi: 10.1016/j.nbd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo M, Giuffrida R, Palmeri A, Sapienza S. Excitatory amino acids as neurotransmitters of corticostriatal projections: immunocytochemical evidence in the rat. Arch Ital Biol. 1998;136:215–223. [PubMed] [Google Scholar]
- 5.Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- 6.Broening HW, Morford LL, Vorhees CV. Interactions of dopamine D1 and D2 receptor antagonists with D-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse. 2005;56:84–93. doi: 10.1002/syn.20130. [DOI] [PubMed] [Google Scholar]
- 7.Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- 8.Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res. 1985;334:227–233. doi: 10.1016/0006-8993(85)90214-8. [DOI] [PubMed] [Google Scholar]
- 10.Friend DM, Keefe KA. Glial Reactivity in Resistance to Methamphetamine-Induced Neurotoxicity. J Neurochem. 2013 doi: 10.1111/jnc.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friend DM, Son JH, Keefe KA, Fricks-Gleason AN. Expression and activity of nitric oxide synthase isoforms in methamphetamine-induced striatal dopamine toxicity. J Pharmacol Exp Ther. 2013;344:511–521. doi: 10.1124/jpet.112.199745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- 13.Gross NB, Duncker PC, Marshall JF. Cortical ionotropic glutamate receptor antagonism protects against methamphetamine-induced striatal neurotoxicity. Neuroscience. 2011;199:272–283. doi: 10.1016/j.neuroscience.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross NB, Duncker PC, Marshall JF. Striatal dopamine D1 and D2 receptors: Widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse. 2011;65:1144–1155. doi: 10.1002/syn.20952. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko T, Mizuno N. Immunohistochemical study of glutaminase-containing neurons in the cerebral cortex and thalamus of the rat. J Comp Neurol. 1988;267:590–602. doi: 10.1002/cne.902670411. [DOI] [PubMed] [Google Scholar]
- 16.Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur J Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- 17.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson LF, Faull RL, Waldvogel HJ, Dragunow M. GABA and GABAA receptor changes in the substantia nigra of the rat following quinolinic acid lesions in the striatum closely resemble Huntington's disease. Neuroscience. 1995;66:507–521. doi: 10.1016/0306-4522(94)00607-7. [DOI] [PubMed] [Google Scholar]
- 21.O'Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J Neurochem. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- 23.Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 24.Steiner H, Kitai ST. Regulation of rat cortex function by D1 dopamine receptors in the striatum. J Neurosci. 2000;20:5449–5460. doi: 10.1523/JNEUROSCI.20-14-05449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmerman W, Westerink BH. Electrical stimulation of the substantia nigra reticulata: detection of neuronal extracellular GABA in the ventromedial thalamus and its regulatory mechanism using microdialysis in awake rats. Synapse. 1997;26:62–71. doi: 10.1002/(SICI)1098-2396(199705)26:1<62::AID-SYN7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]