Abstract
PTPN22 encodes a tyrosine phosphatase that is expressed by haematopoietic cells and functions as a key regulator of immune homeostasis by inhibiting T-cell receptor signalling and by selectively promoting type I interferon responses after activation of myeloid-cell pattern-recognition receptors. A single nucleotide polymorphism of PTPN22, 1858C>T (rs2476601), disrupts an interaction motif in the protein, and is the most important non-HLA genetic risk factor for rheumatoid arthritis and the second most important for juvenile idiopathic arthritis. PTPN22 exemplifies a shared autoimmunity gene, affecting the pathogenesis of systemic lupus erythematosus, vasculitis and other autoimmune diseases. In this Review, we explore the role of PTPN22 in autoimmune connective tissue disease, with particular emphasis on candidate-gene and genome-wide association studies and clinical variability of disease. We also propose a number of PTPN22-dependent functional models of the pathogenesis of autoimmune diseases.
Introduction
Recognition that the PTPN22 gene is a major risk factor for autoimmunity began with the association of a missense single nucleotide polymorphism (SNP) (1858C>T, rs2476601) with an increased risk of type 1 diabetes mellitus,1 rheumatoid arthritis (RA)2 and systemic lupus erythematosus (SLE).3 Since then, this susceptibility locus has been found to affect multiple connective tissue and autoimmune diseases. At the genome-wide level, PTPN22 1858C>T ranks as the most important non-MHC single-gene contributor to RA susceptibility and the second most important for juvenile idiopathic arthritis (JIA).4,5
The frequency of the PTPN22 1858T allele varies among different populations. In Europe, a northeast to southwest gradient exists, with the highest frequencies in northern and eastern Europe (>10%) and the lowest in southern Europe (2–3%).6–8 Approximately 6–10% of US, Australian and New Zealand white populations, and 4–5% of Hispanic populations, have the 1858T allele.6,8 PTPN22 1858T is rare in Native American (<1%), African (<1%), Middle Eastern (0–3%) and Asian populations (<1%).6–10
In addition to autoimmune diseases, PTPN22 1858C>T also affects susceptibility to infectious diseases. Carriers of the 1858T allele are at increased risk of bacterial infections, including invasive pneumococcal infections, bacterial pulmonary infections in patients with chronic muco cutaneous candidiasis, and lepromatous and tuberculoid leprosy.7 Remarkably, carriers of the 1858T allele are resistant to the development of pulmonary tuberculosis,11,12 and the allele has not been found to affect susceptibility to brucellosis, Chagas disease or hepatitis C.7
Reviews on the immunological function and molecular regulation of PTPN22 are available.7,13 Here we briefly review the functions of Tyrosine-protein phosphatase nonreceptor type 22 (PTPN22; also known as lymphoid phosphatase) in T cells, B cells and myeloid cells. We then describe the role of PTPN22 in autoimmunity, with emphasis on clinical manifestations of connective tissue autoimmune diseases, including RA, JIA, SLE, systemic sclerosis (SSc) and vasculitis. We also describe how the autoimmune-associated PTPN22 1858T variant affects the function of immune cells, and describe current mechanistic models for its role in the pathogenesis of autoimmunity.
PTPN22 structure and function
PTPN22 belongs to a family of genes encoding protein tyrosine phosphatases (PTPs).14 PTPs function as regulators of tyrosine phosphorylation-based cell-signal transduction by removing phosphate groups from tyrosine residues on intracellular proteins. PTPs are the natural counterparts of protein tyrosine kinases, which catalyse the addition of phosphate groups on tyrosine residues. PTPN22 encodes a nonreceptor PTP expressed only by haematopoietic cells. PTPN22 contains three domains, including: an N-terminal PTP catalytic domain; an interdomain region; and a C-terminal domain with four proline-rich regions that function as motifs for interaction with other proteins (Figure 1). The autoimmune-associated SNP PTPN22 1858C>T encodes an arginine to tryptophan substitution at amino acid 620 (Arg620Trp) in the first proline-rich motif of the PTPN22 protein.1
Figure 1.
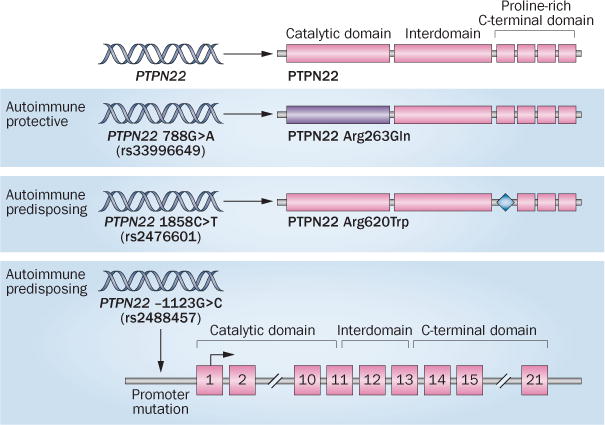
Variants of human PTPN22. PTPN22 encodes a tyrosine phosphatase with an N-terminal catalytic domain, an interdomain region and a C-terminal domain with four proline-rich regions. Several SNPs in the PTPN22 gene are associated with autoimmune disease. The autoimmune-protective 788G>A SNP (rs33996649) causes an Arg263Gln substitution in the PTPN22 catalytic domain (purple). The autoimmune-predisposing 1858C>T SNP (rs2476601) causes an Arg620Trp substitution in the first proline-rich motif (blue diamond). The autoimmune-predisposing −1123G>C SNP (rs2488457) is in the promoter region of the PTPN22 gene. Abbreviations: PTPN22, tyrosine-protein phosphatase nonreceptor type 22; SNP, single-nucleotide polymorphism.
PTPN22 has dual roles in the regulation of immune cell signalling (Figure 2).7,13 In the adaptive immune system, PTPN22 inhibits T-cell activation by restricting signalling downstream of the T-cell receptor (TCR). By contrast, in the innate immune system, PTPN22 selectively promotes myeloid-cell type I interferon production by enhancing signalling downstream of pattern recognition receptors.15
Figure 2.
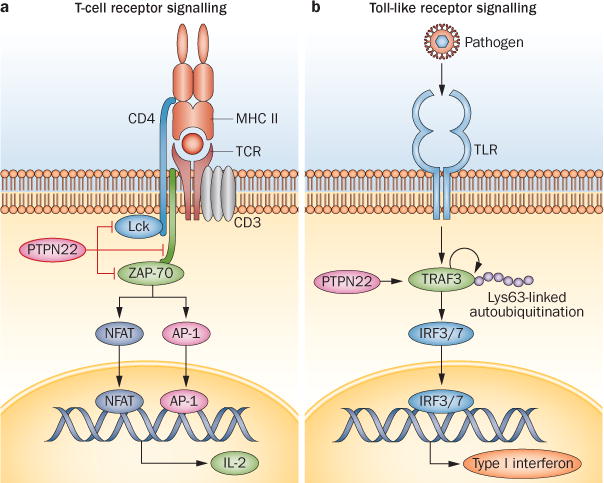
PTPN22 regulation of cell signalling. PTPN22 has dual roles in the regulation of immune-cell signalling. a | In T cells, PTPN22 restricts signalling by inhibitory tyrosine dephosphorylation of key promoters of signalling downstream of the TCR: the SRC family tyrosine protein kinase Lck; the SYK family kinase ZAP-70 and the TCR-associated CD3ζ-chain. The catalytic activity of PTPN22 is essential for this role. b | In myeloid cells, PTPN22 acts as a selective promoter of the type I interferon response by promoting the Lys63-linked autoubiquitination of TRAF3 and phosphorylation of IRF3 and IRF7 downstream of pattern-recognition receptors. In contrast to lymphocytes, the catalytic activity of PTPN22 is not substantially involved in the promotion of TLR signalling in myeloid cells. Instead, myeloid cells rely on scaffolding properties within the C-terminal domain of the protein. Abbreviations: IRF3, interferon regulatory factor 3; IRF7, interferon regulatory factor 7; NFAT, nuclear factor of activated T cells; PTPN22, tyrosine-protein phosphatase nonreceptor type 22; TCR, T-cell receptor; TLR, Toll-like receptor; TRAF3, TNF-receptor-associated factor 3.
T cells
Studies of human and mouse cells show that PTPN22 is a potent inhibitor of T-cell activation by inhibitory dephosphorylation of key mediators of signal transduction immediately downstream of the TCR (Figure 2a). T-cell activation by TCR engagement requires a ‘wave’ of tyrosine-based phosphorylation events mediated by kinases of the Src family (Lck and Fyn being the most important) and the Syk family (ZAP-70 being the most important). These kinases cause phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) of the TCR-associated CD3ζ-chain and trigger multiple intracellular signalling pathways.14 PTPN22 inhibits early TCR signalling by dephosphorylating the activation loops of Lck, Fyn, ZAP-70 and ITAMs of the CD3ζ-chain. PTPN22 knock-down or pharmacological inhibition in human cells and deletion in mouse cells enhances TCR signalling.2,16–19 In T cells, PTPN22 forms a high-stoichiometry complex with tyrosine-protein kinase CSK (also known as C-terminal SRC kinase), which is also an inhibitor of TCR signalling. This complex is mediated by a proline-rich motif of PTPN22 and the SH3 domain of CSK, both of which bind PTPN22 to its substrates and regulate its activity.18,20,21
The effect of PTPN22 on TCR signalling might differ between T-cell subpopulations. Studies showed that compared with wild-type mice, Ptpn22−/− mice had increased numbers of effector memory CD4+ and CD8+ T cells that are hyper-responsive to TCR engagement,16 and increased numbers of follicular helper T cells that produce more IL-21.22 CD4+CD25+Foxp3+ regulatory T (TREG) cells are also affected by deletion of Ptpn22 —compared with Ptpn22-sufficient mice, Ptpn22−/− mice have more TREG cells,19,23 and those cells are more suppressive and adhesive.19
B cells
The function of PTPN22 in B cells is not as clear as it is for T cells. Ptpn22 deficiency in mice has been shown to have no effect on B-cell receptor (BCR) signalling or B-cell development.16,24 Increased spontaneous germinal-centre formation in the spleen and Peyer’s patches was attributed to increased T-cell help;16,22 however, primary B cells from humans with the PTPN22 Arg620Trp substitution and from mice with the homologous mutation, Ptpn22 Arg619Trp, had alterations in BCR signalling and the B-cell repertoire, suggesting PTPN22 regulates these processes.25–30 The lack of effect of Ptpn22 deficiency on B-cell function in the knockout mouse could be due to compensation by other phosphatases during development, or to features of the C57BL/6 strain from which the knockout was generated.
Myeloid cells
PTPN22 is a selective promoter of type I interferon production by pattern-recognition receptor-activated human and mouse macrophages and dendritic cells (DCs; Figure 2b).15 Efficient type I interferon induction after TLR engagement requires activation of a signalling cascade involving phosphorylation of interferon response factors (IRFs), IRF3 and IRF7. This cascade is dependent upon the Lys63-linked autoubiquitination of the E3 ubiquitin ligase TNF receptor-associated factor 3 (TRAF3). PTPN22 bound TRAF3 and selectively promoted TRAF3 Lys63-linked autoubiquitination in bone marrow-derived macrophages (BMDMs) after engagement of TLRs, enabling PTPN22 to mediate production of type I interferon without affecting expression of proinflammatory cytokines, such as IL-1β and TNF.15 In contrast to TCR signalling, the effect of PTPN22 in the TLR signalling pathway is not mediated by PTPN22 catalytic activity, but instead is reliant upon scaffolding properties within the C-terminal domain of the protein. Knockdown of PTPN22 in human blood monocyte-derived macrophages, mouse BMDMs and mouse DCs, and Ptpn22-deficiency in mouse BMDMs and DCs, impaired type I interferon production after TLR engagement.15 However, treatment of mouse BMDMs and a macrophage cell line with pharmacological inhibitors of Ptpn22 had no effect on TLR-stimulated type I interferon production. Although Ptpn22 deletion in mice did not lead to spontaneous autoimmunity or other pathology,16 Ptpn22−/− mice had impaired type I interferon responses to TLR ligands in vivo and had reduced host responses to infection with lymphochoriomeningitis virus.15 Ptpn22−/− mice have also been shown to have diminished type I interferon-dependent suppression of colitis and arthritis-related inflammation after TLR activation.15
PTPN22 1858C>T disease associations
PTPN22 is associated with many, but not all, connective tissue autoimmune diseases (Box 1). PTPN22 1858C>T is not associated with ankylosing spondylitis,31,32 a single case–control study reported no association with primary antiphospholipid syndrome,33 and meta-analysis of two case–control studies showed no association with primary Sjögren’s syndrome.8
Box 1. PTPN22 1858C>T disease association*.
Increased risk (OR)
-
■
Addison disease (1.43)
-
■
Alopecia areata (1.38)
-
■
Giant cell arteritis (1.62)84
-
■
Graves disease (1.59)
-
■
Granulomatosis with polyangiitis (1.91)88
-
■
Hashimoto thyroiditis (1.63)119
-
■
Idiopathic inflammatory myopathy (1.77)
-
■
Idiopathic thrombocytopenic purpura (1.93)
-
■
Juvenile idiopathic arthritis (1.54)
-
■
Myasthenia gravis (1.53)
-
■
Psoriatic arthritis (1.22)
-
■
Rheumatoid arthritis (1.65)
-
■
Systemic lupus erythematosus (1.46)
-
■
Systemic sclerosis (1.16)
-
■
Type 1 diabetes mellitus (1.84)
-
■
Vitiligo (1.98)
Decreased risk (OR)
-
■
Behçet disease (0.42)91
-
■
Crohn disease (0.84)
Not affected‡
-
■
Celiac disease
-
■
Multiple sclerosis
-
■
Psoriasis
-
■
Ulcerative colitis
Probably not affected§
-
■
Ankylosing spondylitis
-
■
Aplastic anaemia120
-
■
Chronic urticaria121
-
■
Churg–Strauss syndrome88
-
■
Microscopic polyangiitis88
-
■
Pemphigus
-
■
Primary antiphospholipid syndrome33
-
■
Primary biliary cirrhosis
-
■
Primary sclerosing cholangitis
-
■
Primary Sjögren’s syndrome
-
■
Takayasu arteritis85
-
■
Uveitis
*Unless otherwise indicated, data were obtained from Zheng et al.8
‡Diseases categorized as “not affected” did not have a statistically significant association with PTPN22 1858C>T in a meta-analysis of multiple studies.
§Diseases categorized as “probably not affected” were addressed by single studies, and did not have a statistically significant association with PTPN22 1858C>T.8
Rheumatoid arthritis
In connective tissue autoimmune disease research, the majority of genetic studies of PTPN22 1858C>T have examined its association with RA. Begovich et al.2 reported the first association of PTPN22 with RA, showing in a case–control study that the PTPN22 1858T allele increased the risk of rheumatoid factor (RF)-seropositive disease (OR 1.65) in white American individuals. Subsequent haplotype analyses in RA cohorts confirmed that PTPN22 1858C>T is the SNP that confers disease risk.34–36 Notably, this SNP is responsible for the association between RA and a locus on chromosome 1p13 that had been previously identified by genetic linkage analysis.37 Although the low frequency of homozygous individuals in most studies means the confidence intervals of the odds ratios often overlap with the odds ratios for heterozygous individuals, a single copy of the 1858T variant approximately doubles the risk of RA compared with 1858C homozygocity (OR 1.5–2.0),2,38–42 indicating that the variant is a co-dominant allele.
Autoantibody status
Although PTPN22 1858C>T is associated with both autoantibody seropositive and seronegative RA, most studies have reported stronger associations of PTPN22 with RF-positive or anti-cyclic citrullinated peptide (CCP) antibody-positive RA.2,43–49 A stratified meta-analysis validated the association of PTPN22 1858T with both RF and anti-CCP autoantibody-positive RA.8 PTPN22 1858T is more common in RF-positive than in RF-negative patients (OR 1.21), and is also more common in patients with anti-CCP antibodies than those without (OR 1.45).8 Ultimately, a genome-wide association study (GWAS) of patients with RA revealed the risk effect of PTPN22 1858T is only of genome-wide significance in patients who test positive for anti-citrullinated peptide antibodies (ACPAs).50
Prediction of RA development
Studies of European early arthritis and inception cohorts revealed that, by itself, the PTPN22 1858T allele is not a good predictor of progression from undifferentiated arthritis to RA. For example, in one study, PTPN22 1858T was not associated with progression from undifferentiated arthritis to RA during a 1-year follow-up period; however, a trend of increased progression of disease in ACPA-positive cases was detected, but was not proven to be statistically significant.51 In other studies, with mixed cohorts of patients with undifferentiated arthritis and other defined arthritis syndromes, no differences were found in PTPN22 1858T allele frequency between RA and non-RA cases diagnosed at study entry or within a 2-year,52 or longer,53 follow-up period. In one report, the combination of PTPN22 1858T and anti-CCP antibody seropositivity was highly specific for RA, as only 1 of 184 patients with an arthritis other than RA was positive for this combination.53 Findings were similar in another case–control study, in which blood samples were taken from patients with RA prior to disease onset.46 PTPN22 1858T carriage alone had 80.3% specificity for the development of RA; however, PTPN22 1858T and anti-CCP antibody seropositivity were not co-present in healthy individuals (n = 368, 100% specificity). PTPN22 genotyping alone is not as effective as anti-CCP antibody analysis (98.6% specificity) in predicting RA,46 yet the strong association of PTPN22 1858T with anti-CCP antibody positive RA indicates that genotyping PTPN22 might enhance the sensitivity of testing and enable better prediction of progression to RA in patients with early signs of arthritis. Most studies showed an earlier (2–7.5 years) age at onset of RA in carriers of the PTPN22 1858T allele,38,44,48,54,55 but not all studies showed the same effect.45,56,57 This heterogeneity might result from lack of stratification of the RA cases by clinical variability, as the 1858T allele might exert a stronger effect on the age of onset of autoantibody-positive RA. Although some studies have detected an effect of PTPN22 on the presence of radiographic erosions or the rate of joint destruction in RA,47,57,58 a meta-analysis indicated no such association in either anti-CCP antibody seropositive or seronegative individuals.59
Response to therapy
Most studies report no effect of PTPN22 1858T on the response of patients with RA to treatments including methotrexate,58 rituximab,60 anti-TNF biologic agents (adalimumab, etanercept or infliximab),61 other DMARDs or prednisolone.55 Only one study has shown an effect of PTPN22 genotype on the likelihood of patients being maintained on methotrexate mono therapy; however, this study included only a small number of patients homozygous for PTPN22 1858T.62
Juvenile idiopathic arthritis
With a Norwegian case–control study, Viken et al.63 reported the first association of PTPN22 1858C>T with JIA (OR 1.41). Several other studies confirmed the genome-wide significance of PTPN22 1858T as a risk factor for oligoarticular and RF-negative polyarticular JIA in white European, American and Australian individuals, and ranked it as the second most important non-HLA genetic contributor to the risk of developing JIA.5,64,65 When patients with JIA were stratified by subgroup classification,66 PTPN22 1858T was associated with oligoarticular JIA, but not with systemic-onset or enthesitis-related JIA.39,67 It should be noted that the lack of association might reflect the limited power of these studies; only a small number of patients with rare subtypes of disease were included. Meta-analysis showed the presence of PTPN22 1858T increased the risk of both RF-positive and RF-negative polyarticular JIA.39,67
Psoriatic arthritis
PTPN22 1858C>T has a weak association with psoriatic arthritis (PsA) in white Canadian and Swedish populations (OR 1.22, shown by meta-analysis).8,68,69 A case–control study in Sweden showed the association of the PTPN22 1858T with PsA was stronger in RF-negative or anti-CCP antibody-negative patients, and that carriers had more numerous deformed joints and were more likely to have been diagnosed with dactylitis.69 In this study, the frequency of PTPN22 1858T was similar in patients with monoarthritic, oligoarthritic and polyarthritic disease.
Systemic lupus erythematosus
The first report of an association of PTPN22 1858T with SLE was from a case–control study of white North American individuals by Kyogoku et al.3 The association was replicated in case–control analyses of white and Hispanic populations (OR 1.46–1.56),8,70 and was shown to be of genome-wide significance in white North American and Swedish populations (OR 1.35).71 Consistent with the role of PTPN22 as a major autoimmunity gene, the 1858T allele seems to be a risk factor for comorbid SLE and autoimmune thyroid disease.72,73
Several studies have found no association between PTPN22 1858C>T and manifestations of SLE;3,56,74–76 however, in a case-only analysis, patients with SLE and the PTPN22 1858T allele had a greater risk of renal disorder than those homozygous for PTPN22 1858C (OR = 1.93).77 Furthermore, a GWAS to identify SNPs associated with anti-double-stranded DNA (dsDNA) antibody seropositive SLE found an association of PTPN22 1858T with seropositive SLE in a case-only analysis.78 Another study found a positive association between PTPN22 1858T and anti-cardiolipin IgG, and a trend towards an increased frequency of PTPN22 1858T in patients with lupus nephritis or in individuals seropositive for anti-dsDNA autoantibodies, although this trend was not shown to be statistically significant.73
Systemic sclerosis
A weak association occurs between PTPN22 1858C>T and SSc (OR 1.15–1.16, by meta-analysis of European individuals),8,79,80 but has no genome-wide significance.81–83 Unlike in RA studies,8 the association with SSc is not affected by the presence of autoantibodies, as meta-analysis did not reveal a difference in allele frequency when comparing anti-centromere antibody seropositive and seronegative or anti-topoisomerase I autoantibody seropositive and seronegative SSc.8,80
Vasculitis syndromes
PTPN22 1858C>T is associated with only some forms of vasculitis. One study showed a strong association (OR 1.62) between PTPN22 1858T and biopsy-proven giant cell arteritis in white European individuals.84 No specific association was found when patients were stratified by the presence of polymyalgia rheumatica, visual ischaemic manifestations or irreversible occlusive disease. Another study showed no association of PTPN22 1858C>T with Takayasu arteritis in Turkish individuals.85 Among the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV), PTPN22 1858T is associated with granulomatosis with polyangiitis (GPA) (OR 1.91), but not with eosinophilic granulomatosis with polyangiitis (also known as Churg–Strauss syndrome) or microscopic polyangiitis.86–88 In a GWAS of white individuals, PTPN22 was associated with AAV in a discovery cohort (the association was not of genome-wide significance), but no such association was found in a replication cohort. However, this study included a group of patients with either GPA or microscopic polyangiitis.89 The association with GPA is stronger in patients with organ pathology (lung, kidney, eye or peripheral nervous system).86,88 A single case–control study of white Spanish individuals showed no association of PTPN22 1858T with IgA vasculitis.90 Intriguingly, two studies reported that PTPN22 1858T can protect against Behçet disease (OR 0.65).91,92
Idiopathic inflammatory myopathy
A single case–control study reported an association of PTPN22 1858C>T with idiopathic inflammatory myopathy in white individuals from the UK (OR 1.8).93 Although the sample sizes were small when stratified into subgroups, the study suggested the association was restricted to polymyositis and juvenile dermatomyositis, and not to dermatomyositis or myositis overlapping with another connective tissue disease.93 PTPN22 1858T was not associated with dermatomyositis in a GWAS of patients with adult or juvenile dermatomyositis.94
Other immune-mediated disorders
The discovery that PTPN22 1858C>T contributes to genetic susceptibility to many, but not all, autoimmune diseases suggests fundamental similarities and differences underlie their pathogenesis. Aside from the connective tissue diseases, PTPN22 1858T is associated with many other autoimmune diseases (Box 1).6–8 Intriguingly, a number of autoimmune diseases are not associated with the 1858T allele, and the allele is protective against two autoinflammatory disorders, Crohn disease and Behçet disease.95 Generally, PTPN22 has stronger associations with autoimmune disorders in which autoantibodies have a major role in pathogenesis. An interesting hypothesis proposed by Zheng et al.8 is that the effect of PTPN22 depends upon the tissue where the autoimmunity manifests as pathology. Autoimmune diseases affecting connective tissues, joints, muscles, blood, pancreas, kidney or thyroid show a stronger association with PTPN22 than diseases of the gastrointestinal tract or immune-privileged sites, such as the central nervous system and the eye.8
Functional models for PTPN22 in disease
At the molecular level the functional effect of PTPN22 1858C>T is still under investigation. In human lymphocytes the SNP disrupts the interaction between PTPN22 and CSK,1,18,20 a potent inhibitor of TCR signalling that phosphorylates inhibitory tyrosine residues of Lck and Fyn.21,96 The interaction with CSK is believed to modulate the inhibitory function of PTPN22 in TCR signalling; however, whether the effect of CSK is to promote or inhibit the action of PTPN22, and the molecular mechanism by which CSK regulates PTPN22, is still a matter of debate.18,20,96 The effect of the PTPN22 Arg620Trp substitution on TCR signalling is controversial, with some studies suggesting increased suppression of signalling by the Trp620 variant,18,20,28 and other studies suggesting the opposite.24,30
At the cellular level, the mechanism of action of PTPN22 Trp620 is also under investigation. Reports of genotyped human primary cells and newly-described mouse models mimicking the human PTPN22 Trp620 variant have suggested several overlapping models of how PTPN22 Trp620 causes autoimmunity (Figure 3). It should be noted that translating information from mouse models to human PTPN22 biology must be considered with caution as no clear characterization of the functional differences between the human PTPN22 and mouse Ptpn22 proteins exists.
Figure 3.
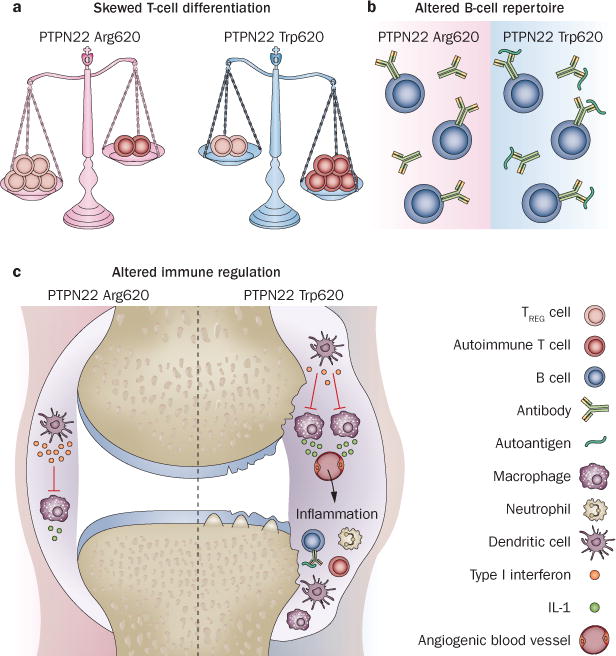
Models of PTPN22-regulated autoimmune disease. a | The PTPN22 ArgTrp620 promotes the expansion of pathogenic, autoimmune T cells. b | PTPN22 ArgTrp620 alters the B-cell repertoire, promoting autoantibody production. c | PTPN22 ArgTrp620 impairs type I interferon production by myeloid cells, which during homeostasis functions to antagonize the effect of proinflammatory cytokines, for example, to protect against arthritis in the synovium. Abbreviations: PTPN22, tyrosine-protein phosphatase nonreceptor type 22; TREG cell, regulatory T cell.
Skewed T-cell differentiation model
One functional explanation for the role of PTPN22 Trp620 in the pathogenesis of autoimmune diseases is that it alters the balance of effector T-cell and TREG-cell compartments (Figure 3a). Experiments on Ptpn22−/− mice showed that the loss of Ptpn22 causes alterations in the number and function of TREG cells and follicular helper T cells.19,22,23 The complexity of the effect of Ptpn22 in T cells is demonstrated by data from nonobese diabetic (NOD) mouse studies. Overexpression of T-cell-specific, transgenic wild type Ptpn22,97 or knockdown of Ptpn22,98 protected NOD mice from diabetes. Although these findings seem contradictory, they suggest that the balance between or within autoimmune-promoting effector T-cell and autoimmune-protecting TREG-cell compartments is finely regulated by PTPN22 expression or activity. A study of primary human T cells showed that individuals homozygous for PTPN22 Trp620 had increased type 1 T helper (TH1) cell-mediated IFN-γ responses and reduced suppression of TH1 cells by TREG cells.99
Altered B-cell repertoire model
PTPN22 ArgTrp620 could contribute to the generation of autoreactive B cells (Figure 3b). Ptpn22 Trp619 (homologous to human PTPN22 Trp620) knock-in mice have been made with various genetic backgrounds.25,30 Although the Ptpn22 Trp619 mouse on an inbred C57BL/6 background does not develop spontaneous autoimmunity, mice on a mixed C57BL/6×129 background develop spontaneous systemic autoimmunity characterized by circulating autoantibodies and immune-cell infiltration in multiple tissues, including the lungs and liver.25 Importantly, conditional overexpression of Ptpn22 Trp619 only in B cells was sufficient to cause spontaneous autoimmunity in the mixed background,25 suggesting a critical effect of Ptpn22 in regulating B-cell function that is disrupted by the Trp619 variant. In mice with a C57BL/6 or mixed background, the Arg619Trp mutation led to hyper-responsiveness of peripheral B cells to anti-IgM antibody stimulation.25,30 Considering that some of the strongest associations of PTPN22 1858C>T are with autoimmune diseases characterized by production of circulating autoantibodies (RA, SLE and type 1 diabetes), dysregulation of B-cell clonal deletion and receptor editing is likely to contribute to PTPN22-associated autoimmune diseases.100 It was reported that, compared with those without the allele, individuals with PTPN22 1858T have expanded anergic B-cell numbers, as well as transitional and naive B cells with diminished BCR signalling and resistance to BCR-stimulated apoptosis.27 The same study showed expanded transitional B-cell numbers in PTPN22 1858T carriers,27 although this effect was not found in another study.101 These data suggest the PTPN22 Trp620 variant contributes to the escape of autoreactive B cells from deletion at developmental checkpoints. Furthermore, a SNP (rs34933034) augmenting CSK expression that leads to enhanced activation of mature B cells is also a risk factor for SLE, highlighting that alterations in the PTPN22–CSK complex and signalling pathway might disrupt B-cell homeostasis and promote pathogenic alterations in the B-cell repertoire.102 Finally, PTPN22 1858C>T is associated with an increased risk of chronic lymphocytic leukaemia (CLL).103 PTPN22 is proposed to inhibit antigen-induced apoptosis of human CLL blasts;104 therefore, PTPN22 Trp620 might promote both CLL and autoimmunity by protecting autoreactive B cells from antigen-receptor-induced death.
Altered immune regulation model
PTPN22 Trp620 has also been shown to impair production of type I interferon by myeloid cells (Figure 3c).15 Type I interferons might be critical for maintaining immune homeostasis by antagonizing the action of IL-1β and TNF.105 Human cells expressing PTPN22 Trp620 have deficient TLR-induced type I interferon production, and in a model of IL-1β-dependent synovial inflammation, overexpression of transgenic human PTPN22 Trp620 in mice impaired amelioration of inflammatory arthritis by treatment with polyinosinic-polycytidylic acid, a type I interferon-inducing TLR agonist.15
Other PTPN22 polymorphisms
PTPN22 788G>A
SNPs of PTPN22 other than 1858C>T (Figure 1) have been associated with connective tissue autoimmune diseases. PTPN22 788G>A (rs33996649) is a rare missense SNP does that not co-occur with PTPN22 1858C>T and, intriguingly, it reduces the risk of both RA106 and SLE107 but is not associated with SSc80 or giant cell arteritis.84 PTPN22 788G>A encodes a loss-of-function Arg263Gln substitution in the PTPN22 catalytic domain, which changes the conformation of the active site and reduces the phosphatase activity of the protein.107
PTPN22 –1123G>C
PTPN22 –1123G>C (rs2488457) is a SNP in the promoter region of the PTPN22 gene and its function has not yet been characterized. In white Europeans, PTPN22 –1123G>C is often coexpressed with PTPN22 1858C>T, and genetic studies showed that in this group of individuals PTPN22 –1123G>C is only a minor contributor to RA risk.108 However, this SNP might be an important risk factor in Asian populations, as in case–control studies it has been shown to increase the risk of RA in Chinese individuals109,110 and ankylosing spondylitis in a Taiwanese population.111
Future directions
More molecular work is required to understand the function of PTPN22 in immune-cell homeostasis, and to clarify the effect of the PTPN22 Trp620 variant in immune-cell signalling. Autoimmune pathogenesis promoted by PTPN22 1858C>T probably involves concerted anomalies in the differentiation of T-cell subsets, B-cell repertoire and the balance between immunoregulatory and proinflammatory cytokine production. Selective expression of the PTPN22 Trp620 variant in lymphocyte subsets and other cell types in autoimmune disease models will help to clarify the cellular mechanism of action of PTPN22 in disease, and indicate whether the effect on disease pathogenesis varies between autoimmune diseases.
Studies also suggest that PTPN22 has important novel functions in myeloid cells, an area that should be further explored. For example, a report showed that neutrophils from individuals with PTPN22 1858T release increased levels of reactive oxygen species (when primed with TNF) and Ca2+ after stimulation with a chemotactic bacterial peptide mimic.112 PTPN22 might also promote IFN-γ-dependent Janus kinase–signal transducer and activator of transcription (JAK–STAT) signalling in myeloid cells, as knockdown of human THP-1 monocytes decreased STAT1 and STAT3 phosphorylation after stimulation with IFN-γ.113
Further experiments should also test whether PTPN22 is clinically relevant as a therapeutic target or a biomarker for autoimmunity. Although PTPN22 1858C>T does not seem to be a predictive marker for disease development, several studies have suggested that PTPN22 expression profiles could be used as biomarkers for RA, SLE or vasculitis. In one study, the ratio between the full-length PTPN22 and a shorter isoform (called LYP2) was higher in peripheral blood mononuclear cells from patients with RA than from healthy individuals.114 Another study showed that transcript levels encoding an isoform of PTPN22 that lacks catalytic activity (PTPN22.6) correlated with RA disease activity, as assessed by C-reactive protein-based 28-joint disease activity score (DAS28-CRP3).115 Additionally, altered histone H3 lysine 4 trimethylation of PTPN22,116 and higher levels of the PTPN22 transcript,117 have been detected in patients with SLE, compared with healthy individuals. Additionally, high PTPN22 transcript numbers in CD8+ T cells correlated with poor prognosis of SLE and AAV.118
Conclusions
PTPN22 is a major genetic risk factor for multiple connective tissue and other autoimmune diseases, including RA, JIA, PsA, SLE, SSc and some forms of vasculitis. In RA, PTPN22 is the strongest non-HLA genetic predisposition factor. Carriers of the 1858T variant are more likely to develop ACPA-negative or RF-positive disease and experience disease onset at an earlier age. Patients with early arthritis who have both the 1858T allele and ACPA seropositivity are highly likely to develop RA. Once RA has developed, the presence of the PTPN22 1858T allele does not substantially affect disease progression or severity, or whether patients will respond to anti-TNF therapy or other treatments. PTPN22 1858C>T is also a strong genetic risk factor for SLE. Carriers of the 1858T allele are at increased risk of co-occurrence of SLE and autoimmune thyroid disease. PTPN22 does not seem to affect clinical manifestations of SLE, with the possible exceptions of the development of anti-dsDNA autoantibodies and renal disorder,73 an area for further investigation. Further exploration of the immunological functions of PTPN22 will hopefully explain how it confers risk of disease and reveal any potential as a therapeutic or prognostic target for connective tissue diseases.
Key points.
-
■
PTPN22 encodes a protein tyrosine phosphatase that inhibits antigen-receptor signalling in T cells and promotes pattern-recognition receptor-induced type I interferon production by myeloid cells
-
■
PTPN22 1858C>T is a risk factor for connective tissue autoimmune diseases, including rheumatoid arthritis (RA), juvenile idiopathic arthritis, psoriatic arthritis, systemic lupus erythematosus, systemic sclerosis and some forms of vasculitis
-
■
In white populations, PTPN22 1858C>T is the most important non-HLA genetic risk factor for RA and the second most important for juvenile idiopathic arthritis
-
■
Individuals with PTPN22 1858T are more likely to develop RA with seropositivity for anti-citrullinated protein antibodies or rheumatoid factor, and have this disease at an earlier age than those without the variant
-
■
Interactions between the protein encoded by PTPN22 1858T and tyrosine-protein kinase CSK are impaired, the functional consequences of which are still under investigation
-
■
Autoimmune pathogenesis promoted by PTPN22 1858C>T probably involves the differentiation of T-cell subsets, the B-cell repertoire and the balance between immunoregulatory and proinflammatory cytokine production
Review criteria.
PubMed was searched for articles and abstracts from 2004 to the present, using the terms “PTPN22” and “lymphoid tyrosine phosphatase”. Studies relevant to autoimmunity were identified using the search terms “autoimmunity”, “autoimmune disease”, “arthritis”, “lupus”, “sclerosis” and “vasculitis”. Only English-language reports were included. Full-length papers were downloaded and reviewed and their reference lists were scanned for further relevant references. The list was last updated in April 2014.
Acknowledgments
N.B. is supported by an NIH grant (NIH R01AI070544). S.M.S. is supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation. The authors are grateful to M. Bottini for help with image preparation. This is manuscript #1684 from the La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
N.B. and S.M.S. contributed equally to researching data for the article, discussing its content, writing the article and to the review and/or editing of the manuscript before submission.
References
- 1.Bottini N, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 2.Begovich AB, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyogoku C, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viatte S, Plant D, Raychaudhuri S. Genetics and epigenetics of rheumatoid arthritis. Nat Rev Rheumatol. 2013;9:141–153. doi: 10.1038/nrrheum.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinks A, et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45:664–669. doi: 10.1038/ng.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett. 2011;585:3689–3698. doi: 10.1016/j.febslet.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Ibrahim S, Petersen F, Yu X. Meta-analysis reveals an association of PTPN22 C1858T with autoimmune diseases, which depends on the localization of the affected tissue. Genes Immun. 2012;13:641–652. doi: 10.1038/gene.2012.46. [DOI] [PubMed] [Google Scholar]
- 9.Lins TC, Vieira RG, Grattapaglia D, Pereira RW. Allele and haplotype frequency distribution in PTPN22 gene across variable ethnic groups: Implications for genetic association studies for autoimmune diseases. Autoimmunity. 2010;43:308–316. doi: 10.3109/08916930903405883. [DOI] [PubMed] [Google Scholar]
- 10.Lindenau JD, et al. Distribution patterns of variability for 18 immune system genes in Amerindians—relationship with history and epidemiology. Tissue Antigens. 2013;82:177–185. doi: 10.1111/tan.12183. [DOI] [PubMed] [Google Scholar]
- 11.Gomez LM, Anaya JM, Martin J. Genetic influence of PTPN22 R620W polymorphism in tuberculosis. Hum Immunol. 2005;66:1242–1247. doi: 10.1016/j.humimm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Lamsyah H, et al. Association of PTPN22 gene functional variants with development of pulmonary tuberculosis in Moroccan population. Tissue Antigens. 2009;74:228–232. doi: 10.1111/j.1399-0039.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 13.Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- 14.Stanford SM, Rapini N, Bottini N. Regulation of TCR signalling by tyrosine phosphatases: from immune homeostasis to autoimmunity. Immunology. 2012;137:1–19. doi: 10.1111/j.1365-2567.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, et al. The autoimmunity-associated gene PTPN22 potentiates Toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39:111–122. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa K, et al. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 17.Stanford SM, et al. Discovery of a novel series of inhibitors of lymphoid tyrosine phosphatase with activity in human T cells. J Med Chem. 2011;54:1640–1654. doi: 10.1021/jm101202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vang T, et al. LYP inhibits T-cell activation when dissociated from CSK. Nat Chem Biol. 2012;8:437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brownlie RJ, et al. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5:ra87. doi: 10.1126/scisignal.2003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorillo E, et al. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. 2010;285:26506–26518. doi: 10.1074/jbc.M110.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cloutier JF, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 22.Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J Immunol. 2014;192:1415–1424. doi: 10.4049/jimmunol.1302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maine CJ, et al. PTPN22 alters the development of regulatory T cells in the thymus. J Immunol. 2012;188:5267–5275. doi: 10.4049/jimmunol.1200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zikherman J, et al. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai X, et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arechiga AF, et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib T, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188:487–496. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieck M, et al. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 29.Menard L, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–907. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 31.Orozco G, et al. Lack of association between ankylosing spondylitis and a functional polymorphism of PTPN22 proposed as a general susceptibility marker for autoimmunity. Ann Rheum Dis. 2006;65:687–688. doi: 10.1136/ard.2005.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Australo-Anglo-American Spondyloarthritis Consortium (TASC) Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro-Marrero J, Balada E, Vilardell-Tarrés M, Ordi-Ros J. The PTPN22*R620W polymorphism does not confer genetic susceptibility to antiphospholipid syndrome in the Spanish population. Int J Immunogenet. 2011;38:529–531. doi: 10.1111/j.1744-313X.2011.01038.x. [DOI] [PubMed] [Google Scholar]
- 34.Wesoly J, et al. The 620W allele is the PTPN22 genetic variant conferring susceptibility to RA in a Dutch population. Rheumatology (Oxford) 2007;46:617–621. doi: 10.1093/rheumatology/kel381. [DOI] [PubMed] [Google Scholar]
- 35.Hinks A, Eyre S, Barton A, Thomson W, Worthington J. Investigation of genetic variation across the protein tyrosine phosphatase gene in patients with rheumatoid arthritis in the UK. Ann Rheum Dis. 2007;66:683–686. doi: 10.1136/ard.2006.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin JE, et al. Evidence for PTPN22 R620W polymorphism as the sole common risk variant for rheumatoid arthritis in the 1p13.2 region. J Rheumatol. 2011;38:2290–2296. doi: 10.3899/jrheum.110361. [DOI] [PubMed] [Google Scholar]
- 37.Michou L, et al. Linkage proof for PTPN22, a rheumatoid arthritis susceptibility gene and a human autoimmunity gene. Proc Natl Acad Sci USA. 2007;104:1649–1654. doi: 10.1073/pnas.0610250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steer S, Lad B, Grumley JA, Kingsley GH, Fisher SA. Association of R602W in a protein tyrosine phosphatase gene with a high risk of rheumatoid arthritis in a British population: evidence for an early onset/disease severity effect. Arthritis Rheum. 2005;52:358–360. doi: 10.1002/art.20737. [DOI] [PubMed] [Google Scholar]
- 39.Hinks A, et al. Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene. Arthritis Rheum. 2005;52:1694–1699. doi: 10.1002/art.21049. [DOI] [PubMed] [Google Scholar]
- 40.Simkins HM, et al. Association of the PTPN22 locus with rheumatoid arthritis in a New Zealand Caucasian cohort. Arthritis Rheum. 2005;52:2222–2225. doi: 10.1002/art.21126. [DOI] [PubMed] [Google Scholar]
- 41.van Oene M, et al. Association of the lymphoid tyrosine phosphatase R620W variant with rheumatoid arthritis, but not Crohn’s disease, in Canadian populations. Arthritis Rheum. 2005;52:1993–1998. doi: 10.1002/art.21123. [DOI] [PubMed] [Google Scholar]
- 42.Lee AT, et al. The PTPN22 R620W polymorphism associates with RF positive rheumatoid arthritis in a dose-dependent manner but not with HLA-SE status. Genes Immun. 2005;6:129–133. doi: 10.1038/sj.gene.6364159. [DOI] [PubMed] [Google Scholar]
- 43.Wesoly J, et al. Association of the PTPN22 C1858T single-nucleotide polymorphism with rheumatoid arthritis phenotypes in an inception cohort. Arthritis Rheum. 2005;52:2948–2950. doi: 10.1002/art.21294. [DOI] [PubMed] [Google Scholar]
- 44.Plenge RM, et al. Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet. 2005;77:1044–1060. doi: 10.1086/498651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison P, Pointon JJ, Farrar C, Brown MA, Wordsworth BP. Effects of PTPN22 C1858T polymorphism on susceptibility and clinical characteristics of British Caucasian rheumatoid arthritis patients. Rheumatology (Oxford) 2006;45:1009–1011. doi: 10.1093/rheumatology/kei250. [DOI] [PubMed] [Google Scholar]
- 46.Johansson M, Arlestig L, Hallmans G, Rantapää-Dahlqvist S. PTPN22 polymorphism and anti-cyclic citrullinated peptide antibodies in combination strongly predicts future onset of rheumatoid arthritis and has a specificity of 100% for the disease. Arthritis Res Ther. 2006;8:R19. doi: 10.1186/ar1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marinou I, et al. Association of interleukin-6 and interleukin-10 genotypes with radiographic damage in rheumatoid arthritis is dependent on autoantibody status. Arthritis Rheum. 2007;56:2549–2556. doi: 10.1002/art.22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlson EW, et al. Associations between human leukocyte antigen, PTPN22, CTLA4 genotypes and rheumatoid arthritis phenotypes of autoantibody status, age at diagnosis and erosions in a large cohort study. Ann Rheum Dis. 2008;67:358–363. doi: 10.1136/ard.2007.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viatte S, et al. Genetic markers of rheumatoid arthritis susceptibility in anti-citrullinated peptide antibody negative patients. Ann Rheum Dis. 2012;71:1984–1990. doi: 10.1136/annrheumdis-2011-201225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padyukov L, et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. 2011;70:259–265. doi: 10.1136/ard.2009.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feitsma AL, et al. Risk of progression from undifferentiated arthritis to rheumatoid arthritis: the effect of the PTPN22 1858T-allele in anti-citrullinated peptide antibody positive patients. Rheumatology (Oxford) 2007;46:1092–1095. doi: 10.1093/rheumatology/kem006. [DOI] [PubMed] [Google Scholar]
- 52.Goeb V, et al. Contribution of PTPN22 1858T, TNFRII 196R and HLA-shared epitope alleles with rheumatoid factor and anti-citrullinated protein antibodies to very early rheumatoid arthritis diagnosis. Rheumatology (Oxford) 2008;47:1208–1212. doi: 10.1093/rheumatology/ken192. [DOI] [PubMed] [Google Scholar]
- 53.Orozco G, et al. Auto-antibodies, HLA and PTPN22: susceptibility markers for rheumatoid arthritis. Rheumatology (Oxford) 2008;47:138–141. doi: 10.1093/rheumatology/kem343. [DOI] [PubMed] [Google Scholar]
- 54.Pierer M, et al. Association of PTPN22 1858 single-nucleotide polymorphism with rheumatoid arthritis in a German cohort: higher frequency of the risk allele in male compared to female patients. Arthritis Res Ther. 2006;8:R75. doi: 10.1186/ar1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokkonen H, Johansson M, Innala L, Jidell E, Rantapää-Dahlqvist S. The PTPN22 1858C/T polymorphism is associated with anti-cyclic citrullinated peptide antibody-positive early rheumatoid arthritis in northern Sweden. Arthritis Res Ther. 2007;9:R56. doi: 10.1186/ar2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orozco G, et al. Association of a functional single-nucleotide polymorphism of PTPN22, encoding lymphoid protein phosphatase, with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2005;52:219–224. doi: 10.1002/art.20771. [DOI] [PubMed] [Google Scholar]
- 57.Lie BA, et al. Associations between the PTPN22 1858C>T polymorphism and radiographic joint destruction in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis. 2007;66:1604–1609. doi: 10.1136/ard.2006.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Majorczyk E, Pawlik A, Kusnierczyk P. PTPN22 1858C>T polymorphism is strongly associated with rheumatoid arthritis but not with a response to methotrexate therapy. Int Immunopharmacol. 2010;10:1626–1629. doi: 10.1016/j.intimp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Taylor LH, et al. Metaanalysis of the association of smoking and PTPN22 R620W genotype on autoantibody status and radiological erosions in rheumatoid arthritis. J Rheumatol. 2013;40:1048–1053. doi: 10.3899/jrheum.120784. [DOI] [PubMed] [Google Scholar]
- 60.Daien CI, et al. TGF β1 polymorphisms are candidate predictors of the clinical response to rituximab in rheumatoid arthritis. Joint Bone Spine. 2012;79:471–475. doi: 10.1016/j.jbspin.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Potter C, et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis. 2009;68:69–74. doi: 10.1136/ard.2007.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plant D, et al. A genetic marker at the OLIG3/TNFAIP3 locus associates with methotrexate continuation in early inflammatory polyarthritis: results from the Norfolk Arthritis Register. Pharmacogenomics J. 2012;12:128–133. doi: 10.1038/tpj.2010.80. [DOI] [PubMed] [Google Scholar]
- 63.Viken MK, et al. Association analysis of the 1858C>T polymorphism in the PTPN22 gene in juvenile idiopathic arthritis and other autoimmune diseases. Genes Immun. 2005;6:271–273. doi: 10.1038/sj.gene.6364178. [DOI] [PubMed] [Google Scholar]
- 64.Hinks A, et al. Overlap of disease susceptibility loci for rheumatoid arthritis and juvenile idiopathic arthritis. Ann Rheum Dis. 2010;69:1049–1053. doi: 10.1136/ard.2009.110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellis JA, et al. Independent replication analysis of genetic loci with previous evidence of association with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2013;11:12. doi: 10.1186/1546-0096-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petty RE, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 67.Kaalla MJ, et al. Meta-analysis confirms association between TNFA-G238A variant and JIA, and between PTPN22-C1858T variant and oligoarticular, RF-polyarticular and RF-positive polyarticular JIA. Pediatr Rheumatol Online J. 2013;11:40. doi: 10.1186/1546-0096-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butt C, et al. Association of functional variants of PTPN22 and tp53 in psoriatic arthritis: a case-control study. Arthritis Res Ther. 2006;8:R27. doi: 10.1186/ar1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juneblad K, Johansson M, Rantapää-Dahlqvist S, Alenius GM. Association between the PTPN22 +1858 C/T polymorphism and psoriatic arthritis. Arthritis Res Ther. 2011;13:R45. doi: 10.1186/ar3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lea WW, Lee YH. The association between the PTPN22 C1858T polymorphism and systemic lupus erythematosus: a meta-analysis update. Lupus. 2011;20:51–57. doi: 10.1177/0961203310381774. [DOI] [PubMed] [Google Scholar]
- 71.Gateva V, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu H, et al. Association analysis of the R620W polymorphism of protein tyrosine phosphatase PTPN22 in systemic lupus erythematosus families: increased T allele frequency in systemic lupus erythematosus patients with autoimmune thyroid disease. Arthritis Rheum. 2005;52:2396–2402. doi: 10.1002/art.21223. [DOI] [PubMed] [Google Scholar]
- 73.Namjou B, et al. PTPN22 association in systemic lupus erythematosus (SLE) with respect to individual ancestry and clinical sub-phenotypes. PLoS ONE. 2013;8:e69404. doi: 10.1371/journal.pone.0069404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Budarf ML, et al. A targeted association study in systemic lupus erythematosus identifies multiple susceptibility alleles. Genes Immun. 2011;12:51–58. doi: 10.1038/gene.2010.47. [DOI] [PubMed] [Google Scholar]
- 75.Ramirez M, et al. The PTPN22 C1858T variant as a risk factor for rheumatoid arthritis and systemic lupus erythematosus but not for systemic sclerosis in the Colombian population. Clin Exp Rheumatol. 2012;30:520–524. [PubMed] [Google Scholar]
- 76.Sanchez E, et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1752–1757. doi: 10.1136/ard.2011.154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reddy MV, et al. The R620W C/T polymorphism of the gene PTPN22 is associated with SLE independently of the association of PDCD1. Genes Immun. 2005;6:658–662. doi: 10.1038/sj.gene.6364252. [DOI] [PubMed] [Google Scholar]
- 78.Chung SA, et al. Differential genetic associations for systemic lupus erythematosus based on anti-dsDNA autoantibody production. PLoS Genet. 2011;7:e1001323. doi: 10.1371/journal.pgen.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dieudé P, et al. The PTPN22 620W allele confers susceptibility to systemic sclerosis: findings of a large case-control study of European Caucasians and a meta-analysis. Arthritis Rheum. 2008;58:2183–2188. doi: 10.1002/art.23601. [DOI] [PubMed] [Google Scholar]
- 80.Diaz-Gallo LM, et al. Analysis of the influence of PTPN22 gene polymorphisms in systemic sclerosis. Ann Rheum Dis. 2011;70:454–462. doi: 10.1136/ard.2010.130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radstake TR, et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet. 2010;42:426–429. doi: 10.1038/ng.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allanore Y, et al. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet. 2011;7:e1002091. doi: 10.1371/journal.pgen.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gorlova O, et al. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet. 2011;7:e1002178. doi: 10.1371/journal.pgen.1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serrano A, et al. Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Ann Rheum Dis. 2013;72:1882–1886. doi: 10.1136/annrheumdis-2013-203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahin N, et al. PTPN22 gene polymorphism in Takayasu’s arteritis. Rheumatology (Oxford) 2008;47:634–635. doi: 10.1093/rheumatology/ken106. [DOI] [PubMed] [Google Scholar]
- 86.Jagiello P, et al. The PTPN22 620W allele is a risk factor for Wegener’s granulomatosis. Arthritis Rheum. 2005;52:4039–4043. doi: 10.1002/art.21487. [DOI] [PubMed] [Google Scholar]
- 87.Carr EJ, et al. Confirmation of the genetic association of CTLA4 and PTPN22 with ANCA-associated vasculitis. BMC Med Genet. 2009;10:121. doi: 10.1186/1471-2350-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martorana D, et al. PTPN22 R620W polymorphism in the ANCA-associated vasculitides. Rheumatology (Oxford) 2012;51:805–812. doi: 10.1093/rheumatology/ker446. [DOI] [PubMed] [Google Scholar]
- 89.Lyons PA, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orozco G, Miranda-Filloy JA, Martin J, Gonzalez-Gay MA. Lack of association of a functional single nucleotide polymorphism of PTPN22, encoding lymphoid protein phosphatase, with susceptibility to Henoch-Schonlein purpura. Clin Exp Rheumatol. 2007;25:750–753. [PubMed] [Google Scholar]
- 91.Baranathan V, et al. The association of the PTPN22 620W polymorphism with Behcet’s disease. Ann Rheum Dis. 2007;66:1531–1533. doi: 10.1136/ard.2007.073866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahin N, Bicakcigil M, Atagunduz P, Direskeneli H, Saruhan-Direskeneli G. PTPN22 gene polymorphism in Behcet’s disease. Tissue Antigens. 2007;70:432–434. doi: 10.1111/j.1399-0039.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 93.Chinoy H, et al. The protein tyrosine phosphatase N22 gene is associated with juvenile and adult idiopathic inflammatory myopathy independent of the HLA 8.1 haplotype in British Caucasian patients. Arthritis Rheum. 2008;58:3247–3254. doi: 10.1002/art.23900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller FW, et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 2013;65:3239–3247. doi: 10.1002/art.38137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galeazzi M, et al. Autoinflammatory syndromes. Clin Exp Rheumatol. 2006;24:S79–S85. [PubMed] [Google Scholar]
- 96.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeh LT, et al. Different modulation of Ptpn22 in effector and regulatory T cells leads to attenuation of autoimmune diabetes in transgenic nonobese diabetic mice. J Immunol. 2013;191:594–607. doi: 10.4049/jimmunol.1203380. [DOI] [PubMed] [Google Scholar]
- 98.Zheng P, Kissler S. PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant. Diabetes. 2013;62:896–904. doi: 10.2337/db12-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vang T, et al. The autoimmune-predisposing variant of lymphoid tyrosine phosphatase favors T helper 1 responses. Hum Immunol. 2013;74:574–585. doi: 10.1016/j.humimm.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cambier JC. Autoimmunity risk alleles: hotspots in B cell regulatory signaling pathways. J Clin Invest. 2013;123:1928–1931. doi: 10.1172/JCI69289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson WS, et al. Multi-parametric flow cytometric and genetic investigation of the peripheral B-cell compartment in human type 1 diabetes. Clin Exp Immunol. 2014 doi: 10.1111/cei.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manjarrez-Orduño N, et al. CSK regulatory polymorphism is associated with systemic lupus erythematosus and influences B-cell signaling and activation. Nat Genet. 2012;44:1227–1230. doi: 10.1038/ng.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hebbring SJ, et al. Genetic evidence of PTPN22 effects on chronic lymphocytic leukemia. Blood. 2013;121:237–238. doi: 10.1182/blood-2012-08-450221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Negro R, et al. Overexpression of the autoimmunity-associated phosphatase PTPN22 promotes survival of antigen-stimulated CLL cells by selectively activating AKT. Blood. 2012;119:6278–6287. doi: 10.1182/blood-2012-01-403162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corr M, et al. Interleukin 1 receptor antagonist mediates the beneficial effects of systemic interferon beta in mice: implications for rheumatoid arthritis. Ann Rheum Dis. 2011;70:858–863. doi: 10.1136/ard.2010.141077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodriguez-Rodriguez L, et al. The PTPN22 R263Q polymorphism is a risk factor for rheumatoid arthritis in Caucasian case-control samples. Arthritis Rheum. 2011;63:365–372. doi: 10.1002/art.30145. [DOI] [PubMed] [Google Scholar]
- 107.Orru V, et al. A loss-of-function variant of PTPN22 is associated with reduced risk of systemic lupus erythematosus. Hum Mol Genet. 2009;18:569–579. doi: 10.1093/hmg/ddn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dieudé P, et al. Testing for linkage and association with rheumatoid arthritis a Ptpn22 promoter polymorphism reported to be associated and linked with type 1 diabetes in the Caucasian population. Ann Rheum Dis. 2008;67:900–901. doi: 10.1136/ard.2007.077180. [DOI] [PubMed] [Google Scholar]
- 109.Feng X, et al. Association of the PTPN22 gene (–1123G>C) polymorphism with rheumatoid arthritis in Chinese patients. Tissue Antigens. 2010;76:297–300. doi: 10.1111/j.1399-0039.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- 110.Huang JJ, et al. A PTPN22 promoter polymorphism –1123G>C is associated with RA pathogenesis in Chinese. Rheumatol Int. 2012;32:767–771. doi: 10.1007/s00296-010-1705-x. [DOI] [PubMed] [Google Scholar]
- 111.Huang CH, et al. Associations of the PTPN22 and CTLA-4 genetic polymorphisms with Taiwanese ankylosing spondylitis. Rheumatol Int. 2013;34:683–691. doi: 10.1007/s00296-013-2894-x. [DOI] [PubMed] [Google Scholar]
- 112.Bayley R, et al. The autoimmune-associated genetic variant PTPN22 R620W enhances neutrophil activation and function in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204796. http://dx.doi.org/10.1136/annrheumdis-2013-204796. [DOI] [PubMed]
- 113.Spalinger MR, et al. Loss of protein tyrosine phosphatase nonreceptor type 22 regulates interferon-γ-induced signaling in human monocytes. Gastroenterology. 2013;144:978–988. doi: 10.1053/j.gastro.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 114.Ronninger M, et al. The balance of expression of PTPN22 splice forms is significantly different in rheumatoid arthritis patients compared with controls. Genome Med. 2012;4:2. doi: 10.1186/gm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang HH, et al. PTPN22.6, a dominant negative isoform of PTPN22 and potential biomarker of rheumatoid arthritis. PLoS ONE. 2012;7:e33067. doi: 10.1371/journal.pone.0033067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dai Y, Zhang L, Hu C, Zhang Y. Genome-wide analysis of histone H3 lysine 4 trimethylation by ChIP-chip in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Clin Exp Rheumatol. 2010;28:158–168. [PubMed] [Google Scholar]
- 117.Chang HH, Tseng W, Cui J, Costenbader K, Ho IC. Altered expression of protein tyrosine phosphatase, non-receptor type 22 isoforms in systemic lupus erythematosus. Arthritis Res Ther. 2014;16:R14. doi: 10.1186/ar4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKinney EF, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586–591. doi: 10.1038/nm.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Criswell LA, et al. Analysis of families in the Multiple Autoimmune Disease Genetics Consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graf SA, Calado RT, Young NS. PTPN22 620W allele is not associated with aplastic anemia. Am J Hematol. 2007;82:291–292. doi: 10.1002/ajh.20768. [DOI] [PubMed] [Google Scholar]
- 121.Brzoza Z, Grzeszczak W, Trautsolt W, Moczulski D. Protein tyrosine phosphatase-22 (PTPN-22) polymorphism in the pathogenesis of chronic urticaria. Allergy. 2011;66:1392–1393. doi: 10.1111/j.1398-9995.2011.02651.x. [DOI] [PubMed] [Google Scholar]


