Abstract
AIM: To investigate whether transarterial chemoembolization (TACE) before liver transplantation (LT) improves long-term survival in hepatocellular carcinoma (HCC) patients.
METHODS: A retrospective study was conducted among 204 patients with HCC who received LT from January 2002 to December 2010 in PLA General Hospital. Among them, 88 patients received TACE before LT. Prognostic factors of serum α-fetoprotein (AFP), intraoperative blood loss, intraoperative blood transfusion, disease-free survival time, survival time with tumor, number of tumor nodules, tumor size, tumor number, presence of blood vessels and bile duct invasion, lymph node metastasis, degree of tumor differentiation, and preoperative liver function were determined in accordance with the Child-Turcotte-Pugh (Child) classification and model for end-stage liver disease. We also determined time of TACE before transplant surgery and tumor recurrence and metastasis according to different organs. Cumulative survival rate and disease-free survival rate curves were prepared using the Kaplan-Meier method, and the log-rank and χ2 tests were used for comparisons.
RESULTS: In patients with and without TACE before LT, the 1, 3 and 5-year cumulative survival rate was 70.5% ± 4.9% vs 91.4% ± 2.6%, 53.3% ± 6.0% vs 83.1% ± 3.9%, and 46.2% ± 7.0% vs 80.8% ± 4.5%, respectively. The median survival time of patients with and without TACE was 51.857 ± 5.042 mo vs 80.930 ± 3.308 mo (χ2 = 22.547, P < 0.001, P < 0.05). The 1, 3 and 5-year disease-free survival rates for patients with and without TACE before LT were 62.3% ± 5.2% vs 98.9% ± 3.0%, 48.7% ± 6.7% vs 82.1% ± 4.1%, and 48.7% ± 6.7% vs 82.1% ± 4.1%, respectively. The median survival time of patients with and without TACE before LT was 50.386 ± 4.901 mo vs 80.281 ± 3.216 mo (χ2 = 22.063, P < 0.001, P < 0.05). TACE before LT can easily lead to pulmonary or distant metastasis of the primary tumor. Although there was no significant difference between the two groups, the chance of metastasis of the primary tumor in the group with TACE was significantly higher than that of the group without TACE.
CONCLUSION: TACE pre-LT for HCC patients increased the chances of pulmonary or distant metastasis of the primary tumor, thus reducing the long-term survival rate.
Keywords: Liver transplantation, Hepatocellular carcinoma, Transarterial chemoembolization, Long-term survival rate, Disease-free survival rate
Core tip: Hepatocellular carcinoma (HCC) has a high prevalence in China. Patients always have a long-term history of liver cirrhosis, varying degrees of portal hypertension symptoms, and the tumor volume exceeds the Milan criteria when they receive treatment. Whether it is necessary to adopt transarterial chemoembolization (TACE), which is more commonly used in China pre-transplantation, when the patients in waiting state. This study assessed the influence of preoperative TACE on long-term survival in liver transplantation (LT). TACE pre-LT in patients with HCC increased the chances of pulmonary or distant metastasis of the primary tumor, thus reducing long-term survival.
INTRODUCTION
About 600000 people die of hepatocellular carcinoma (HCC) annually, making it one of the most common malignancies worldwide[1]. Only about 10%-30% of patients have the opportunity for surgery[2], which is mainly liver resection and liver transplantation (LT). There is considerable controversy about whether liver transplant patients in the waiting period should receive other adjuvant therapy, such as transarterial chemoembolization (TACE) and radiofrequency ablation (RFA)[3-10]. Some researchers propose that TACE before LT can help patients meet the strict Milan criteria and thus prolong postoperative long-term survival[11]. However, other researchers propose that it is inappropriate to perform adjuvant treatment before LT for patients with HCC because spread and metastasis may occur at the liver puncture site[12,13]. It has also been reported that incomplete local treatment may cause sarcomatous changes in patients with HCC[14]. Kim et al[15] reported that treatment before LT has a significant impact on the prognosis if the volume ratio of the active tumor is > 10%. Wong et al[16] reported that the chance of necrosis was < 75% in five patients, due to local/non-local recurrence, and the use of RFA, TACE, or cisplatin gelatin injection.
In China, the tumor volume in the vast majority of patients with HCC already exceeds the Milan criteria when they receive treatment. Also, these patients always have a long-term history of liver cirrhosis, and varying degrees of portal hypertension symptoms are also seen in some patients. For such patients, there is still no consensus about whether it is necessary to adopt adjuvant therapy, such as TACE, which is more commonly used in China before transplantation. Thus, a retrospective analysis was carried out to assess the influence of preoperative TACE on long-term survival rates in liver transplant patients.
MATERIALS AND METHODS
Clinical data
We analyzed retrospectively 204 patients with HCC and cirrhosis receiving LT from January 2002 to December 2010.
Patients constitution
Two hundred and four patients met the inclusion criterion, 180 male and 24 female, with ages ranging from 31 to 68 years [average: 50 (50.23 ± 7.88) years]. There were 88 and 116 patients in the groups with and without TACE, respectively. The duration of follow-up was 96 mo with a follow-up rate of 100%. All donors made voluntary donations, including 155 cases of cardiac death and 49 living donors. No donor was a condemned donor. The PLA General Hospital Ethics Committee reviewed and supervised the entire donation process to ensure compliance with these requirements.
Preoperative examination
Preoperative diagnosis of HCC relied on imaging examinations such as ultrasound, computed tomography (CT) and enhanced nuclear magnetic resonance imaging (MRI). All patients received radionuclide bone scans, chest CT, and/or positron emission tomography-CT examinations to exclude extrahepatic metastases.
Inclusion criteria
Patients who had no lung or abdominal metastases or lymph node metastasis, as determined by chest and abdominal CT or MRI examination; patients who had no metastases, as determined by whole-body bone scan; patients whose HCC was confirmed by postoperative pathology; and patients who had long-term follow-up.
Exclusion criteria
Patients who had serious perioperative complications or died, and patients who died of non-liver-related diseases.
Patient information
Clinical data included age (years), serum α-fetoprotein (AFP), blood loss, intraoperative blood transfusion, disease-free survival time, and survival time with tumor. AFP was divided into four groups: normal range ≤ 20 ng/L, 20-400 ng/L, 400-1000 ng/L, and > 1000 ng/L. Tumor size was divided into three groups: meeting the Milan criteria (≤ 5 cm), complying with the UCSF criteria (5-8 cm), and beyond the UCSF criteria (≥ 8 cm). The degree of tumor differentiation was in accordance with the Edmondson grade: level I was highly differentiated (G1); II and III were moderately differentiated (G2); and IV was low differentiation (G3). Grades I and II were classified as well-differentiated, and III and IV as poorly differentiated. Numbers of lesions, determined on the basis of preoperative CT, MRI, and pathological examinations, were divided into single and multiple groups. Preoperative liver function was determined in accordance with the Child classification and the model for end stage liver disease (MELD). Based on time of TACE from transplant surgery, the patients were divided into three groups: ≤ 1 mo, 1-3 mo, and ≥ 3 mo. Survival state was divided into live and dead groups. Liver surgery was divided into whole liver transplantation and living donor transplantation groups. The patients were divided into tumor recurrence and no tumor recurrence groups. According to postoperative complications, patients were divided into four groups: no complications, biliary complications, vascular complications, and infection. Tumor recurrence and metastasis were divided according to different organs: liver, lung and bone.
Liver resection and tumor histopathological evaluation
Numbers of tumor nodules, tumor size, tumor number, presence of blood vessels and bile duct invasion, and lymph node metastasis were determined. Tumor grade was determined according to the standard Edmondson classification. For multiple tumors, total tumor diameter was the maximum diameter of each tumor.
Postoperative immunosuppressive and other therapies
Cyclosporine A + mycophenolate mofetil + methylprednisolone (CsA + MMF + Pred) and tacrolimus (FK506 + MMF + Pred) were used as the main postoperative regimens. Intravenous infusion of hepatitis B immunoglobulin, combined with oral administration of lamivudine and other antiviral drugs, was adopted after surgery to prevent any recurrence of hepatitis B.
TACE before LT
Femoral artery puncture was carried out according to the Seldinger method. A catheter was inserted into the target vessel branches (usually the hepatic or superior mesenteric artery and other arteries) for imaging to determine the location, size, number, and artery of the tumor. After catheter infusion chemotherapy, a vessel was inserted to the supplying arteries (proper hepatic artery, left and right hepatic artery, or branch artery) of the tumor, and iodized oil, chemotherapy drug suspensions, and gelatin sponge were injected for thrombosis.
Follow-up
All patients were followed up after surgery. Methods of follow-up included inpatient and outpatient follow-up and telephone calls. Liver transplant patients were checked monthly in the first 6 mo, and then every 2 mo in the second 6 mo. After that, patients were examined every 3-6 mo. The patient’s condition changes were recorded, and routine examinations of blood, liver and kidney function, blood drug concentrations, and qualitative and quantitative examination of hepatitis B virus were performed. Tumor recurrence and metastasis were monitored by AFP, CT, color Doppler ultrasound, chest radiography, whole-body bone ECT, and other tests. The time and location of tumor recurrence and the time and cause of death were recorded.
Statistical methods
Measurement data are presented as mean ± SD or median, and the t test or χ2 test was used for comparisons between the groups. Survival analysis was performed using the Kaplan-Meier method, and the log-rank test was used to compare the survival probability. The cumulative survival rate was expressed as rate ± SE, and P < 0.05 was considered to indicate a significant difference. SPSS version 16.0 (SPSS, Chicago, IL, United States) was used.
RESULTS
Patient data
Basic data for the 204 cases are shown in Table 1. There were 88 cases with preoperative TACE and 116 without preoperative TACE. The basic data of the two groups were similar. There was no statistically significant difference between age, Child classification or MELD score, AFP values, intraoperative blood loss, or intraoperative autotransfusion between the groups. However, disease-free survival time and survival time with tumor between the two groups did show significant differences (P < 0.05).
Table 1.
Statistical data of the groups with and without preoperative transarterial chemoembolization
| Demographics | TACE (n = 88) | No TACE (n = 116) | t | P value |
| Age (yr) | 49.34 ± 8.06 | 50.90 ± 7.70 | -1.401 | 0.163 |
| Child | 7.33 ± 2.69 | 7.23 ± 2.46 | 0.267 | 0.789 |
| MELD | 8.36 ± 7.16 | 8.07 ± 5.74 | 0.324 | 0.747 |
| IBL (mL) | 2944.32 ± 4008.34 | 2528.79 ± 2681.56 | 0.886 | 0.377 |
| IOA (mL) | 1910.34 ± 2902.87 | 1539.53 ± 2532.91 | 0.972 | 0.332 |
| AFP (ng/L) | 3439.08 ± 6623.56 | 1922.59 ± 5180.01 | 1.835 | 0.068 |
| Disease-free survival time (mo) | 20.77 ± 17.52 | 32.79 ± 22.63 | -4.131 | < 0.001a |
| Survival time with tumor (mo) | 1.88 ± 3.10 | 0.68 ± 2.10 | 3.278 | 0.001a |
P < 0.05, TACE vs no TACE. TACE: Transarterial chemoembolization; Child: Child-Turcotte-Pugh classification; MELD: Model for end-stage liver disease; AFP: α-fetoprotein; IBL: Intraoperative blood loss; IOA: Intraoperative autotransfusion.
Categorical data between the groups with and without preoperative TACE
Tumor recurrence and AFP in the two groups showed significant differences (P < 0.05). The differences in sex, MELD score, Child classification, surgical approach, and postoperative complications between the two groups were not significant (P > 0.05). The results are shown in Table 2.
Table 2.
Categorical data of the groups with and without preoperative transarterial chemoembolization treatment
| Variables and stratification | TACE (n = 88) | No TACE (n = 116) | χ2 | P value |
| Sex | 0.352 | 0.553 | ||
| Male | 79 | 101 | ||
| Female | 9 | 15 | ||
| MELD | 5.269 | 0.072 | ||
| < 15 | 80 | 104 | ||
| 15-25 | 5 | 12 | ||
| > 25 | 3 | 0 | ||
| Child | 0.207 | 0.902 | ||
| A | 56 | 77 | ||
| B | 14 | 18 | ||
| C | 18 | 21 | ||
| Operation method | 1.874 | 0.171 | ||
| CDLT | 71 | 84 | ||
| LDLT | 17 | 32 | ||
| AFP | 14.141 | 0.003a | ||
| < 20 | 21 | 47 | ||
| ≤ 400 | 22 | 38 | ||
| 400-1000 | 18 | 9 | ||
| > 1000 | 27 | 22 | ||
| Tumor recurrence | 21.983 | < 0.001a | ||
| No | 46 | 96 | ||
| Yes | 42 | 20 | ||
| Complications | 3.064 | 0.382 | ||
| No | 66 | 97 | ||
| Complication of bile duct | 8 | 9 | ||
| Complication of vessel | 7 | 6 | ||
| Infection | 7 | 4 |
P < 0.05, TACE vs no TACE. TACE: Transarterial chemoembolization; Child: Child-Turcotte-Pugh classification; MELD: Model for end-stage liver disease; AFP: α-fetoprotein; CDLT: Cadaveric donor liver transplantation; LDLT: Living donor liver transplantation.
Postoperative pathological data
Postoperative pathological data for the two groups of patients are shown in Table 3. Tumor size was divided into three groups: meeting the Milan criteria (≤ 5 cm), complying with the UCSF criteria (5-8 cm), and beyond the UCSF criteria (≥ 8 cm). The degree of tumor differentiation was in accordance with the Edmondson grade: a moderate-high differentiation group and a moderate-low differentiation group; the two groups showed no significant difference (P > 0.05). The size, number, and vascular invasion in the two groups showed significant differences (P < 0.05).
Table 3.
Postoperative pathological data for the two groups of patients
| Variables and stratification | TACE (n = 88) | No TACE (n = 116) | χ2 | P value |
| Tumor size | 6.569 | 0.037a | ||
| Within Milan criteria | 38 | 70 | ||
| Within UCSF criteria | 22 | 24 | ||
| Beyond UCSF criteria | 28 | 22 | ||
| Vascular invasion | 12.138 | < 0.001a | ||
| No | 48 | 90 | ||
| Yes | 40 | 26 | ||
| Tumor Edmondson grade | 2.521 | 0.112 | ||
| Well-moderate | 57 | 87 | ||
| Moderate-poor | 31 | 29 | ||
| Number of tumors | 15.632 | < 0.001a | ||
| Solitary | 38 | 82 | ||
| Multiple | 50 | 34 | ||
P < 0.05, TACE vs no TACE. TACE: Transarterial chemoembolization.
Influence of TACE before LT on long-term and disease-free survival
The 1, 3 and 5-year cumulative survival rates of the patients with TACE before LT were 70.5% ± 4.9%, 53.3% ± 6.0%, and 46.2% ± 7.0%, respectively, while for patients without TACE treatment before LT, they were 91.4% ± 2.6%, 83.1% ± 3.9%, and 80.8% ± 4.5%, respectively. The median survival times of the patients with and without TACE before LT were 51.857 ± 5.042 and 80.930 ± 3.308 mo, respectively (χ2 = 22.547, P < 0.001, P < 0.05), and the cumulative survival curves are shown in Figure 1. The 1, 3 and 5-year disease-free survival rates of the patients with TACE before LT were 62.3% ± 5.2%, 48.7% ± 6.7%, and 48.7% ± 6.7%, respectively, while for patients without TACE treatment before LT, they were 98.9% ± 3.0%, 82.1% ± 4.1%, and 82.1% ± 4.1%, respectively, and cumulative disease-free survival curves are shown in Figure 2. The median survival times of patients with and without TACE treatment before LT were 50.386 ± 4.901 and 80.281 ± 3.216 mo, respectively (χ2 = 22.063, P < 0.001, P < 0.05).
Figure 1.
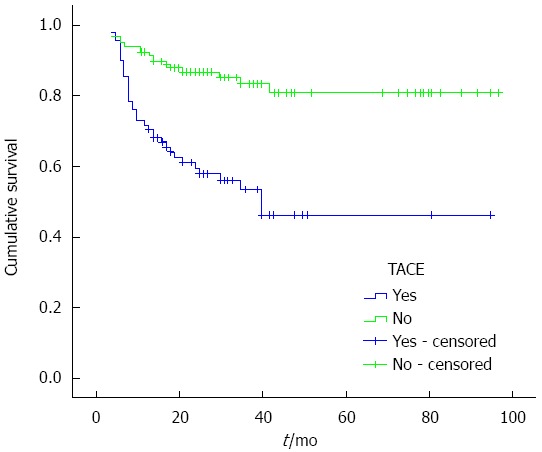
Cumulative survival curves of patients plotted using the Kaplan-Meier method. Green line: Cumulative survival curve of the 116 patients with TACE before LT; Blue line: Cumulative survival curve of the 88 patients without TACE before LT. The cumulative survival rates of the two groups showed a significant difference (P < 0.05, log-rank test). TACE: Transarterial chemoembolization; LT: Liver transplantation.
Figure 2.
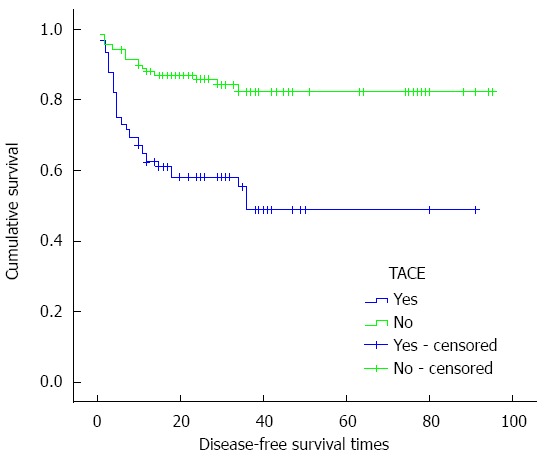
Cumulative disease-free survival curves of patients plotted using the Kaplan-Meier method. Green line: Cumulative disease-free survival curve of the 116 patients with TACE before LT; Blue line: Cumulative disease-free survival curve of the 88 patients without TACE before LT. The cumulative disease-free survival rates of the two groups showed a significant difference (P < 0.05, log-rank test). TACE: Transarterial chemoembolization; LT: Liver transplantation.
Influence of time of TACE from LT on location and recurrence of the tumor
The 62 patients with tumor recurrence were divided into three groups according to tumor size: those meeting the Milan criteria, complying with UCSF criteria, and beyond the UCSF criteria. Based on the time of TACE treatment from transplant surgery, the patients were divided into three groups: ≤ 1, 1-3 and ≥ 3 mo. As seen in Table 4, the results showed that the groups with and without TACE treatment showed significant differences (P < 0.05).
Table 4.
Comparison of the groups with and without transarterial chemoembolization treatment at different times from the liver transplantation
|
TACE |
No TACE | χ2 | P value | |||
| ≤ 1 mo | 1-3 mo | ≥ 3 mo | ||||
| Milan criteria | 2 | 1 | 8 | 5 | 16 | 0.001a |
| UCSF criteria | 1 | 1 | 7 | 6 | 15 | 0.002a |
| BUCSF criteria | 8 | 9 | 5 | 9 | 31 | < 0.001a |
P < 0.05, TACE vs no TACE. TACE: Transarterial chemoembolization.
Tumor recurrence and metastases were divided according to different organs: liver, lung and bone, and multiple metastases. The results showed that for the groups beyond USCF with and without TACE, χ2 = 10.459, P = 0.015 and P <0.05, respectively, while for the groups meeting the Milan criterion and USCF with and without TACE treatment, there was no significant difference (P > 0.05). As seen in Table 5, the number of cases with pulmonary and distant metastasis in the TACE groups was higher than that of the groups without TACE.
Table 5.
Tumor recurrence and metastasis in the groups with and without transarterial chemoembolization
|
TACE |
No TACE |
χ2 | P value | |||||||
| Liver | Lung | Bone | M | Liver | Lung | Bone | M | |||
| Milan criteria | 2 | 7 | 0 | 2 | 1 | 3 | 0 | 1 | 0.019 | 0.990 |
| UCSF criteria | 0 | 7 | 0 | 2 | 1 | 3 | 0 | 2 | 2.083 | 0.353 |
| BUCSF criteria | 3 | 11 | 0 | 8 | 5 | 3 | 1 | 0 | 10.459 | 0.015a |
P < 0.05, TACE vs no TACE. TACE: Transarterial chemoembolization.
DISCUSSION
LT as a curative and effective therapy for HCC can remove the tumor and cirrhosis of the liver tissue and avoid malignant changes in residual disease in liver tissue[17,18]. However, whether TACE before LT is suitable for patients with HCC is still controversial. Grasso and others have suggested that preoperative adjuvant therapy has no effect on the recurrence rate when the Milan criteria are not followed[19]. Yao et al[20] have proposed that proper preoperative treatment is necessary for patients exceeding the Milan criteria. Roayaie and others have suggested that preoperative TACE combined with postoperative doxorubicin chemotherapy has satisfactory therapeutic effects for large liver cancers and LT[21]. Aggressive preoperative treatment may have a positive role in reducing neoplasm stage, and thus reduce the rate of tumor recurrence.
Our results suggest that sex, MELD score, Child classification, surgical approach, postoperative complications, and other general clinical data in the groups with and without TACE made no significant difference. The 1-, 3- and 5-year cumulative survival rates of the patients with and without TACE were significantly different (χ2 = 22.547, P < 0.001, P < 0.05). The cumulative disease-free survival rates of the two groups were also significantly different (χ2 = 22.063, P < 0.001, P < 0.05). These results indicate that preoperative TACE had no positive role on the long-term or disease-free survival rates for HCC. Indeed, on the contrary, they may reduce the lifetime of the patients. Docaens and others have proposed that preoperative TACE does not prolong long-term survival[3]. Sarasin and others noted that only when liver cancer patients waited > 8 mo for LT did the tumor show unfavorable prognostic factors for LT[22]. If the waiting time for LT is 1-2 mo, TACE may have no effect. If the waiting time for LT is a few months, TACE may make the tumor remain at the earliest state, and thus may be beneficial in controlling tumor growth during the period of waiting for a donor.
In the 62 cases of patients with tumor recurrence, tumor size (meeting the Milan criteria, complying with UCSF criteria, and beyond the UCSF criteria) and the time of TACE from transplant surgery (≤ 1, 1-3 and ≥ 3 mo) in the groups with and without TACE did show significant differences (χ2 = 16.0, P = 0.001; χ2 = 15.0, P = 0.002; χ2 = 31.0, P < 0.001, P < 0.05, respectively). Tumor recurrence and metastasis were divided according to different organs: liver, lung and bone, and multiple metastases. Comparisons between the TACE and non-TACE groups showed that TACE before LT may cause pulmonary and distant metastases. The number of cases meeting the USCF criteria in the TACE group was higher than in the non-TACE group, although there was no significant difference between them. For patients beyond the USCF criteria, larger tumors were more common in the TACE group, and comparison of the two groups showed a significant difference (P < 0.05). The mechanism remains unclear.
The current study shows that residual liver cancer cells and normal liver tissue undergo gene expression changes after TACE; that is, TACE can promote angiogenesis factor expression in residual tumor cells. Animal experiments have demonstrated that after TACE, in tumor remnants, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, microvascular density, and proliferative activity of the non-embolized tumor cells increased[23]. An et al[24] found that VEGF levels in the peripheral blood of patients with HCC after TACE increased, and expression of VEGF by cancer cells and noncancerous liver cells in surgical specimens from a two-stage operation after TACE also increased. Li et al[25] found that part of the non-embolized liver appears to undergo compensatory hyperplasia and increased proliferative activity after TACE. The possibility for recurrence and metastasis of residual HCC cells increases after TACE. The remnants of normal liver tissue may lead to recurrence of HCC, due to cirrhosis and an increase in compensatory hyperplasia and proliferative activity after TACE. Furthermore, recent studies have shown that the establishment of collateral circulation after TACE is a major factor for HCC recurrence and metastasis, which may lead to more vulnerable growth in other parts of the tumor cells with high metastatic potential. The specific mechanisms remain to be studied further. However, at least based on current findings, there is no conclusive evidence that preoperative TACE before LT can prolong long-term survival after LT in patients with HCC[21,26-33].
In conclusion, for the patients with HCC before LT, especially for patients who can undergo LT in < 3 mo, preoperative TACE is not appropriate and may result in lung and/or distant metastases. However, whether this conclusion applies to other transplant centers needs further study.
COMMENTS
Background
Hepatocellular carcinoma (HCC) has a high prevalence in Asian countries, particularly China. These patients always have a long-term history of liver cirrhosis, varying degrees of portal hypertension symptoms, and the tumor volume exceeds the Milan criteria when they receive treatment. So, there is still no consensus about whether it is necessary to adopt transarterial chemoembolization (TACE), which is more commonly used in China before liver transplantation (LT). This study assessed the influence of preoperative TACE on long-term survival rates in liver transplant patients.
Research frontiers
A retrospective study included 204 patients with HCC and cirrhosis who received LT from January 2002 to December 2010. The numbers of patients in the groups with and without TACE before LT were 88 and 116, respectively. All patients were in the PLA General Hospital.
Innovations and breakthroughs
The results demonstrated TACE before LT for patients with HCC had no positive influence on long-term survival or disease-free survival rates, but increased the chances of pulmonary or distant metastasis of the primary tumor, thus reducing the long-term survival rate.
Applications
Preoperative TACE before LT for patients with HCC had no positive influence on long-term or disease-free survival rates, however, it increased the chances of pulmonary or distant metastasis of the primary tumor.
Peer-review
This study aimed to evaluate outcomes whether TACE treatment before LT can improve long-term survival rates in HCC patients. The results are interesting and suggest that preoperative TACE treatment before LT for the patients with HCC had no positive influence on long-term survival or disease-free survival rates, however increased the chances of pulmonary metastasis or distant metastasis of the primary tumor. It can be reduced the long-term survival rate.
Footnotes
Supported by National Science and Technology Major Project for Infectious Diseases of China, No. 2012ZX10002-017; National Key Technology Research And Development Program of China, No. 2012BAI06B01; and the Grants from Natural Science Foundation of China, No. 81302123.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 13, 2014
First decision: August 15, 2014
Article in press: October 21, 2014
P- Reviewer: Bell E, Ordi J S- Editor: Yu J L- Editor: Kerr C E- Editor: Ma S
References
- 1.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 2.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 3.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 4.Ravaioli M, Grazi GL, Ercolani G, Fiorentino M, Cescon M, Golfieri R, Trevisani F, Grigioni WF, Bolondi L, Pinna AD. Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation. 2004;78:1780–1786. doi: 10.1097/01.tp.0000145892.97114.ee. [DOI] [PubMed] [Google Scholar]
- 5.Liou TC, Shih SC, Kao CR, Chou SY, Lin SC, Wang HY. Pulmonary metastasis of hepatocellular carcinoma associated with transarterial chemoembolization. J Hepatol. 1995;23:563–568. doi: 10.1016/0168-8278(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 6.Bharat A, Brown DB, Crippin JS, Gould JE, Lowell JA, Shenoy S, Desai NM, Chapman WC. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg. 2006;203:411–420. doi: 10.1016/j.jamcollsurg.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R, Kerlan R, Venook A, Ascher NL, Emond JC, Roberts JP. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 8.Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, Margreiter R, Vogel W. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. doi: 10.1002/lt.21033. [DOI] [PubMed] [Google Scholar]
- 9.Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim do Y, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Shin SW, Choo SW, Do YS, Rhee JC. Milan criteria are useful predictors for favorable outcomes in hepatocellular carcinoma patients undergoing liver transplantation after transarterial chemoembolization. World J Gastroenterol. 2006;12:6992–6997. doi: 10.3748/wjg.v12.i43.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 13.Ishii H, Okada S, Okusaka T, Yoshimori M, Nakasuka H, Shimada K, Yamasaki S, Nakanishi Y, Sakamoto M. Needle tract implantation of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1998;82:1638–1642. [PubMed] [Google Scholar]
- 14.Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res. 2003;27:163–167. doi: 10.1016/s1386-6346(03)00207-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Choi BI, Lee JY, Kim SJ, So YH, Eun HW, Lee JM, Han JK. Diagnostic accuracy of multi-/single-detector row CT and contrast-enhanced MRI in the detection of hepatocellular carcinomas meeting the milan criteria before liver transplantation. Intervirology. 2008;51 Suppl 1:52–60. doi: 10.1159/000122598. [DOI] [PubMed] [Google Scholar]
- 16.Wong LL, Tanaka K, Lau L, Komura S. Pre-transplant treatment of hepatocellular carcinoma: assessment of tumor necrosis in explanted livers. Clin Transplant. 2004;18:227–234. doi: 10.1111/j.1399-0012.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 17.Steinmüller T, Jonas S, Neuhaus P. Review article: liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17 Suppl 2:138–144. doi: 10.1046/j.1365-2036.17.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 18.Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: an update of the International Tumor Registry. Liver Transpl. 2002;8:736–748. doi: 10.1053/jlts.2002.34879. [DOI] [PubMed] [Google Scholar]
- 19.Grasso A, Stigliano R, Morisco F, Martines H, Quaglia A, Dhillon AP, Patch D, Davidson BR, Rolles K, Burroughs AK. Liver transplantation and recurrent hepatocellular carcinoma: predictive value of nodule size in a retrospective and explant study. Transplantation. 2006;81:1532–1541. doi: 10.1097/01.tp.0000209641.88912.15. [DOI] [PubMed] [Google Scholar]
- 20.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 21.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998;28:436–442. doi: 10.1002/hep.510280222. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Influence of transarterial chemoembolization on angiogenesis and expression of vascular endothelial growth factor and basic fibroblast growth factor in rat with Walker-256 transplanted hepatoma: an experimental study. World J Gastroenterol. 2003;9:2445–2449. doi: 10.3748/wjg.v9.i11.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2000;126:153–160. doi: 10.1007/s004320050025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Hu DY, Chu Q, Wu JH, Gao C, Zhang YQ, Huang YR. Cell apoptosis and regeneration of hepatocellular carcinoma after transarterial chemoembolization. World J Gastroenterol. 2004;10:1876–1880. doi: 10.3748/wjg.v10.i13.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldhafer KJ, Chavan A, Frühauf NR, Flemming P, Schlitt HJ, Kubicka S, Nashan B, Weimann A, Raab R, Manns MP, et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol. 1998;29:953–959. doi: 10.1016/s0168-8278(98)80123-2. [DOI] [PubMed] [Google Scholar]
- 27.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701-3. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veltri A, Grosso M, Martina MC, Ciancio A, David E, Salizzoni M, Soldano U, Galli J, Fava C. Effect of preoperative radiological treatment of hepatocellular carcinoma before liver transplantation: a retrospective study. Cardiovasc Intervent Radiol. 1998;21:393–398. doi: 10.1007/s002709900286. [DOI] [PubMed] [Google Scholar]
- 29.Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 30.Harnois DM, Steers J, Andrews JC, Rubin JC, Pitot HC, Burgart L, Wiesner RH, Gores GJ. Preoperative hepatic artery chemoembolization followed by orthotopic liver transplantation for hepatocellular carcinoma. Liver Transpl Surg. 1999;5:192–199. doi: 10.1002/lt.500050307. [DOI] [PubMed] [Google Scholar]
- 31.Venook AP, Ferrell LD, Roberts JP, Emond J, Frye JW, Ring E, Ascher NL, Lake JR. Liver transplantation for hepatocellular carcinoma: results with preoperative chemoembolization. Liver Transpl Surg. 1995;1:242–248. doi: 10.1002/lt.500010409. [DOI] [PubMed] [Google Scholar]
- 32.Spreafico C, Marchianò A, Regalia E, Frigerio LF, Garbagnati F, Andreola S, Milella M, Lanocita R, Mazzaferro V. Chemoembolization of hepatocellular carcinoma in patients who undergo liver transplantation. Radiology. 1994;192:687–690. doi: 10.1148/radiology.192.3.8058934. [DOI] [PubMed] [Google Scholar]
- 33.Morino M, Miglietta C, Grosso M, De Giuli M, Bismuth H. Preoperative chemoembolization for hepatocellular carcinoma. J Surg Oncol Suppl. 1993;3:91–93. doi: 10.1002/jso.2930530525. [DOI] [PubMed] [Google Scholar]


