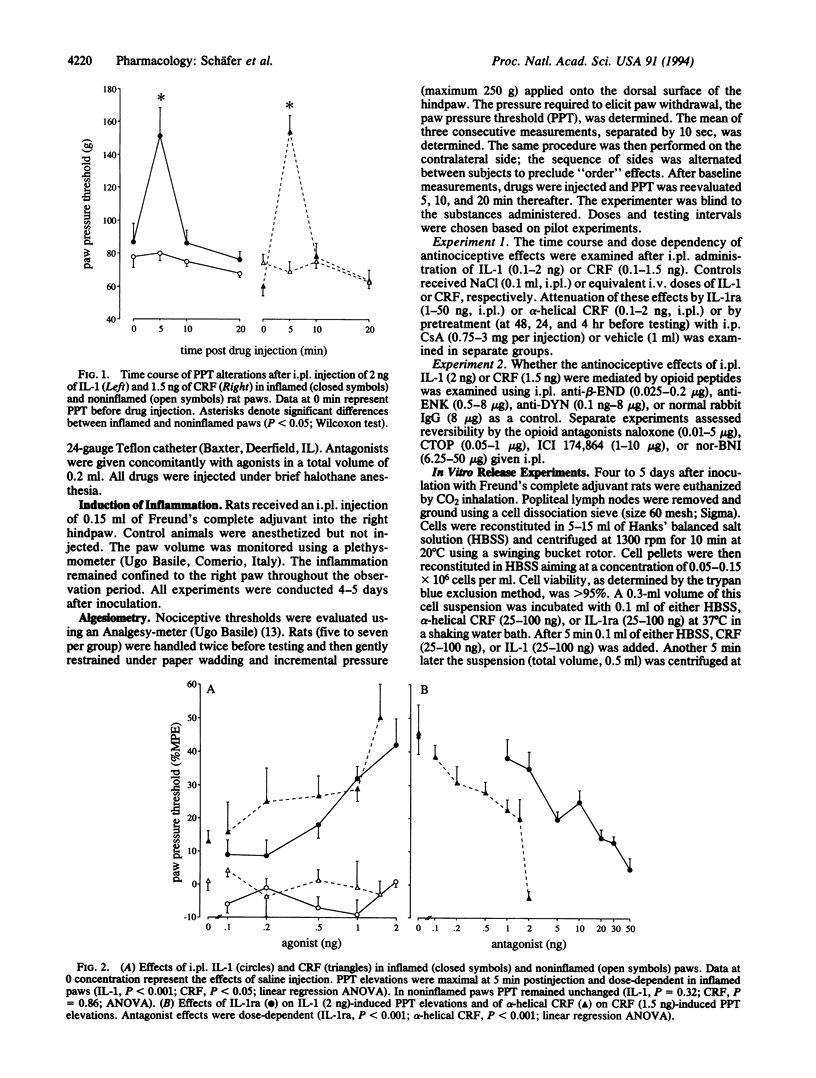
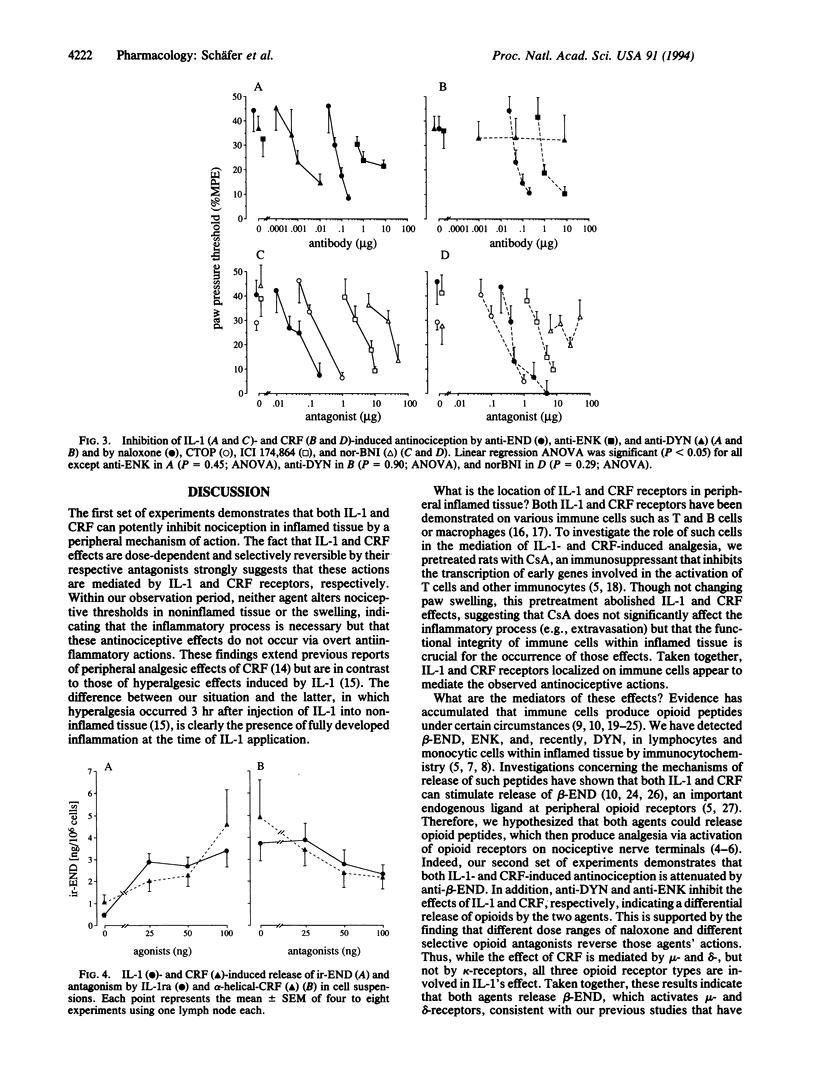
Abstract
Local analgesic effects of exogenous opioid agonists are particularly prominent in painful inflammatory conditions and are mediated by opioid receptors on peripheral sensory nerves. The endogenous ligands of these receptors, opioid peptides, have been demonstrated in resident immune cells within inflamed tissue of animals and humans. Here we examine in vivo and in vitro whether interleukin 1 beta (IL-1) or corticotropin-releasing factor (CRF) is capable of releasing these endogenous opioids and inhibiting pain. When injected into inflamed rat paws (but not intravenously), IL-1 and CRF produce antinociception, which is reversible by IL-1 receptor antagonist and alpha-helical CRF, respectively, and by the immunosuppressant cyclosporine A. In vivo administration of antibodies against opioid peptides indicates that the effects of IL-1 and CRF are mediated by beta-endorphin and, in addition, by dynorphin A and [Met]enkephalin, respectively. Correspondingly, IL-1 effects are inhibited by mu-, delta-, and kappa-opioid antagonists, whereas CRF effects are attenuated by all except a kappa-antagonist. Finally, IL-1 and CRF produce acute release of immunoreactive beta-endorphin in cell suspensions freshly prepared from inflamed lymph nodes. This effect is reversible by IL-1 receptor antagonist and alpha-helical CRF, respectively. These findings suggest that IL-1 and CRF activate their receptors on immune cells to release opioids that subsequently occupy multiple opioid receptors on sensory nerves and result in antinociception. beta-Endorphin, mu- and delta-opioid receptors play a major role, but IL-1 and CRF appear to differentially release additional opioid peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A., Gottschlich R. Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev. 1992 Sep;12(5):525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- Barthó L., Stein C., Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn Schmiedebergs Arch Pharmacol. 1990 Dec;342(6):666–670. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- Buzzetti R., McLoughlin L., Lavender P. M., Clark A. J., Rees L. H. Expression of pro-opiomelanocortin gene and quantification of adrenocorticotropic hormone-like immunoreactivity in human normal peripheral mononuclear cells and lymphoid and myeloid malignancies. J Clin Invest. 1989 Feb;83(2):733–737. doi: 10.1172/JCI113940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Lorenzetti B. B., Bristow A. F., Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988 Aug 25;334(6184):698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Hargreaves K. M., Dubner R., Costello A. H. Corticotropin releasing factor (CRF) has a peripheral site of action for antinociception. Eur J Pharmacol. 1989 Nov 7;170(3):275–279. doi: 10.1016/0014-2999(89)90550-5. [DOI] [PubMed] [Google Scholar]
- Hassan A. H., Ableitner A., Stein C., Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993 Jul;55(1):185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Hassan A. H., Pzewłocki R., Herz A., Stein C. Dynorphin, a preferential ligand for kappa-opioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the rat. Neurosci Lett. 1992 Jun 8;140(1):85–88. doi: 10.1016/0304-3940(92)90688-4. [DOI] [PubMed] [Google Scholar]
- Heijnen C. J., Kavelaars A., Ballieux R. E. Beta-endorphin: cytokine and neuropeptide. Immunol Rev. 1991 Feb;119:41–63. doi: 10.1111/j.1600-065x.1991.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Karalis K., Sano H., Redwine J., Listwak S., Wilder R. L., Chrousos G. P. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991 Oct 18;254(5030):421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Kavelaars A., Ballieux R. E., Heijnen C. J. The role of IL-1 in the corticotropin-releasing factor and arginine- vasopressin-induced secretion of immunoreactive beta-endorphin by human peripheral blood mononuclear cells. J Immunol. 1989 Apr 1;142(7):2338–2342. [PubMed] [Google Scholar]
- Lolait S. J., Clements J. A., Markwick A. J., Cheng C., McNally M., Smith A. I., Funder J. W. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J Clin Invest. 1986 Jun;77(6):1776–1779. doi: 10.1172/JCI112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewłocki R., Hassan A. H., Lason W., Epplen C., Herz A., Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48(2):491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- Rosen H., Behar O., Abramsky O., Ovadia H. Regulated expression of proenkephalin A in normal lymphocytes. J Immunol. 1989 Dec 1;143(11):3703–3707. [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Sibinga N. E., Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Morrill A. C., Meyer W. J., 3rd, Blalock J. E. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. 1986 Jun 26-Jul 2Nature. 321(6073):881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Stein C., Gramsch C., Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J Neurosci. 1990 Apr;10(4):1292–1298. doi: 10.1523/JNEUROSCI.10-04-01292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Lehrberger K., Giefing J., Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993 Aug 7;342(8867):321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Przewłocki R., Gramsch C., Peter K., Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Millan M. J., Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988 Oct;31(2):445–451. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993 Jan;76(1):182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- Wall P. D. Vigilance in defense of animal welfare. International Association for the Study of Pain. Pain. 1993 Sep;54(3):239–239. doi: 10.1016/0304-3959(93)90026-L. [DOI] [PubMed] [Google Scholar]
- Webster E. L., Tracey D. E., Jutila M. A., Wolfe S. A., Jr, De Souza E. B. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990 Jul;127(1):440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]
- van Woudenberg A. D., Hol E. M., Wiegant V. M. Endorphin-like immunoreactivities in uncultured and cultured human peripheral blood mononuclear cells. Life Sci. 1992;50(10):705–714. doi: 10.1016/0024-3205(92)90473-3. [DOI] [PubMed] [Google Scholar]



