Abstract
AIM: To evaluate variation of the concentration of thiopurine metabolites after 5-aminosalicylate (5-ASA) interruption and the role of genetic polymorphisms of N-acetyl transferase (NAT) 1 and 2.
METHODS: Concentrations of thioguanine nucleotides (TGN) and methymercaptopurine nucleotides (MMPN), metabolites of thiopurines, were measured by high performance liquid chromatography in 12 young patients (3 females and 9 males, median age 16 years) with inflammatory bowel disease (6 Crohn’s disease and 6 ulcerative colitis) treated with thiopurines (7 mercaptopurine and 5 azathioprine) and 5-ASA. Blood samples were collected one month before and one month after the interruption of 5-ASA. DNA was extracted and genotyping of NAT1, NAT2, inosine triphosphate pyrophosphatase (ITPA) and thiopurine methyl transferase (TPMT) genes was performed using PCR assays.
RESULTS: Median TGN concentration before 5-ASA interruption was 270 pmol/8 x 108 erythrocytes (range: 145-750); after the interruption of the aminosalicylate, a 35% reduction in TGN mean concentrations (absolute mean reduction 109 pmol/8 × 108 erythrocytes) was observed (median 221 pmol/8 × 108 erythrocytes, range: 96-427, P value linear mixed effects model 0.0011). Demographic and clinical covariates were not related to thiopurine metabolites concentrations. All patients were wild-type for the most relevant ITPA and TPMT variants. For NAT1 genotyping, 7 subjects presented an allele combination corresponding to fast enzymatic activity and 5 to slow activity. NAT1 genotypes corresponding to fast enzymatic activity were associated with reduced TGN concentration (P value linear mixed effects model 0.033), putatively because of increased 5-ASA inactivation and consequent reduced inhibition of thiopurine metabolism. The effect of NAT1 status on TGN seems to be persistent even after one month since the interruption of the aminosalicylate. No effect of NAT1 genotypes was shown on MMPN concentrations. NAT2 genotyping revealed that 6 patients presented a genotype corresponding to fast enzymatic activity and 6 to slow activity; NAT2 genotypes were not related to thiopurine metabolites concentration in this study.
CONCLUSION: NAT1 genotype affects TGN levels in patients treated with thiopurines and aminosalicylates and could therefore influence the toxicity and efficacy of these drugs; however the number of patients evaluated is limited and this has to be considered a pilot study.
Keywords: Thiopurines, Aminosalicylates, Inflammatory bowel diseases, N-acetyl transferase, Pharmacogenomics
Core tip: During treatment of inflammatory bowel disease with thiopurines and aminosalicylates, interruption of the aminosalicylate results in a significant decrease in thiopurines’ thioguanine nucleotides (TGN) active metabolites. Genetic polymorphisms in genes involved in aminosalicylates biotransformation (NAT1 genotype) affects TGN levels in patients treated with thiopurines and aminosalicylates and could therefore influence the toxicity and efficacy of these drugs.
INTRODUCTION
Thiopurines and aminosalicylates are the two most widely used drugs in inflammatory bowel disease (IBD) and are often used in combination. The thiopurines 6-mercaptopurine (6MP) and its prodrug azathioprine (AZA) are effective in inducing and maintaining remission and are considered steroid sparing agents. 6MP is metabolized by a multistep enzymatic pathway, initiated by hypoxanthine phosphoribosyl transferase that leads to formation of thioguanine nucleotides (TGNs). These active metabolites act as purine antagonist and inhibit DNA, RNA and protein synthesis, inducing cytotoxicity and immunosuppression. Blood levels of thiopurine metabolites have been correlated with the efficacy and toxicity of these drugs in patients with IBD: TGN levels higher than 235 pmol/8 x 108 red blood cells are considered therapeutic, and methyl mercaptopurine nucleotides (MMPNs) levels above 5700 pmol/8 x 108 red blood cells have been associated with hepatotoxicity[1-3].
The aminosalicylate 5-aminosalicylic acid (mesalazine, 5-ASA) is used in the induction and maintenance of remission in ulcerative colitis[4,5]. In Crohn’s disease, the use of aminosalicylates is controversial, however studies suggest that they could have a role in the postoperative maintenance of remission also in this IBD[6]. In addition, a chemopreventive role of 5-ASA in IBD against colon cancer has been suggested[7].
An increase in mean TGN blood levels has been reported in patients on 6MP or AZA co-treated with 5-ASA[2,8-12]. Even more important, a higher rate of myelotoxicity was observed in patients treated with this combination in comparison with those treated with the thiopurine alone[2,9,13].
6MP is inactivated by the enzyme thiopurine methyltransferase (TPMT, EC 2.1.1.67) that catalyzes its S-methylation to 6-methylmercaptopurine and, at least in part by inosine triphosphate pyrophosphatase (ITPA, EC 3.6.1.19). In vitro studies have shown that aminosalicylates and their metabolites can inhibit the activity of TPMT[14,15], however, this observation has not been confirmed in vivo[2,8,9].
The enzymes N-acetyltransferases (NAT1 and NAT2, EC 2.3.1.5) are responsible for the N-acetylation of a number of xenobiotics and drugs including the aminosalicylates. Even the activity of NAT1 and NAT2 is genetically determined and subjects are classified as rapid, intermediate or slow acetylators. Although NAT1 and NAT2 polymorphisms have been associated with the incidence of some diseases, no significant effect has been reported for IBD[16]. 5-ASA is inactivated primarily by the NAT1 isoform in the colonic mucosa, and the drug and its metabolites are excreted in the urine[17-19]. The inheritance of a slow acetylator genotype for NAT1 could therefore lead to a reduced inactivation of 5-ASA, and hence to higher blood levels of the drug.
The aim of this study was to measure variation of the concentration of thiopurine metabolites after 5-ASA interruption and to evaluate the role of genetic polymorphisms of NAT1 and NAT2 on this phenomenon.
MATERIALS AND METHODS
Patients and inclusion criteria
Twelve patients with IBD were enrolled by the Gastroenterology Unit of the Pediatric Hospital “Burlo Garofolo” in Trieste, and by the Research Children’s Hospital “Meyer”, Florence, Italy. These patients have been retrospectively selected considering the following criteria: previous diagnosis of IBD and treatment with AZA or 6MP plus 5-ASA for at least three months. 5-ASA therapy was interrupted, and a minimum of two blood samples for thiopurine metabolites measurement were taken one month before and one month after 5-ASA interruption. The study was approved by the local ethical committees and appropriate informed consent was obtained from all patients or their parents or guardians.
Measurement of azathioprine metabolites
Azathioprine metabolites (TGN and MMPN) were measured in patients’ erythrocytes using an HPLC assay by Dervieux and Boulieu[20] within few weeks from the sample collection. The ratio between TGN and the dose of azathioprine was calculated to account for the respective dose each patient was taking on the day that the metabolite testing was performed.
Genotypes
Genomic DNA was extracted from peripheral blood samples using a commercial kit (SIGMA, Milan, Italy), in order to characterize genetic polymorphisms in the candidate genes NAT1, NAT2, TPMT and ITPA. The considered genotypes and method of analysis are described in Table 1.
Table 1.
Genotypes and methods of analysis considered in this study
| Gene | Polymorphism | Method | References |
| NAT1 | T1088A | Sequencing | With primer forward: 5’-TGCCCAAACATGGTGATAGATTT-3’ |
| With primer reverse: 5’-CCATAAAACTTTTCTAGGAATTCAACAAT-3’ | |||
| NAT1 | C1095A | Sequencing | As above |
| NAT2 | C282T | PCR-RFLP | [23,24] |
| NAT2 | T341C | PCR-RFLP | [23,24] |
| TPMT | G238C | PCR-ASO | [27] |
| TPMT | G460A | PCR-RFLP | [27] |
| TPMT | A719G | PCR-RFLP | [27] |
| ITPA | C94A | TaqMan | TaqMan SNP genotyping assay from |
| Applied biosystems (C_27465000_10) |
NAT acetylator status determination
NAT acetylator status (i.e., rapid or slow) was assessed from the genotyping results. In particular, for NAT1, patients with an A nucleotide at both 1088 and 1095 nucleotides, corresponding to NAT1*10 allele, were considered as fast NAT1 acetylators while all other genotype combinations were considered as slow NAT1 acetylators[21,22]. For NAT2, patients homozygous for the wild-type allele at either the 282 or 341 position or patients heterozygous for the variant allele at just one of these two positions were considered as fast NAT2 acetylators, all other genotypes combinations were considered as slow NAT2 acetylators[23,24].
Statistical analysis
Statistical analysis was performed using the software R (version 3.0.1).
The primary intended outcome of this study was to evaluate variations of the concentration of thiopurine metabolites after 5-ASA interruption and the role of genetic polymorphisms of NAT 1 and 2.
Power analyses on preliminary data available indicate that given the difference in means and the distribution’s standard deviation, the minimum sample size to identify a statistically significant (P = 0.05, power 80%) result is 9 for the paired test comparing azathioprine metabolites during aminosalicylate treatment and after the suspension. For the analysis comparing thiopurine metabolites concentration in NAT1 fast acetylators compared to slow acetylators, the minimum number of patients to detect a statistically significant (P = 0.05, power 80%) result is 5 for each NAT1 activity status.
The association between pharmacological phenotypes of interest (i.e., TGN metabolites concentrations, MMPN metabolites concentrations) and the considered demographic variables, IBD type, co-treatment with aminosalicylate or genotypes in a univariate analysis, was evaluated by considering for each phenotype and patient the individual observations and evaluating the effect of each covariate by calculating the P value from a linear mixed effects model built using the phenotype as the dependent variable, each covariate as the fixed effect and the patients as the random effect in the model. Multivariate analysis was done to test the independence of the effects of the covariates significant in the univariate analysis on the phenotypes considered by using linear mixed effects models with the phenotype of interest as the independent variables and the covariates selected in the univariate analysis as the dependent variables. For all parametric analyses (i.e., linear mixed effects models used in the univariate analysis and the multivariate analysis), normality of the phenotype was tested by the Shapiro test and log10 transformation was applied if needed, in order to achieve normality of the distribution.
RESULTS
Patients enrolled and samples collected
The present study recruited 12 young patients (3 females and 9 males, median age 16 years) with IBD (6 Crohn’s disease and 6 ulcerative colitis). Seven patients were treated with 6MP (median dosage 1.0 mg/kg, range: 0.5-1.0, equivalent to a median AZA dose of 2.08 mg/kg, range: 1.04-2.08) and 5 with AZA (median dosage 2.2 mg/kg, range: 1.6-2.6). All patients were co-treated with 5-ASA for at least three months at standard doses (50 mg/kg). A total of 36 samples of peripheral blood were collected to measure azathioprine metabolites; on average, 3 samples for patient were collected (range: 2-4). For 8 patients it was not possible to collect two samples before 5-ASA interruption and 2 samples after 5-ASA interruption, because of clinical reasons: therefore 12 samples (5 before and 7 after) were missing; however each patient had at least one sample before and one after 5-ASA interruption. Among these, 19 were obtained during treatment with the thiopurines and 5-ASA, before 5-ASA interruption, and 17 after interruption of 5-ASA and therefore during treatment with the thiopurine alone. The reason for 5-ASA interruption was clinical, mostly simplification of therapy to increase compliance, which is particularly useful in pediatric patients. Samples were taken from the same patient with an interval of at least one month.
Measurement of azathioprine metabolites
Median TGN concentrations before 5-ASA interruption was 270 pmol/8 x 108 erythrocytes (range: 145-750); after the interruption of the aminosalicylate, a 35% reduction (mean absolute value 109 pmol/8 x 108 erythrocytes) in TGN mean concentrations was observed [median 221 pmol/8 × 108 erythrocytes, range: 96-427, coefficient = -0.18, 95%CI: -0.27-(-0.09), P value linear mixed effects model 0.0011]. MMPN concentration were not affected significantly by interruption of the aminosalicylate, with a median value of 1059 pmol/8 x 108 erythrocytes, range 246-17943 before the interruption in comparison to a median of 1071 pmol/8 × 108 erythrocytes, range 209-4531 after the interruption (coefficient = -0.13, 95%CI: -0.29-(-0.03), P value linear mixed effects model 0.14). There was a significant correlation between TGN and MMPN concentrations (coefficient 0.3, 95%CI: 0.15-0.45, linear mixed effect P value 0.0007) while the dose of thiopurine did not correlate with TGN and MMPN concentrations in these patients. The dose of thiopurine did not change before and after the interruption of the aminosalicylate (median value of azathioprine dose and range 2.08 mg/kg, range: 1.04-2.6).
Demographic and clinical covariates and azathioprine dose and metabolites
For the demographic (gender and age) and clinical (type of IBD and treatment duration) covariates considered, none showed a fully significant effect on the median TGN or MMPN concentrations in a univariate analysis (Table 2).
Table 2.
Demographic, clinical and pharmacological data for the 12 patients enrolled
| Patient | Age at enrollment (yr) | Disease | Thiopurine dose (mg/kg per day) | 5-ASA dose (mg/d) |
TGN concentration1 |
% TGN change | NAT1 status | |
| Before | After | |||||||
| 1 | 7.7 | CD | AZA 2.6 | 50 | 244 | 218 | -11% | Rapid |
| 2 | 17.3 | CD | AZA 2.2 | 50 | 2102 | 176 | -16% | Rapid |
| 3 | 17.8 | CD | AZA 1.6 | 50 | 2762 | 101 | -64% | Rapid |
| 4 | 14.7 | CD | 6MP 1.0 | 50 | 2882 | 1422 | -51% | Rapid |
| 5 | 10.4 | UC | 6MP 0.6 | 50 | 3102 | 2062 | -34% | Rapid |
| 6 | 14.5 | UC | 6MP 0.5 | 50 | 3302 | 1882 | -43% | Rapid |
| 7 | 11.9 | UC | 6MP 1.0 | 50 | 228 | 243 | +7% | Rapid |
| 8 | 17.4 | CD | AZA 2.2 | 50 | 2172 | 221 | +2% | Slow |
| 9 | 6.3 | UC | AZA 2.3 | 50 | 375 | 140 | -63% | Slow |
| 10 | 17.5 | UC | 6MP 1.0 | 50 | 6472 | 4012 | -38% | Slow |
| 11 | 17.3 | UC | 6MP 1.0 | 50 | 264 | 278 | +5% | Slow |
| 12 | 16.6 | CD | 6MP 0.5 | 50 | 501 | 2682 | -47% | Slow |
Pmol/8 × 108 erythrocytes;
This value is the average of two measurements. 5-ASA: 5-aminosalicylate; TGN: 6-thioguanine nucleotides; NAT1: N-acetyltransferase 1; CD: Crohn’s disease; UC: Ulcerative colitis; 6MP: 6-mercaptopurine; AZA: Azathioprine.
Genotyping
All polymorphisms considered were respecting Hardy-Weinberg equilibrium and their distribution is comparable to what has been reported in the literature for patients of Caucasian ethnicity. All patients were wild-type for the most relevant ITPA and TPMT variants. For NAT1 genotyping, 7 presented an allele combination corresponding to fast enzymatic activity and 5 to slow activity. NAT2 genotyping revealed that 6 patients presented a genotype corresponding to fast enzymatic activity and 6 to slow activity.
Genotypes and thiopurine metabolites
NAT1 genotypes corresponding to fast enzymatic activity was associated with reduced TGN concentration [coefficient = -0.159, 95%CI: -0.28-(-0.04), P value linear mixed effects model 0.033]: mean values were 269.3 and 400.8 respectively in patients with fast and slow NAT1 status before 5-ASA interruption, and 181.9 and 261.6 after 5-ASA interruption. Unexpectedly, the effect of NAT1 status on TGN seems to be persistent even one month since the interruption of the aminosalicylate (Figure 1). No effect of NAT1 genotypes was shown on MMPN concentrations. NAT2 genotypes were not related to thiopurine metabolites' concentration in this study.
Figure 1.
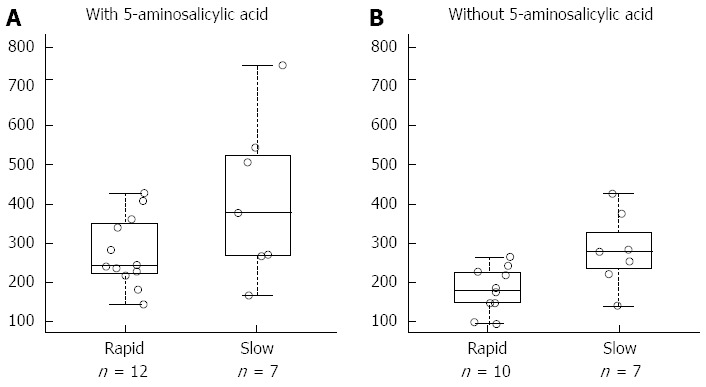
6-thioguanine nucleotides concentration and N-acetyl transferase 1 acetylator status during co-treatment of azathioprine with 5-aminosalicylic acid and after the interruption of the aminosalicylate. A: With 5-aminosalicylic acid; B: Without 5-aminosalicylic acid. A total of 36 samples of peripheral blood were collected from 12 patients to measure azathioprine metabolites; on average, 3 samples for patient were collected (range: 2-4). Among these, 19 were obtained during treatment with the thiopurines and 5-aminosalicylic acid (panel A) and 17 during treatment with the thiopurine alone (panel B). Samples were taken from the same patient with an interval of at least one month. 6-thioguanine nucleotides concentration is expressed as pmol/8 × 108 erythrocytes.
DISCUSSION
The clinical use of thiopurines in IBDs has increased substantially in recent years; these drugs have indeed a steroid sparing effect[25] and their use in combination with infliximab has been also advocated[26]. Coprescription of 5-ASA is also common, and up to 60% of patients on thiopurines are also treated with an aminosalicylate.
Thiopurines are generally well tolerated, however, 15%-20% of patients develop side effects such as leukopenia, hepatitis and pancreatitis[27-30]. Two key enzymes, TPMT and ITPA, are important for 6MP metabolism: TPMT catalyzes the S-methylation to 6MP and genetic polymorphisms in the TPMT gene are associated with a reduced enzymatic activity and an increased production of the active TGNs; indeed, patients with the homozygous mutation are at high risk of severe and sometimes fatal immunosuppression. For this reason TPMT genotyping or phenotyping is recommended prior to the initiation of therapy. Another important enzyme in thiopurines' metabolism is ITPA; a polymorphism in this gene leads to accumulation of the metabolite 6-thioinosine triphosphate and has been associated with an increased risk of toxicity, in particular pancreatitis, flu like symptoms, rash and gastrointestinal toxicity[31]; this observation was however not confirmed by other studies[32,33]. All patients included in our study had a normal TPMT genotype and were wild-type for the most common mutation of ITPA, hence excluding bias due to the influence of these genotypes.
In IBD patients treated with thiopurines an additional risk results from the co-administration of other drugs, such as the aminosalicylates. In the present study we confirm previous observations[2,8,9,11,13,34] of a significant decrease in TGN levels after discontinuation of 5-ASA. Furthermore, a dose dependent effect was previously reported for two different 5-ASA doses on thiopurine metabolites levels[35]. In our study all patients were treated with a dose of 5-ASA of 50 mg/kg, equivalent to the higher dose reported by de Graaf et al[35]. Consistently with this study, after interruption of 5-ASA we observed an effect on TGN and not on MMPN concentration. This may be due to the different populations considered: TPMT activity indeed is significantly higher in wild-type children (0.08-17 years) than in wild-type adults (aged 18-68 years)[36].
The mechanism of this interaction is however still unclear. It has been demonstrated that the aminosalicylates inhibit the activity of recombinant TPMT in vitro[37,38], with IC50 values of 78 and 1240 µmol/L for sulfasalazine and 5-ASA respectively. In in vivo studies, an increase in TGN levels and in the prevalence of leukopenia was observed in patients treated with azathioprine and 5-ASA; however, short term investigations in patients with IBD[8-11] did not demonstrate any significant change in TPMT activity. A long term study in patients treated for one year with a high dose (4 g/d) of 5-ASA again did not show any in vivo effect on TPMT activity[39]. It can therefore be concluded that the interaction between aminosalicylates and thiopurines seems not based on inhibition of TPMT, and other pharmacokinetic and/or pharmacodynamic aspects have to be investigated.
5-ASA is orally administered, is poorly absorbed by the gastrointestinal tract and is in part inactivated in the colonic mucosa by NATs[17]. These enzymes are widely distributed in tissues[40] and among species[41], and have important physiological functions; they are also responsible for the N-acetylation of a number of xenobiotics and drugs including the aminosalicylates. The activity of NAT1 and NAT2 is genetically determined; both genes are located on chromosome 8p22 and a number of polymorphisms have been reported, allowing subjects to be classified as rapid or slow acetylators. The isozymes NAT1 and NAT2 have distinct substrate specificity and the NAT1 isozyme is more important (19000-fold more active) than NAT2 in 5-ASA acetylation in vitro[42]. Interestingly, in our study, patients with the NAT1 slow metabolizer phenotype had significantly higher TGN levels in comparison with rapid metabolizers. The inheritance of a slow acetylator genotype for NAT1 could therefore lead to a reduced inactivation of 5-ASA, and hence to higher blood levels of the aminosalicylate. This could result in a reduction of 6MP inactivation, via a still unclear mechanism, with consequent increase in TGN levels. Quite unexpectedly however, this difference was maintained when measurements were performed one month after 5-ASA discontinuation. This may be due to the long half-life of TGN and the fact that a longer period is needed to overcome the reduction in TGN concentrations determined by the increased metabolism of 5-ASA. It is however possible that NAT1 influences TGN concentrations by a different mechanism, not involving 5-ASA metabolism.
As expected, no effect of the NAT2 polymorphism was observed in these patients.
In conclusion, co-administration of 5-ASA and thiopurines is common and probably this association will continue to be prescribed in light of the demonstrated chemopreventive activity for IBD associated colorectal cancer[7].
Since the number of patients enrolled in this study is limited, this has to be considered a pilot study and more research should be performed to evaluate if the difference in TGN levels observed in patients with the NAT1 slow acetylator phenotype are also related to an increased incidence of thiopurine induced side effects. If this were true, it might be useful to assess the NAT1 genotype before starting therapy and, in those patients with a slow acetylator genotype, it might be prudent to start therapy with a reduced dose of AZA. Moreover, further studies should be performed to evaluate a dose dependent effect of 5-ASA or thiopurine dose on the association between NAT1 status and the pharmacokinetic interaction between 5-ASA and thiopurines: indeed patients with adverse NAT1 status may be treated with low doses of aminosalicylates, maintaining their chemopreventive effect. The effect of NAT1 acetylator status on TGN concentration in patients treated only with thiopurine could also be investigated.
NAT1 genotyping, in addition to careful clinical monitoring and evaluation of thiopurine metabolites, might be a useful guide in those patients receiving azathioprine and aminosalicylates.
COMMENTS
Background
Thiopurines and aminosalicylates are the two most widely used drugs in inflammatory bowel disease (IBD) and are often used in combination. A significant pharmacokinetic interaction has been described for these medications, since this association increases the concentration of thiopurines’ active metabolites (TGN).
Research frontiers
Treatment of IBD with thiopurines and aminosalicylates displays significant inter-patient variability in terms of efficacy and incidence of adverse events. Identification of determinants to predict effects of these treatments, such as genetic polymorphisms of enzymes involved in thiopurines and aminosalicylates biotransformation, is of clinical interest.
Innovations and breakthroughs
This article confirms that after interruption of aminosalicylate, concentration of TGNs decrease significantly. Moreover, N-acetyl-transferase 1 (NAT1) acetylator status, relevant for aminosalicylates biotransformation, influences TGN concentration during co-treatment and after the interruption of the aminosalicylate.
Applications
If supported by further clinical studies, NAT1 may be incorporated in multilocus signatures of genotypes useful to predict the efficacy and safety of thiopurine and aminosalicylate co-treatment in young patients with IBD.
Terminology
TGN, the active metabolites of thiopurines, formed after biotransformation of mercaptopurine by enzymes of nucleotides salvage pathway. NAT1, an enzyme that catalyzes the acetylation of amino groups of aminosalicylates such as 5-ASA.
Peer-review
This is an interesting and clinically relevant paper. Obviously the study has a small sample size and thus it is best described as a pilot study. The results of this small study are of interest in that there seems to be some pharmacokinetic interaction between 5-aminosalicylate and thiopurines, there also seems to be a potentially important and previously unexplored effect of NAT polymorphisms.
Footnotes
Supported by Italian Ministry of Health, and Fondazione Benefica Alberto e Kathleen Casali.
Ethics approval: The study was reviewed and approved by the Institute for Maternal and Child Health I.R.C.C.S. Burlo Garofolo Medical Etics Review Board, approval Trieste (n. 58/05, 12/12/2005) and by the Institutional Research Board (#23/05).
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: All authors declare they have no conflict of interest.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 3, 2014
First decision: October 29, 2014
Article in press: January 16, 2015
P- Reviewer: Beales ILP, van Langenberg DR, Zouiten-Mekki L S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 2.Lowry PW, Franklin CL, Weaver AL, Szumlanski CL, Mays DC, Loftus EV, Tremaine WJ, Lipsky JJ, Weinshilboum RM, Sandborn WJ. Leucopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine, or balsalazide. Gut. 2001;49:656–664. doi: 10.1136/gut.49.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541–1549. doi: 10.1002/ibd.21221. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland L, Roth D, Beck P, May G, Makiyama K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2002;(4):CD000544. doi: 10.1002/14651858.CD000544. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland L, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2003;(3):CD000543. doi: 10.1002/14651858.CD000543. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:60–65. doi: 10.1111/j.1365-2036.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 7.Rubin DT, Cruz-Correa MR, Gasche C, Jass JR, Lichtenstein GR, Montgomery EA, Riddell RH, Rutter MD, Ullman TA, Velayos FS, et al. Colorectal cancer prevention in inflammatory bowel disease and the role of 5-aminosalicylic acid: a clinical review and update. Inflamm Bowel Dis. 2008;14:265–274. doi: 10.1002/ibd.20297. [DOI] [PubMed] [Google Scholar]
- 8.Dewit O, Vanheuverzwyn R, Desager JP, Horsmans Y. Interaction between azathioprine and aminosalicylates: an in vivo study in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16:79–85. doi: 10.1046/j.1365-2036.2002.01156.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Zhang FB, Ding L, Liu H, Wang XD, Chen BL, Bi HC, Xiao YL, Zhao LZ, Chen MH, et al. The potential influence of 5-aminosalicylic acid on the induction of myelotoxicity during thiopurine therapy in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2012;24:958–964. doi: 10.1097/MEG.0b013e3283545ae3. [DOI] [PubMed] [Google Scholar]
- 10.de Boer NK, Wong DR, Jharap B, de Graaf P, Hooymans PM, Mulder CJ, Rijmen F, Engels LG, van Bodegraven AA. Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol. 2007;102:2747–2753. doi: 10.1111/j.1572-0241.2007.01511.x. [DOI] [PubMed] [Google Scholar]
- 11.Gilissen LP, Bierau J, Derijks LJ, Bos LP, Hooymans PM, van Gennip A, Stockbrügger RW, Engels LG. The pharmacokinetic effect of discontinuation of mesalazine on mercaptopurine metabolite levels in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2005;22:605–611. doi: 10.1111/j.1365-2036.2005.02630.x. [DOI] [PubMed] [Google Scholar]
- 12.Lennard L. Clinical implications of thiopurine methyltransferase--optimization of drug dosage and potential drug interactions. Ther Drug Monit. 1998;20:527–531. doi: 10.1097/00007691-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Shah JA, Edwards CM, Probert CS. Should azathioprine and 5-aminosalicylates be coprescribed in inflammatory bowel disease?: an audit of adverse events and outcome. Eur J Gastroenterol Hepatol. 2008;20:169–173. doi: 10.1097/MEG.0b013e3282f16d50. [DOI] [PubMed] [Google Scholar]
- 14.Lowry PW, Szumlanski CL, Weinshilboum RM, Sandborn WJ. Balsalazide and azathiprine or 6-mercaptopurine: evidence for a potentially serious drug interaction. Gastroenterology. 1999;116:1505–1506. doi: 10.1016/s0016-5085(99)70524-x. [DOI] [PubMed] [Google Scholar]
- 15.Woodson LC, Ames MM, Selassie CD, Hansch C, Weinshilboum RM. Thiopurine methyltransferase. Aromatic thiol substrates and inhibition by benzoic acid derivatives. Mol Pharmacol. 1983;24:471–478. [PubMed] [Google Scholar]
- 16.Mahid SS, Colliver DW, Crawford NP, Martini BD, Doll MA, Hein DW, Cobbs GA, Petras RE, Galandiuk S. Characterization of N-acetyltransferase 1 and 2 polymorphisms and haplotype analysis for inflammatory bowel disease and sporadic colorectal carcinoma. BMC Med Genet. 2007;8:28. doi: 10.1186/1471-2350-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allgayer H, Ahnfelt NO, Kruis W, Klotz U, Frank-Holmberg K, Söderberg HN, Paumgartner G. Colonic N-acetylation of 5-aminosalicylic acid in inflammatory bowel disease. Gastroenterology. 1989;97:38–41. doi: 10.1016/0016-5085(89)91412-1. [DOI] [PubMed] [Google Scholar]
- 18.Sim E, Abuhammad A, Ryan A. Arylamine N-acetyltransferases: from drug metabolism and pharmacogenetics to drug discovery. Br J Pharmacol. 2014;171:2705–2725. doi: 10.1111/bph.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westwood IM, Kawamura A, Fullam E, Russell AJ, Davies SG, Sim E. Structure and mechanism of arylamine N-acetyltransferases. Curr Top Med Chem. 2006;6:1641–1654. doi: 10.2174/156802606778108979. [DOI] [PubMed] [Google Scholar]
- 20.Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998;44:551–555. [PubMed] [Google Scholar]
- 21.Bell DA, Badawi AF, Lang NP, Ilett KF, Kadlubar FF, Hirvonen A. Polymorphism in the N-acetyltransferase 1 (NAT1) polyadenylation signal: association of NAT1*10 allele with higher N-acetylation activity in bladder and colon tissue. Cancer Res. 1995;55:5226–5229. [PubMed] [Google Scholar]
- 22.Zhangwei X, Jianming X, Qiao M, Xinhua X. N-Acetyltransferase-1 gene polymorphisms and correlation between genotype and its activity in a central Chinese Han population. Clin Chim Acta. 2006;371:85–91. doi: 10.1016/j.cca.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Cascorbi I, Brockmöller J, Mrozikiewicz PM, Bauer S, Loddenkemper R, Roots I. Homozygous rapid arylamine N-acetyltransferase (NAT2) genotype as a susceptibility factor for lung cancer. Cancer Res. 1996;56:3961–3966. [PubMed] [Google Scholar]
- 24.Cascorbi I, Roots I. Pitfalls in N-acetyltransferase 2 genotyping. Pharmacogenetics. 1999;9:123–127. doi: 10.1097/00008571-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Travis SP, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y, Colombel JF, D’Haens G, Ghosh S, Marteau P, et al. European evidence-based Consensus on the management of ulcerative colitis: Current management. J Crohns Colitis. 2008;2:24–62. doi: 10.1016/j.crohns.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Travis S. Infliximab and azathioprine: bridge or parachute? Gastroenterology. 2006;130:1354–1357. doi: 10.1053/j.gastro.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Stocco G, Martelossi S, Barabino A, Fontana M, Lionetti P, Decorti G, Malusà N, Bartoli F, Fezzi M, Giraldi T, et al. TPMT genotype and the use of thiopurines in paediatric inflammatory bowel disease. Dig Liver Dis. 2005;37:940–945. doi: 10.1016/j.dld.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–567. doi: 10.1002/pds.926. [DOI] [PubMed] [Google Scholar]
- 29.Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–1800. doi: 10.1111/j.1572-0241.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 30.Lees CW, Maan AK, Hansoti B, Satsangi J, Arnott ID. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:220–227. doi: 10.1111/j.1365-2036.2007.03570.x. [DOI] [PubMed] [Google Scholar]
- 31.Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, Shobowale-Bakre el-M, Escuredo E, Fairbanks LD, Sanderson JD. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase) Pharmacogenetics. 2004;14:181–187. doi: 10.1097/00008571-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Allorge D, Hamdan R, Broly F, Libersa C, Colombel JF. ITPA genotyping test does not improve detection of Crohn’s disease patients at risk of azathioprine/6-mercaptopurine induced myelosuppression. Gut. 2005;54:565. doi: 10.1136/gut.2004.055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gearry RB, Roberts RL, Barclay ML, Kennedy MA. Lack of association between the ITPA 94C& gt; A polymorphism and adverse effects from azathioprine. Pharmacogenetics. 2004;14:779–781. doi: 10.1097/00008571-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TM, Le Gall C, Lachaux A, Boulieu R. High thiopurine metabolite concentrations associated with lymphopenia in inflammatory bowel disease (IBD) pediatric patients receiving aminosalicylates combined with azathioprine. Int J Clin Pharmacol Ther. 2010;48:275–281. doi: 10.5414/cpp48275. [DOI] [PubMed] [Google Scholar]
- 35.de Graaf P, de Boer NK, Wong DR, Karner S, Jharap B, Hooymans PM, Veldkamp AI, Mulder CJ, van Bodegraven AA, Schwab M. Influence of 5-aminosalicylic acid on 6-thioguanosine phosphate metabolite levels: a prospective study in patients under steady thiopurine therapy. Br J Pharmacol. 2010;160:1083–1091. doi: 10.1111/j.1476-5381.2010.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serpe L, Calvo PL, Muntoni E, D’Antico S, Giaccone M, Avagnina A, Baldi M, Barbera C, Curti F, Pera A, et al. Thiopurine S-methyltransferase pharmacogenetics in a large-scale healthy Italian-Caucasian population: differences in enzyme activity. Pharmacogenomics. 2009;10:1753–1765. doi: 10.2217/pgs.09.103. [DOI] [PubMed] [Google Scholar]
- 37.Szumlanski CL, Weinshilboum RM. Sulphasalazine inhibition of thiopurine methyltransferase: possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol. 1995;39:456–459. doi: 10.1111/j.1365-2125.1995.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis LD, Benin A, Szumlanski CL, Otterness DM, Lennard L, Weinshilboum RM, Nierenberg DW. Olsalazine and 6-mercaptopurine-related bone marrow suppression: a possible drug-drug interaction. Clin Pharmacol Ther. 1997;62:464–475. doi: 10.1016/S0009-9236(97)90125-9. [DOI] [PubMed] [Google Scholar]
- 39.Dilger K, Schaeffeler E, Lukas M, Strauch U, Herfarth H, Müller R, Schwab M. Monitoring of thiopurine methyltransferase activity in postsurgical patients with Crohn’s disease during 1 year of treatment with azathioprine or mesalazine. Ther Drug Monit. 2007;29:1–5. doi: 10.1097/FTD.0b013e3180312b9a. [DOI] [PubMed] [Google Scholar]
- 40.Chung JG, Levy GN, Weber WW. Distribution of 2-aminofluorene and p-aminobenzoic acid N-acetyltransferase activity in tissues of C57BL/6J rapid and B6.A-NatS slow acetylator congenic mice. Drug Metab Dispos. 1993;21:1057–1063. [PubMed] [Google Scholar]
- 41.Vatsis KP, Weber WW, Bell DA, Dupret JM, Evans DA, Grant DM, Hein DW, Lin HJ, Meyer UA, Relling MV. Nomenclature for N-acetyltransferases. Pharmacogenetics. 1995;5:1–17. doi: 10.1097/00008571-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Hein DW, Doll MA, Rustan TD, Gray K, Feng Y, Ferguson RJ, Grant DM. Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis. 1993;14:1633–1638. doi: 10.1093/carcin/14.8.1633. [DOI] [PubMed] [Google Scholar]


