Abstract
Verbal information is better retained when it is self-generated rather than when it is received passively. The application of self-generation procedures has been found to improve memory in healthy elderly and in individuals with impaired cognition. Overall, the available studies support the notion that active participation in verbal encoding engages memory mechanisms that supplement those used during passive observation. Thus, the objective of this study was to investigate the age-related changes in the neural mechanisms involved in the encoding of paired-associates using a self-generation method that has been shown to improve memory performance across the lifespan. Subjects were 113 healthy right-handed adults (Edinburgh Handedness Inventory >50; 67 females) ages 18–76, native speakers of English with no history of neurological or psychiatric disorders. Subjects underwent fMRI at 3 T while performing didactic learning (“read”) or self-generation learning (“generate”) of 30 word pairs per condition. After fMRI, recognition memory for the second word in each pair was evaluated outside of the scanner. On the post-fMRI testing more “generate” words were correctly recognized than “read” words (p < 0.001) with older adults recognizing the “generated” words less accurately (p < 0.05). Independent component analysis of fMRI data identified task-related brain networks. Several components were positively correlated with the task reflecting multiple cognitive processes involved in self-generated encoding; other components correlated negatively with the task, including components of the default-mode network. Overall, memory performance on generated words decreased with age, but the benefit from self-generation remained consistently significant across ages. Independent component analysis of the neuroimaging data revealed an extensive set of components engaged in self-generation learning compared with didactic learning, and identified areas that were associated with age-related changes independent of performance.
Highlights
-
•
Verbal information is better retained when self-generated vs. received passively.
-
•
Application of self-generation is associated with better retention across ages.
-
•
Generated words were retained better than read words.
-
•
Several components of network for word generation were identified.
-
•
Age-associated changes within the network are discussed.
1. Introduction
Verbal information is better retained when it is self-generated rather than received passively (Backman and Mantyla, 1988; Basso et al., 1994; Craik, 2002; Kanfer and Schefft, 1988; McDaniel et al., 1988; Olofsson and Nilsson, 1992; Schefft and Biederman, 1990; Slamecka and Graf, 1978). Specifically, self-generation involves an individual's production of verbal information based on a cue or set of cues (semantic, phonological, or visual), as opposed to hearing or reading the full phonological or orthographic form. In the clinical setting, the application of self-generation procedures has been found to improve memory in both nondemented elderly individuals and patients with Alzheimer's disease (Barrett et al., 2000; Lipinska et al., 1994; Multhaup and Balota, 1997; Souliez et al., 1996), frontal lobe dementia (Souliez et al., 1996), and in a number of other conditions (Barrett et al., 2000; Chiaravalloti and Deluca, 2002; Marshall et al., 1992; Schefft et al., 2008a; Schefft et al., 2008b; Smith, 1996; Vinogradov et al., 1997). Overall, these clinical studies support the notion that active participation during verbal encoding engages memory mechanisms that supplement those used during passive observation, leading to improvements in memory performance (Barrett et al., 2000; Lipinska et al., 1994; Multhaup and Balota, 1997; Schefft et al., 2008a; Schefft et al., 2008b; Souliez et al., 1996).
The efficacy of self-generation encoding procedures likely lies in the fact that the individual takes an active role in producing material to be remembered rather than passively responding to stimuli provided. Memories are enhanced as a result of self-generation of information because there is an increase in distinctiveness in the to-be-remembered items (Mantyla and Nilsson, 1988; McDaniel et al., 1988), and also, the strategy enforces processing information at a deeper semantic level, which causes verbal information to be better remembered (Backman and Mantyla, 1988; Craik, 2002; Lespinet-Najib et al., 2004).
For older adults, the memory benefit associated with self-generation of words compared to simply reading is as large as it is for younger adults, but overall memory performance decreases with age (Rabinowitz, 1989). Some differences in the generation effect for older and younger adults have been observed; for example, older adults do not receive as much memory benefit as younger adults from simply reading words aloud compared to silent reading (Lin and MacLeod, 2012), suggesting that the memory benefit for generating words may derive from the deep semantic processing associated with the generation process. In addition, while older adults see a memory benefit for self-generated items, they may not remember features of these items (Rabinowitz, 1989) to the extent that younger adults do.
The changes that take place in the neural mechanism underlying self-generation with age are not well defined. In young adults, neuroimaging studies of verbal encoding, which have made use of a variety of tasks and materials, have revealed a general pattern suggesting involvement of a multi-lobar network of brain regions. In general, “deeper” semantic processing at encoding, may be associated with additional participation of the frontal and medial temporal regions when contrasted with shallower encoding (Nyberg, 2002; Otten et al., 2001). Frontal mechanisms for “deeper” encoding have also been suggested to be left-lateralized (HERA model; Tulving et al., 1994). The self-generation task also depends on encoding and retrieval of paired verbal associates, which has been found to involve parahippocampal regions, visual integration areas, bilateral prefrontal cortex and cingulate gyrus, in both encoding and retrieval (Krause et al., 1999; Mottaghy et al., 1999). Studies of “subsequent memory effects”, which examine patterns of activation during encoding of information that is later successfully remembered are also relevant, since the generation effect promotes more successful encoding. A recent meta-analysis showed that left inferior frontal cortex/insula, bilateral fusiform cortex, and left mesial temporal regions were most often engaged during successful encoding of verbal associates (H. Kim, 2011). Using the self-generation encoding task also used in the present study, we previously found increased activation for self-generation encoding of words compared to reading in inferior/middle frontal gyri, anterior cingulate, caudate nucleus, and the temporo-parietal–occipital junction bilaterally (Vannest et al., 2012). A largely overlapping pattern of results was recently observed by Rosner et al. in another similar self-generation task (Rosner et al., 2013). On the whole, these studies support a frontal–temporal–occipital network that is necessary for successful verbal encoding. However, none of these studies have focused on the change in the verbal encoding network that occurs with aging.
A few recent studies examining age-related changes in the verbal encoding network have shown effects of age on the involvement of temporal/parietal versus frontal regions in older adults. Older adults, compared to younger adults, show increased engagement of prefrontal cortex, and decreased engagement of medial temporal regions during encoding tasks, though they exhibit the same level of performance (Dennis et al., 2007; Sambataro et al., 2012). Age-related shifts in activation from temporal or parietal to frontal regions are suggested to indicate increased reliance on attentional and/or executive processes in older adults as a “compensatory” strategy (Rajah and D'Esposito, 2005; Sambataro et al., 2012). This compensation may also go hand-in-hand with dedifferentiation — the theory that in aging, brain networks become less dedicated to specialized functions. For example, frontal regions dedicated to executive function or attention earlier in life support other functions such as memory encoding later (Geerligs et al., 2014; Rajah and D'Esposito, 2005; Sambataro et al., 2012; St-Laurent et al., 2011).
Functional connectivity analyses of fMRI have further contributed to our understanding of age-related changes in task-specific brain networks supporting cognitive function, including verbal encoding. Studies of verbal encoding have found that in older adults medial temporal regions become more connected with dorsolateral frontal regions (Daselaar et al, 2006; Grady et al, 2003) and that there is age-related decrease in connectivity between hippocampal and temporo-parietal regions (Daselaar et al., 2006). These results are consistent with the age-related shift toward increased prefrontal engagement found in other fMRI studies. However, findings of age-related decreases in connectivity in parietal regions are not universal. For example, Matthaus et al. found that, networks engaged during memory tasks showed increased parietal connectivity in older adults (Matthaus et al., 2012).
Default-mode network connectivity has also been shown to decrease with age (Grady et al., 2010). This has been observed in default-mode suppression during a task. For example, Geerligs et al. (2014) found decreased default-mode connectivity in older adults compared to younger adults during a sustained attention task. They also found decreased connectivity in older adults compared to younger adults in dorsal attention/somato-motor networks that were active during the task. Age-related decreases in default-mode connectivity have also been observed in a number of resting-state studies (Mevel et al., 2013; Mowinckel et al., 2012; see Ferreira and Busatto, 2013; Goh, 2011 for reviews). These decreases have been described as a biomarker of cognitive decline in aging (Ferreira and Busatto, 2013; Grady et al., 2010; Mevel et al., 2013; Worsley and Friston, 1995) and are perhaps related to the increased distractibility in older adults (Grady et al., 2010); they are suggested to reflect dedifferentiation as networks are decreasing in specificity with age (Geerligs et al., 2014; Goh, 2011; Sambataro et al., 2012).
In the present study we aimed to investigate age-related changes in the neural mechanisms involved in the encoding of paired-associates using a self-generation method that has been shown to improve memory performance across the lifespan (Barrett et al., 2000; Lipinska et al., 1994; Multhaup and Balota, 1997; Schefft et al., 2008a; Schefft et al., 2008b; Souliez et al., 1996). Our hypothesis was that with increasing age there would be a decrease in memory performance, but no change in the degree of performance improvement associated with self-generation. We also expected age-related changes in the network supporting self-generation encoding (Rosner et al., 2013; Vannest et al., 2012), as examined with fMRI and independent component analysis (Ferreira and Busatto, 2013; Goh, 2011). Finally, we also expected decreased connectivity with age in both task-related networks, particularly those that involve temporal and parietal regions, as well as decreased connectivity in task-negative “default mode” networks.
2. Methods
2.1. Subjects
Subjects were 113 healthy right-handed adults (67 females) ages 18–76, native speakers of English with no history of neurological or psychiatric disorders. Some of these subjects were included in our previous publications (Siegel et al., 2012; Vannest et al., 2012). Age and gender distribution of the examined cohort are included in Fig. 1. Subjects were recruited from a local Cincinnati community via print and word-of-mouth advertising as part of a larger study (Allendorfer et al., 2012; Siegel et al., 2012; Szaflarski et al., 2013; Vannest et al., 2012). The project was reviewed and approved by the Institutional Review Boards at the University of Cincinnati, the Cincinnati Children's Hospital Medical Center and the University of Alabama at Birmingham, and all subjects were provided written informed consent.
Fig. 1.
Age and gender distribution of subjects included in the study.
2.2. Materials
Materials were 60 pairs of related familiar words consisting of 3–6 letters as in previous studies (Basso et al., 1994; Schefft et al., 2008a; Siegel et al., 2012; Vannest et al., 2012). Relationships between pair members were characterized in terms of 5 rules (12 pairs per rule): associates (e.g., lock–key), category members (e.g., saucer–bowl), synonyms (e.g., street–road), antonyms (e.g., hot–cold) and rhymes (e.g., care–dare) (Siegel et al., 2012; Vannest et al., 2012). Thirty pairs (6 per rule) were assigned to the didactic learning condition (“read” condition) and the remaining 30 to the self-generation learning condition (“generate” condition). There was an equal number of trials from each rule in the “generate” and “read” conditions. One word in the pair was always the first word and the word order within the pair did not vary across subjects.
2.3. Procedure-encoding phase
fMRI scanning was preceded by a 15-item practice session as described in Vannest et al. (2012). In both the practice session and the scanning session, each word pair was visually presented for 5000 ms centered in a 36-point white font on a black background (using DirectRT software; http://www.empirisoft.com). Subjects were instructed to produce aloud the second word in the pair, and could make their verbal response at any time during the visual presentation. In the “read” condition, both words were fully presented, but in the “generate” condition, the second word was presented with the first letter only with the remaining letters replaced by asterisks (e.g., salt – p*****). Subjects were instructed that they would later be asked to recognize the second word of each pair. During fMRI scanning, 60 pairs were randomly presented in an event-related design, and verbal responses were recorded via an in-scanner microphone. A sparse acquisition approach (Allendorfer et al., 2012; Schmithorst and Holland, 2004b; Vannest et al., 2009) was used so that verbal responses could be produced and recorded without scanner noise. During image acquisition, after the 5000 ms visual presentation of the word pair, the word STOP appeared on the screen for 6000 ms (Siegel et al., 2012; Vannest et al., 2012).
2.4. MRI acquisition methods
As described in Vannest et al. (2012), fMRI scanning was performed on a 3 T Philips Achieva MRI scanner with the following parameters: TR/TE = 2000/38 ms, FOV 24.0 × 24.0 cm, matrix 64 × 64, slice thickness = 4 mm. This resulted in a voxel size of 4 × 4 × 4 mm and 32 axial slices. Three image volumes were collected during each sparse acquisition phase following each stimulus presentation for a total of 180 volumes. High-resolution T1-weighted anatomical images were also obtained using the following parameters: TR/TE = 8.1/3.7 ms, FOV 25.0 × 21.1 × 18.0 cm, matrix 252 × 211 and slice thickness = 1 mm.
2.5. Recognition post-test
After fMRI scanning, recognition memory for the second word in each pair was evaluated outside of the scanner (Siegel et al., 2012; Vannest et al., 2012). All 60 of the “read” or “generated” words were presented in a three-item forced-choice recognition task. The target item and two foils were presented simultaneously on the computer screen. Both foils were semantically related to the target in 30 trials, unrelated in 15 trials, rhymes in 10 trials, and in the remaining 5 trials one foil was associated with the target and one was not (semantic or rhyme). No words presented during the encoding phase were used as foils; all foils were 3–7 letters. Subjects responded with a self-paced key press to indicate which of the three words they recognized from the preceding in-scanner task.
2.6. Functional MRI data analysis
Processing of 3D anatomical and fMRI image data were done using routines written in-house in the Interactive Data Language (IDL; ITT Visual Information Solutions, Boulder, CO) programming language and the Cincinnati Children's Hospital Image Processing Software (CCHIPS©) using methods similar to those used in our previous studies (Schmithorst et al., 2000; Szaflarski et al., 2008; Szaflarski et al., 2004). Briefly, several pre-processing steps were performed including removal of ghosting and geometric distortion artifacts using a multi-echo reference technique (Schmithorst et al., 2001), motion correction using a pyramid iterative algorithm (Thevenaz et al., 1998), and affine spatial transformation of the anatomical and functional images to align them with the standard Montreal Neurological Institute (MNI) coordinate reference frame.
2.7. Independent component analysis
Group spatial independent component analysis (Schmithorst and Holland, 2004a) was implemented using GIFT software (Calhoun et al., 2001). Principal component analysis (PCA) reduction was carried out in three stages, once for each image volume in the sparse acquisition (3 image volumes per trial). This allows for the greatest sensitivity to fluctuations in BOLD response that occur at various latencies following the stimulus and response. PCA reduction was first applied to each of the three image volumes for each subject concatenated across time to obtain 59 principal components (PCs). These first-level PCs were then concatenated within each subject, and a second round of subject-wise PCA reduction was applied to obtain 51 PCs. All subjects' second-level PCs were concatenated, and a third and final round of PCA reduction was applied to obtain 41 PCs for the group. This final number was estimated using the minimum description length (MDL) criteria (Li et al., 2007; Schmithorst and Holland, 2004a) for a representative subject/image volume. Following ICA, group components were backprojected onto individual subjects using dual regression (Filippini et al., 2009). Backprojected timecourses for each image volume within each independent component (IC) were correlated with a binary task timecourse (1 = “generate”, 0 = “read”). For further analyses, we chose to focus on those ICs that were most related to the task; specifically, we quantified task-relatedness in terms of degree of correlation (correlation coefficient r) of the IC with the task timecourse across all subjects. We selected only those ICs that were highly correlated with the task timecourse across subjects (those with an average correlation coefficient of |r| > 0.50 in at least one of the three image volumes; if more than one image volume was correlated at |r| > 0.50, the most highly correlated volume was selected). These 13 ICs were retained for further analyses. Intra-component connectivity was assessed by thresholding each group component t-map at t > 5 create a binary mask of each of the 13 ICs. These masks represent groups of voxels whose activations are maximally dependent on the task across the entire group of subjects. Each of these group component masks was then applied to each subject's corresponding back-projected component. For each subject, the mean of the voxels within each mask was extracted as a measure of intra-component connectivity. Specifically, this extracted measure represents how tightly intercorrelated the voxels in the selected component are for a given subject. This analysis resulted in values for intra-component connectivity for each of the 113 subjects for each of the 13 ICs.
2.8. Relationships between connectivity, age, and performance
For the 13 task-related components, the effects of age and post-test performance were assessed with a multiple regression analysis. Age and post-test performance in the “read” and “generate” conditions were used as predictors of intra-component connectivity at the single-subject level. Adjustment for multiple testing over the 13 components was done using the False Discovery Rate (FDR) methodology. FDR procedures are designed to control the expected proportion of incorrectly rejected null hypotheses (Benjamini and Hochberg, 2000).
3. Results
3.1. Performance data
In-scanner performance data during the encoding task were recorded, transcribed and scored. If a participant generated a related word that fit the appropriate number of letters but was not the expected target this was counted as a correct response, though such responses were rare. As expected, more words were correctly read than correctly generated (t(112) = 20.19, p < 0.001), though performance levels were very high in both conditions (98.9% correct responses for read words, 80.1% correct responses for generated words). On the recognition post-test, more words in the generate condition were correctly recognized than words in the read condition (78.4% correct recognition responses for the generate condition, 70.0% for the read condition; t(112) = 7.77, p < 0.001). Note that post-test performance was not conditionalized on correctly reading or generating the word during the encoding phase; subjects frequently recognized words that they did not correctly produce during the encoding phase (Siegel et al., 2012) indicating subliminal processing even when they were unable to generate the correct words.
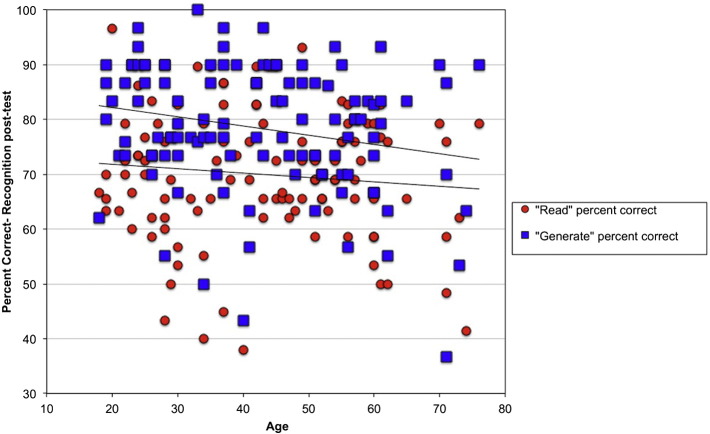
Age was not a significant predictor of in-scanner performance, but it was a predictor of post-test recognition performance for words in the generate condition, where older adults performed less accurately (r = −0.22, p = 0.021). This was not the case for words in the read condition (r = −0.10, p = 0.28). There was a significant difference in the age-related change in these two conditions (t(225) = 15.07, p < 0.001) However, if we examine the difference in recognition scores for “generated” versus “read” words, this does not differ with age (r = −0.12, p = 0.22), i.e. the performance “boost” for generated words compared to read was consistent across age. Post-test recognition performance as a function of age is shown in Fig. 2.
Fig. 2.
Percentage correct responses on recognition post-test for “read” and “generate” conditions by age.
3.2. fMRI results — independent component analysis
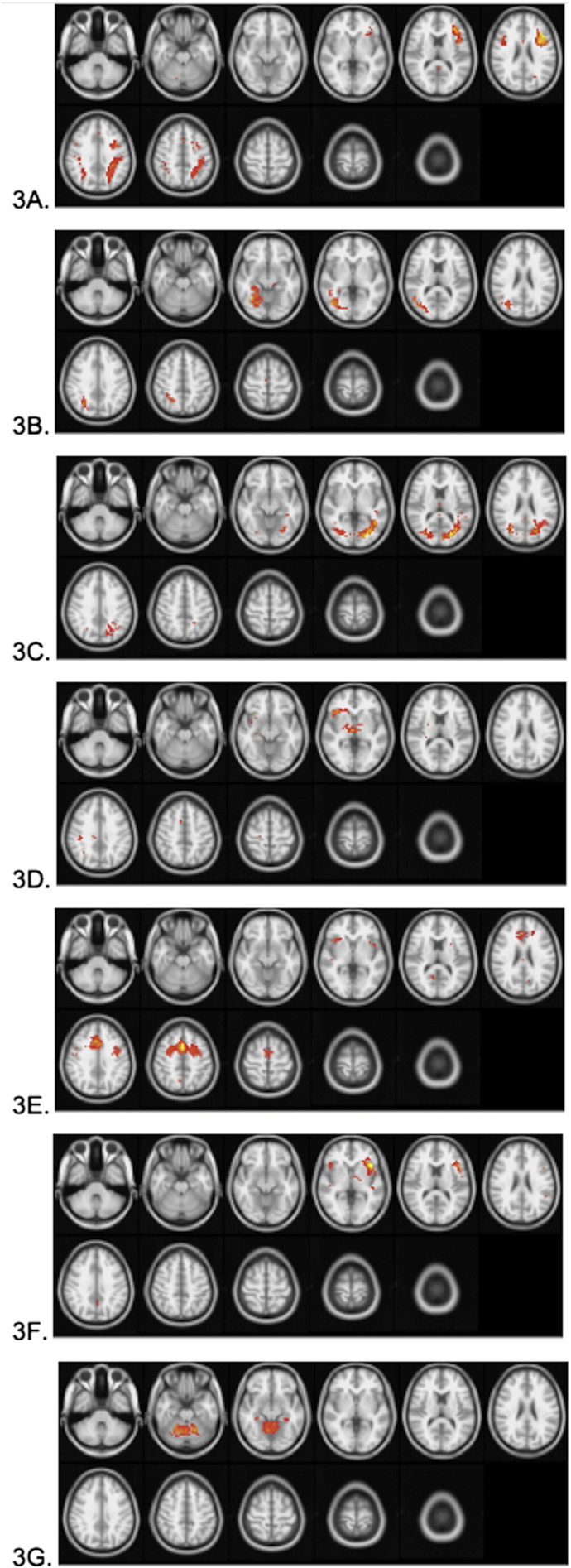
Seven components were identified as task-positive (“generate” > “read”); these components correlated positively with the task above the threshold of r > 0.50 (“generate” > “read”). These components are displayed in Fig. 3, and locations for each of the clusters in each component are given in Table 1.
Fig. 3.
Group average independent components positively correlated (r > 0.5) with the task timecourse. Images are presented in radiological orientation (right in the figure is left in the brain). A) Bilateral fronto-parietal; B) right fusiform gyrus; C) bilateral occipital; D) right inferior frontal/insula and thalamus; E) right anterior insula, anterior cingulate and precentral/dorsomedial frontal cortex; F) bilateral (left-lateralized) inferior frontal gyrus; and G) cerebellum. Components in A and B showed decreasing intra-component connectivity with increasing age. The exact location of the BOLD signal changes or each component is located in Table 1.
Table 1.
MNI coordinates and extent for each cluster in each task-related component.
| Component | Location | X | Y | Z | Extent (voxels) |
|---|---|---|---|---|---|
| Components positively correlated with the task (Fig. 3) | |||||
| 3A | Left inferior frontal gyrus | −36.9 | 15.7 | 22.4 | 294 |
| 3A | Left superior parietal lobule/ supramarginal gyrus | −31.1 | −44.9 | 40.2 | 240 |
| 3A | Right superior parietal lobule/ supramarginal gyrus | 35.2 | −43.3 | 40.9 | 77 |
| 3A | Right inferior frontal gyrus | 41.9 | 9.2 | 26.5 | 38 |
| 3A | Anterior cingulate | −0.3 | 20.1 | 43.9 | 13 |
| 3A | Anterior cingulate | 3.4 | 2.6 | 29.1 | 13 |
| 3B | Right fusiform gyrus | 32.2 | −61.0 | 7.9 | 405 |
| 3C | Intracalcarine cortex | −12.0 | −69.5 | 11.5 | 670 |
| 3D | Thalamus | 14.2 | −9.1 | 1.7 | 63 |
| 3D | Right insula | 33.7 | 22.6 | −3.7 | 61 |
| 3E | Anterior cingulate/dorsomedial frontal | −0.6 | 9.3 | 43.1 | 531 |
| 3E | Right insula | 35.3 | 19.5 | 3.2 | 32 |
| 3E | Left insula | −33.3 | 13.4 | 2.5 | 13 |
| 3F | Left inferior frontal gyrus | −38.9 | 19.1 | 2.3 | 155 |
| 3F | Right inferior frontal gyrus | 39.9 | 22.4 | −3.4 | 23 |
| 3G | Cerebellum | 2.8 | −53.0 | −18.3 | 365 |
| Components negatively correlated with the task (Fig. 4) | |||||
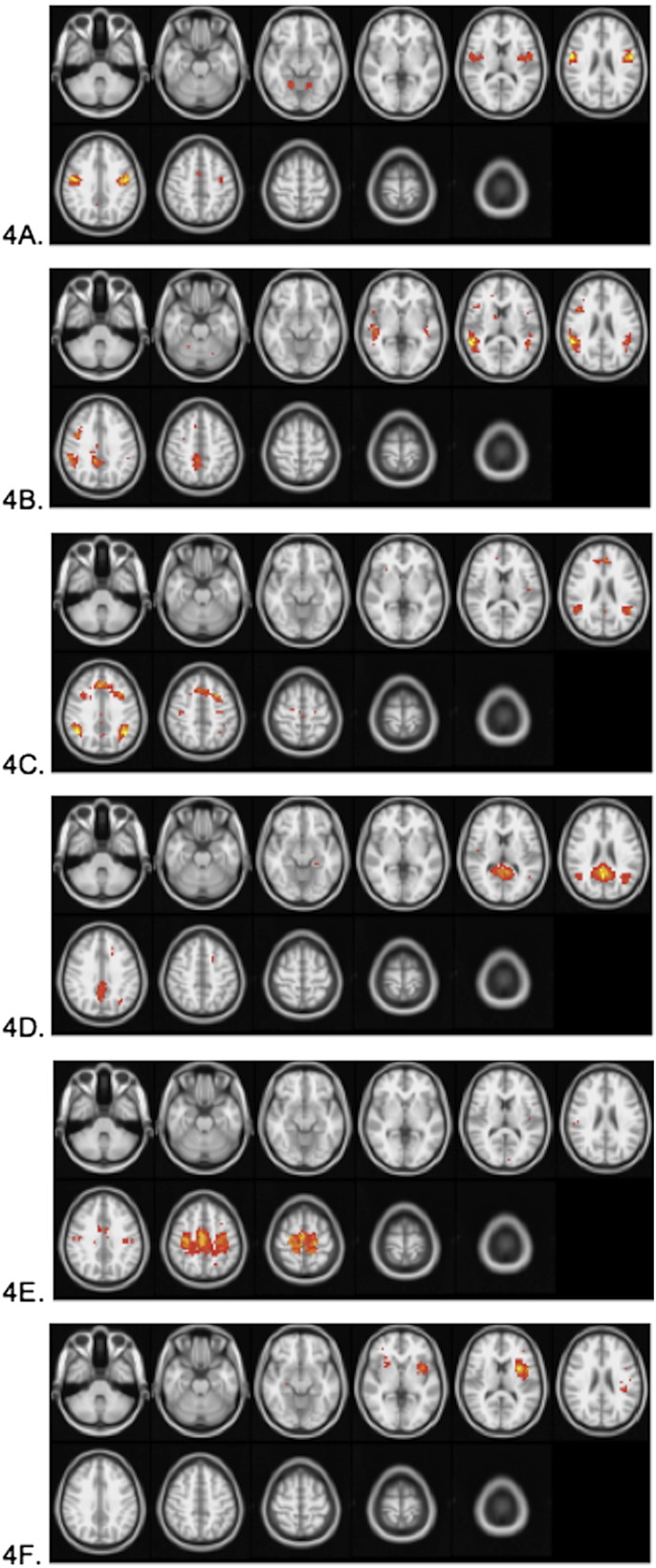
| 4A | Left postcentral gyrus | −44.1 | −8.1 | 27.4 | 221 |
| 4A | Right postcentral gyrus | 48.2 | −8.1 | 27.2 | 169 |
| 4A | Right cerebellum | 18.0 | −56.1 | −13.1 | 23 |
| 4A | Anterior cingulate/dorsomedial frontal | 3.4 | 1.4 | 49.7 | 15 |
| 4A | Left cerebellum | −13.4 | −55.1 | −14.4 | 14 |
| 4B | Right supramarginal gyrus | 50.5 | −41.4 | 17.4 | 317 |
| 4B | Posterior cingulate | 7.1 | −39.9 | 42.1 | 140 |
| 4B | Left supramarginal/superior temporal | −45.9 | −42.6 | 18.0 | 104 |
| 4B | Right precentral | 40.5 | 9.8 | 28.9 | 67 |
| 4B | Right caudate | 14.7 | 3.8 | 14.9 | 11 |
| 4C | Anterior cingulate | −5.3 | 21.7 | 38.4 | 291 |
| 4C | Left angular/supramarginal gyrus | −38.3 | −52.5 | 33.7 | 126 |
| 4C | Right angular/supramarginal gyrus | 45.3 | −49.6 | 32.6 | 99 |
| 4C | Precentral gyrus | −28.2 | −17.2 | 52.2 | 15 |
| 4D | Posterior cingulate | 1.9 | −49.8 | 20.9 | 479 |
| 4D | Left angular gyrus/superior occipital cortex | −37.2 | −60.1 | 24.2 | 92 |
| 4D | Right angular gyrus/superior occipital cortex | 45.7 | −57.0 | 23.1 | 22 |
| 4D | Left superior frontal gyrus | −18.0 | 17.4 | 42.3 | 15 |
| 4E | Precentral gyrus/anterior cingulate | 1.1 | −23.2 | 50.8 | 953 |
| 4F | Left insula | −36.4 | 1.5 | 10.7 | 222 |
| 4F | Right insula | 26.1 | 15.1 | −1.0 | 23 |
Six task-negative (“read” > “generate”) components, including those associated with default mode networks, were identified; they correlated negatively with the task below the threshold of r < −0.50. These components are displayed in Fig. 4, and locations for each of the clusters in each component are also given in Table 1.
Fig. 4.
Group average independent components negatively correlated (r < −0.5) with the task timecourse. Images are presented in radiological orientation (right in the figure is left in the brain). A) Bilateral postcentral gyrus, cerebellum, anterior cingulate/dorsomedial frontal cortex; B) bilateral supramarginal gyrus, posterior cingulate, right precentral, right caudate; C) anterior cingulate, bilateral angular/supramarginal gyrus; D) posterior cingulate, bilateral angular gyrus/superior occipital cortex, left superior frontal gyrus; E) bilateral anterior cingulate/precentral gyrus; and F) bilateral insula. Components shown in 4A and 4B showed decreasing intra-component connectivity with increasing age. The exact location of the BOLD signal changes for each component is located in Table 1.
3.3. Relationships between performance and connectivity
There were no components where there was a significant relationship between connectivity and memory performance in either the “read” condition, “generate” condition, or overall (collapsed across the read and generate conditions). This was the case with or without including age as a covariate.
3.4. Relationships between age and connectivity
Results of the multiple regression analysis examining age (including overall post-test performance, as a covariate) are included in Table 2. Two task-positive (“generate” > “read”) components showed decreasing intra-component connectivity with increasing age; the components shown in Fig. 3A (consisting of middle/inferior frontal and inferior parietal regions bilaterally; p < 0.05 corrected), and Fig. 3B (right fusiform gyrus; p < 0.05 corrected). Two task-negative (“read” > “generate”) components showed decreasing intra-component connectivity with increasing age; the components shown in Fig. 4A (cerebellum and pre/postcentral gyrus bilaterally; p < 0.005, corrected); Fig. 4B (posterior cingulate cortex, bilateral temporo-parietal regions; p < 0.05 corrected). The component in 4D also showed a decrease in intra-component connectivity with age, but this did not survive correction for multiple testing.
Table 2.
Regression results for effects of age (adjusting for performance) in all 13 task-correlated components.
| Linear model — effect of age (connectivity as a function of age, overall performance) |
|||
|---|---|---|---|
| Component | t | p | FDR-corrected p |
| Components positively correlated with the task (Fig. 3) | |||
| 3A | −2.49 | 0.014 | 0.047 |
| 3B | −3.12 | 0.002 | 0.015 |
| 3C | −1.79 | 0.076 | 0.165 |
| 3D | 1.15 | 0.255 | 0.331 |
| 3E | 0.70 | 0.487 | 0.576 |
| 3F | 1.45 | 0.149 | 0.215 |
| 3G | 1.59 | 0.114 | 0.204 |
| Components negatively correlated with the task (Fig. 4) | |||
| 4A | −2.61 | 0.010 | 0.044 |
| 4B | −3.92 | 0.000 | 0.002 |
| 4C | −1.54 | 0.126 | 0.204 |
| 4D | −2.01 | 0.047 | 0.122 |
| 4E | 0.26 | 0.799 | 0.799 |
| 4F | 0.40 | 0.690 | 0.748 |
Components marked in bold showed a significant relationship with age (p<0.05).
4. Discussion
Our results confirm the behavioral finding that memory for self-generated verbal information is more accurate than memory for verbal information that is read (Rabinowitz, 1989). While overall memory performance decreased with age, the benefit from self-generation remained consistent across age — more self-generated words were remembered than read words in young and older adults alike. Overall, the results of the present study reveal a set of networks engaged during self-generation of words, as well as networks more active during the baseline reading task (“task-negative”). Within many of these networks, we observed age-related decreases in connectivity. However, this reduced connectivity did not correspond directly with the decrease in memory performance for generated words that was observed in the behavioral results. Further, independent component analysis of neuroimaging data revealed an extensive set of components engaged in self-generation compared with reading aloud. This was consistent with previous results that were limited to younger adults (Vannest et al., 2012), where large regions of bilateral frontal and temporo-parietal–occipital cortex were more active for self-generation than reading (Rosner et al., 2013; Vannest et al., 2012). The functional connectivity analysis in the present study expands on these findings and allows us to examine networks or regions with common spatiotemporal patterns during the task (sub-regions of those found in the standard GLM analysis) that may represent functional components of the network. Importantly, we chose the independent component analysis approach because it allowed us to examine these data without any assumption about the temporal dynamics of the hemodynamic response function (Calhoun et al., 2009; McKeown et al., 1998).
Each task-positive (“generate” > “read”) component may support the multiple cognitive processes engaged during this task. The bilateral fronto-parietal component depicted in Fig. 3A has been shown to support working memory and executive functions that are crucial for the performance of this task (Dosenbach et al., 2007; Gordon et al., 2012; Grady et al., 2010; Sridharan et al., 2008) with the left-lateralization of this component consistent with the HERA model of memory encoding, in which left prefrontal cortical regions are responsible for retrieval of information from semantic memory and simultaneous encoding of novel aspects of the retrieved information (Tulving et al., 1994). The component shown in Fig. 3B, the right fusiform gyrus, is known to participate in visual imagery associated with the semantic processing involved in the generation task (D'Esposito et al., 1997; Lambert et al., 2002), also found in previous studies of successful verbal encoding (H. Kim, 2011). The network component depicted in Fig. 3C, the bilateral occipital cortex, likely supports processing of the visually presented stimuli (Nenert et al., 2014; Wandell et al., 2012). The right-lateralized inferior frontal/subcortical component shown in Fig. 3D may be involved in the process of selecting the appropriate response and inhibiting inappropriate ones (Hampshire et al., 2010). The component shown in Fig. 3E, primarily composed of the right anterior insula, anterior cingulate and precentral/dorsomedial frontal cortex, likely represents a component that is involved in attentional control (Eckert et al., 2009; Huang et al., 2012; Touroutoglou et al., 2012) and also consistent with other studies involving memory for paired associates (Krause et al., 1999; Mottaghy et al., 1999). The component shown in Fig. 3F, a bilateral but left-lateralized component in inferior frontal gyrus, has been shown to support the generation of verbal responses, as well as semantic processing (K.K. Kim et al., 2011; Karunanayaka et al., 2010; Vannest et al., 2012; Wende et al., 2012). Finally, the cerebellum, in Fig. 3G, is known to play a role in speech production (Ackermann and Riecker, 2004; Nagels et al., 2012; Szaflarski et al., 2013; Wende et al., 2012).
Several task- negative components were also found (Fig. 4). Specifically, these components were engaged more during the “read” condition than when words were self-generated. Most of these are recognized default mode components that are engaged during rest or during a less cognitively demanding task (Fox et al., 2005; Fox and Raichle, 2007; Geerligs et al., 2014; Grady et al., 2010; Kay et al., 2012; Mevel et al., 2013; Morgan et al., 2008; Mowinckel et al., 2012; Paret al.k, 2010). Interestingly, components that involve regions of the sensorimotor system, those depicted in Fig. 4A, cerebellum and pre/postcentral gyrus bilaterally (Damoiseaux et al., 2006; Golfinopoulos et al., 2010; Peeva et al., 2010) and 4F, anterior insula (Ackermann and Riecker, 2004), were also correlated negatively with the task. While these are not components typically observed as task-negative (Fox et al., 2005), we note that our “read” control task is an active baseline rather than a passive or resting state, and, therefore, it may engage additional networks that the “generate” task does not. We speculate that the engagement of the motor components 4A and 4F may be due to the participants' ability to better execute robust and consistent motor speech responses in the “read” condition where the to-be-produced word was given on the screen and no semantic processing was necessary.
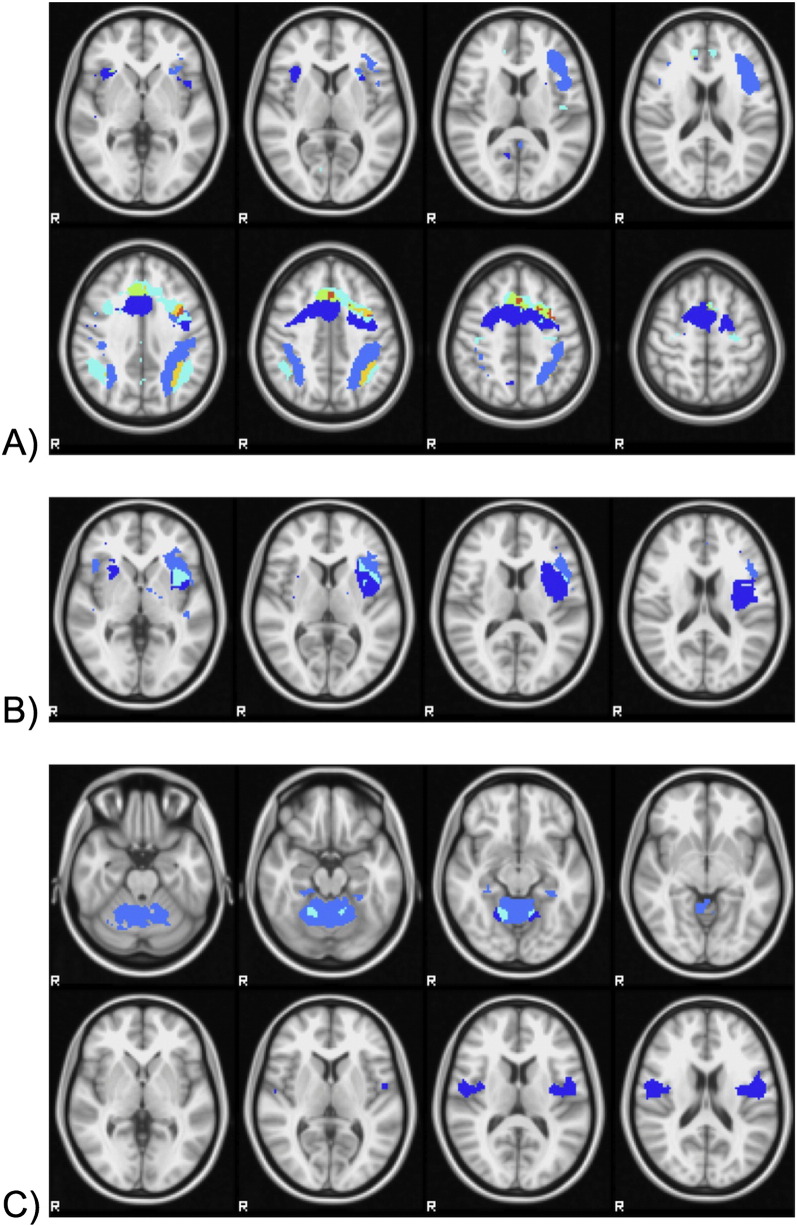
There were also a number of regions of overlap between task-positive (“generate” > “read”) and task-negative (“read” > “generate”) components. The bilateral fronto-parietal component shown in Fig. 3A and the component shown in Fig. 3E, composed of the right anterior insula, anterior cingulate and frontal cortex, overlapped with the “default mode” component in 4C, in part of the bilateral supramarginal gyrus and anterior cingulate /dorsomedial frontal cortex respectively. This overlap highlights the complexity of attentional/executive resources involved in both the “generate” and “read” conditions, both of which involve active responses. In line with this, there is also an overlap between “generate” > “read” and “read” > “generate” components in areas of the speech motor system. Specifically, the components shown in Figs. 3F and 4F overlap in the left insula (where 3F extends posteriorly from the inferior frontal gyrus) and the components shown Figs. 3G and 4A overlap in the dorsal cerebellum. See Fig. 5 for an illustration of this overlap.
Fig. 5.
Regions of overlap among task-positive (“generate” > “read”) and task-negative (“read” > “generate”) components. A) Components 3A, 3E, and 4C, with overlap in bilateral supramarginal gyrus and anterior cingulate /dorsomedial frontal cortex B) Components 3F and 4F with overlap in the left insula (where 3F extends posteriorly from the inferior frontal gyrus) C) Components 3G and 4A with overlap in the dorsal cerebellum.
In this study, several components showed an age-related decrease in intra-component connectivity even after accounting for post-test performance. Two task-positive (“generate” > “read”) components showed these decreases — the fronto-parietal working memory/executive network in Fig. 3A, and the right fusiform gyrus (Fig. 3B). This is consistent with several other studies that have found age-related decreases in connectivity in task-positive networks that involve posterior regions (Daselaar et al., 2006; Grady et al., 2003; Rieckmann et al., 2011). Because our connectivity measure reflects the degree of intercorrelation among the voxels in the component, decreases in this connectivity measure are likely to reflect dedifferentiation, i.e., the network becoming less specialized. We also found age-related decreases in connectivity in task-negative (“read” > “generate”) components, particularly posterior components of the default-mode network, involving posterior cingulate cortex and bilateral temporo-parietal regions (Fig. 4B). These results confirm a number of other studies showing decreased default-mode connectivity in older adults (Geerligs et al., 2014; Grady et al., 2010; Mevel et al., 2013; Mowinckel et al., 2012), in some cases associated with poorer cognitive performance, as we find in the present study (Geerligs et al., 2014; Mevel et al., 2013). The sensorimotor/cerebellar component (Fig. 4A), which was more active during the “read” baseline than the generation task, decreased in connectivity with age. Decreased cortico-cerebellar connectivity in older adults has been observed in other studies (Bernard et al., 2013).
In contrast to other studies (Dennis et al., 2007; Grady et al., 2003; Sambataro et al., 2012), we did not find compensatory increases in connectivity associated with age in the prefrontal regions. However, those task-positive (“generate” > “read”) components that primarily involved frontal regions (3D, 3E, 3F) did not decrease in connectivity with age, suggesting that these networks may be less vulnerable to age-related dedifferentiation.
5. Conclusions
Overall, we found that, as in other studies of verbal memory and aging, there were widespread age-related decreases in connectivity in many regions of the verbal encoding network engaged during self-generation of words, as well as changes in task-negative (“read” > “generate”) and default mode networks more active during the baseline reading task. These changes occurred independently of a small, but significant, decrease in memory performance for generated words with age. However, a self-generation strategy gave a “boost” to memory performance regardless of age. In contrast to other studies that have shown compensatory increases in the engagement of frontal regions (Dennis et al., 2007; Grady et al., 2003; Sambataro et al., 2012), no networks were observed that increased in connectivity with age (though connectivity in frontal task-positive networks remained stable with age). Thus, our results support the hypothesis that older adults engage a less connected network of brain regions, and this corresponds to a significant decrease in memory performance for generated words. However, this decrease is approximately 10%, suggesting that there is some continued support memory processes in this less connected network (Dennis et al., 2007; Nagels et al., 2012; Sambataro et al., 2012).
Acknowledgments
This study was presented in part at the Human Brain Mapping Conference in Seattle, WA in 2013 and in part at the American Academy of Neurology Annual Meeting in Philadelphia, PA in 2014. This study was supported, in part, by R01 NS048281 (NIH/NINDS).
References
- Ackermann H., Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89(2):320–328. doi: 10.1016/S0093-934X(03)00347-X. 15068914 [DOI] [PubMed] [Google Scholar]
- Allendorfer J.B., Lindsell C.J., Siegel M., Banks C.L., Vannest J., Holland S.K., Szaflarski J.P. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex. 2012;48:1218–1233. doi: 10.1016/j.cortex.2011.05.014. 21676387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L., Mäntylä T. Effectiveness of self-generated cues in younger and older adults: the role of retention interval. Int. J. Aging Hum. Dev. 1988;26(4):241–248. doi: 10.2190/TQWD-W1AQ-1NV2-P73G. 3170014 [DOI] [PubMed] [Google Scholar]
- Barrett A.M., Crucian G.P., Schwartz R.L., Heilman K.M. Testing memory for self-generated items in dementia: method makes a difference. Neurology. 2000;54(6):1258–1264. doi: 10.1212/wnl.54.6.1258. 10746595 [DOI] [PubMed] [Google Scholar]
- Basso M.R., Schefft B.K., Hoffmann R.G. Mood-moderating effects of affect intensity on cognition: sometimes euphoria is not beneficial and dysphoria is not detrimental. J. Pers. Soc. Psychol. 1994;66(2):363–368. doi: 10.1037//0022-3514.66.2.363. 8195991 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Statist. 2000;25(1):60–83. [Google Scholar]
- Bernard J.A., Peltier S.J., Wiggins J.L., Jaeggi S.M., Buschkuehl M., Fling B.W., Kwak Y., Jonides J., Monk C.S., Seidler R.D. Disrupted cortico-cerebellar connectivity in older adults. Neuroimage. 2013;83:103–119. doi: 10.1016/j.neuroimage.2013.06.042. 23792980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. 11559959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1 Suppl):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. 19059344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti N.D., Deluca J. Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch. Phys. Med. Rehabil. 2002;83(8):1070–1079. doi: 10.1053/apmr.2002.33729. 12161827 [DOI] [PubMed] [Google Scholar]
- Craik F.I. Levels of processing: past, present. and future? Memory. 2002;10(5-6):305–318. doi: 10.1080/09658210244000135. 12396643 [DOI] [PubMed] [Google Scholar]
- D’Esposito M., Detre J.A., Aguirre G.K., Stallcup M., Alsop D.C., Tippet L.J., Farah M.J. A functional MRI study of mental image generation. Neuropsychologia. 1997;35(5):725–730. doi: 10.1016/s0028-3932(96)00121-2. 9153035 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. 16945915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S.M., Fleck M.S., Dobbins I.G., Madden D.J., Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb. Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. 16421332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N.A., Daselaar S., Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol. Aging. 2007;28(11):1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. 16919850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. 17576922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M.A., Menon V., Walczak A., Ahlstrom J., Denslow S., Horwitz A., Dubno J.R. At the heart of the ventral attention system: the right anterior insula. Hum. Brain Mapp. 2009;30(8):2530–2541. doi: 10.1002/hbm.20688. 19072895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L.K., Busatto G.F. Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 2013;37(3):384–400. doi: 10.1016/j.neubiorev.2013.01.017. 23333262 [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. 19357304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. 15976020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L., Maurits N.M., Renken R.J., Lorist M.M. Reduced specificity of functional connectivity in the aging brain during task performance. Hum. Brain Mapp. 2014;35:319–330. doi: 10.1002/hbm.22175. 22915491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J.O. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2011;2(1):30–48. 21461180 [PMC free article] [PubMed] [Google Scholar]
- Golfinopoulos E., Tourville J.A., Guenther F.H. The integration of large-scale neural network modeling and functional brain imaging in speech motor control. Neuroimage. 2010;52(3):862–874. doi: 10.1016/j.neuroimage.2009.10.023. 19837177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Stollstorff M., Vaidya C.J. Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Hum. Brain Mapp. 2012;33(7):1536–1552. doi: 10.1002/hbm.21306. 21761505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C.L., McIntosh A.R., Craik F.I. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13(5):572–586. doi: 10.1002/hipo.10114. 12921348 [DOI] [PubMed] [Google Scholar]
- Grady C.L., Protzner A.B., Kovacevic N., Strother S.C., Afshin-Pour B., Wojtowicz M., Anderson J.A., Churchill N., McIntosh A.R. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb. Cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. 19789183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. 20056157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Belliveau J.W., Tengshe C., Ahveninen J. Brain networks of novelty-driven involuntary and cued voluntary auditory attention shifting. PLOS One. 2012;7(8):e44062. doi: 10.1371/journal.pone.0044062. 22937153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer F.H., Schefft B.K. Guiding the Process of Therapeutic Change. Research Press; Champaign, IL: 1988. [Google Scholar]
- Karunanayaka P., Schmithorst V.J., Vannest J., Szaflarski J.P., Plante E., Holland S.K. A group independent component analysis of covert verb generation in children: a functional magnetic resonance imaging study. Neuroimage. 2010;51(1):472–487. doi: 10.1016/j.neuroimage.2009.12.108. 20056150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B.P., Meng X., Difrancesco M.W., Holland S.K., Szaflarski J.P. Moderating effects of music on resting state networks. Brain Res. 2012;1447:53–64. doi: 10.1016/j.brainres.2012.01.064. 22365746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54(3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. 20869446 [DOI] [PubMed] [Google Scholar]
- Kim K.K., Karunanayaka P., Privitera M.D., Holland S.K., Szaflarski J.P. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011;20(4):613–622. doi: 10.1016/j.yebeh.2010.11.010. 21296027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B.J., Horwitz B., Taylor J.G., Schmidt D., Mottaghy F.M., Herzog H., Halsband U., Müller-Gärtner H. Network analysis in episodic encoding and retrieval of word-pair associates: a PET study. Eur. J. Neurosci. 1999;11(9):3293–3301. doi: 10.1046/j.1460-9568.1999.00723.x. 10510193 [DOI] [PubMed] [Google Scholar]
- Lambert S., Sampaio E., Scheiber C., Mauss Y. Neural substrates of animal mental imagery: calcarine sulcus and dorsal pathway involvement — an fMRI study. Brain Res. 2002;924(2):176–183. doi: 10.1016/s0006-8993(01)03232-2. 11750903 [DOI] [PubMed] [Google Scholar]
- Lespinet-Najib V., N’Kaoua B., Sauzéon H., Bresson C., Rougier A., Claverie B. Levels of processing with free and cued recall and unilateral temporal lobe epilepsy. Brain Lang. 2004;89(1):83–90. doi: 10.1016/S0093-934X(03)00303-1. 15010240 [DOI] [PubMed] [Google Scholar]
- Li Y.O., Adali T., Calhoun V.D. Estimating the number of independent components for functional magnetic resonance imaging data. Hum. Brain Mapp. 2007;28(11):1251–1266. doi: 10.1002/hbm.20359. 17274023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin O.Y., MacLeod C.M. Aging and the production effect: a test of the distinctiveness account. Can. J. Exp. Psychol. 2012;66(3):212–216. doi: 10.1037/a0028309. 22686153 [DOI] [PubMed] [Google Scholar]
- Lipinska B., Bäckman L., Mäntylä T., Viitanen M. Effectiveness of self-generated cues in early Alzheimer's disease. J. Clin. Exp. Neuropsychol. 1994;16(6):809–819. doi: 10.1080/01688639408402695. 7890817 [DOI] [PubMed] [Google Scholar]
- Mantyla T., Nilsson L. Distinctiveness and forgetting: effectiveness of self-generated retrieval cues in delayed recall. J. Exp. Psychol. Learn. Mem. Cogn. 1988;14(3):502–509. [Google Scholar]
- Marshall R.C., Neuburger S.I., Phillips D.S. Effects of facilitation and cueing on labelling of ‘novel’ stimuli by aphasic subjects. Aphasiology. 1992;6(6):567–583. [Google Scholar]
- Matthäus F., Schmidt J.P., Banerjee A., Schulze T.G., Demirakca T., Diener C. Effects of age on the structure of functional connectivity networks during episodic and working memory demand. Brain Connect. 2012;2(3):113–124. doi: 10.1089/brain.2012.0077. 22698449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel M.A., Waddill P.J., Einstein G.O. A contextual account of the generation effect: a three-factor theory. J. Mem. Lang. 1988;27(5):521–536. [Google Scholar]
- McKeown M.J., Makeig S., Brown G.G., Jung T.P., Kindermann S.S., Bell A.J., Sejnowski T.J. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. 9673671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel K., Landeau B., Fouquet M., La Joie R., Villain N., Mézenge F., Perrotin A., Eustache F., Desgranges B., Chételat G. Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiol. Aging. 2013;34(4):1292–1301. doi: 10.1016/j.neurobiolaging.2012.08.018. 23084083 [DOI] [PubMed] [Google Scholar]
- Morgan V.L., Gore J.C., Szaflarski J.P. Temporal clustering analysis: what does it tell us about the resting state of the brain? Med. Sci. Monit. 2008;14(7):CR345–CR352. 18591915 [PMC free article] [PubMed] [Google Scholar]
- Mottaghy F.M., Shah N.J., Krause B.J., Schmidt D., Halsband U., Jäncke L., Müller-Gärtner H.W. Neuronal correlates of encoding and retrieval in episodic memory during a paired-word association learning task: a functional magnetic resonance imaging study. Exp. Brain Res. 1999;128(3):332–342. doi: 10.1007/s002210050853. 10501805 [DOI] [PubMed] [Google Scholar]
- Mowinckel A.M., Espeseth T., Westlye L.T. Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. Neuroimage. 2012;63(3):1364–1373. doi: 10.1016/j.neuroimage.2012.08.004. 22992492 [DOI] [PubMed] [Google Scholar]
- Multhaup K.S., Balota D.A. Generation effects and source memory in healthy older adults and in adults with dementia of the Alzheimer type. Neuropsychol. 1997;11(3):382–391. doi: 10.1037//0894-4105.11.3.382. 9223142 [DOI] [PubMed] [Google Scholar]
- Nagels A., Kircher T., Dietsche B., Backes H., Marquetand J., Krug A. Neural processing of overt word generation in healthy individuals: the effect of age and word knowledge. Neuroimage. 2012;61(4):832–840. doi: 10.1016/j.neuroimage.2012.04.019. 22521476 [DOI] [PubMed] [Google Scholar]
- Nenert R., Allendorfer J.B., Szaflarski J.P. A model for visual memory encoding. PLOS One. 2014;9(10):e107761. doi: 10.1371/journal.pone.0107761. 25272154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L. Levels of processing: a view from functional brain imaging. Memory. 2002;10(5–6):345–348. doi: 10.1080/09658210244000171. 12396647 [DOI] [PubMed] [Google Scholar]
- Olofsson U., Nilsson L.G. The generation effect in primed word-fragment completion reexamined. Psychol. Res. 1992;54(2):103–109. doi: 10.1007/BF00937138. 1620793 [DOI] [PubMed] [Google Scholar]
- Otten L.J., Henson R.N., Rugg M.D. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124(2):399–412. doi: 10.1093/brain/124.2.399. 11157567 [DOI] [PubMed] [Google Scholar]
- Park D.C., Polk T.A., Hebrank A.C., Jenkins L.J. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front. Hum. Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. 20126437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva M.G., Guenther F.H., Tourville J.A., Nieto-Castanon A., Anton J.L., Nazarian B., Alario F.X. Distinct representations of phonemes, syllables, and supra-syllabic sequences in the speech production network. Neuroimage. 2010;50(2):626–638. doi: 10.1016/j.neuroimage.2009.12.065. 20035884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J.C. Judgments of origin and generation effects: comparisons between young and elderly adults. Psychol. Aging. 1989;4(3):259–268. doi: 10.1037//0882-7974.4.3.259. 2803618 [DOI] [PubMed] [Google Scholar]
- Rajah M.N., D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128(9):1964–1983. doi: 10.1093/brain/awh608. 16049041 [DOI] [PubMed] [Google Scholar]
- Rieckmann A., Karlsson S., Fischer H., Bäckman L. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J. Neurosci. 2011;31(40):14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011. 21976513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner Z.A., Elman J.A., Shimamura A.P. The generation effect: activating broad neural circuits during memory encoding. Cortex. 2013;49:1901–1909. doi: 10.1016/j.cortex.2012.09.009. 23079490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Safrin M., Lemaitre H.S., Steele S.U., Das S.B., Callicott J.H., Weinberger D.R., Mattay V.S. Normal aging modulates prefrontoparietal networks underlying multiple memory processes. Eur. J. Neurosci. 2012;36(11):3559–3567. doi: 10.1111/j.1460-9568.2012.08254.x. 22909094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefft B.K., Biederman J.J. Emotional effects of self- generated behavior and the influence of resourcefulness and depressed mood. J. Soc. Clin. Psychol. 1990;9(3):354–366. [Google Scholar]
- Schefft B.K., Dulay M.F., Fargo J.D. The use of a self-generation memory encoding strategy to improve verbal memory and learning in patients with traumatic brain injury. Appl. Neuropsychol. 2008;15(1):61–68. doi: 10.1080/09084280801917806. 18443942 [DOI] [PubMed] [Google Scholar]
- Schefft B.K., Dulay M.F., Fargo J.D., Szaflarski J.P., Yeh H.S., Privitera M.D. The use of self-generation procedures facilitates verbal memory in individuals with seizure disorders. Epilepsy Behav. 2008;13(1):162–168. doi: 10.1016/j.yebeh.2008.01.012. 18343201 [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Dardzinski B.J., Holland S.K. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. I.E.E.E. Trans. Med. Imaging. 2001;20(6):535–539. doi: 10.1109/42.929619. 11437113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K. Comparison of three methods for generating group statistical inferences from independent component analysis of functional magnetic resonance imaging data. J. Magn. Reson. Imaging. 2004;19(3):365–368. doi: 10.1002/jmri.20009. 14994306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magn. Reson. Med. 2004;51(2):399–402. doi: 10.1002/mrm.10706. 14755667 [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Dardzinski B.J. CCHIPS: Cincinnati Children's Hospital Imaging Processing Software. 2000. https://irc.cchmc.org/software/cchips.php. [Google Scholar]
- Siegel M., Allendorfer J.B., Lindsell C.J., Vannest J., Szaflarski J.P. The effects of linguistic relationships among paired associates on verbal self-generation and recognition memory. Brain Behav. 2012;2(6):789–795. doi: 10.1002/brb3.98. 23170241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamecka N.J., Graf P. The generation effect: delineation of a phenomenon. Journal of Experimental Psychology: Human Learning & Memory. 1978;4(6):592–604. [Google Scholar]
- Smith M.L. Recall of frequency of occurrence of self-generated and examiner-provided words after frontal or temporal lobectomy. Neuropsychologia. 1996;34(6):553–563. doi: 10.1016/0028-3932(95)00139-5. 8736568 [DOI] [PubMed] [Google Scholar]
- Souliez L., Pasquier F., Lebert F., Leconte P., Petit H. Generation effect in short-term verbal and visuospatial memory: comparisons between dementia of Alzheimer type and dementia of frontal lobe type. Cortex. 1996;32(2):347–356. doi: 10.1016/s0010-9452(96)80056-6. 8800620 [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. 18723676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M., Abdi H., Burianová H., Grady C.L. Influence of aging on the neural correlates of autobiographical, episodic, and semantic memory retrieval. J. Cogn. Neurosci. 2011;23(12):4150–4163. doi: 10.1162/jocn_a_00079. 21671743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Allendorfer J.B., Banks C., Vannest J., Holland S.K. Recovered vs. not-recovered from post-stroke aphasia: the contributions from the dominant and non-dominant hemispheres. Restor. Neurol. Neurosci. 2013;31:347–360. doi: 10.3233/RNN-120267. 23482065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Jacola L.M., Lindsell C., Privitera M.D., Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12(1):74–83. doi: 10.1016/j.yebeh.2007.07.015. 17964221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Schmithorst V.J., Dunn R.S., Privitera M.D. High-resolution functional MRI at 3 T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004;5(2):244–252. doi: 10.1016/j.yebeh.2004.01.002. 15123027 [DOI] [PubMed] [Google Scholar]
- Thévenaz P., Ruttimann U.E., Unser M. A pyramid approach to subpixel registration based on intensity. I. E.E.E. Trans. Image Process. 1998;7(1):27–41. doi: 10.1109/83.650848. 18267377 [DOI] [PubMed] [Google Scholar]
- Touroutoglou A., Hollenbeck M., Dickerson B.C., Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60(4):1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. 22361166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Craik F.I., Moscovitch M., Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc. Natl. Acad. Sci. U S A. 1994;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. 8134342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J., Eaton K.P., Henkel D., Siegel M., Tsevat R.K., Allendorfer J.B., Schefft B.K., Banks C., Szaflarski J.P. Cortical correlates of self-generation in verbal paired associate learning. Brain Res. 2012;1437:104–114. doi: 10.1016/j.brainres.2011.12.020. 22227457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J.J., Karunanayaka P.R., Altaye M., Schmithorst V.J., Plante E.M., Eaton K.J., Rasmussen J.M., Holland S.K. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J. Magn. Reson. Imaging. 2009;29(4):971–976. doi: 10.1002/jmri.21694. 19306445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S., Willis-Shore J., Poole J.H., Marten E., Ober B.A., Shenaut G.K. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. Am. J. Psychiatry. 1997;154(11):1530–1537. doi: 10.1176/ajp.154.11.1530. 9356560 [DOI] [PubMed] [Google Scholar]
- Wandell B.A., Rauschecker A.M., Yeatman J.D. Learning to see words. Annu. Rev. Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. 21801018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende K.C., Straube B., Stratmann M., Sommer J., Kircher T., Nagels A. Neural correlates of continuous causal word generation. Neuroimage. 2012;62(3):1399–1407. doi: 10.1016/j.neuroimage.2012.06.003. 22691614 [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Friston K.J. Analysis of fMRI time-series revisited — again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. 9343600 [DOI] [PubMed] [Google Scholar]