Abstract
Background
There is little known about cardiorespiratory fitness and its association with volumes of the thalamus, hippocampus, and basal ganglia in multiple sclerosis (MS). Such inquiry is important for identifying a possible behavioral approach (e.g., aerobic exercise training) that might change volumes of deep gray matter (DGM) structures associated with cognitive and motor functions in MS.
Purpose
This study examined the association between cardiorespiratory fitness and volumes of the thalamus, hippocampus, and basal ganglia in MS.
Method
We enrolled 35 persons with MS who underwent a maximal exercise test for measuring cardiorespiratory fitness as peak oxygen consumption (VO2peak) and brain MRI. Volumes of the thalamus, hippocampus, caudate, putamen, and pallidum were calculated from 3D T1-weighted structural brain images. We examined associations using partial (pr) correlations controlling for demographic and clinical variables.
Results
VO2peak was significantly associated with composite scaled volumes of the caudate(pr = .47, p < .01), putamen (pr = .44, p < .05), pallidum (pr = .40, p < .05), and hippocampus (pr = .42, p < .05), but not thalamus (pr = .31, p = .09), when controlling for sex, age, disability, and duration of MS.
Conclusion
Our results provide novel evidence that cardiorespiratory fitness is associated with volumes of DGM structures that are involved in motor and cognitive functions in MS.
Keywords: Brain, Multiple sclerosis, Exercise, MRI, Physical activity
Highlights
-
•
We examine the association between cardiorespiratory fitness and deep gray matter structures in multiple sclerosis.
-
•
Cardiorespiratory fitness was positively associated with volumes of basal ganglia nuclei in multiple sclerosis.
-
•
Researchers should examine aerobic exercise training for improving brain health in multiple sclerosis.
1. Introduction
There is increasing evidence for the beneficial effects of aerobic exercise training on motor and cognitive outcomes in persons with multiple sclerosis (MS). For example, one recent randomized controlled trial (RCT) reported beneficial effects of cycle ergometry on cardiorespiratory fitness, walking performance, and cognition in persons with progressive MS (Briken et al., 2014). Other RCTs of exercise training have similarly reported improvements in cardiorespiratory fitness and walking performance in persons with MS (Latimer-Cheung et al., 2013; Motl, 2014). The improvements in walking and cognitive function might be associated with the effects of cardiorespiratory fitness on deep gray matter (DGM) structures within the brain including the hippocampus, thalamus, and basal ganglia (Dalgas and Stenager, 2012; Motl and Pilutti, 2012; White and Castellano, 2008).
Such an argument is based, in part, on consistent evidence for an association between cardiorespiratory fitness and hippocampal volume across the lifespan (Chaddock et al., 2011; Erickson et al., 2014). For example, researchers have reported that cardiorespiratory fitness was associated with hippocampal volume in a sample of 156 non-demented older adults (Erickson et al., 2009). That evidence informed the design of RCTs for examining the effects of aerobic exercise training on hippocampal volume in older adults and persons with neurological conditions (e.g., Schizophrenia and Alzheimer's). For example, one group of researchers conducted an RCT of aerobic exercise training in 120 older adults, and reported that aerobic exercise training increased hippocampal volume by 2% compared with an attention/social contact control condition (Erickson et al., 2011). Another group of researchers reported an increase in hippocampal volume that accounted for improvements in short-term memory following aerobic exercise training in persons with Schizophrenia (Pajonk et al., 2010).
Some evidence suggests that cardiorespiratory fitness might be associated with other DGM structures within the brain such as the thalamus and basal ganglia. For example, one study demonstrated that cardiorespiratory fitness was weakly associated with volumes of the caudate and pallidum, but not the putamen, in older adults (Verstynen et al., 2012). Another study of preadolescent children demonstrated that cardiorespiratory fitness was associated with volumes of the caudate, putamen, and pallidum (Chaddock et al., 2010). Such research extends the focus of aerobic fitness and exercise training effects beyond the hippocampus and into other DGM brain structures that are associated with motor outcomes (Draganski et al., 2008).
We are aware of one study that examined the association between cardiorespiratory fitness and sub-cortical gray matter volume in persons with MS (Prakash et al., 2010). That study enrolled 21 persons with relapsing–remitting MS and 15 controls who were matched on age and education. All participants underwent an assessment of cardiorespiratory fitness using a maximal, incremental exercise test with spirometry and 3T MRI imaging of the brain. The exploratory analysis identified gray matter differences in the post-central gyrus, medial frontal gyrus, anterior cingulate cortex, and precuneus between MS and control participants, and further indicated that cardiorespiratory fitness, defined as peak oxygen consumption (VO2peak), was associated with volumes of those sub-cortical gray matter structures in the sample with MS. That study did not focus on the association between cardiorespiratory fitness and volumes of the hippocampus, thalamus, and basal ganglia. Importantly, there is evidence for atrophy of those DGM structures in MS (Batista et al., 2012; Sicotte et al., 2008), and such atrophy has been associated with worsening of motor and cognitive outcomes (Schoonheim et al., 2012).
The present study examined the association between cardiorespiratory fitness and volumes of the hippocampus, thalamus, and basal ganglia in persons with MS. Such an examination is important for identifying cardiorespiratory fitness as a possible target of interventions for slowing or reversing the effects of MS pathogenesis on atrophy of DGM brain structures (i.e., promoting brain health). Indeed, rodent research has indicated that voluntary wheel running influences the basal ganglia and other DGM structures through production and secretion of brain derived neurotrophic factor (BDNF) in striatum and hippocampus (Berchtold et al., 2005; Marais et al., 2009) through a molecular PGC-1α/FNDC5 pathway associated with skeletal muscle contract during physical activity (Wrann et al., 2013). Based on previous research in older adults and preadolescent children (Chaddock et al., 2011; Erickson et al., 2014), we hypothesized that persons with MS who had higher levels of cardiorespiratory fitness would have larger volumes of the hippocampus, thalamus, and basal ganglia, after controlling for sex, age, disability, and duration of MS.
2. Methods
2.1. Participants and procedures
The methods for this study were approved by an institutional review board (IRB; ethics committee approval), and all participants provided a written informed consent before enrollment. We enrolled 35 patients with clinically definite MS (Polman et al., 2005) who were recruited through targeted advertisements dispersed in central Illinois. The sample size of 35 patients was based on a power analysis performed in G*Power 3.1 for detecting a correlation of ρ ≥ .40, based on a previous research in MS (Prakash et al., 2010), with an alpha value of .05 and a beta value of .80. The inclusion criteria were diagnosis of MS based on physician verification using the accepted criteria (Polman et al., 2005), relapse-free for the past 30 days, age between 18 and 64 years, ability to walk with or without an assistive device, willingness and ability to complete in-person assessments, minimal risk for engaging in physical activity (i.e., reported ‘yes’ to less than two questions on the Physical Activity Readiness Questionnaire (PAR-Q); Thomas et al., 1992), and physician approval for undertaking exercise testing. The sample was primary female (71%) with a mean age of 50.8 (SD = 9.8) years, and the clinical course was relapsing–remitting MS (RRMS) in 26 patients (74%). The mean disease duration of those with MS was 11.4 (SD = 7.5) years, and the median Expanded Disability Status Scale (EDSS) score was 5.0 (IQR = 3.5). All participants with RRMS were receiving an ongoing disease modifying therapy, but none of the participants were currently receiving short-term medications for an ongoing relapse. All participants completed an assessment of cardiorespiratory fitness and then underwent an MRI within 14 days of the exercise testing session.
2.2. Cardiorespiratory fitness
The assessment of cardiorespiratory fitness was performed by researchers who were uninvolved in the MRI acquisition and analyses. Cardiorespiratory fitness was measured as VO2peak using an incremental exercise test on a recumbent seated stepper (Nustep T5XR, Nustep Inc., Ann Arbor, MI) and a calibrated open-circuit spirometry system (TrueOne, Parvo Medics, Sandy, UT) for analyzing expired gases (Motl and Fernhall, 2012; Sandroff et al., 2015). The recumbent seated stepper is ideal for persons across the disability spectrum as it does not require balance for maintaining a seated posture, and includes both arms and legs thereby yielding a greater extent of muscle activation for estimating cardiorespiratory capacity. The recumbent seated stepper is preferable compared with a cycle ergometer as persons with advanced disability often have difficulty with maintaining an appropriate pedaling cadence, even with a submaximal test. The participants were initially fitted to the stepper and were read standardized instructions for completing the incremental exercise test. After being fitted with a mouthpiece (Hans Rudolph, Kansas City, MO) for collecting expired gases, the participants performed a 1-minute warm-up at 15 W. This was the initial work rate for the test, and the work rate continuously increased at a rate of 5–10 W/min until the participant reached volitional fatigue; we established the protocol through pilot testing with persons who had a range of MS disability. Oxygen consumption (VO2) and respiratory exchange ratio (RER) were measured continuously by the open-circuit spirometry system and expressed as 20-s averages. Heart rate (HR) was continuously displayed using a Polar heart rate monitor (Polar Electro Oy, Finland). HR and ratings of perceived exertion (RPE) were recorded every minute during the test. VO2peak was expressed in ml × kg−1 × min−1 based on the highest recorded 20-s VO2 value when 2 of the 4 criteria were satisfied: (1) plateau of VO2 despite a continued increase in work rate; (2) RER ≥ 1.10; (3) peak HR within 10 beats/min of age-predicted maximum (i.e., 220–age); and (4) peak RPE ≥ 17. All the participants achieved the criteria for a valid assessment of VO2peak. Researchers have reported that this protocol is valid for measuring cardiorespiratory capacity in MS across a broad range of disability, and the resulting VO2peak scores have been associated with outcomes such as cognitive functioning in MS (Sandroff et al., 2015).
2.3. MRI acquisition and analysis of DGM structures
The MRI acquisition and analysis were conducted by personnel who were not involved in the assessment of cardiorespiratory fitness. Using a whole body Siemens Trio 3 Tesla MRI scanner (Erlangen, Germany), we acquired high-resolution 3D T1-weighted structural brain images using an MPRAGE sequence with the following parameters: 23 cm FOV, 256 × 256 × 192 matrix size with 0.9 mm isotropic resolution, echo time (TE)/repetition time (TR)/inversion time (TI) of 2.32/1900/900 ms, a flip angle of 9°, and a GRAPPA accelerated factor of 2 with 24 reference lines. We then extracted the brain and excluded the skull by deleting non-brain tissue from our T1 whole-brain images using the Brain Extraction Tool (Smith, 2002) from FMRIB's Software Library (FSL). We used bias field correction in order ensure accurate brain extraction. We segmented the 3D T1 image into 3 tissue types, white matter, gray matter, and cerebrospinal fluid, using FMRIB's Automated Segmentation Tool (FAST) (Zhang et al., 2001) from FSL. Using the 3D T1 images, we calculated volumes of the right and left hippocampi, thalami, and basal ganglia nuclei using FMRIB's Integrated Registration and Segmentation Tool (FIRST) (Patenaude et al., 2011). This segments the T1 images into subcortical structures and measures the volumes. We computed a volume scaling factor by linear registration of the skull and brain masks using FSL's Linear Image Registration Tool (FLIRT), and extracting scaling parameters from the resulting affine transformation matrix, as is done in FSL's SIENAX (Jenkinson et al., 2002; Jenkinson and Smith, 2001; Smith et al., 2002). We then normalized the volumes based on intracranial volume by multiplication with this volume scaling factor, and then normalized right and left volumes were averaged for inclusion as composites in the statistical analyses. This was based on strong correlations between the right and left volumes (e.g., thalami: r = .90, p < .0001) and aimed to reduce possible inflated error rates with multiple comparisons. We further note that this process has been undertaken in other research on DGM volumes in MS (Batista et al., 2012).
2.4. Data analysis
The data were analyzed in SPSS Statistics Version 22.0 for a Mac OS X operating system. Descriptive statistics were provided as mean (standard deviation) for the overall sample, and by subgroups based on the MS clinical course (RRMS vs. progressive MS) and disability status (mild-to-moderate, EDDS ≤ 5.5 vs. severe, EDSS ≥ 6.0). We compared the subgroups on cardiorespiratory fitness and MRI variables using independent samples t-tests. We examined the associations among aerobic fitness and hippocampal, thalamic, and basal ganglia scaled volumes using Pearson product-moment partial correlation coefficients (pr) collectively controlling for sex (0 = female, 1 = male), age (years), EDSS, and disease duration (years).
3. Results
3.1. Descriptive statistics
The mean values for cardiorespiratory fitness (VO2peak) and composite scaled volumes for the thalamus, hippocampus, caudate, pallidum, and putamen are provided in Table 1 for the overall sample and the subgroups. There were no differences in cardiorespiratory fitness or composite scaled volumes of DGM brain structures between subgroups with relapsing–remitting or progressive clinical course of MS (all ps ≥ .23). There were significant differences between disability groups in cardiorespiratory fitness (t = 3.51, p < .001) and composite scaled volumes of the thalamus (t = 2.15, p < .05) and pallidum (t = 2.62, p < .05), but not the caudate, putamen, and hippocampus (all ps ≥ .14). Those with severe disability had lower cardiorespiratory fitness and smaller volumes of the thalamus and pallidum than those with mild-to-moderate disability.
Table 1.
Descriptive statistics for cardiorespiratory fitness (ml/kg/min) and scaled composite volumes (mm3) of the thalamus, hippocampus, and basal ganglia in 35 people with multiple sclerosis.
| Variable | Overall sample (n = 35) | MS clinical course |
Disability status |
||
|---|---|---|---|---|---|
| RRMS (n = 26) | Progressive MS (n = 9) | Mild & moderate (EDSS ≤ 5.5, n = 15) | Severe (EDSS > 6.0, n = 20) | ||
| VO2peak | 17.6 (6.8) | 18.2 (7.3) | 15.8 (4.9) | 21.7 (7.4) | 14.6 (4.4) |
| Thalamus | 8581 (1216) | 8574 (1253) | 8600 (1181) | 9060 (1178) | 8202 (1136) |
| Hippocampus | 4339 (702) | 4251 (714) | 4583 (641) | 4341 (878) | 4337 (551) |
| Caudate | 3898 (740) | 3866 (806) | 3986 (545) | 4111 (893) | 3729 (562) |
| Pallidum | 1829 (315) | 1828 (348) | 1832 (214) | 1976 (311) | 1713 (272) |
| Putamen | 5181 (920) | 5157 (945) | 5249 (897) | 5424 (835) | 4990 (960) |
Note. Values are mean (standard deviation). MS = multiple sclerosis; RRMS = relapsing–remitting MS; EDSS = Expanded Disability Status Scale; VO2peak = peak oxygen consumption.
3.2. Partial correlations in MS
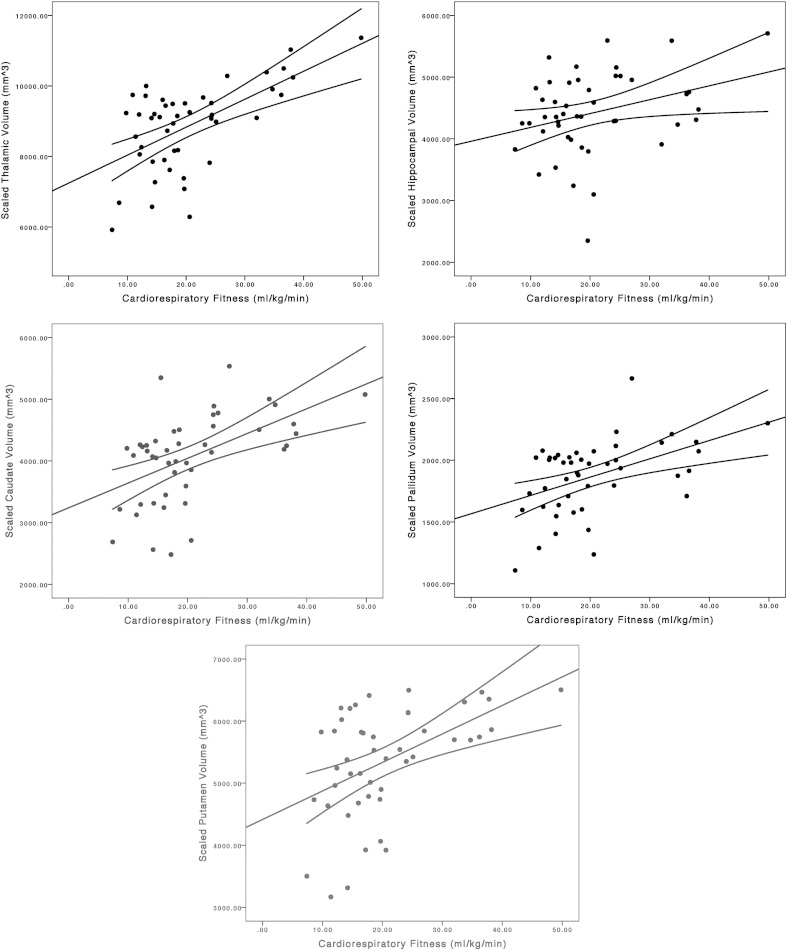
Scatter plots of the association between cardiorespiratory fitness and scaled volumes of DGM structures are provided in Fig. 1. The partial correlations indicated significant associations between cardiorespiratory fitness and composite scaled volumes for the caudate (pr = .47, p < .01, 95% CI = .16, .69), putamen (pr = .44, p < .05, 95% CI = .13, .67), pallidum (pr = .40, p < .05, 95% CI = .08, .65), and hippocampus (pr = .42, p < .05, 95% CI = .11, .66), but not thalamus (pr = .31, p = .09, 95% CI = −.03. 58), when controlling for sex, age, EDSS, and disease duration. Persons with MS who had higher levels of cardiorespiratory fitness had larger scaled volumes of the hippocampus and basal ganglia structures.
Fig. 1.
Scatter plots along with line of best fit and 95% confidence interval for the association between cardiorespiratory fitness (ml/kg/min) and scaled composite volumes (mm3) of the thalamus, hippocampus, caudate, pallidum, and putamen.
4. Discussion
This study examined the associations between cardiorespiratory fitness and volumes of DGM structures in the brains of persons with MS that correspond with cognitive and motor functions. The primary result was a robust association between cardiorespiratory fitness and volumes of the basal ganglia and hippocampus. Those with higher levels of cardiorespiratory fitness had larger volumes of the caudate, putamen, pallidum, and hippocampus, and the associations were independent of sex, age, disability status, and disease duration. There was less substantial evidence regarding the association between cardiorespiratory fitness and the volume of the thalamus. Importantly, volumes of the caudate, putamen, pallidum, and hippocampus have been associated with cognitive and motor outcomes in MS (Batista et al., 2012; Sicotte et al., 2008; Schoonheim et al., 2012), and the associations with cardiorespiratory fitness reported herein might support the design and delivery of an aerobic exercise training intervention for changing those DGM structures and cognitive and motor outcomes such as learning and memory, information processing speed, and walking performance in MS. Indeed, the results of associations between DGM structures and cardiorespiratory fitness in older adults, for example, supported the subsequent design and execution of successful RCTs of aerobic exercise training for improving brain health and cognition (Erickson et al., 2011). Collectively, such an examination is timely for persons with MS, particularly considering that the disease pathology is associated with atrophy of DGM structures, walking dysfunction, and cognitive impairment, and those functional consequences are currently poorly managed in this chronic, often progressive disease of the central nervous system.
Our results regarding cardiorespiratory fitness and basal ganglia are generally consistent with previous research in older adults and preadolescent children and extend previous research in MS. For example, one study demonstrated that cardiorespiratory fitness was associated with volumes of the caudate and pallidum, but not the putamen, in older adults (Verstynen et al., 2012). Another study of preadolescent children demonstrated that cardiorespiratory fitness was associated with volumes of the caudate, putamen, and pallidum (Chaddock et al., 2010). We essentially report similar, but novel, observations in this sample of persons with MS. To date, one study has reported associations between cardiorespiratory fitness and sub-cortical gray matter volumes of the post-central gyrus, medial frontal gyrus, anterior cingulate cortex, and precuneus in a largely exploratory analysis of persons with MS (Prakash et al., 2010). We provide the first evidence that the association between cardiorespiratory fitness and volumes of the basal ganglia extends into those with MS.
Our results regarding cardiorespiratory fitness and hippocampal volume are encouraging and consistent with previous research in older adults, preadolescent children, and persons with Schizophrenia and MS. We observed that cardiorespiratory fitness was significantly associated with hippocampal volume when controlling for covariates that have joint associations with both variables. We note that other researchers have generally reported positive associations between cardiorespiratory fitness and aerobic exercise training with hippocampal volume in preadolescents, older adults, and persons with Schizophrenia (Chaddock et al., 2011; Erickson et al., 2014; Erickson et al., 2009; Erickson et al., 2011; Pajonk et al., 2010). One case study has reported that aerobic exercise training improved hippocampal volume and memory in a person with MS (Leavitt et al., 2014). Subsequent research might examine the association between cardiorespiratory fitness and sub-regions within the structure of the hippocampus that demonstrate regional atrophy (Sicotte et al., 2008).
We fully recognize that our data only involved a cross-sectional assessment of cardiorespiratory fitness and volume of DGM structures in persons with MS, and the directional nature of the association is not clear from our data. Cardiorespiratory fitness could influence the structure of DGM regions, or the integrity of the DGM regions might influence one's capacity for engaging in a maximal exercise test directly (i.e., reverse causality) or indirectly through cognitive or motor functions. Accordingly, we believe that an RCT is necessary for examining the effect of aerobic exercise training on the volumes of the basal ganglia and hippocampus, and perhaps the thalamus, in MS. This will provide a first step in the possibility that aerobic exercise can induce neuroplasticity in MS, particularly given that such a possibility has repeatedly been proposed without supporting evidence (Dalgas and Stenager, 2012; Motl and Pilutti, 2012; White and Castellano, 2008).
The current study has limitations. We did not assess other MRI variables such as MR spectroscopy, magnetization transfer, or diffusion tensor imaging nor quantify the regions of the motor cortex. We further did not control for neocortical volume and thickness. We did not assess other domains of fitness such as muscle strength or body composition nor include measures of cognitive and motor/walking function. The study only included cross-sectional data and analyses. The results have not been replicated in a separate sample by an independent team of researchers. We controlled for age, sex, disability, and disease duration, but other variables that could confound the examination of associations between cardiorespiratory capacity and volumes of DGM structures may exist. The progression of work rate during the incremental exercise test was designed based on pilot work and has been included in previous research among persons with MS (Sandroff et al., 2015), but might be slow and result in exhaustion thereby yielding underestimates of VO2peak.
Overall, our paper provides novel inquiry and evidence for cardiorespiratory fitness as a correlate of basal ganglia and hippocampal volumes in persons with MS. To the extent that cardiorespiratory fitness is modifiable by aerobic exercise training, our results suggest that MS-related loss in volumes of the basal ganglia and hippocampus may be remediated through regular exercise participation. Such a possibility requires subsequent examination of aerobic exercise training and volumes of DGM structures in MS.
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Acknowledgments
This paper was supported, in part, by a grant from the National Multiple Sclerosis Society (IL0003).
References
- Batista S., Zivadinov R., Hoogs M., Bergsland N., Heininen-Brown M., Dwyer M.G. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J. Neurol. 2012;259(1):139–146. doi: 10.1007/s00415-011-6147-1. 21720932 [DOI] [PubMed] [Google Scholar]
- Berchtold N.C., Chinn G., Chou M., Kesslak J.P., Cotman C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neurosci. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. 15896913 [DOI] [PubMed] [Google Scholar]
- Briken S., Gold S.M., Patra S., Vettorazzi E., Harbs D., Tallner A. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Mult. Scler. 2014;20(3):382–390. doi: 10.1177/1352458513507358. 24158978 [DOI] [PubMed] [Google Scholar]
- Chaddock L., Erickson K.I., Prakash R.S., VanPatter M., Voss M.W., Pontifex M.B. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev. Neurosci. 2010;32(3):249–256. doi: 10.1159/000316648. 20693803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L., Pontifex M.B., Hillman C.H., Kramer A.F. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J. Int. Neuropsychol. Soc. 2011;17(6):975–985. doi: 10.1017/S1355617711000567. 22040896 [DOI] [PubMed] [Google Scholar]
- Dalgas U., Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther. Adv. Neurol. Disord. 2012;5(2):81–95. doi: 10.1177/1756285611430719. 22435073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Kherif F., Klöppel S., Cook P.A., Alexander D.C., Parker G.J. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J. Neurosci. 2008;28(28):7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. 18614684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Leckie R.L., Weinstein A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging. 2014;35(Suppl. 2):S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. 24952993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Hu L., Morris K.S. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. 19123237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. 21282661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. 12377157 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. 11516708 [DOI] [PubMed] [Google Scholar]
- Latimer-Cheung A.E., Pilutti L.A., Hicks A.L., Martin Ginis K.A., Fenuta A.M., MacKibbon K.A. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013;94(9):1800–1828. doi: 10.1016/j.apmr.2013.04.020. 23669008 [DOI] [PubMed] [Google Scholar]
- Leavitt V.M., Cirnigliaro C., Cohen A., Farag A., Brooks M., Wecht J.M. Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase. 2014;20(6):695–697. doi: 10.1080/13554794.2013.841951. 24090098 [DOI] [PubMed] [Google Scholar]
- Marais L., Stein D.J., Daniels W.M. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab. Brain Dis. 2009;24(4):587–597. doi: 10.1007/s11011-009-9157-2. 19844781 [DOI] [PubMed] [Google Scholar]
- Motl R.W. Benefits, safety, and prescription of exercise in persons with multiple sclerosis. Expert Rev. Neurother. 2014;14(12):1429–1436. doi: 10.1586/14737175.2014.983904. 25413175 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Fernhall B. Accurate prediction of cardiorespiratory fitness using cycle ergometry in minimally disabled persons with relapsing–remitting multiple sclerosis. Arch. Phys. Med. Rehabil. 2012;93(3):490–495. doi: 10.1016/j.apmr.2011.08.025. 22225573 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Pilutti L.A. The benefits of exercise training in multiple sclerosis. Nat. Rev. Neurol. 2012;8(9):487–497. doi: 10.1038/nrneurol.2012.136. 22825702 [DOI] [PubMed] [Google Scholar]
- Pajonk F.G., Wobrock T., Gruber O., Scherk H., Berner D., Kaizl I. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. 20124113 [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. 21352927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Edan G., Filippi M., Hartung H.P., Kappos L. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. 16283615 [DOI] [PubMed] [Google Scholar]
- Prakash R.S., Snook E.M., Motl R.W., Kramer A.F. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 2010;1341:41–51. doi: 10.1016/j.brainres.2009.06.063. 19560443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandroff B.M., Pilutti L.A., Benedict R.H.B., Motl R.W. Association between physical fitness and cognitive function in multiple sclerosis: does disability status matter? Neurorehabil. Neural Repair. 2015;29(3):214–223. doi: 10.1177/1545968314541331. [DOI] [PubMed] [Google Scholar]
- Schoonheim M.M., Popescu V., Rueda Lopes F.C., Wiebenga O.T., Vrenken H., Douw L. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology. 2012;79(17):1754–1761. doi: 10.1212/WNL.0b013e3182703f46. 23019265 [DOI] [PubMed] [Google Scholar]
- Sicotte N.L., Kern K.C., Giesser B.S., Arshanapalli A., Schultz A., Montag M. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(4):1134–1141. doi: 10.1093/brain/awn030. 18375977 [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. 12391568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. 12482100 [DOI] [PubMed] [Google Scholar]
- Thomas S., Reading J., Shephard R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can. J. Sport Sci. 1992;17(4):338–345. 1330274 [PubMed] [Google Scholar]
- Verstynen T.D., Lynch B., Miller D.L., Voss M.W., Prakash R.S., Chaddock L. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J. Aging Res. 2012;2012:939285. doi: 10.1155/2012/939285. 22900181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L.J., Castellano V. Exercise and brain health—implications for multiple sclerosis: part 1—neuronal growth factors. Sports Med. 2008;38(2):91–100. doi: 10.2165/00007256-200838020-00001. 18201113 [DOI] [PubMed] [Google Scholar]
- Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. 24120943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. I. E.E.E. Trans. Med. Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. 11293691 [DOI] [PubMed] [Google Scholar]