Abstract
Background
Krabbe disease is a fatal neurodegenerative disease caused by rapid demyelination of the central and peripheral nervous systems. The only available treatment, unrelated umbilical cord blood transplantation, is effective only if performed before clinical symptoms appear. Phenotypic expressions of disease-causing mutations vary widely, but genotype–phenotype relationships are unclear. Therefore, we evaluated diffusion tensor imaging (DTI) tractography with volumetric analysis as a biomarker of early white matter changes and functional disability in presymptomatic infants.
Methods
We obtained DTI and structural scans of newborns with early-infantile Krabbe disease (n = 9) diagnosed by family history or newborn screening. We compared white matter fiber tract properties to those of normal controls (n = 336) and assessed the ability of tract-based properties to predict longitudinal development in four functional domains (cognitive, fine motor, gross motor, adaptive behavior) after treatment with unrelated umbilical cord blood transplantation. We also assessed the relationship between the standard evaluation (modified Loes score) and DTI results, and the volumetric differences between the Krabbe subjects and normal controls.
Findings
Reductions in fractional anisotropy were significant in the corticospinal tract in the Krabbe patients compared to controls, which strongly correlated with motor and cognitive outcomes after transplantation. Significant regional differences were observed in the splenium and uncinate fasciculus in Krabbe patients and these differences correlated only with cognitive outcomes. Regional brain volumes of Krabbe patients were slightly larger than controls. Loes scores did not correlate with DTI results.
Interpretation
Neonatal microstructural abnormalities correlate with neurodevelopmental treatment outcomes in patients treated for infantile Krabbe disease. DTI with quantitative tractography is an excellent biomarker for evaluating infants with Krabbe disease identified through newborn screening.
Highlights
-
•
Reductions in FA and increases in MD and RD in the 6 tracts assessed in Krabbe neonates strongly correlate with motor and cognitive outcomes after cord blood transplantation.
-
•
Significant regional differences in the splenium and uncinate fasciculus correlated only with cognitive outcomes.
-
•
Regional brain volumes of Krabbe patients were slightly larger than controls.
-
•
Loes scores did not correlate with DTI results.
-
•
DTI tractography can be a key component of Krabbe newborn screening.
1. Introduction
Krabbe disease (globoid cell leukodystrophy) is a rare autosomal recessive disorder caused by a deficiency of the lysosomal enzyme galactocerebrosidase (GALC), which is essential for normal catabolism of the principal lipid component of myelin. Galactolipid accumulation triggers a pathogenic defect in myelinating glial cells that causes inflammation and rapid demyelination, resulting in progressive deterioration of the central and peripheral nervous systems (Wenger et al., 2001). This is a very rare disorder with an incidence of 1 in 70–100,000 live births. In most patients with Krabbe disease, rapidly progressive demyelination begins before 6 months of age (early-infantile onset). Symptoms include irritability, seizures, muscle spasticity, mental deterioration, blindness, and deafness. Death usually occurs before 2 years of age (Wenger et al., 2001). The phenotypic expressions of disease-causing mutations vary, and in some cases Krabbe disease presents as the late-infantile, juvenile, or adult onset forms. However, affected siblings follow the same disease course.
Diagnosis of Krabbe disease is based on family history, genetic testing and clinical symptoms. In addition, a test for GALC enzyme activity has been developed to screen newborns. In 2006 New York was the first state to include Krabbe disease in its newborn screening program, with more states to follow (Duffner et al., 2009). Screening involves a serologic test for the missing GALC enzyme followed by mutational analysis to confirm the diagnosis. However, neither genotype nor GALC activity can reliably distinguish the early-infantile form of the disease from the juvenile and adult forms. Furthermore, some individuals with low levels of GALC activity may never develop clinical symptoms (Wenger et al., 2000).
Umbilical cord blood stem cell transplantation is the only available treatment for early infantile Krabbe disease and is effective only if performed before symptoms develop (Escolar et al., 2005). Outcomes of patients who undergo transplantation are significantly better than those of untreated children. Among patients with early-onset Krabbe disease, those treated as presymptomatic infants show normal cognitive function; however, they develop progressive motor disability, growth failure, and speech difficulties (Escolar et al., 2005). Lower survival rates and more severe disability have been observed in infants with early-onset disease who underwent transplantation in later stages of the disease, demonstrating that treatment within the first few weeks of life is critical (Escolar et al., 2005).
Infants currently identified by newborn screening may develop early-onset disease, remain healthy, or have late-onset disease, which is clinically silent until late childhood or even adulthood. An objective measure of disease progression is urgently needed to determine whether an asymptomatic baby will develop early-infantile disease requiring immediate treatment. Once clinical symptoms appear, it is too late to derive benefits from treatment. As previously reported, conventional MR images and neurophysiologic tools cannot predict outcome, and results do not correlate with disease stage, because these tools are not standardized for use in the neonatal period (Escolar et al., 2006).
Because transplantation is effective only in the presymptomatic stages of the disease, it is important to identify a biomarker that can detect changes in the brain before clinical symptoms develop, help determine the optimal time of treatment, and predict disease onset and expected phenotype. This information is crucial to clinicians counseling parents about the treatment of a neonate who screens positive for Krabbe disease. The purpose of this research is to test whether DTI with tractography can be used as such biomarker.
DTI is an MRI technique that measures the effective diffusion of water molecules in biological tissues. DTI provides insight into white matter development (myelination), reflecting changes in axonal fiber size, density, tract coherence, as well as membrane structure and permeability (Provenzale et al., 2007; Hermoye et al., 2006; Mukherjee et al., 2001). The diffusion information within each voxel is synthesized in the form of a diffusion tensor. By assembling these tensors into tracts using tractography methods, white matter fiber tracts are defined and grouped into DTI-derived fiber bundles based on anatomical knowledge. Because direct interpretation of the 3D image data of diffusion tensors (represented by 3 × 3 matrix at each voxel) is difficult, simpler scalar parameters are commonly used for analysis and comparisons. The trace of the tensor, a measure of the magnitude of diffusion, is rotationally invariant and referred to as mean diffusivity (MD). The most widely used invariant measure of anisotropy, fractional anisotropy (FA), is quite sensitive to a broad spectrum of pathological conditions. Parameters like axial diffusivity (AD) and radial diffusivity (RD) are related to the eigenvalue or a combination of eigenvalues of the diffusion tensor matrix and have demonstrated more specific relationships to white matter pathology. RD appears to be modulated by myelin in white matter and has been shown to be more specific for demyelination (Song et al., 2002), whereas AD is more specific to axonal degeneration. Consequently all four DTI parameters are considered along the various fiber bundles to provide insight into tissue microstructure and architectural organization in different regions of the brain.
FA measurements have been shown to be abnormal in early-infantile Krabbe disease (McGraw et al., 2005). The FA difference in the corticospinal tracts of presymptomatic newborns with Krabbe when compared with normal controls is an indicator of early neurological changes (Escolar et al., 2009). Therefore, this technique may be useful to determine which infants identified through screening have the early-infantile form of the disease, requiring immediate treatment. In addition, pretreatment FA changes may predict gross motor function after transplantation (Escolar et al., 2009).
2. Subjects and methods
2.1. Subjects
Newborns with early-infantile Krabbe disease identified by family history or newborn screening were referred to the Program for the Study of Neurodevelopment in Rare Disorders (NDRD) for assessment of baseline neurological function before undergoing unrelated umbilical cord blood transplantation. Patients were scanned in the first 6 weeks of life (range 20–49 days). The controls age ranged from 0 to 120 days and were scanned with the same protocol.
2.2. Brain MRI
Neonates with Krabbe disease referred for transpant evaluations were scanned using a structural and DTI protocol. We analyzed the four DTI parameters along six early myelinating white matter fiber tracts – corticospinal (left and right), corpus callosum (genu and splenium), and uncinate fasciculus (left and right) – for nine neonates with early-infantile Krabbe disease who subsequently underwent transplantation. We compared these DTI values with those of 336 age- and gender-matched controls. To study whether the DTI parameters can predict long-term outcomes in Krabbe, we evaluated relationships between DTI parameters with functional development at each patient's last evaluation. We tested whether effects we previously reported in gross motor function, were also present in other domains of function (cognitive, fine motor, and adaptive behavior). To explore the hypothesis that in neonatal Krabbe, microstructural differences may precede the development of volumetric differences, we compared volumes of white matter, gray matter, and cerebrospinal fluid in 27 non-overlapping regions including the lateral ventricles and tested for structural differences between Krabbe and controls. To evaluate the strength and sensitivity of the DTI measurements against visual assessment of the MR images (current practice), we evaluated the relationship between modified Loes score (Provenzale et al., 2009; Loes 1999) and DTI.
2.3. Scanning protocol details
Neonatal controls were recruited as part of a separate study and were scanned using an identical protocol. All neonates (control and Krabbe subjects) were scanned without sedation at the UNC Biomedical Research Imaging Center and the Presbyterian Center at the Children's Hospital of Pittsburgh either on a Siemens Allegra 3T head-only (UNC) or a Siemens Tim Trio 3T full-body MR scanner (UNC, Pittsburgh). The scan protocol consisted of four main imaging sequences — a magnetization prepared rapid gradient echo (MP-RAGE) T1-weighted (TR = 1820 ms, TI = 1100 ms, TE = 3.75 ms, flip angle = 7°, 1 × 1 × 1 mm resolution), a T2-weighted sequence, a resting-state functional MRI BOLD sequence and a single shot echo planar (EPI) DTI sequence (42 directions). Compatibility of the different scan protocols and across the two scanners was successfully evaluated with respect to both volumetric and DTI analyses based on 8 pediatric control subjects scanned on both scanners. This study was approved by the institutional review boards at the University of North Carolina and Children's Hospital of Pittsburgh. Written informed consent was obtained from the parents of the infants.
2.4. DTI processing
The processing protocol consisted of multiple repeated diffusion weighted acquisitions, which were corrected for eddy current and motion artifacts and bad acquisitions were removed via the automatic DTI quality control tool DTIPrep (Gilmore et al., 2007; Mullen, 1995) The analysis is performed by registration of the Krabbe cases into a DTI atlas built from normal neonate controls. In order to build the normative DTI atlas, we used an unbiased diffeomorphic atlas building method based on a non-linear fluid deformation method (Wang Y Gupta 2011; Hasan et al., 2009). The DTI derived intensity-histogram normalized FA is selected as the feature for atlas building (Mukherjee et al., 2002). All the tensor images are reoriented into the unbiased space using finite strain approximation. The atlas is then computed by averaging all the reoriented tensor images in log-Euclidean space. Having achieved an unbiased DTI atlas space, all tensor images were remapped into this atlas using a full tensor registration method called DTI-ToolKit, a non-parametric, diffeomorphic deformable image registration that has shown superior performance in Krabbe neonates compared to seven other non-linear registration methods (Wang et al., 2011). The Krabbe cases were mapped into the atlas using that same registration method to allow for direct comparison of DTI properties in the DTI atlas space of Krabbe and control subjects. For the analysis, the fiber circuitries of interest were determined in collaboration with a neuroanatomist. The DTI properties along the fiber tracts were extracted for all cases in their original DTI image space by inverse application of the atlas registration deformation field. Using a prior definition of a tract origin plane in atlas space, which defines a curvilinear re-parameterization of the tracts, corresponding average fiber bundle property profiles of FA, AD, RD and MD are extracted from the fiber tracts. Fig. 1 shows the details of DTI analysis.
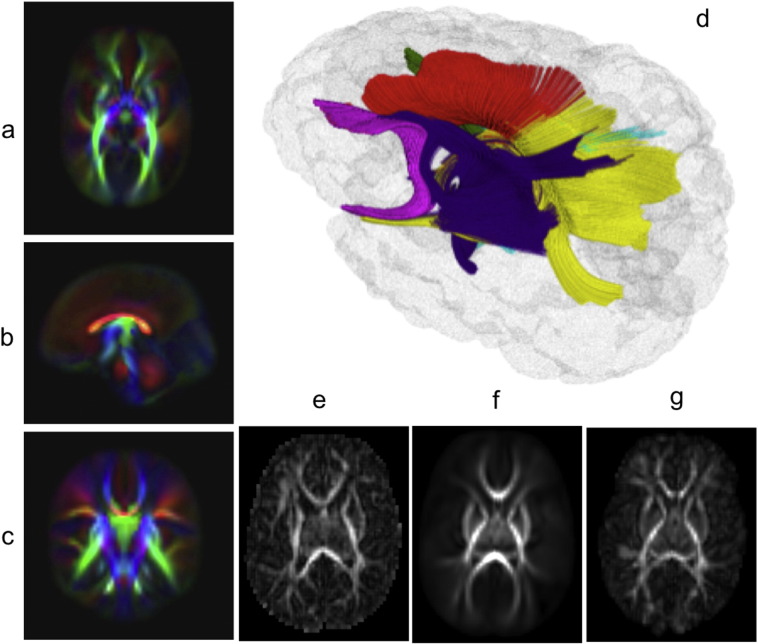
Fig. 1.
Axial, coronal, and sagittal slices of the normal control diffusion tensor imaging atlas from 336 controls (a, b, c). Three-dimensional visualization of target fiber tracts colored by regions of interest (genu, red; splenium, yellow; left and right internal capsule, celeste and smalt; left and right uncinate, peachblow and green) (d). Krabbe disease patient, the atlas, and Krabbe disease patient registered to the atlas (e, f, g).
An experienced neuroradiologist blinded to the DTI results scored the MR images using the modified Loes score. For this, an experienced neuroradiologist blinded to the DTI results scored the MR images using the modified Loes score.
2.5. Neurodevelopmental assessments
The Krabbe neonates underwent a detailed neurodevelopmental evaluation within 2 days of the brain MRI and every 6–12 months. The median follow-up time was 22.8 months with 4–10 follow-up evaluations (median = 9). Cognitive, fine and gross motor evaluations were done using well-standardized and validated assessments for this age population. These included the Peabody Developmental Motor Scales (Folio and Fewell, 1983) (PDMS-2) and the Mullen Scales of Early Learning (Mullen, 1995). Neonatal DTI measures were tested for their ability to predict functional outcomes at their last follow-up evaluation.
2.6. Statistical analysis
To compare DTI results of Krabbe patients with control subjects, we computed expected DTI value based on age at scanning, gestational age, gender, and birth weight using a general linear model that was fit to 336 typically developing neonates. A ratio of observed to expected value was calculated and converted to standard, normalized z-scores to create the FA, MD, AD, and RD scores. To determine the relationship between multiple DTI measures along the fiber tracts and covariates (group, age, and gender), we used functional analysis of diffusion tensor tract statistics (FADTTS), which provides a multivariate varying coefficient model to characterize relationships between fiber tract DTI properties and the set of covariates (Zhu et al., 2011). The average value between the groups for each tract was compared using general linear models. White matter, gray matter, and CSF volumes of Krabbe patients and controls were compared using model-estimated coefficients. Functional outcome measures at the last evaluation for each patient were converted to a functional ratio (calculated as age equivalence score divided by chronologic age). Pearson correlations were used to determine whether DTI results were associated with Loes scores or long-term functional outcomes.
3. Results
3.1. DTI parameters
After adjusting for gestational age, birth weight, gender, and race (Table 1), the Krabbe group had significantly lower FA for all six tracts compared with controls (p < 0.001) (Fig. 2), with no significant differences in hemispheric asymmetry (Table 2). Similarly, MD and RD of Krabbe patients were significantly higher for all six tracts, and AD was slightly higher for corticospinal internal capsule, right uncinate, and genu. Fig. 3 shows the group coefficient plots from the linear model fit of FADTTS data, illustrating the size and directionality of group differences along the fiber. Krabbe patients showed the following differences when compared to controls: FA values were significantly lower along the length of the corticospinal and genu tracts of Krabbe patients. Focal regional differences were detected in splenium and uncinate fasciculus. MD and RD were significantly higher in the corticospinal, uncinate and genu tracts. AD was slightly higher in the corticospinal tracts.
Table 1.
Demographics of transplanted Krabbe disease patients and normal controls.
| Krabbe disease patients (n = 9) |
Controls (n = 336) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Birth weight, g | 3212.4 | 596.26 | 2280 | 4130 | 2773.6 | 742.0 | 790 | 4701 |
| Gestational age, weeks (weeks) | 38.8 | 1.9 | 34.5 | 40.0 | 37.0 | 3.0 | 27.4 | 42.1 |
| Age at MRI, weeks | 15.3 | 8.3 | 7.0 | 34.0 | 26.5 | 25.2 | 0 | 120.0 |
| Gender (% male) | 56% | 51% | ||||||
| Race | ||||||||
| Asian | 0% | 2% | ||||||
| Black | 0% | 22% | ||||||
| American Indian | 0% | 1% | ||||||
| White | 100% | 76% | ||||||
| Ethnicity | ||||||||
| Hispanic | 11% | 15% | ||||||
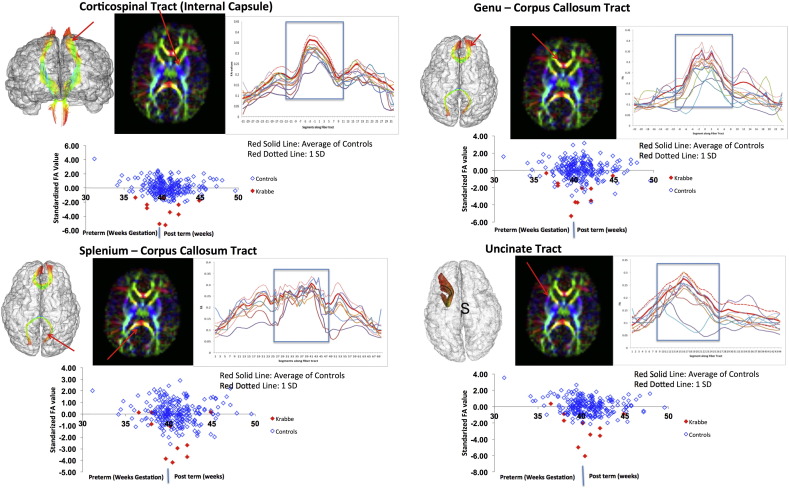
Fig. 2.
Figure shows the fiber tract (colored by fractional anisotropy [FA] values) in the brain and on the FA scalar image (derived from diffusion tensor imaging), FA profiles along the length of the tract of the Krabbe subjects with mean and standard deviation when compared to controls, and scatter plot of normalized FA values plotted against gestational age (in weeks) for the corticospinal internal capsule (left), genu, splenium, and uncinate tracts (left). The yellow window in the FA tract profile indicates values considered for statistical analysis.
Table 2.
Differences in effect size of diffusion tensor imaging parameters between patients with Krabbe disease (n = 9) and age- and gender-matched controls (n = 336).
| FA |
MD |
RD |
AD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diff | SE | p | Diff | SE | p | Diff | SE | p | Diff | SE | p | |
| L CSIC | −2.63 | 0.34 | <.001 | 2.31 | 0.34 | <.001 | 2.54 | 0.34 | <.001 | 1.57 | 0.35 | <.001 |
| R CSIC | −2.85 | 0.34 | <.001 | 2.24 | 0.35 | <.001 | 2.55 | 0.35 | <.001 | 1.32 | 0.35 | 0.002 |
| L Unc | −2.46 | 0.35 | <.001 | 1.37 | 0.34 | <.001 | 1.74 | 0.34 | <.001 | 0.54 | 0.34 | 0.115 |
| R Unc | −2.17 | 0.35 | <.001 | 1.51 | 0.34 | <.001 | 1.77 | 0.34 | <.001 | 0.86 | 0.34 | 0.012 |
| Genu | −2.15 | 0.34 | <.001 | 1.51 | 0.34 | <.001 | 1.81 | 0.34 | <.001 | 0.71 | 0.34 | 0.036 |
| Splenium | −1.67 | 0.35 | <.001 | 0.69 | 0.34 | 0.041 | 0.99 | 0.34 | 0.004 | 0.09 | 0.34 | 0.798 |
AD, axial diffusivity; CSIC, corticospinal internal capsule; Diff, difference; FA, fractional anisotropy; L, left; MD, mean diffusivity; R, right; RD, radial diffusivity; Unc, uncinate.
Fig. 3.
Plot showing correlation between the fractional anisotropy score (normalized mean ratio) within the internal capsule left tract and the gross motor and cognitive function scores.
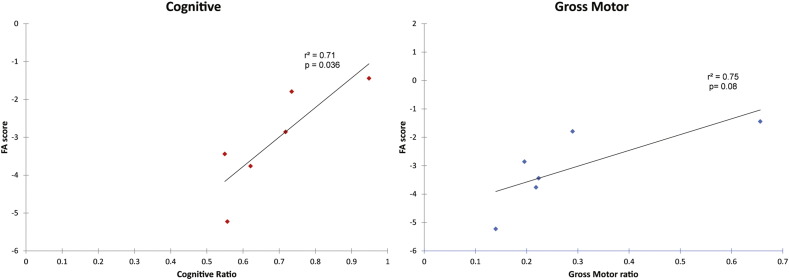
3.2. Correlation of DTI parameters with functional outcomes
There was a strong positive correlation of the neonatal FA score in the corticospinal tracts of the six surviving patients with their long-term neurodevelopmental function (cognitive, fine motor, and gross motor) (range 3–8 years post-transplant) (Table 3; Fig. 3). In contrast, FA and AD in splenium and uncinate fasciculus only correlated strongly with cognitive function. Of the two patients with the highest FA scores, one is able to walk and run at 9 years of age, and the other can walk with a walker at 48 months. The patient with the lowest neonatal FA score shows the greatest motor delay and is not able to walk independently at 25 months of age. Another patient with low FA is not able to walk independently at 9 years and has the gross motor development of a typical 10-month-old.
Table 3.
Pearson correlations between neurodevelopment at the last follow-up evaluation and diffusion tensor imaging parameters at baseline for the six surviving Krabbe disease patients.
| DTI parameter | Fiber tract | Cog (r) |
AB (r) |
GM (r) |
FM (r) |
|---|---|---|---|---|---|
| FA | CSIC | 0.96* | 0.37 | 0.79 | 0.70 |
| Genu | 0.22 | −0.61 | −0.22 | −0.36 | |
| Splenium | 0.79 | −0.06 | 0.45 | 0.32 | |
| Uncinate | 0.84 | 0.03 | 0.53 | 0.40 | |
| MD | CSIC | −0.12 | −0.09 | 0.01 | 0.12 |
| Genu | 0.30 | 0.46 | 0.55 | 0.65 | |
| Splenium | −0.10 | 0.46 | 0.27 | 0.40 | |
| Uncinate | 0.36 | −0.28 | 0.23 | 0.25 | |
| RD | CSIC | −0.27 | −0.14 | −0.12 | 0.00 |
| Genu | 0.25 | 0.53 | 0.54 | 0.65 | |
| Splenium | −0.46 | 0.29 | −0.08 | 0.07 | |
| Uncinate | 0.04 | −0.23 | 0.05 | 0.13 | |
| AD | CSIC | 0.29 | 0.06 | 0.36 | 0.43 |
| Genu | 0.43 | 0.23 | 0.53 | 0.60 | |
| Splenium | 0.97* | 0.20 | 0.75 | 0.65 | |
| Uncinate | 0.72 | −0.26 | 0.42 | 0.35 |
AB, adaptive behavior; AD, axial diffusivity; Cog, cognitive function; CSIC, corticospinal internal capsule; DTI, diffusion tensor imaging; FA, fractional anisotropy; FM, fine motor function; GM, gross motor function; MD, mean diffusivity; RD, radial diffusivity.
p < .05.
3.3. Volumetric differences between subject groups
Compared to controls, Krabbe patients showed slightly larger volumes (10%–15%) of gray and white matter within frontal and parietal regions, and 17% higher white matter volume in the right occipital region. The CSF volume was increased by 41% (Table 4). Examination of the lateral ventricles revealed a laterality effect, with the left ventricle showing greater enlargement than the right (83% vs. 22%).
Table 4.
Age- and gender-adjusted mean brain volumes for Krabbe disease patients (n = 8) and normal controls (n = 336).
| Region | Tissue | Controls |
Patients |
Difference | % | p* | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||||
| Frontal | Gray | 70.6 | 0.48 | 77.8 | 3.65 | 7.2 | 10% | 0.052 |
| Right | 37.8 | 0.26 | 41.6 | 1.95 | 3.7 | 10% | 0.059 | |
| Left | 32.7 | 0.23 | 36.2 | 1.76 | 3.4 | 11% | 0.054 | |
| Frontal | White | 62.4 | 0.43 | 69.9 | 3.40 | 7.4 | 12% | 0.031 |
| Right | 30.3 | 0.21 | 34.1 | 1.67 | 3.8 | 13% | 0.024 | |
| Left | 32.2 | 0.23 | 35.8 | 1.78 | 3.6 | 11% | 0.045 | |
| Occipital | Gray | 68.6 | 0.51 | 72.0 | 3.84 | 3.4 | 5% | 0.382 |
| Right* | 33.0 | 0.26 | 35.6 | 1.94 | 2.6 | 8% | 0.183 | |
| Left* | 35.6 | 0.27 | 36.4 | 1.99 | 0.8 | 2% | 0.697 | |
| Occipital | White | 42.0 | 0.35 | 47.3 | 2.99 | 5.3 | 13% | 0.082 |
| Right* | 19.6 | 0.17 | 23.0 | 1.47 | 3.4 | 17% | 0.023 | |
| Left* | 22.4 | 0.19 | 24.3 | 1.59 | 1.9 | 8% | 0.242 | |
| Parietal | Gray | 60.1 | 0.40 | 69.1 | 3.25 | 9.0 | 15% | 0.006 |
| Right | 31.0 | 0.21 | 34.9 | 1.67 | 3.9 | 13% | 0.020 | |
| Left | 29.1 | 0.21 | 34.2 | 1.65 | 5.1 | 18% | 0.002 | |
| Parietal | White | 48.0 | 0.34 | 54.2 | 2.93 | 6.2 | 13% | 0.036 |
| Right | 24.0 | 0.17 | 26.7 | 1.48 | 2.6 | 11% | 0.078 | |
| Left | 23.9 | 0.17 | 27.5 | 1.48 | 3.6 | 15% | 0.017 | |
| Cerebellum | Gray | 22.8 | 0.15 | 23.4 | 1.17 | 0.5 | 2% | 0.649 |
| Cerebellum | White | 1.5 | 0.15 | 3.0 | 1.17 | 1.5 | 100% | 0.198 |
| Corpus callosum | White | 1.7 | 0.03 | 1.8 | 0.26 | 0.08 | 5% | 0.752 |
| Brainstem and midbrain | White | 3.8 | 0.08 | 4.2 | 0.76 | 0.4 | 10% | 0.6265 |
| Lateral ventricles | ||||||||
| Right* | 2.4 | 0.07 | 2.93 | 0.47 | 0.53 | 22% | 0.267 | |
| Left* | 2.25 | 0.07 | 4.12 | 0.44 | 1.87 | 83% | <.001 | |
| Total cerebrospinal fluid | 52.7 | 0.56 | 74.3 | 5.33 | 21.6 | 41% | <.001 | |
Significant difference between group effects for the left versus right hemisphere (p < 0.05).
3.4. Relationship between Loes scoring and DTI parameters
DTI scores did not correlate with overall Loes scores (refer to Supplementary material).
4. Discussion
Our studies show that in Krabbe patients the DTI parameters (FA, MD, and RD) differed significantly with those of age- and gender-matched normal controls in the six fiber tracts assessed. In contrast, AD values differed only in the corticospinal tracts. FA has the highest correlation coefficient with the myelin water fraction (Mädler et al., 2008). The significantly lower FA in the six fiber tracts of Krabbe patients compared to normal controls may be a strong indicator of poor myelination. However, FA is also sensitive to non-myelin-related structural and biochemical changes. For example, in compact white matter structures such as the genu of the corpus callosum, the highly organized directionality of the fiber bundle would result in a higher FA value compared with a less compact white matter structure. In addition to poor myelination, disorganization of the fiber bundles may also be responsible for the lower FA values in Krabbe disease. Histological studies and myelin-specific MRI sequences have demonstrated that myelination begins in the cerebellum, pons and internal capsule, proceeding caudocranially to the splenium of the corpus callosum and optic radiations, to the occipital and parietal lobes, and finally to the genu of the corpus callosum and frontal and temporal lobes. In neonates, this spatiotemporal progression of myelination may affect DTI parameters along the different fiber tracts. Hence, in fiber tracts like the uncinate, lower FA values could be attributed to the early stage of life when this tract is less myelinated even in typically developing infants.
Krabbe patients showed higher values for RD, which is a specific marker of demyelination and dysmyelination (Song et al., 2002). However, inflammation around the axons could also contribute to increased RD. Interpretation of RD is difficult because any process affecting fiber diameter or membrane permeability could alter perpendicular diffusivity. In terms of AD, two processes have been shown to directly affect its values: inflammation around the axonal structures with tissue swelling, and axonopathy or axonal damage. Recent studies have reported axonopathy in the absence of demyelination (Castelvetri et al., 2011) and inflammation around axonal structures with endoneural edema in mouse models of Krabbe disease (Potter et al., 2013). In our studies, AD for the corticospinal tracts, right uncinate, and genu was significantly higher in Krabbe patients, whereas increases in AD in the left uncinate and splenium tracts were not significant. These differences in AD for different tracts are assumed to be due to a combination of axonopathy, a condition similar to disorganization, which lowers AD, and inflammation around axonal structures, which increases AD. The higher MD for Krabbe disease patients can be attributed to demyelination and inflammation of axons. Thus, a study of the four DTI parameters suggests active demyelination, reduced myelination, and ongoing inflammation in the brain of neonates with Krabbe disease. Certainly, the above differences in all the four DTI parameters between the Krabbe and the normal controls show the potential of using DTI as a biomarker for detecting early changes in the brain before clinical symptoms develop. DTI could also help predict timing of disease onset and expected phenotype in babies who screen positive for Krabbe disease. Strong correlations between neonatal DTI metrics and functional outcomes indicate that DTI can help determine the optimal time of treatment and predict functional outcomes in the absence of transplant-related complications. Predicting outcomes with neonatal DTI measures is more objective, and the methodology is easier to disseminate to sites that lack experience in evaluating these patients.
In contrast to DTI findings, Loes scores do not correlate with functional outcomes. The reason for this may be that at term-age equivalent, the neonatal brain is difficult to assess because the cerebral white matter is still not myelinated. The corresponding high T2 signal intesity can be difficult to distinguish from pathological signal intensity. In addition, qualitative assessment of volume loss and atrophy of neonatal cerebral structures (including corpus callosum) can be inconsistent among neuroradiologists. The difficulty involved in visual assessment and the inconsistency between neuroradiologists' readings prompted us to develop a consistent quantitative biomarker that is not subjective.
An interesting finding is that in addition to the white matter microstructural differences, Krabbe seems to have volumetric differences in both gray and white matter, with surprisingly enlarged volumes in the lateral ventricles and the total cerebrospinal fluid. This could be the result of inflammation of the more central, early myelinating structures. The left lateral ventricle was more enlarged than the right in Krabbe, which may be due to differences in myelination rates of the left and right periventricular areas in newborns. However, in newborns with Krabbe disease the left periventricular area may have already undergone myelination and subsequent demyelination, resulting in atrophy by the time the baby is born. Whether this is the reason for greater enlargement of the left ventricle would be an interesting question to address in future research.
One limitation of our study is the small number of infants with Krabbe disease; however, this represents a large portion of this population, and the results are statistically significant. Although a random sampling technique was not used, we used sequential enrollment (i.e., each child diagnosed with early-infantile Krabbe disease who presented at our center underwent imaging). During the study period, our center saw nearly all presymptomatic children in the United States suspected of having early-infantile Krabbe disease; therefore, there is no reason to believe that these results would differ from those obtained from a random sample.
5. Conclusion
Differences observed in FA in the corticospinal tract differentiate between babies who will develop early-infantile disease from those with later-onset disease. This DTI-based approach produces consistent results and detects small regional differences and age-related changes in the neonatal period. For this reason, DTI with quantitative tractography is an excellent technology for studying infantile Krabbe disease and can be a key component of Krabbe newborn screening to help determine which infants should receive treatment before symptoms develop. This tool has the potential to have extended applications in predicting motor and cognitive involvement in other leukodystrophies.
We have developed a quantitative DTI based tractography tool that can be used for predicting functional outcomes in asymptomatic neonates with Krabbe disease diagnosed because of family history or newborn screening.
Author contributions
Dr. Escolar is the PI of the study, designed the study, evaluated the patients, provided significant input to the manuscript draft and edited the manuscript.
Dr. Gupta wrote the manuscript and did the DTI & structural image analysis. Dr. Poe was responsible for the statistical design and analysis of the data. Dr. Ashok Panigrahy performed the Loes scoring on the MR images and provided significant reviews on the manuscript. Dr. Martin Styner provided significant reviews to the manuscript and guidance on the image analysis.
Funding
DTI As A Tool To Identify Infants With Krabbe Disease In Need Of Urgent Treatment (Funding Agency: NIH/NINDS 1R01NS061965-01), The DANA Foundation and the Legacy of Angels.
Acknowledgement
We would like to acknowledge the families that participated in the study, the nursing staff, research assistants and technicians who helped in the image acquisition and processing, the NDRD clinical team and collaborators at the Neurodevelopmental Research Center at the University of North Carolina at Chapel Hill and Duke University Division of Pediatric Bone Marrow Transplantation.
Footnotes
Supplementary material for this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.09.014.
References
- Castelvetri L.C., Givogri M.I., Zhu H. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathologica. 2011;122:35–48. doi: 10.1007/s00401-011-0814-2. 21373782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner P.K., Caggana M., Orsini J.J. Newborn screening for Krabbe disease: the New York State model. Pediatric Neurology. 2009;40:245–252. doi: 10.1016/j.pediatrneurol.2008.11.010. 19302934 [DOI] [PubMed] [Google Scholar]
- Escolar M.L., Poe M.D., Martin H.R. A staging system for infantile Krabbe disease to predict outcome after unrelated umbilical cord blood transplantation. Pediatrics. 2006;118:e879–e889. doi: 10.1542/peds.2006-0747. 16923928 [DOI] [PubMed] [Google Scholar]
- Escolar M.L., Poe M.D., Smith J.K. Diffusion tensor imaging detects abnormalities in the corticospinal tracts of neonates with infantile Krabbe disease. AJNR. American Journal of Neuroradiology. 2009;30:1017–1021. doi: 10.3174/ajnr.A1476. 19386732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar M.L., Poe M.D., Provenzale J.M. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. New England Journal of Medicine. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. 15901860 [DOI] [PubMed] [Google Scholar]
- Folio R., Fewell R. Peabody Developmental Motor Scales and Activity Cards. DLM Teaching Resources; Allen, TX: 1983. [Google Scholar]
- Gilmore J.H., Lin W., Corouge I. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR. American Journal of Neuroradiology. 2007;28:1789–1795. doi: 10.3174/ajnr.A0751. 17923457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K.M., Iftikhar A., Kamali A. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. 19393229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoye L., Saint-Martin C., Cosnard G. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. 16194615 [DOI] [PubMed] [Google Scholar]
- Mädler B., Drabycz S.A., Kolind S.H., Whittall K.P., MacKay A.L. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magnetic Resonance Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. 18524521 [DOI] [PubMed] [Google Scholar]
- McGraw P., Liang L., Escolar M. Krabbe disease treated with hematopoietic stem cell transplantation: serial assessment of anisotropy measurements — Initial experience. Radiology. 2005;236:221–230. doi: 10.1148/radiol.2353040716. 15987975 [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Miller J.H., Shimony J.S. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. 11687675 [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Miller J.H., Shimony J.S. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR. American Journal of Neuroradiology. 2002;23:1445–1456. 12372731 [PMC free article] [PubMed] [Google Scholar]
- Mullen E. The Mullen Scales of Early Learning: AGS Edition. American Guidance Service; Circle Pines, NM: 1995. [Google Scholar]
- Potter G.B., Santos M., Davisson M.T. Missense mutation in mouse GALC mimics human gene defect and offers new insights into Krabbe disease. Human Molecular Genetics. 2013;22:3397–3414. doi: 10.1093/hmg/ddt190. 23620143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzale J.M., Peddi S., Kurtzberg J., Poe M.D., Mukundan S., Escolar M. Correlation of neurodevelopmental features and MRI findings in infantile Krabbe's disease. American Journal of Roentgenology. 2009;192(1):59–65. doi: 10.2214/ajr.07.3885. [DOI] [PubMed] [Google Scholar]
- Provenzale J.M., Liang L., DeLong D., White L.E. Diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. American Journal of Roentgenology. 2007;189:476–486. doi: 10.2214/AJR.07.2132. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. 12414282 [DOI] [PubMed] [Google Scholar]
- Wang Y., Gupta A., Liu Z. DTI registration in atlas based fiber analysis of infantile Krabbe disease. Neuroimage. 2011;55:1577–1586. doi: 10.1016/j.neuroimage.2011.01.038. 21256236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D.A., Rafi M.A., Luzi P. Krabbe disease: genetic aspects and progress toward therapy. Molecular Genetics and Metabolism. 2000;70:1–9. doi: 10.1006/mgme.2000.2990. 10833326 [DOI] [PubMed] [Google Scholar]
- Wenger D.A., Suzuki K., Suzuki Y. Galactosylceramide lipidosis: globoid-cell leukodystrophy (Krabbe disease). The metabolic and molecular bases of inherited disease. In: Scriver C.R., Sly W.S., editors. McGraw-Hill; New York: 2001. pp. 3669–3694. [Google Scholar]
- Zhu H., Kong L., Li R. FADTTS: functional analysis of diffusion tensor tract statistics. Neuroimage. 2011;56:1412–1425. doi: 10.1016/j.neuroimage.2011.01.075. 21335092 [DOI] [PMC free article] [PubMed] [Google Scholar]