Abstract
AIM: To analyze whether prompt and appropriate empirical antibiotic (AEA) use is associated with mortality in cirrhotic patients with bacteremia.
METHODS: A total of 102 episodes of bacteremia in 72 patients with cirrhosis were analyzed. AEA was defined as a using or starting an antibiotic appropriate to the isolated pathogen at the time of bacteremia. The primary endpoint was 30-d mortality.
RESULTS: The mortality rate at 30 d was 30.4% (31/102 episodes). Use of AEA was associated with better survival at 30 d (76.5% vs 46.9%, P = 0.05), and inappropriate empirical antibiotic (IEA) use was an independent factor associated with increased mortality (OR = 3.24; 95%CI: 1.50-7.00; P = 0.003, adjusted for age, sex, Child-Pugh Class, gastrointestinal bleeding, presence of septic shock). IEA use was more frequent when the isolated pathogen was a multiresistant pathogen, and when infection was healthcare-related or hospital-acquired.
CONCLUSION: AEA use was associated with increased survival of cirrhotic patients who developed bacteremia. Strategies for AEA use, tailored according to the local epidemiological patterns, are needed to improve survival of cirrhotic patients with bacteremia.
Keywords: Liver cirrhosis, Bacteremia, Appropriate antibiotics, Survival, Multiresistant pathogen
Core tip: Appropriate empirical antibiotic use was associated with improved survival in cirrhotic patients with bacteremia, indicating the importance of initial antibiotic selection.
INTRODUCTION
Bacterial infections are very frequent in patients with advanced cirrhosis[1]. Patients with cirrhosis have altered and impaired immunity, which favors bacterial translocation, and may explain the high incidence of bacterial infection[2]. Patients with cirrhosis are not only at increased risk of developing bacterial infection[3], but are also at increased risk of death from bacterial infection compared with individuals without cirrhosis[4,5]. Bacterial infection is a major cause of death in cirrhosis patients[6]. Therefore, early diagnosis and treatment of bacterial infection is pivotal in the management of these patients[6]. In a heterogeneous patient population with septic shock, early initiation of appropriate empirical antibiotic (AEA) therapy was associated with a higher survival rate[7-9]. However, little data exist on the association between AEA use and outcome in patients with cirrhosis. Therefore, in this study, we assessed whether AEA use is associated with survival in cirrhotic patients who developed bacteremia.
MATERIALS AND METHODS
Study design, setting and participants
A retrospective, historical cohort was identified by reviewing the database of Sanggye Paik Hospital, Inje University School of Medicine, Seoul, South Korea, between January 2008 and December 2011. During the study period, a total of 114 episodes of bacteremia were identified. Cirrhosis was defined clinically, when indicators of cirrhosis were present, including thrombocytopenia (platelet count < 150 × 103/L), splenomegaly (by cross-sectional images), ascites (by cross-sectional images or the use of diuretics for control of ascites), varices (by upper endoscopy, cross-sectional images, or a history of variceal bleeding), and a cirrhotic liver in cross-sectional imaging studies (nodular liver surface or caudate lobe hypertrophy)[10]. We excluded 12 episodes of bacteremia that were transferred to other hospitals within 30 d. Finally, a total of 102 episodes of bacteremia were included and analyzed. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and was approved by the Institutional Review Board at Sanggye Paik Hospital.
Variables and definitions
From the medical records, we collected data on age, sex, height, weight, admission date, causes of cirrhosis, presence of gastrointestinal bleeding, ascites, encephalopathy, discharge date, and mortality. We also collected data on the isolated pathogen, and antibiotics used, as well as systolic blood pressure, diastolic blood pressure, heart rate, body temperature, respiration rate, white blood cell (WBC) count, neutrophil count, platelet count, prothrombin time, bilirubin, albumin, creatinine, sodium, aspartate aminotransferase, alanine aminotransferase, and C-reactive protein on the day of bacteremia. The type of infection was defined as community-acquired, if diagnosed within 48 h of admission without hospitalization in the previous 6 mo; healthcare-associated, if diagnosed within 48 h of admission in patients hospitalized for at least 2 d in the previous 6 mo; and hospital-acquired if diagnosed 48 h after admission[11].
Infections were defined as follows[11,12]: (1) spontaneous bacteremia; positive blood cultures without a source of infection; (2) spontaneous bacterial peritonitis (SBP): ascetic fluid polymorphonuclear cells > 250/μL with/without a positive fluid culture; (3) lower respiratory tract infections: new pulmonary infiltrate in the presence of: (a) at least one respiratory symptom (cough, sputum production, dyspnea, peluritic pain) with (b) at least one finding on auscultation (rales or crepitation) or one sign of infection (core body temperature > 38 °C or < 36 °C) in the absence of antibiotics; (4) skin infection: fever with cellulitis; (5) urinary tract infection (UTI): urine WBC > 15/high-power field with either positive urine Gram stain or culture in a symptomatic patients; and (6) other sources of infection; (e.g., intraabdominal abscess, cholecystitis, secondary peritonitis).
Systemic inflammatory response syndrome (SIRS) was defined when 2 or more of the following criteria were present: (1) a core temperature ≥ 38 °C or ≤ 36 °C; (2) a heart rate ≥ 90 beats/min; (3) tachypnea ≥ 20 breaths/min or partial carbon monoxide pressure ≤ 32 mmHg or the need of mechanical ventilation; and (4) a WBC count ≥ 12 × 109/L or ≤ 4 × 109/L or > 10% of immature neutrophils[13]. Septic shock was defined as patients with SIRS plus persistent hypotension requiring therapy with vasopressors[14]. AEA use was defined as an antimicrobial with in vitro activity appropriate for the isolated pathogen[14]. Otherwise, the initial therapy was considered inappropriate empirical antibiotics (IEA) therapy.
Multiresistant pathogens were defined as the following in the current study: extended-spectrum beta-lactamase-producing bacteria (e.g., Escherichia coli and Klebsiellan pneumonia) or derepressed chromosomic AmpC beta-lactamase producing Enterobacteriaceae (e.g., Enterobacter or Citrobacter spp), Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Acinetobacter baumanii, Achromobacter spp., methicillin-resistant Staphylococus aureus, and Enterococcus faecium.
Statistical analysis
The primary endpoint variable was survival at 30 d. The difference in 30-d survival rate was compared between patients who received AEA and those who did not, using the Kaplan-Meier method with the log-rank test. Cox regression analysis was conducted to identify factors associated with 30-d mortality. Multivariable Cox-regression analysis was conducted to determine the independent factors using variables with P < 0.05 in the univariate analysis. Age and sex were included in the multivariable model, irrespective of the P value in the univariable analysis. A P-value < 0.05 was considered significant. The statistical methods in this study were reviewed by Dong Hyun Sinn, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
RESULTS
Baseline characteristics
During the study period, 102 episodes of bacteremia in 72 patients (52 male, 20 female; mean age: 57.6 ± 11.0 years) were identified. Thirty patients had multiple episodes (range: 2-4) of bacteremia during the study period. Alcohol was a major cause of cirrhosis (34/72, 47.2%) followed by hepatitis B (25/72, 35.7%), non-B, non-C cirrhosis (8/72, 11.1%) and hepatitis C (5/72, 6.9%). Four patients had hepatocellular carcinoma.
Infection type, site, isolated pathogen
Of the 102 episodes of bacteremia, 23 (22.5%) were community-acquired, 3 (2.9%) were healthcare-associated, and 69 (67.6%) were hospital-acquired. SIRS was noted in 68 episodes (66.7%). Septic shock was present in 27 episodes (26.5%). The most frequent origin of bacteremia was spontaneous bacteremia (33.3%), followed by SBP (30.4%), UTI (16.7%), lower respiratory tract infection (8.8%), soft tissue infection (4.9%), intra-abdominal abscess (3.0%), cholecystitis (2.0%), and secondary peritonitis (1.0%). The isolated pathogens are shown in Table 1.
Table 1.
Isolated bacteria n (%)
| n = 102 | |
| Gram (+) | |
| Staphylococcus aureus | 13 (13) |
| Coagulase negative staphylococci | 21 (21) |
| Streptococcus species | 7 (7) |
| Enterococcus species | 8 (8) |
| 1Other Gram (+) pathogens | 3 (3) |
| Gram (-) | |
| Escherichia coli | 16 (16) |
| Klebsiella pneumoniae | 18 (18) |
| Pseudomonas aeruginosa | 3 (3) |
| Acinetobacter baumannii | 5 (5) |
| 2Other Gram (-) pathogens | 8 (8) |
Other Gram (+) pathogens were Micrococcus luteus (n = 2), and Leuconostoc (n = 1);
Other Gram (-) pathogens were Aeromonas (n = 3), Citrobacter (n = 1), Flavobacterium (n = 1), Morganella (n = 1), Serratia (n = 1), Vibrio vulnificus (n = 1).
AEA use and 30-d mortality
The mortality rate at 30-d was 30.4% (31/102 episodes). The 30-day mortality was significantly lower in patients treated with AEA (18.5% vs 43.8%, P = 0.006, Figure 1). In the univariable analysis, use of AEA, Child-Pugh class (C vs A/B), gastrointestinal bleeding (yes vs no), and presence of septic shock (yes vs no) were also factors associated with 30-d mortality (Table 2). Age, sex, multiresistant pathogen, infection type (hospital- or healthcare-acquired vs community-acquired), and presence of SIRS were not associated with 30-d mortality. In the multivariable analysis, Child-Pugh Class (C vs A/B), AEA use, gastrointestinal bleeding, and presence of septic shock were independent factors associated with 30-d mortality (Table 2).
Figure 1.
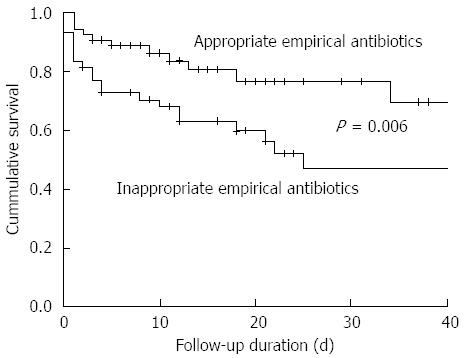
30-d survival rate according to the use of appropriate empirical antibiotics. The 30-d survival rate was significantly higher in patients treated with appropriate empirical antibiotics (81.5% vs 56.2%, P = 0.006).
Table 2.
Factors associated with 30-d mortality
| Factors | Univariate | P value | Multivariate | P value |
| Age (per years) | 1.00 (0.97-1.03) | 0.89 | ||
| Male ( vs female) | 0.92 (0.42-2.1) | 0.84 | ||
| Child-Pugh class (C vs A/B) | 7.93 (2.40-26.1) | 0.01 | 7.18 (2.07-24.91) | 0.002 |
| Multiresistant pathogen | 1.49 (0.73-3.06) | 0.26 | ||
| Inappropriate antibiotic use | 2.00 (0.97-4.13) | 0.06 | 3.24 (1.50-7.00) | 0.003 |
| Gastrointestinal bleeding | 2.12 (1.01-4.43) | 0.05 | 2.92 (1.33-6.38) | 0.007 |
| Infection type (community vs non-community) | 0.76 (0.35-1.64) | 0.49 | ||
| Systemic inflammatory response syndrome | 1.79 (0.77-4.16) | 0.17 | ||
| Septic shock | 4.26 (2.10-8.66) | < 0.01 | 3.39 (1.56-7.37) | 0.002 |
AEA use and 30-d mortality in subgroups
In subgroup analysis, the 30-d mortality was significantly higher for no AEA use in patients with Child-Pugh Class C [66.7% (18/27) vs 30.3% (10/33), P = 0.005], with SIRS [53.1% (17/32) vs 19.4% (7/36), P = 0.004], or with septic shock [85.7% (12/14) vs 30.8% (4/13), P = 0.006]. The 30-d mortality was also higher in Child-Pugh Class A/B [14.3% (3/21) vs 0% (0/21), P = 0.23], in patients without SIRS [25.0% (4/16) vs 16.7% (3/18), P = 0.68], and in patients without septic shock [26.5% (9/34) vs 14.6% (6/41), P = 0.25], although the difference was not statistically significant.
Factors associated with AEA use
IEA use was much higher in hospital-acquired infection (55.1%, 38/69) and healthcare-associated infection (66.7%, 2/3) than in community-acquired infection (26.7%, 8/30, P = 0.02). IEA use was also much higher when the isolated organism was a multiresistant pathogen [80.0%, (36/45) vs 21.1%, (12/57), P = 0.01], but was not different according to infection site, presence of SIRS, septic shock, or Child-Pugh Class.
DISCUSSION
In this study, the 30-d mortality rate in cirrhotic patients with bacteremia was 30.4%. The independent predictors for mortality were liver function (Child-Pugh Class C), gastrointestinal bleeding, presence of septic shock, and IEA use. IEA use was significantly associated with mortality, especially for patients with poor liver function (Child-Pugh class C), with SIRS or with septic shock. The major reason for IEA use was a multiresistant pathogen, usually in the setting of hospital-acquired or healthcare-associated infection.
Patients with liver cirrhosis are known to have impaired immunity and are predisposed to infection[15]. Furthermore, when infections develop, they are known to increase mortality 4-fold in these patients[16]. Compared to non-cirrhotic patients, cirrhotic patients showed poor prognosis in community-acquired bacteremia[17,18], community-acquired pneumonia[19], and any bacteremia[5]. In this study, we also observed a high mortality rate in our series (30.4%) when cirrhotic patients developed bacteremia. In this study, advanced liver disease (Child-Pugh Class C) was found to be an independent prognostic factor for mortality, indicating the importance of underlying liver function as a prognostic marker. Consistent with our finding, several studies also showed that fatal outcomes were more frequent in patients with advanced cirrhosis[15,17,20]. Certainly, liver function is an important predictor in mortality. However, in addition to liver function, gastrointestinal bleeding, presence of septic shock, and IEA use were independent factors associated with mortality in this study. Of these factors, a modifiable, physician-dependent factor is AEA use. Indeed, several previous studies have shown the importance of AEA use in the setting of septic shock[7-9,14]. In line with a previous study in cirrhosis patients[14], we also noted that AEA use was a crucial component in improving the outcome of cirrhotic patients with bacteremia.
Generally, intravenous third generation cephalosporins are recommended as an empirical antibiotic therapy for cirrhotic patients[6]. However, increased incidences of Gram-positive and drug-resistant organisms have been reported, particularly in hospital-acquired infections and in patients receiving quinolone prophylaxis[21]. Nosocomial infection is a well-known predictor of multiresistant bacteria, along with long-term norfloxacin prophylaxis, recent infection by multiresistant bacteria, and recent use of beta-lactams[22]. Hospitalized patients with cirrhosis have the highest risk of developing infections[23], and secondary infections that develop in a hospital setting are a predictor of mortality in cirrhotic patients[11]. We also observed that IEA use was mainly due to multiresistant pathogens, mainly in the setting of hospital-acquired or healthcare-related infection.
Although the data are limited by being a retrospective study with a small sample size, they demonstrate the importance of AEA use on the outcome of cirrhotic patients with bacteremia. The impact of AEA on survival of patients with bacteremia was significant, especially those with Child-Pugh Class C, SIRS, or septic shock. IEA use was mainly due to multiresistant pathogens. Strategies for AEA use, tailored according to the local epidemiological patterns, are needed to improve survival of cirrhotic patients with bacteremia.
COMMENTS
Background
Bacterial infections are very frequent in patients with advanced cirrhosis. Patients with cirrhosis are not only at increased risk of developing bacterial infection, but are also at increased risk of death from bacterial infection compared with individuals without cirrhosis. Currently, little data exist on the association between appropriate empirical antibiotic (AEA) use and outcome in patients with cirrhosis.
Research frontiers
The association between AEA use and survival of cirrhotic patients who developed bacteremia was analyzed.
Innovations and breakthroughs
AEA use was associated with better survival at 30 d and inappropriate empirical antibiotic (IEA) use was an independent factor associated with increased mortality. IEA use was more frequent when the isolated pathogen was multiresistant, and when the infection was healthcare-related or hospital-acquired infection.
Applications
This data suggest that AEA use is an important factor in improving the outcome of cirrhotic patients with bacteremia. Strategies for AEA use, tailored according to the local epidemiological patterns, are needed to improve survival of cirrhotic patients with bacteremia.
Terminology
AEA use was defined if an the antimicrobial had in vitro activity appropriate for the isolated pathogen.
Peer-review
Bacterial infections are the major cause of death in cirrhotic patients. This manuscript described the retrospective investigation of the importance of appropriate empirical antibiotics in treatment of cirrhotic patients with bacteremia. Although this story is lack of novelty due to the publications of several other studies on the similar subject, the experiments conducted in this study have rationally been designed and carried out, and the elementary internal connections between use of appropriate empirical antibiotics and outcome in patients with cirrhosis have been revealed in this study.
Footnotes
Ethics approval: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and was reviewed and approved by the Institutional Review Board at Sanggye Paik Hospital.
Informed consent: Waived by the Institutional Review Board.
Conflict-of-interest: The authors (Park H, Jang KJ, Jang W, Park SH, Park JY, Jeon TJ, Oh TH, Shin WC, Choi WC and Sinn DH) declare no conflict of interest relevant to this study.
Biostatistics statement: The statistical methods of this study were reviewed by Dong Hyun Sinn, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 29, 2014
First decision: November 26, 2014
Article in press: January 8, 2015
P- Reviewer: Yu Y S- Editor: Qi Y L- Editor: Cant MR E- Editor: Wang CH
References
- 1.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 2.Pleguezuelo M, Benitez JM, Jurado J, Montero JL, De la Mata M. Diagnosis and management of bacterial infections in decompensated cirrhosis. World J Hepatol. 2013;5:16–25. doi: 10.4254/wjh.v5.i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–2033. doi: 10.1002/hep.23264. [DOI] [PubMed] [Google Scholar]
- 4.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016–1020. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 5.Kang CI, Song JH, Chung DR, Peck KR, Yeom JS, Ki HK, Son JS, Lee JS, Kim YS, Jung SI, et al. Liver cirrhosis as a risk factor for mortality in a national cohort of patients with bacteremia. J Infect. 2011;63:336–343. doi: 10.1016/j.jinf.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38:1773–1785. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 10.Yang JD, Kim WR, Coelho R, Mettler TA, Benson JT, Sanderson SO, Therneau TM, Kim B, Roberts LR. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475–482. doi: 10.1016/j.jhep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 13.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 14.Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, et al. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305–2315. doi: 10.1002/hep.25931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HJ, Lee YM, Bang KM, Park SY, Moon SM, Park KH, Chong YP, Kim SH, Lee SO, Choi SH, et al. Clinical significance of Staphylococcus aureus bacteremia in patients with liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2012;31:3309–3316. doi: 10.1007/s10096-012-1697-4. [DOI] [PubMed] [Google Scholar]
- 16.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–156, 1246-156. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Chen SY, Tsai CL, Lin CH, Lee CC, Chiang WC, Wang JL, Ma MH, Chen SC, Chen WJ, Chang SC. Impact of liver cirrhosis on mortality in patients with community-acquired bacteremia. Diagn Microbiol Infect Dis. 2009;64:124–130. doi: 10.1016/j.diagmicrobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Linderoth G, Jepsen P, Schønheyder HC, Johnsen SP, Sørensen HT. Short-term prognosis of community-acquired bacteremia in patients with liver cirrhosis or alcoholism: A population-based cohort study. Alcohol Clin Exp Res. 2006;30:636–641. doi: 10.1111/j.1530-0277.2006.00074.x. [DOI] [PubMed] [Google Scholar]
- 19.Viasus D, Garcia-Vidal C, Castellote J, Adamuz J, Verdaguer R, Dorca J, Manresa F, Gudiol F, Carratalà J. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 2011;90:110–118. doi: 10.1097/MD.0b013e318210504c. [DOI] [PubMed] [Google Scholar]
- 20.Choi SH, Park HG, Jun JB, Lee SO, Choi SH, Woo JH, Kim YS. Clinical characteristics and outcomes of pneumococcal bacteremia in adult patients with liver cirrhosis. Diagn Microbiol Infect Dis. 2009;63:160–164. doi: 10.1016/j.diagmicrobio.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Bunchorntavakul C, Chavalitdhamrong D. Bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. World J Hepatol. 2012;4:158–168. doi: 10.4254/wjh.v4.i5.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 23.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]


