Abstract
In this review we focus on lymphoepithelioma-like hepatocellular carcinomas (LEL-HCC) and lymphoepithelioma-like cholangiocarcinomas (LEL-ICC). Despite their rarity, these tumors are of general interest because of their epidemiological and clinical features, and because they represent a distinct model of interaction between the immune system and neoplastic cells. Approximately half of LEL-HCC arise in the context of chronic hepatitis C virus (HCV) infection and have been described both in Eastern and Western patients. By contrast, LEL-ICC is associated in almost all cases with Epstein-Barr virus (EBV) infection and exhibits the same epidemiological features of EBV related malignancies. Compared with classical hepatocellular carcinoma and intrahepatic cholangiocarcinoma of corresponding stage, both LEL-HCC and LEL-ICC are characterized by lower rates of recurrence after surgery and better overall survival. How this behavior is related to distinct genetic alterations and tumor microenvironment is unclear. The pathophysiological mechanisms of lymphoid infiltrations seem to be different among the two groups of tumors. In fact, LEL-HCC frequently arises in the context of inflammatory changes driven by HCV infection, and has been recognized as a variant of classical hepatocellular carcinoma. At variance, lymphocyte recruitment of LEL-ICC is similar to that described in nasopharyngeal carcinoma and gastric LEL, and possibly depends on the expression pattern of latent EBV infection.
Keywords: Lymphoepithelioma, Lymphoepithelioma-like carcinoma, Nasopharyngeal carcinoma, Lymphoepithelioma-like hepatocellular carcinoma, Lymphoepithelioma-like intrahepatic cholangiocarcinoma, Epstein-Barr virus infection
Core tip: Despite their rarity, lymphoepithelioma-like hepatic carcinomas are of general interest because of their peculiar epidemiological and clinical features, and because they represent a distinct model of interaction between the immune system and neoplastic cells. Compared with classical hepatocellular carcinoma and intrahepatic cholangiocarcinoma of corresponding stage, lymphoepithelioma-like hepatic carcinomas are characterized by lower rates of recurrence after surgery and better overall survival. Whether these differences are related to distinct genetic alterations or to the tumor microenvironment is unclear. Here we review the features of these tumors and the mechanisms of lymphoid infiltration.
INTRODUCTION
The term lymphoepithelioma denotes a subgroup of nasopharyngeal carcinomas. These tumors are mostly related to Epstein-Barr virus (EBV) infection, and are characterized by the concomitance of undifferentiated carcinoma cells and polyclonal lymphocyte infiltration[1-4]. Tumors with similar morphological features, lymphoepithelioma-like (LEL) carcinomas, have been described in salivary glands[5], lungs[6], thymus[7], stomach[8], colon[9], uterus[10], ovaries[11], bladder and urinary tract[12], breast[13], and skin[14]. Although the association with EBV infection was confirmed in LEL carcinomas of the digestive tract[15], lung[16,17], and thymus[18], it was not found in LEL carcinomas of the breast[19] and uterus[20]. In the liver, this type of tumor is extremely rare. Thus far, 29 cases of LEL hepatocellular carcinoma (HCC) and 24 cases of LEL intrahepatic cholangiocarcinoma (ICC) have been described.
LEL-HCC
In 24 of 29 cases[21-29], the diagnosis of LEL-HCC was based on the retrospective analysis of three different series: one from Japan[21], one from France[22], and one from the United States[23]. These studies were conducted in different periods and differ substantially in design and patient clinicopathological features. Considering these caveats, all three of the studies suggest that patients with LEL-HCC have a better outcome compared with patients with classical hepatocellular carcinoma. The study from Japan dates back to 1994 and was aimed at evaluating the features and the long-term outcome of 11 hepatocellular carcinomas heavily infiltrated by inflammatory cells. The investigation was limited to tumors of less than 3 cm in diameter. The rate of recurrence at 5 years after surgery was 9.1 percent in these patients and 47.5 percent in 116 controls matched for the etiology of the associated liver disease and tumor size. The study from France evaluated the features and the disease course after liver transplantation in 5 patients with hepatocellular carcinoma with lymphoid infiltration. The 5-year survival was significantly better when compared with 163 patients transplanted for hepatocellular carcinoma. Finally, the study from the United States compared 8 cases of resected tumors classified as inflammatory hepatocellular carcinoma with 18 undifferentiated hepatocellular carcinomas. The rates of local recurrence and distant metastases were 25% and 33.3% respectively, for those patients with inflammatory hepatocellular carcinoma, and 12.5% and 22.5%, respectively for those patients with undifferentiated hepatocellular carcinoma. When the survival data from all 29 patients with LEL-HCC were pooled together, it was evident that 19 patients were alive and free of disease 15 mo to 10 years after surgery (median: 43 mo). Six patients were alive with disease recurrence, and 4 patients died of recurrent disease.
The retrospective design of the above studies implicates that the pre-operative features of these patients are largely unknown. In particular, descriptions of the radiological findings are available in only two cases. In one patient, both computed tomography (CT) and magnetic resonance imaging (MRI) scans showed the features of classical hepatocellular carcinoma, as enhancement in the arterial phase and wash-out in the portal phase were observed[24]. In the second patient, the CT scan did not show arterial enhancement, whereas the contrast phase of MRI showed peripheral rim enhancement and hyper-intensity of the nodule in T2 sequences[25]. LEL-HCC was associated with liver cirrhosis in 13 cases, with hepatitis C virus (HCV) infection in 16 cases, hepatitis B virus (HBV) in 3 cases, and EBV in 1 case. Histopathological analysis of LEL-HCC showed that in 16 cases the tumors were poorly differentiated (Figure 1). Well-differentiated or moderately-differentiated tumors were observed in the remaining cases. Interestingly, in 3 cases the same tumor exhibited different grades of differentiation. Positivity for HepPar 1 was described in 3 of 3 tested cases. Cytokeratin 7 and 19 were positive in 4 cases. The overexpression of p16 protein was found in 2 cases. Nests of epithelial cells were surrounded by polyclonal lymphoid cells. Lymphocyte subset analysis showed that CD3-positive cells were 10 times more frequent than B cells. The majority of T cells were CD8-positive in 13 cases, whereas FoxP3 positive cells represented a minority in the single tested case. Remarkably, and similar to the metastases of undifferentiated NPC, the nodal metastatic lesions from LEL-HCC did not show lymphocytic infiltration. The molecular changes associated with LEL-HCC are unknown.
Figure 1.
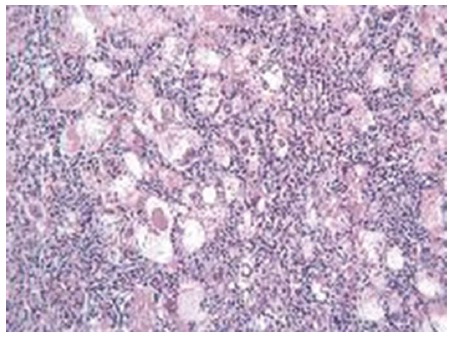
Histological picture of lymphoepitelioma-like hepatocellular carcinoma. Undifferentiated neoplastic cells are surrounded by lymphoid stroma (magnification × 250) (Obtained from Nemolato et al[29], reprinted with permission).
LEL-ICC
Twenty-two of the 24 patients with LEL-ICC were from South-East Asia. In seven of them, the diagnosis was made in surgically resected patients in a single center in Hong-Kong, between 1999 and 2008, and accounted for 5% of all ICC in that period[30]. The 5-year survival rate of these patients was 100 per cent and was significantly better compared with the 13.2 percent observed in 11 matched controls (classical cholangiocarcinomas). Five cases were from a single center in Taiwan[31]. The remaining patients were from a single case report[32-42]. At the time of writing, fifteen patients were alive without disease 2 to 165 mo after surgery (median recurrence free survival: 39 mo), and three patients were alive with recurrent disease (median survival: 56 mo). In addition, four patients died of recurrent disease (median survival: 48 mo), one patient died of post-operative complications and, in another case, no information on survival is available. Descriptions of the CT scans were available only in five cases and were limited to the unenhanced phase[30,32,35,42]. In these cases, hypodense lesions ranging from 3 to 10 cm in diameter were described. Dynamically enhanced MR T1 imaging was available only in one case, and (similar to ICC) showed centrifugal enhancement of the neoplastic lesion[30]. LEL-ICC was associated with cirrhosis in 6 cases. HBV infection was documented in 6 cases and HCV infection in two cases. EBV infection was found in 17 cases. Histopathology showed the features of adenocarcinoma with different grades of glandular differentiation and polyclonal lymphocytic infiltration. Interestingly, in at least seven cases, two different components were recognized within the tumor[31,32,34,37,38,40]. One component consisted of undifferentiated lymphoepithelioma-like cholangiocarcinoma, whereas the other consisted of an adenocarcinoma without lymphocytic infiltration. CK AE1/A3, CK7, and CK19 immunoreactivity was detected in all of the cases but one. The latter tumor was in fact HepPar1 positive, raising the possibility of a misdiagnosed HCC, or a combined hepatocellular and cholangiocarcinoma[38]. Immunolabeling for markers of stemness, such as CD133 and EpCam, was positive in 5 of 5 evaluated cases[30]. In situ hybridization for detection of EBV non coding RNA, using antisense oligoprobes, was positive within the epithelial cells in 17 cases. Lymphocytic infiltration consisted of CD3 and CD20 positive cells, and was consistently negative for markers of active or latent EBV infection. One single study[30] addressed the gene methylation status and the mutations of KRAS and EGFR genes in LEL-ICC. Loci coding for CRBPI (cellular retinol binding protein I) and CRBPIV (cellular retinol binding protein IV) showed significantly higher methylation status than in ICC. This finding is in agreement with the epigenetic changes occurring in EBV-related nasopharyngeal carcinoma. Wild-type KRAS and EGFR genes were detected in all of the cases.
INTRAHEPATIC LYMPHOEPITHELIOMA-LIKE CARCINOMA: WORKING HYPOTHESES
Hepatic LEL carcinomas, similar to gastric and lung LEL carcinomas, are characterized by a significantly better survival than classical HCC and ICC counterparts of corresponding stage. Whether the more favorable outcome of hepatic LEL carcinomas depends on distinct genetic and epigenetic changes, or whether the tumor infiltrating lymphocytes play a prominent role in improving the outcome of these patients remains unclear. In turn, the baseline features and the outcome of hepatic LEL carcinomas are also related to the clinical setting in which the tumor arises. Notably, the patients with LEL-HCC and LEL-ICC (Table 1) differ with regard to age, tumor size, presence of cirrhosis, and rate of HCV and EBV infections. The World Health Organization recently recognized LEL-HCC as a variant of HCC[43]. Indeed, HCC is characterized by different grades of lymphoid infiltration[44,45] and LEL-HCC represents an end of this spectrum. The molecular mechanisms linking inflammation and cancer depend on the complex network of chemokine and cognate receptor axes[46-53]. In the setting of HCC, it has been shown that the CXCL12[54,55] CXCL8[56], CCL3[57] CCL20[58] and CCL22[59] ligands, and the aberrant expression of their receptors affect tumor development and progression, angiogenesis and metastasis. Several lines of evidence, however, note that the chemokine system also has a role in tumor control. Myeloid cell infiltration is associated with a poor prognosis, whereas T helper 1 infiltration directly correlates with a reduced risk of tumor recurrence[60]. In addition, a pro-inflammatory microenvironment characterized by high expression of the innate immune genes TNF, IL6, and CCL2 is a predictor of survival[61]. A validated model of a 14 immune gene signature, in patients resected for early HCC, is also associated with a better prognosis[62]. Among the genes of this signature with increased expression of particular interest include CXCL10, CCL5, and CCL2. These chemokines are related with Th1 and NK cell recruitment. Potentially tumors with the features of LEL-HCC are characterized by a similar chemokine profile.
Table 1.
Characteristics of the patients with lymphoepitelioma-like hepatic carcinomas n (%)
| LEL-HCC (n = 29) | LEL-ICC (n = 24) | |
| Age at diagnosis, median (range) | 61 (39-79) | 53 (19-71) |
| HBV infection (positive) | 3 | 7 |
| HCV infection (positive) | 16 | 2 |
| EBV infection (positive) | 3 | 17 |
| Cirrhosis | 13 | 6 |
| Maximum diameter of the tumor (mm) | 25 (13-130) | 45 (16-160) |
| Surgical treatment (OLT/resection) | 6/23 | 0/24 |
| Rate of recurrence | 6 (20.6) | 8 (36.3) |
LEL-HCC: Lymphoepithelioma-like hepatocellular carcinomas; LEL-ICC: Lymphoepithelioma-like cholangiocarcinomas; EBV: Epstein-Barr virus; OLT: Orthotopic liver transplantation; HCV: Hepatitis C virus; HBV: Hepatitis B virus.
LEL-HCC arise in nearly half of the cases in the context of HCV-related cirrhosis. In these patients, chemokine-driven inflammatory changes related to HCV chronic hepatitis may play a role in LEL-HCC development and in lymphocyte recruitment[63-66]; however, LEL-HCC also arises in apparently normal livers. Whether lymphocyte recruitment is driven by the same genetic alterations in both groups of LEL-HCC remains unknown.
In comparison with LEL-HCC, LEL-ICC contains a higher proportion of tumorous cells with features of stemness. Indeed, 7 of 7 samples were positive for EpCam and CD133 (Table 2). In addition, in at least 2 cases the tumor exhibited the features of hepatocellular and cholangiocarcinoma. In contrast to LEL-HCC, LEL-ICC is associated in almost all cases with EBV infection and exhibits the same epidemiological features of EBV related malignancies[67]. Consequently, the mechanistic of LEL-ICC should be analyzed considering this background. EBV infection is detected in nearly all patients with endemic Burkitt lymphoma[68]. By contrast, the association of EBV infection with other malignancies such as Hodgkin and non-Hodgkin lymphomas[69], post-transplant lympho-proliferative disorders[70], gastric adenocarcinomas[71], leiomyosarcomas[72], and NPC[73] is variable and is influenced by several factors, including age of infection, ethnicity, genetic susceptibility, socio-economic status, and immune function[74,75]. Intriguingly, EBV-related NPC has a geographical and racial distribution similar to that of intra-hepatic LEL-ICC. In contrast to LEL-ICC, the genetic alterations occurring in NPC have been extensively studied[49-53,76-80]. Several chromosomal abnormalities, including copy number changes on chromosomes 3p, 9p, 11q, 12p and 14q, and gene alterations, such as CDKN2A deletion and LTBR (lymphotoxin beta receptor) amplification, together with epigenetic changes, such as RASSF1A and TSLC promoter hypermethylation, have been described. According to the Catalogue of Somatic Mutations in Cancer (COSMIC), the most frequent mutations in NPC affect the CDKN2A gene (11% of tested samples), which encodes for the p16INK4A protein, and PIK3CA[81]. The prominent lymphocyte infiltration of NPC is a model of the complex interactions between tumor and immune system[82-86]. Most of the infiltrating lymphocytes consist of CD3 positive T cells; among them, the majority show the morphology of small resting lymphocytes. The ratio of CD4 to CD8 ranges from 0.4 to 2.2. Regulatory CD4 and CD25high and FoxP3 positive cells represent 12% of all T cells. Two distinct subpopulations of CD8 positive lymphocytes were described: pro-inflammatory interleukin (IL)-17 secreting cells, and regulatory CD8 cells, whereas NK and B lymphocytes represent a minority of the tumor infiltrating lymphocytes. In addition, it has been shown that malignant NPC cells constitutively produce several cytokines, including IL-1α, IL-1β, and IL-18[87,88], and chemokines including CCL20 and CXCL10[89,90]. Infiltrating leucocytes in turn further amplify the inflammatory process by positive regulatory loops. Clinical and experimental findings indicate that NPC associated lymphocytes do not control tumor growth. Conversely, inflammation promotes tumor progression, as cytokines behave as tumoral growth factors. Immune escape is related to CCL20 dependent Treg expansion[89] and resistance to interferon γ as a result of small EBV-encoded RNAs (EBERs) and latent membrane protein (LMP) 2A and B secretion[91]. The complex interactions described in NPC possibly differ in other types of LEL carcinomas, depending on the burden of EBV intermediates and the latent gene expression. Three forms of EBV latency have been recognized[92,93]. Latency I is characterized by the expression of Epstein Barr nuclear antigen (EBNA) 1, EBERs, and BamHI A rightward transcripts (BARTs). This expression pattern is found in Burkitt lymphoma[94]. By contrast, latency II is characterized by the variable expression of LMP1, LMP-2A and LMP-2B, BARTs, and EBERs. This pattern is typical of NPC[95,96]. Latency III is characterized by the expression of EBNA 1, EBNA2, EBNA3, EBNA3B, EBNA3C, EBNA LP, LMP-1/2, BARTs, and EBERs. This expression pattern is found in isolated cell lines, and in the elderly with lymphomas[97,98]. Finally, viral reactivation from latency expresses the BamHI Z leftward open reading frame[99]. In the setting of LEL carcinomas, the evidence that the lymphoid stroma is related to the pattern of EBV latency is provided by the finding that gastric LEL carcinoma, expressing EBNA1 mRNA, BARTs and LPM2, was associated with olygoclonal CD8 positive EBV specific lymphocytes[100]. The evaluation of the EBV pattern of latency in LEL-ICC has been incompletely elucidated. EBERs were positive in 18 of 18 samples, LMP was negative in 8 of the samples by immune-histology[30,40], and the LMP-related gene showed a 30 bp deletion in 2 of 2 cases[35,36]. The EBNA2 gene was found in one of one tested case. Progression of LEL-ICC is possibly associated with further molecular changes. In this respect, it is of interest that in more than one third of LEL-ICC cases, the tumor consisted of LEL-ICC and ICC with different grades of differentiation, and metastases of LEL-ICC usually lost the capacity to recruit inflammatory cells. These histological features implicate the concomitance of two different neoplastic clones. In contrast to HCC, little is known regarding the chemokines associated with ICC. Thus far, the single relevant finding in this regard is the overexpression of CXCL5[101]. In ICC this chemokine has been associated with tumor progression and metastasis. The chemokines involved in HCC- LEL and in ICC-LEL are unknown.
Table 2.
Pathological findings in patients with lymphoepithelioma-like-hepatic carcinomas
| LEL-HCC (n = 29) | LEL-ICC (n = 24) | ||
| Histological features | |||
| Poorly differentiated | 16/29 | 10/24 | |
| Moderately differentiated | 13/29 | 3/24 | |
| Combined LEL-HCC and HCC | None | ||
| Combined LEL-ICC and ICC | 9/24 | ||
| Immunohistology | |||
| HepPAR1 | 3/3 | 1/2 | |
| CK7 and CK19 | 4/11 | 24/24 | |
| EpCAM | ND | 7/7 | |
| CD133 | ND | 7/7 | |
| EBV status | |||
| EBERs | 3/18 | 17/24 | |
| LMP1/2 antigens | ND | 0/8 | |
| LMP1 gene | ND | 2/2 | |
| EBNA2 | ND | 1/1 | |
| Lymphoid infiltration | |||
| CD3/CD20 ratio > 1 | 29/29 | 4/4 | |
| CD4/CD8 ratio < 1 | 11/29 | 1/4 | |
LEL-HCC: Lymphoepithelioma-like hepatocellular carcinomas; LEL-ICC: Lymphoepithelioma-like cholangiocarcinomas; EBV: Epstein-Barr virus; EBERs: EBV-encoded RNAs; LMP: Latent membrane protein; EBNA2: Epstein Barr nuclear antigen 2; ND: Not done.
The features of intrahepatic LEL carcinomas and their molecular differences compared with classical HCC and ICC warrant further study. Analysis of archival samples and prospective whole genome analysis of new cases would provide new insights into this issue. Given their rarity, addressing the above issues would be feasible only through a close cooperation between all the centers following these patients via the generation of a dedicated registry and the commitment of high profile basic researchers.
Footnotes
Conflict-of-interest: The Authors have no conflict of interests to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 15, 2014
First decision: November 14, 2014
Article in press: February 5, 2015
P- Reviewer: Govaere O, Pote N, Ribeiro-Silva A S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Fee WE. Nasopharyngeal carcinoma. Curr Opin Oncol. 1990;2:585–588. [PubMed] [Google Scholar]
- 2.Klein G, Giovanella BC, Lindahl T, Fialkow PJ, Singh S, Stehlin JS. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci USA. 1974;71:4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshizaki T, Kondo S, Wakisaka N, Murono S, Endo K, Sugimoto H, Nakanishi S, Tsuji A, Ito M. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337:1–7. doi: 10.1016/j.canlet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 5.Ma H, Lin Y, Wang L, Rao H, Xu G, He Y, Liang Y. Primary lymphoepithelioma-like carcinoma of salivary gland: sixty-nine cases with long-term follow-up. Head Neck. 2014;36:1305–1312. doi: 10.1002/hed.23450. [DOI] [PubMed] [Google Scholar]
- 6.Huang YC, Hsueh C, Ho SY, Liao CY. Lymphoepithelioma-like carcinoma of the lung: an unusual case and literature review. Case Rep Pulmonol. 2013;2013:143405. doi: 10.1155/2013/143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen PC, Pan CC, Yang AH, Wang LS, Chiang H. Detection of Epstein-Barr virus genome within thymic epithelial tumours in Taiwanese patients by nested PCR, PCR in situ hybridization, and RNA in situ hybridization. J Pathol. 2002;197:684–688. doi: 10.1002/path.1141. [DOI] [PubMed] [Google Scholar]
- 8.Bittar Z, Fend F, Quintanilla-Martinez L. Lymphoepithelioma-like carcinoma of the stomach: a case report and review of the literature. Diagn Pathol. 2013;8:184. doi: 10.1186/1746-1596-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samaha S, Tawfik O, Horvat R, Bhatia P. Lymphoepithelioma-like carcinoma of the colon: report of a case with histologic, immunohistochemical, and molecular studies for Epstein-Barr virus. Dis Colon Rectum. 1998;41:925–928. doi: 10.1007/BF02235379. [DOI] [PubMed] [Google Scholar]
- 10.Mori T, Sawada M, Matsuo S, Kuroboshi H, Tatsumi H, Iwasaku K, Kitawaki J. Lymphoepithelial-like carcinoma of the uterine cervix; a case report. Eur J Gynaecol Oncol. 2011;32:325–327. [PubMed] [Google Scholar]
- 11.Lee S, Park SY, Hong EK, Ro JY. Lymphoepithelioma-like carcinoma of the ovary: a case report and review of the literature. Arch Pathol Lab Med. 2007;131:1715–1718. doi: 10.5858/2007-131-1715-LCOTOA. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Ando T, Nomura T, Sato F, Mimata H. Lymphoepithelioma-like carcinoma of the bladder: a case report and review of the literature. Case Rep Urol. 2013;2013:356576. doi: 10.1155/2013/356576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinniwell R, Hanna WM, Mashhour M, Saad RS, Czarnota GJ. Lymphoepithelioma-like carcinoma of the breast: a diagnostic and therapeutic challenge. Curr Oncol. 2012;19:e177–e183. doi: 10.3747/co.19.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López V, Martín JM, Santonja N, Molina I, Ramón D, Monteagudo C, Jordá E. Lymphoepitelioma-like carcinoma of the skin: report of three cases. J Cutan Pathol. 2011;38:54–58. doi: 10.1111/j.1600-0560.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Kim KM, Min BH, Lee JH, Rhee PL, Kim JJ. Epstein-Barr virus-associated lymphoepithelioma-like early gastric carcinomas and endoscopic submucosal dissection: case series. World J Gastroenterol. 2014;20:1365–1370. doi: 10.3748/wjg.v20.i5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittaluga S, Wong MP, Chung LP, Loke SL. Clonal Epstein-Barr virus in lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol. 1993;17:678–682. doi: 10.1097/00000478-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Román JJ, Martínez MN, Fernández SL, Val-Bernal JF. Epstein-Barr virus-associated adenocarcinomas and squamous-cell lung carcinomas. Mod Pathol. 2009;22:530–537. doi: 10.1038/modpathol.2009.7. [DOI] [PubMed] [Google Scholar]
- 18.Koppula BR, Pipavath S, Lewis DH. Epstein-Barr virus (EBV) associated lymphoepithelioma-like thymic carcinoma associated with paraneoplastic syndrome of polymyositis: a rare tumor with rare association. Clin Nucl Med. 2009;34:686–688. doi: 10.1097/RLU.0b013e3181b53f5a. [DOI] [PubMed] [Google Scholar]
- 19.Nio Y, Tsuboi K, Tamaoki M, Tamaoki M, Maruyama R. Lymphoepithelioma-like carcinoma of the breast: a case report with a special analysis of an association with human papilloma virus. Anticancer Res. 2012;32:1435–1441. [PubMed] [Google Scholar]
- 20.Noel J, Lespagnard L, Fayt I, Verhest A, Dargent J. Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: report of two cases and review of the literature. Hum Pathol. 2001;32:135–138. doi: 10.1053/hupa.2001.20901. [DOI] [PubMed] [Google Scholar]
- 21.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 22.Emile JF, Adam R, Sebagh M, Marchadier E, Falissard B, Dussaix E, Bismuth H, Reynès M. Hepatocellular carcinoma with lymphoid stroma: a tumour with good prognosis after liver transplantation. Histopathology. 2000;37:523–529. doi: 10.1046/j.1365-2559.2000.00952.x. [DOI] [PubMed] [Google Scholar]
- 23.Patel KR, Liu TC, Vaccharajani N, Chapman WC, Brunt EM. Characterization of inflammatory (lymphoepithelioma-like) hepatocellular carcinoma: a study of 8 cases. Arch Pathol Lab Med. 2014;138:1193–1202. doi: 10.5858/arpa.2013-0371-OA. [DOI] [PubMed] [Google Scholar]
- 24.Shinoda M, Kadota Y, Tsujikawa H, Masugi Y, Itano O, Ueno A, Mihara K, Hibi T, Abe Y, Yagi H, et al. Lymphoepithelioma-like hepatocellular carcinoma: a case report and a review of the literature. World J Surg Oncol. 2013;11:97. doi: 10.1186/1477-7819-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HS, Jang KY, Kim YK, Cho BH, Moon WS. Hepatocellular carcinoma with massive lymphoid infiltration: a regressing phenomenon? Pathol Res Pract. 2009;205:648–652. doi: 10.1016/j.prp.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Szekely E. Hepatocellular carcinoma with lymphoid stroma: ‘lymphoepithelioma-like carcinoma’? Histopathology. 2001;39:540. doi: 10.1046/j.1365-2559.2001.1301a.x. [DOI] [PubMed] [Google Scholar]
- 27.Si MW, Thorson JA, Lauwers GY, DalCin P, Furman J. Hepatocellular lymphoepithelioma-like carcinoma associated with epstein barr virus: a hitherto unrecognized entity. Diagn Mol Pathol. 2004;13:183–189. doi: 10.1097/01.pas.0000124336.90615.8d. [DOI] [PubMed] [Google Scholar]
- 28.Chen CJ, Jeng LB, Huang SF. Lymphoepithelioma-like hepatocellular carcinoma. Chang Gung Med J. 2007;30:172–177. [PubMed] [Google Scholar]
- 29.Nemolato S, Fanni D, Naccarato AG, Ravarino A, Bevilacqua G, Faa G. Lymphoepitelioma-like hepatocellular carcinoma: a case report and a review of the literature. World J Gastroenterol. 2008;14:4694–4696. doi: 10.3748/wjg.14.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan AW, Tong JH, Sung MY, Lai PB, To KF. Epstein-Barr virus-associated lymphoepithelioma-like cholangiocarcinoma: a rare variant of intrahepatic cholangiocarcinoma with favourable outcome. Histopathology. 2014;65:674–683. doi: 10.1111/his.12455. [DOI] [PubMed] [Google Scholar]
- 31.Jeng YM, Chen CL, Hsu HC. Lymphoepithelioma-like cholangiocarcinoma: an Epstein-Barr virus-associated tumor. Am J Surg Pathol. 2001;25:516–520. doi: 10.1097/00000478-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Hsu HC, Chen CC, Huang GT, Lee PH. Clonal Epstein-Barr virus associated cholangiocarcinoma with lymphoepithelioma-like component. Hum Pathol. 1996;27:848–850. doi: 10.1016/s0046-8177(96)90460-8. [DOI] [PubMed] [Google Scholar]
- 33.Vortmeyer AO, Kingma DW, Fenton RG, Curti BD, Jaffe ES, Duray PH. Hepatobiliary lymphoepithelioma-like carcinoma associated with Epstein-Barr virus. Am J Clin Pathol. 1998;109:90–95. doi: 10.1093/ajcp/109.1.90. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz MR, Garijo G, Adrados M, López-Bonet E, Acero D, Bernadó L. Epstein-Barr Virus-Associated Cholangiocarcinoma with Lymphoepithelioma-Like Component. Int J Surg Pathol. 2000;8:347–351. doi: 10.1177/106689690000800418. [DOI] [PubMed] [Google Scholar]
- 35.Chen TC, Ng KF, Kuo T. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like component. Mod Pathol. 2001;14:527–532. doi: 10.1038/modpathol.3880342. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Tsung JS, Lin CW, Cheng TY. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like carcinoma component. Ann Clin Lab Sci. 2004;34:476–480. [PubMed] [Google Scholar]
- 37.Henderson-Jackson E, Nasir NA, Hakam A, Nasir A, Coppola D. Primary mixed lymphoepithelioma-like carcinoma and intra-hepatic cholangiocarcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2010;3:736–741. [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YB, Park YN, Han JY, Hong KC, Hwang TS. Biliary lymphoepithelioma-like carcinoma not associated with Epstein-Barr virus. Arch Pathol Lab Med. 1999;123:441–443. doi: 10.5858/1999-123-0441-BLLCNA. [DOI] [PubMed] [Google Scholar]
- 39.Szekely E. Lymphoepithelioma-like cholangiocarcinoma (LELC) not associated with Epstein-Barr virus. Am J Surg Pathol. 2001;25:1464–1466. doi: 10.1097/00000478-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Adachi S, Morimoto O, Kobayashi T. Lymphoepithelioma-like cholangiocarcinoma not associated with EBV. Pathol Int. 2008;58:69–74. doi: 10.1111/j.1440-1827.2007.02192.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee W. Intrahepatic lymphoepithelioma-like cholangiocarcinoma not associated with epstein-barr virus: a case report. Case Rep Oncol. 2011;4:68–73. doi: 10.1159/000324485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hur YH, Kim HH, Koh YS, Seoung JS, Cho CK. Lymphoepithelioma-like cholangiocarcinoma not associated with Epstein-Barr virus. ANZ J Surg. 2011;81:652–653. doi: 10.1111/j.1445-2197.2011.05840.x. [DOI] [PubMed] [Google Scholar]
- 43.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors . Classification of Tumours. 3rd ed. Lyon, France: IARC Press; 2010. [Google Scholar]
- 44.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 45.Sprinzl MF, Galle PR. Immune control in hepatocellular carcinoma development and progression: role of stromal cells. Semin Liver Dis. 2014;34:376–388. doi: 10.1055/s-0034-1394138. [DOI] [PubMed] [Google Scholar]
- 46.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 47.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 48.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 49.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, Coward JI, Schioppa T, Robinson SC, Gallagher WM, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16:1832–1836. doi: 10.3748/wjg.v16.i15.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoong KF, Afford SC, Jones R, Aujla P, Qin S, Price K, Hubscher SG, Adams DH. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100–111. doi: 10.1002/hep.510300147. [DOI] [PubMed] [Google Scholar]
- 53.Fujii H, Itoh Y, Yamaguchi K, Yamauchi N, Harano Y, Nakajima T, Minami M, Okanoue T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res Commun. 2004;322:1052–1058. doi: 10.1016/j.bbrc.2004.07.207. [DOI] [PubMed] [Google Scholar]
- 54.Sutton A, Friand V, Brulé-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poiré A, Saffar L, Kraemer M, Vassy J, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 55.Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am J Transl Res. 2014;6:340–352. [PMC free article] [PubMed] [Google Scholar]
- 56.Ren Y, Poon RT, Tsui HT, Chen WH, Li Z, Lau C, Yu WC, Fan ST. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res. 2003;9:5996–6001. [PubMed] [Google Scholar]
- 57.Yuan Y, Liu J, Liu Z, He Y, Zhang Z, Jiang C, Qian Q. Chemokine CCL3 facilitates the migration of hepatoma cells by changing the concentration intracellular Ca. Hepatol Res. 2010;40:424–431. doi: 10.1111/j.1872-034X.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- 58.Du D, Liu Y, Qian H, Zhang B, Tang X, Zhang T, Liu W. The effects of the CCR6/CCL20 biological axis on the invasion and metastasis of hepatocellular carcinoma. Int J Mol Sci. 2014;15:6441–6452. doi: 10.3390/ijms15046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 61.Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, Loh M, Bolze A, Quek R, Lee VK, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 2010;52:370–379. doi: 10.1016/j.jhep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427–438. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tacke F, Zimmermann HW, Berres ML, Trautwein C, Wasmuth HE. Serum chemokine receptor CXCR3 ligands are associated with progression, organ dysfunction and complications of chronic liver diseases. Liver Int. 2011;31:840–849. doi: 10.1111/j.1478-3231.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 64.Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, Neumann AU, Ferrari C, Missale G, Haagmans BL, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 65.Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594.e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 67.Hsu JL, Glaser SL. Epstein-barr virus-associated malignancies: epidemiologic patterns and etiologic implications. Crit Rev Oncol Hematol. 2000;34:27–53. doi: 10.1016/s1040-8428(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 68.Magrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. Br J Haematol. 2012;156:744–756. doi: 10.1111/j.1365-2141.2011.09013.x. [DOI] [PubMed] [Google Scholar]
- 69.Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, Murray PG. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235:312–322. doi: 10.1002/path.4459. [DOI] [PubMed] [Google Scholar]
- 70.Rouce RH, Louis CU, Heslop HE. Epstein-Barr virus lymphoproliferative disease after hematopoietic stem cell transplant. Curr Opin Hematol. 2014;21:476–481. doi: 10.1097/MOH.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salyakina D, Tsinoremas NF. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum Genomics. 2013;7:23. doi: 10.1186/1479-7364-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tetzlaff MT, Nosek C, Kovarik CL. Epstein-Barr virus-associated leiomyosarcoma with cutaneous involvement in an African child with human immunodeficiency virus: a case report and review of the literature. J Cutan Pathol. 2011;38:731–739. doi: 10.1111/j.1600-0560.2011.01721.x. [DOI] [PubMed] [Google Scholar]
- 73.Han BL, Xu XY, Zhang CZ, Wu JJ, Han CF, Wang H, Wang X, Wang GS, Yang SJ, Xie Y. Systematic review on Epstein-Barr virus (EBV) DNA in diagnosis of nasopharyngeal carcinoma in Asian populations. Asian Pac J Cancer Prev. 2012;13:2577–2581. doi: 10.7314/apjcp.2012.13.6.2577. [DOI] [PubMed] [Google Scholar]
- 74.Houldcroft CJ, Kellam P. Host genetics of Epstein-Barr virus infection, latency and disease. Rev Med Virol. 2015;25:71–84. doi: 10.1002/rmv.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coghill AE, Hildesheim A. Epstein-Barr virus antibodies and the risk of associated malignancies: review of the literature. Am J Epidemiol. 2014;180:687–695. doi: 10.1093/aje/kwu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo KW, Chung GT, To KF. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin Cancer Biol. 2012;22:79–86. doi: 10.1016/j.semcancer.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Lo KW, Teo PM, Hui AB, To KF, Tsang YS, Chan SY, Mak KF, Lee JC, Huang DP. High resolution allelotype of microdissected primary nasopharyngeal carcinoma. Cancer Res. 2000;60:3348–3353. [PubMed] [Google Scholar]
- 78.Hui AB, Or YY, Takano H, Tsang RK, To KF, Guan XY, Sham JS, Hung KW, Lam CN, van Hasselt CA, et al. Array-based comparative genomic hybridization analysis identified cyclin D1 as a target oncogene at 11q13.3 in nasopharyngeal carcinoma. Cancer Res. 2005;65:8125–8133. doi: 10.1158/0008-5472.CAN-05-0648. [DOI] [PubMed] [Google Scholar]
- 79.Or YY, Chung GT, To KF, Chow C, Choy KW, Tong CY, Leung AW, Hui AB, Tsao SW, Ng HK, et al. Identification of a novel 12p13.3 amplicon in nasopharyngeal carcinoma. J Pathol. 2010;220:97–107. doi: 10.1002/path.2609. [DOI] [PubMed] [Google Scholar]
- 80.Lu QL, Elia G, Lucas S, Thomas JA. Bcl-2 proto-oncogene expression in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int J Cancer. 1993;53:29–35. doi: 10.1002/ijc.2910530107. [DOI] [PubMed] [Google Scholar]
- 81. Available from: http://www.cancer.sanger.ac.uk/cosmic.
- 82.Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 2012;22:127–136. doi: 10.1016/j.semcancer.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Herait P, Ganem G, Lipinski M, Carlu C, Micheau C, Schwaab G, De-The G, Tursz T. Lymphocyte subsets in tumour of patients with undifferentiated nasopharyngeal carcinoma: presence of lymphocytes with the phenotype of activated T cells. Br J Cancer. 1987;55:135–139. doi: 10.1038/bjc.1987.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferradini L, Miescher S, Stoeck M, Busson P, Barras C, Cerf-Bensussan N, Lipinski M, von Fliedner V, Tursz T. Cytotoxic potential despite impaired activation pathways in T lymphocytes infiltrating nasopharyngeal carcinoma. Int J Cancer. 1991;47:362–370. doi: 10.1002/ijc.2910470309. [DOI] [PubMed] [Google Scholar]
- 85.Lau KM, Cheng SH, Lo KW, Lee SA, Woo JK, van Hasselt CA, Lee SP, Rickinson AB, Ng MH. Increase in circulating Foxp3+CD4+CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer. 2007;96:617–622. doi: 10.1038/sj.bjc.6603580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Huang ZF, Xiong G, Mo HY, Qiu F, Mai HQ, Chen QY, He J, Chen SP, Zheng LM, et al. Distribution, characterization, and induction of CD8+ regulatory T cells and IL-17-producing CD8+ T cells in nasopharyngeal carcinoma. J Transl Med. 2011;9:189. doi: 10.1186/1479-5876-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Busson P, Braham K, Ganem G, Thomas F, Grausz D, Lipinski M, Wakasugi H, Tursz T. Epstein-Barr virus-containing epithelial cells from nasopharyngeal carcinoma produce interleukin 1 alpha. Proc Natl Acad Sci USA. 1987;84:6262–6266. doi: 10.1073/pnas.84.17.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu H, Tang KF, Chua YN, Lu J, Feng P, Chew CT, Chan SH. Expression of interleukin-18 by nasopharyngeal carcinoma cells: a factor that possibly initiates the massive leukocyte infiltration. Hum Pathol. 2004;35:722–728. doi: 10.1016/j.humpath.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 89.Teichmann M, Meyer B, Beck A, Niedobitek G. Expression of the interferon-inducible chemokine IP-10 (CXCL10), a chemokine with proposed anti-neoplastic functions, in Hodgkin lymphoma and nasopharyngeal carcinoma. J Pathol. 2005;206:68–75. doi: 10.1002/path.1745. [DOI] [PubMed] [Google Scholar]
- 90.Chang KP, Hao SP, Chang JH, Wu CC, Tsang NM, Lee YS, Hsu CL, Ueng SH, Liu SC, Liu YL, et al. Macrophage inflammatory protein-3alpha is a novel serum marker for nasopharyngeal carcinoma detection and prediction of treatment outcomes. Clin Cancer Res. 2008;14:6979–6987. doi: 10.1158/1078-0432.CCR-08-0090. [DOI] [PubMed] [Google Scholar]
- 91.Nanbo A, Takada K. The role of Epstein-Barr virus-encoded small RNAs (EBERs) in oncogenesis. Rev Med Virol. 2002;12:321–326. doi: 10.1002/rmv.363. [DOI] [PubMed] [Google Scholar]
- 92.Murray RJ, Kurilla MG, Brooks JM, Thomas WA, Rowe M, Kieff E, Rickinson AB. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992;176:157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sample J, Brooks L, Sample C, Young L, Rowe M, Gregory C, Rickinson A, Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci USA. 1991;88:6343–6347. doi: 10.1073/pnas.88.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lara J, Cohen M, De Matteo E, Aversa L, Preciado MV, Chabay P. Epstein-Barr virus (EBV) association and latency profile in pediatric Burkitt’s lymphoma: experience of a single institution in Argentina. J Med Virol. 2014;86:845–850. doi: 10.1002/jmv.23737. [DOI] [PubMed] [Google Scholar]
- 95.Rosales-Pérez S, Cano-Valdez AM, Flores-Balcázar CH, Guedea-Edo F, Lino-Silva LS, Lozano-Borbalas A, Navarro-Martín A, Poitevin-Chacón A. Expression of Epstein-Barr virus-encoded latent membrane protein (LMP-1), p16 and p53 proteins in nonendemic nasopharyngeal carcinoma (NPC): a clinicopathological study. Arch Med Res. 2014;45:229–236. doi: 10.1016/j.arcmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 96.Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC) Semin Cancer Biol. 2012;22:144–153. doi: 10.1016/j.semcancer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Rochford R, Mosier DE. Differential Epstein-Barr virus gene expression in B-cell subsets recovered from lymphomas in SCID mice after transplantation of human peripheral blood lymphocytes. J Virol. 1995;69:150–155. doi: 10.1128/jvi.69.1.150-155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castillo JJ, Beltran BE, Miranda RN, Paydas S, Winer ES, Butera JN. Epstein-barr virus-positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16:87–96. doi: 10.1634/theoncologist.2010-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murata T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol Immunol. 2014;58:307–317. doi: 10.1111/1348-0421.12155. [DOI] [PubMed] [Google Scholar]
- 100.Lee JM, Kim H, Noh SH, Lee WY, Kim SJ, Park JH. Expression of Epstein-Barr Virus Gene and Clonality of Infiltrated T Lymphocytes in Epstein-Barr Virus-associated Gastric Carcinoma. Immune Netw. 2011;11:50–58. doi: 10.4110/in.2011.11.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS, Hu ZQ, Huang XY, Yang GH, Shi YH, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597–605. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]


