Abstract
In the current study, we have evaluated the performance of magnetic resonance (MR) T1rho (T1ρ) imaging and CSF biomarkers (T-tau, P-tau and Aβ-42) in characterization of Alzheimer's disease (AD) patients from mild cognitive impairment (MCI) and control subjects. With informed consent, AD (n = 27), MCI (n = 17) and control (n = 17) subjects underwent a standardized clinical assessment and brain MRI on a 1.5-T clinical-scanner. T1ρ images were obtained at four different spin-lock pulse duration (10, 20, 30 and 40 ms). T1ρ maps were generated by pixel-wise fitting of signal intensity as a function of the spin-lock pulse duration. T1ρ values from gray matter (GM) and white matter (WM) of medial temporal lobe were calculated. The binary logistic regression using T1ρ and CSF biomarkers as variables was performed to classify each group. T1ρ was able to predict 77.3% controls and 40.0% MCI while CSF biomarkers predicted 81.8% controls and 46.7% MCI. T1ρ and CSF biomarkers in combination predicted 86.4% controls and 66.7% MCI. When comparing controls with AD, T1ρ predicted 68.2% controls and 73.9% AD, while CSF biomarkers predicted 77.3% controls and 78.3% for AD. Combination of T1ρ and CSF biomarkers improved the prediction rate to 81.8% for controls and 82.6% for AD. Similarly, on comparing MCI with AD, T1ρ predicted 35.3% MCI and 81.9% AD, whereas CSF biomarkers predicted 53.3% MCI and 83.0% AD. Collectively CSF biomarkers and T1ρ were able to predict 59.3% MCI and 84.6% AD. On receiver operating characteristic analysis T1ρ showed higher sensitivity while CSF biomarkers showed greater specificity in delineating MCI and AD from controls. No significant correlation between T1ρ and CSF biomarkers, between T1ρ and age, and between CSF biomarkers and age was observed. The combined use of T1ρ and CSF biomarkers have promise to improve the early and specific diagnosis of AD. Furthermore, disease progression form MCI to AD might be easily tracked using these two parameters in combination.
Keywords: Alzheimer's disease, Mild cognitive impairment, Medial temporal lobe, T1rho, CSF biomarkers
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography; T-tau, total tau; Aβ1-42, amyloid beta 42; T1ρ, T1rho; MTL, medial temporal lobe; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; TR, repetition time; TE, echo time; TSL, total spin lock; FOV, field of view; MPRAGE, magnetization prepared rapid acquisition gradient-echo; TI, inversion time; GM, gray matter; WM, white matter; MTL, medial temporal lobe; ROC, receiver operating characteristic.
Highlights
-
•
Increased T1rho was observed in MCI and AD compared to controls.
-
•
Increased T-tau and P-tau and decreased Aβ1-42 were observed in MCI and AD.
-
•
Combined biomarkers have promise to improve early and specific diagnosis of AD.
-
•
MCI to AD progression might be tracked using these two biomarkers in combination.
1. Introduction
Alzheimer's is a progressive disease, where dementia symptoms gradually worsen over a number of years, and it accounts for 50–80% of dementia cases. Alzheimer's disease (AD) is estimated to affect ~5.2 million Americans — a number expected to swell to as many as 16 million by 2050. Efforts are in progress to find better ways to treat the disease, delay its onset, and prevent it from developing. Many tools are used to look for signs of AD, including a battery of cognitive and behavioral tests (Koppel et al., 2012; Cummings, 2000; Harwood et al., 2000), cerebrospinal fluid (CSF) analysis (Hansson et al., 2006; Mattsson et al., 2009), magnetic resonance imaging (MRI) (Fayed et al., 2012; Frisoni et al., 2010; Chincarini et al., 2011) and positron emission tomography (PET) scans (Pearson and Colby, 2013; Zhang et al., 2012; Kadir et al., 2012). Imaging techniques (MRI and PET) and CSF studies have been pointed as candidates for the diagnostic biomarkers of AD.
It has been found that the CSF measures of total tau (T-tau) and amyloid beta (Aβ1-42) levels are individually sensitive though but with lower specificity for AD against other dementia disorders (Blennow, 2004). Moreover, the CSF Aβ1-42 levels are not easily interpreted because CSF Aβ1-42 is not exclusively brain derived and also its production and clearance are not well characterized. A number of studies have suggested that CSF markers in combination with neuroimaging and neuropsychological tools add to the accuracy of AD diagnosis (Richard et al., 2013; Schuff et al., 2009; Vemuri et al., 2010).
Out of various medical imaging techniques, MRI is the most widely accepted technique to detect the pathological changes in-vivo based on the tissue T2 and T1 contrast relaxation properties. However, to date none of the MRI methods has proven to be an accurate in-vivo marker for early diagnosis of AD. Recently, a new MRI technique has been introduced i.e T1rho (T1ρ), the spin lattice relaxation time constant in the rotating frame, which determines the decay of transfer magnetization in presence of “spin-lock” radio-frequency field (Borthakur et al., 2004, 2006b; Wheaton et al., 2005). In biological tissues, T1ρ may have contribution from several molecular interactions. It is also possible that more than one interaction may contribute to the T1ρ signal at a time; however, their relative contributions may differ. Such interactions include chemical exchange, dipolar interaction, and J-coupling. T1ρ MRI has capability to probe the protein contents in various tissues such as brain, blood and cartilage.
T1ρ MRI has been previously used to measure the T1ρ relaxation time in the normal human brain, and showed higher range of values compared to the T2 relaxation time (Borthakur et al., 2004). Earlier, T1ρ has been used to delineate brain tumors (Aronen et al., 1999), characterize breast cancer tissue (Li et al., 2011), and monitor the level of cartilage degeneration (Regatte et al., 2004; Witschey et al., 2010). Borthakur et al. have shown the feasibility of T1ρ imaging in evaluating the plaques burden in a mouse model of AD (Borthakur et al., 2006a). Previous studies have shown higher T1ρ value in the medial temporal lobe (MTL) of AD compared to those of mild cognitive impairment (MCI) and control (Haris et al., 2011; Borthakur et al., 2008).
In the current study, we aim to evaluate the performance of T1ρ and CSF biomarkers in characterization of AD patients from MCI and control subjects. In addition, we also assess any correlation between T1ρ and CSF biomarkers.
2. Materials and methods
2.1. Participants
Institutional Review Board of the University of Pennsylvania approved the current study protocol. In this study, we have included 27 AD patients (mean age ± SD = 76.8 ± 9.1 years), 17 MCI patients (mean age ± SD = 71.93 ± 8.7 years), and 17 age-matched control subjects (mean age ± SD = 70.2 ± 9.4 years). A standardized clinical assessment including medical history, physical and neurological examination, psychometric evaluation, and brain MRI was performed in all patients. The general cognitive function in each patient was assessed using Mini-Mental State Examination (MMSE) score. Diagnoses was made by a team consisting of neurologist, neuropsychologist, and psychiatrist who performed extensive behavioral, neuropsychological, and neuroimaging assessments. Diagnoses of MCI was made according to the Petersen criteria for MCI (Petersen et al., 2001), while the AD patients were diagnosed according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association criteria (NINCDS–ADRDA) for probable AD (McKhann et al., 1984). Patients with history of irritable bowel syndrome, chronic diarrhea, peptic ulcer, or gastroesophageal reflux disease; cardiac disease; significant electrocardiographic abnormalities; hematologic disorders; hepatic or renal disease; active malignancy within 5 years; or clinically important depressive, neuropsychiatric, cerebrovascular, or respiratory disease were excluded from this study. The control group consisted of patients, who presented to our memory clinic with subjective complaints, and underwent exactly the same diagnostic work-up as the MCI and AD patients.
2.2. Collection of CSF
CSF samples were obtained from all subjects by lumbar puncture following an overnight fast. Spinal fluid was withdrawn by experienced physician through an atraumatic 25-gauge sprotte needle and immediately transferred to a bar code-labeled polypropylene vial and placed in −80 °C freezer.
2.3. Biomarker analysis using multiplex xMAP (Luminex) technology
The 42 β-amyloid (Aβ1-42), T-tau and P-tau181p levels were measured in sample aliquots using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics immunoassay kit-based reagents (INNO-BIA AlzBio3, Ghent, Belgium). Full details of this combination immunoassay have been previously published (Vanderstichele, 2012; Reijn et al., 2007). Briefly, the Innogenetics kit reagents included well characterized capture monoclonal antibodies specific for Aβ1-42 (4D7A3), T-tau (AT120) and P-tau181p (AT270), each chemically bonded to unique sets of color-coded beads, and analyte specific detector antibodies (HT7, 3D6). Calibration curves were produced for each biomarker using aqueous buffered solutions that contained the combination of 3 biomarkers at concentrations ranging from 56 to 1948 pg/mL for recombinant tau, 27–1574 pg/mL for synthetic Aβ1-42 peptide and 8–230 pg/mL for a tau synthetic peptide phosphorylated at the threonine 181 position (i.e. the p-tau181p standard).
2.4. MRI protocol
Written informed consent was obtained from each patient before they underwent for MRI. Brain imaging was performed on a 1.5-Tesla Siemens Sonata clinical-scanner (Siemens Medical Systems, Malvern, PA, USA) using a vendor-supplied head coil. T1ρ images were acquired using a fluid-attenuated T1ρ prepared Turbo Spin-Echo pulse sequence (Borthakur et al., 2004; 2008). The imaging parameters were: TR/TE = 2000 ms/12 ms, TSL (duration of spin lock pulse) = 10, 20, 30, 40 ms, spin lock pulse amplitude of 500 Hz, slice thickness = 2 mm, FOV = 24 × 24 cm2, matrix size = 256 × 128, bandwidth = 130 Hz/pixel, and echo train length = 4 for a total imaging time of 6 min for four T1ρ weighted images. To remove the contribution from CSF to T1ρ weighted MR signal, an inversion time (TI) of 860 ms was used. An oblique coronal T1ρ weighted image of a slice perpendicular to the anterior/posterior commissure (AC/PC) plane was obtained. The slice was chosen to include the head of hippocampus. Immediately after T1ρ MRI, the entire volume of each subject's brain was imaged in the coronal plane using a T1-weighted 3D volumetric magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence with 160 continuous slices. The parameters were TR/TE = 3000 ms/3.5 ms, slice thickness = 1.2 mm, FOV of 240 × 240 cm2 and 192 phase encode steps, and flip angle = 8° for a total imaging time of 10 min.
2.5. Data processing
Images were transformed to a G4 PowerBook computer (Apple Corp., Cupertino, CA) and processed with programs written in the IDL programming language (RSI Corp., Boulder, CO). T1ρ weighted data corresponding to different TSLs were fitted pixel-wise to a mono-exponential decay expression S(TSL) = S(0) × exp (−TSL / T1ρ) (Borthakur et al., 2004; Borthakur et al., 2008). Pixels whose intensities correlated poorly (R2 < 0.95) with the fitting equation were set to zero. Pixels outside of the brain were also set to zero. T1ρ values were automatically calculated from gray matter (GM) and white matter (WM) of the left and right MTL. For brain segmentation, a previously developed method was used to partition the volumetric MPRAGE scans into 92 regions of interest (ROIs) incorporating all major cortical and sub-cortical regions (Davatzikos et al., 2008). For quantitative analysis, 4 regions of interest (ROIs) were defined on T1ρ images i.e. left and right temporal lobes WM and GM. A program written in IDL was used to automatically calculate T1ρ values only from pixels that were classified as GM and WM located either in the left or right MTL.
2.6. Statistical analyses
For statistical analysis T1ρ values from left and right MTL were averaged (separately for GM and WM). Descriptive statistics was performed to calculate the mean value of T1ρ and CSF biomarkers for different cohorts (controls, MCI and AD patients). Mann Whitney U-test was performed to see the difference for T1ρ and CSF biomarkers between controls, MCI and AD patients. Logistic regression with enter method was performed to measure the prediction rate of T1ρ and CSF biomarkers in classification of AD, MCI and controls. Receiver operating characteristic (ROC) analysis was performed to measure the sensitivity and specificity. We used a default cutoff 0.5 to predict the event. Additionally, discriminant analysis using T1ρ and CSF biomarkers was performed when three groups taken together. Pearson correlations between T1ρ versus CSF biomarkers, T1ρ versus age and between T1ρ versus MMSE scores were also performed. p value equal or less than 0.05 was considered statistically significant. All the statistical computations were performed using the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, USA).
3. Results
The mean MMSE scores in control (29.533 ± 1.19), MCI (25.11 ± 2.68) and AD (19.16 ± 5.57) were significantly (p = 0.001, p < 0.001, p = 0.002) different among groups. Mean T1ρ and CSF biomarkers values in three groups (control, MCI and AD) are reported in Table 1.
Table 1.
Quantitative values of T1ρ relaxation times and CSF biomarkers from control, MCI and AD subjects.
| Group | T1ρ (ms) (mean ± SE) |
CSF biomarkers (pg/mL) (mean ± SE) |
|||
|---|---|---|---|---|---|
| Gray matter | White matter | Luminex T-Tau | Luminex P-Tau | Luminex Aβ1-42 | |
| Control | 86.8 ± 1.8 | 80.3 ± 2.1 | 56.9 ± 7.0 | 26.1 ± 3.6 | 230.4 ± 11.1 |
| MCI | 91.9 ± 1.2 | 85.2 ± 1.4 | 74.0 ± 12.2 | 30.6 ± 5.1 | 188.3 ± 16.0 |
| AD | 92.3 ± 1.0 | 88.7 ± 1.9 | 103.3 ± 10.8 | 39.0 ± 4.9 | 144.9 ± 10.4 |
ms = millisecond; SE = standard error; CSF = cerebrospinal fluid; pg = picogram; ml = milliliter; MCI = mild cognitive impairment; AD = Alzheimer's disease.
Fig. 1 shows the overlaid T1ρ maps from MTL region (in color) on fluid-attenuated brain T1ρ weighted images of control, MCI, and AD patient. Pixels with higher T1ρ (red) are more prominent in MTL of AD patient. Increased sulcal space in AD patient suggests greater degree of brain atrophy. A lack of signal from CSF implies that the higher T1ρ values in AD patients are not due to free fluid. The error bars show mean WM T1ρ and GM T1ρ values in controls, MCI and AD patients (Fig. 2).
Fig. 1.
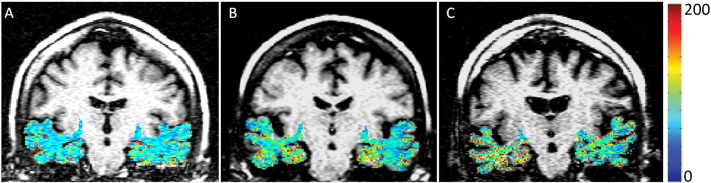
T1rho (T1ρ) contrast from medial temporal lobe overlaid on anatomic fluid attenuated T1ρ weighted images of control (A), medial cognitive impairment (MCI, B), and Alzheimer's disease (AD, C) patients. A progressive increase in T1ρ contrast in medial temporal lobe (MTL) region was observed from control to MCI to AD. No T1ρ contrast from CSF was depicted.
Fig. 2.
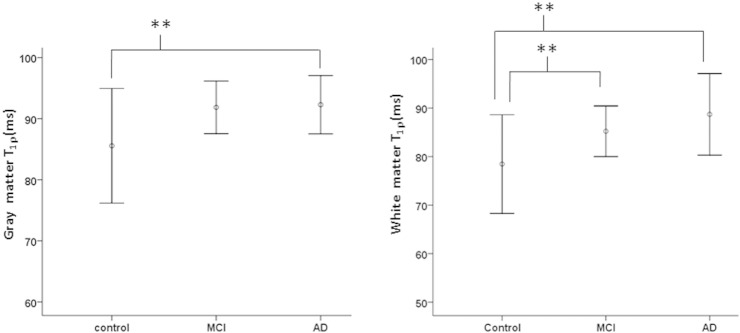
The error bars show the mean GM and WM T1ρ values from medial temporal lobe (MTL) in control, MCI and AD. (**) indicates the significance difference (p < 0.05) for the mean T1ρ values between two groups.
On comparative analysis, MCI subjects showed higher T1ρ, T-tau, P-tau and lower Aβ1-42 compared to control subjects. However, only increase in WM T1ρ (p = 0.05) reached to the statistical significant level (Table 1, Fig. 2). AD patients showed significantly increased GM T1ρ (p = 0.041), WM T1ρ (p = 0.005), T-tau (p = 0.001), P-tau (p = 0.025) and significantly decreased Aβ1-42 (p < 0.001) compared to control subjects (Table 1, Fig. 2). In AD patients, only T-tau concentration was significantly (p = 0.05) increased over MCI subjects.
Binary logistic regression showed that T1ρ (GM and WM) was able to predict 77.3% controls and 40.0% MCI subjects, whereas CSF biomarkers (T-tau, P-tau and Aβ1-42) predicted 81.8% controls and 46.7% MCI subjects accurately. Combination of T1ρ and CSF biomarkers were able to predict 86.4% controls and 66.7% MCI subjects.
T1ρ predicted 68.2% controls and 73.9% AD patients correctly while CSF biomarkers predicted 77.3% controls and 78.3% AD patients. When T1ρ combined with CSF biomarkers the prediction rate was 81.8% for controls and 82.6% for AD patients.
T1ρ predicted 35.3% MCI subjects and 81.9% AD patients, while the prediction rate was 53.3% for MCI subjects and 83.0% for AD patients using the CSF biomarkers. Combined CSF biomarkers and T1ρ were able to predict 57.3% MCI subjects and 84.6% AD patients.
When combined three groups together, T1ρ and CSF biomarkers were able to classify 54.5% controls, 40% MCI subjects and 65.2% AD patients. These two biomarkers misclassified 31.8% controls as MCI subjects and 13.6% controls as AD patients, while 33.3% MCI subjects were falsely predicted as controls and 26.7% MCI subjects as AD patients. There were false prediction of 8.7% AD patients as controls and 26.1% AD patients as MCI subjects.
On ROC analysis, T1ρ showed greater sensitivity (60%) and less specificity (77%) than CSF biomarkers (53% and 82%) in discriminating MCI from control (Table 2). When delineating AD from control T1ρ showed 82% sensitivity and 71% specificity while CSF biomarkers showed 77% sensitivity and 79% specificity (Table 2).
Table 2.
Receiver operating characteristic (ROC) analysis among control, MCI and AD form T1rho and CSF biomarkers.
| Group | Biomarker | AUC | SE | Sensitivity % | Specificity % | 95% CI |
p value | |
|---|---|---|---|---|---|---|---|---|
| LB | UP | |||||||
| Control vs MCI | T1rho | 0.65 | 0.09 | 0.60 | 0.77 | 0.49 | 0.82 | 0.09 |
| CSF biomarker | 0.67 | 0.09 | 0.53 | 0.82 | 0.50 | 0.85 | 0.05 | |
| Control vs AD | T1rho | 0.80 | 0.07 | 0.82 | 0.71 | 0.65 | 0.94 | 0.001 |
| CSF biomarker | 0.83 | 0.06 | 0.77 | 0.79 | 0.72 | 0.95 | 0.001 | |
ROC = receiver operating characteristic; MCI = mild cognitive impairment; AD = Alzheimer's disease; CSF = cerebrospinal fluid; AUC = area under curve; SE = standard error; CI = confidence interval; LB = lower bond; UP = upper bond.
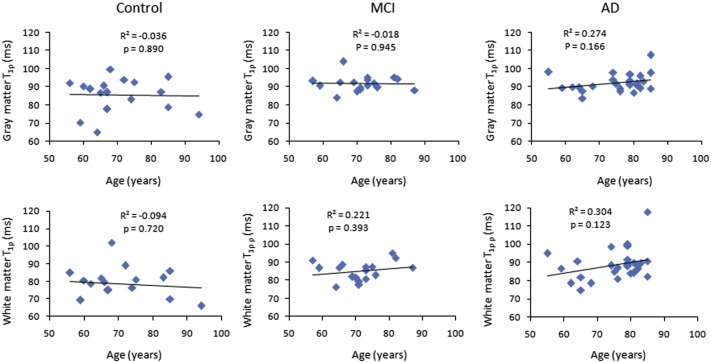
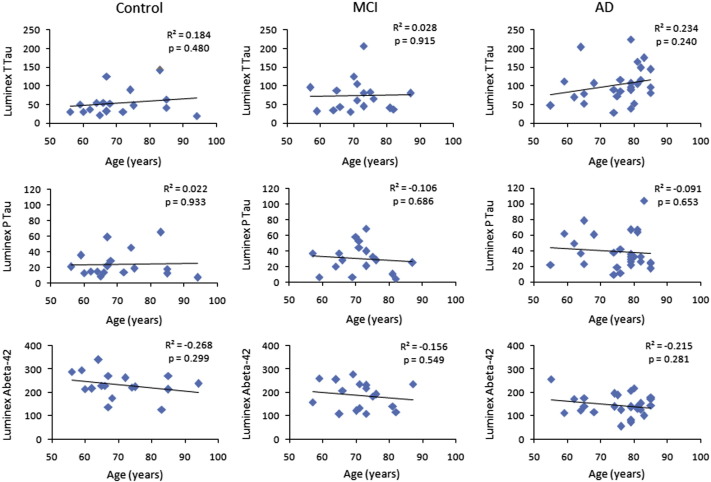
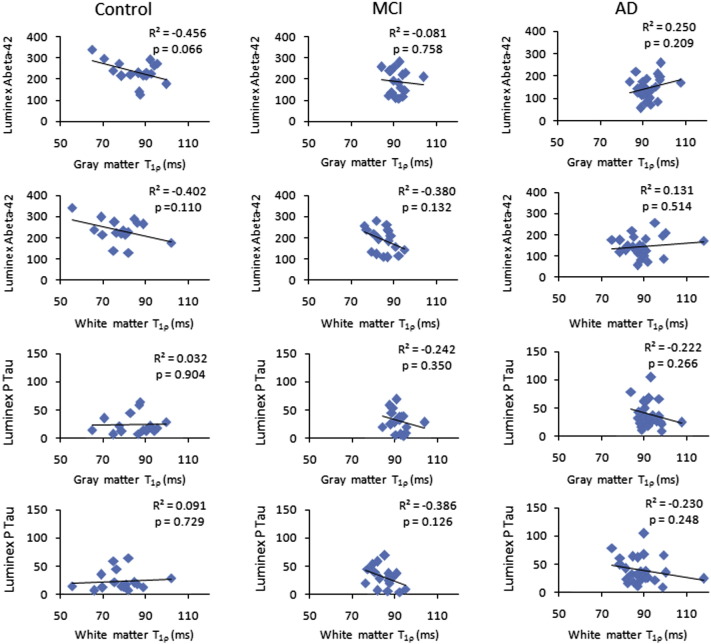
In all three cohorts, scattered maps between age and T1ρ showed no significant change in T1ρ values with age (Fig. 3). However, WM T1ρ in MCI subjects and both GM T1ρ and WM T1ρ in AD patients showed an increasing trend with age (Fig. 3). No significant correlation of CSF biomarkers with age and T1ρ was observed (Figs. 4–5). A negative correlation trend between Aβ1-42 and T1ρ was observed in controls and MCI subjects, while in AD patients this correlation showed a positive trend. On the other hand, correlation between P-tau and T1ρ showed a negative trend both in MCI subjects and AD patients.
Fig. 3.
The scattered maps between age and T1ρ values for controls, MCI subjects and AD patients show no significant correlations.
Fig. 4.
The scattered maps between age and CSF biomarkers show no significant change in the CSF biomarkers with age.
Fig. 5.
The scattered maps between T1ρ values and CSF biomarkers depict no significant correlation in any group.
4. Discussion
In the current study, significantly higher WM T1ρ and GM T1ρ in MTL in brain of AD was observed compared to that of controls, which is in agreement with the previous studies (Haris et al., 2011; Borthakur et al., 2008). MCI subjects showed significantly higher WM T1ρ compared to controls, which suggests that abnormality occur earlier in WM than GM. Significantly higher GM and WM T1ρ in AD compared to controls imply that in AD abnormality persist both in GM and WM. Higher changes in WM T1ρ compared to GM T1ρ suggest greater abnormality in WM than GM.
Earlier, Borthakur et al., have shown decreased T1ρ with increased plaques burden in mouse model of AD (Borthakur et al., 2006a). Over the last two decades, a number of transgenic AD mouse models have been created which differ in their biochemical profiles and disease progression rates (Elder et al., 2010; Chin, 2011). To date it is debatable which model closely relates with the human AD pathology (Elder et al., 2010; Chin, 2011). It is possible that the mouse model used earlier by Borthakur et al., may not clearly depict the human AD pathology. Further, an explanation for higher T1ρ in the human AD patients as observed in the current study and other previous studies (Haris et al., 2011; Borthakur et al., 2008) is only due to the plaques burden is not sufficient. Moreover, in AD, hyperphosphorylation of tau protein leads to the accumulation of neurofibrillary tangles, which results in loss of neurons. Till date no study has been performed to evaluate any relation between phosphorylated tau protein and T1ρ in AD brain. A postmortem study may help to assess correlation between plaques burden, biochemical changes and T1ρ relaxation time in the human brain, and may provide a more precise explanation for increased T1ρ values in the human AD patients.
Majority of studies have reported decreased Aβ1-42, and increased T-tau and P-tau levels in CSF of MCI and AD patients (Hansson et al., 2006; Mattsson et al., 2009; Andreasen et al., 2001, 2003) and our findings are in conformity with those studies. It is widely believed that increased CSF T-tau level reflects neuronal and axonal damage. However, clinical studies have shown that the elevated CSF T-tau level is not specific to AD as it may also be elevated in other neurodegenerative diseases (Arai et al., 1997; Urakami et al., 2001). In a recent study, Hampel's group reported that when compared with T-tau, P-tau showed better specificity for AD (Hampel, and Blennow, 2004). It has also been shown that the P-tau's level consistently elevated in AD when compared with frontotemporal dementia (FTD), lewy body dementia (LBD), and control (Parnetti et al., 2001). An adequate explanation for decreased concentration of Aβ1-42 in CSF of AD patients is still lacking. The suggested explanation that it is due to accumulation of Aβ1-42 in plaques is not sufficient, as decreased Aβ1-42 concentration in CSF of patients with Creutzfeldt–Jakob disease without apparent plaque formation has been reported previously (Wiltfang et al., 2003; Otto et al., 2000). Moreover, considerable uncertainty exists with respect to the influence of ageing on CSF biomarkers levels (de Leon et al., 2004; Hulstaert et al., 1999). Normal ageing studies have depicted both positive and negative age effects (Andreasen et al., 2001; de Leon et al., 2004; Hulstaert et al., 1999). In the current study, no age related changes in CSF biomarkers were observed.
Medial temporal lobe (MTA) atrophy as observed on MRI is considered to be an early and sensitive marker for AD, and is assumed that it reflects underlying neuronal loss in hippocampus and temporal lobe (Clerx et al., 2013; Jack et al., 1997; Duara et al., 2008). However, MTA may also be present in other types of dementia (Tam et al., 2005; Barber et al., 1999) and absence of MTA does not exclude the diagnosis of AD especially in the early stage. Studies have been performed to investigate the cross-sectional relation between CSF biomarkers and atrophy on MRI. Some observed no relation between CSF tau or Aβ1-42 and whole-brain atrophy (Sluimer et al., 2010), while others found a significant inverse relationship between CSF P-tau and hippocampal volume in MCI (Herukka et al., 2008). It has been shown that the increased CSF tau level corresponds to the higher baseline hippocampal volume in AD. The same study has also reported, no significant effect of T-tau level on hippocampal atrophy (Hampel et al., 2005).
In the current study, none of CSF biomarkers were significantly change in MCI compared to controls, while WM T1ρ in MCI was significantly higher than controls. Even if T1ρ was significantly higher in MCI it did not appear as a good predictor of MCI. T1ρ in combination with CSF biomarkers improved the prediction rate for MCI (66.7%). Similarly, combined T1ρ and CSF biomarkers showed better prediction rate for control (81.2%) and AD (82.6%) compared to either T1ρ or CSF biomarkers alone. Combined T1ρ and CSF biomarkers were able to discriminate 57.3% MCI and 84.6% AD. A longitudinal study is needed to elucidate the combined clinical utility of these two biomarkers in progression from MCI to AD.
In the current study, no correlation between T1ρ and CSF biomarkers suggests that these two biomarkers are independent to each other and have differential diagnostic efficacy. Longitudinal studies are needed to understand the relationship between T1ρ and CSF biomarkers. No correlation between T1ρ and age was observed which suggests that the increased T1ρ is due to changes in underlying pathology instead of any age related changes.
No correlation between T1ρ and MMSE suggests that the functional changes as measured by T1ρ probably do not exclusively reflect the cognitive impairment. Moreover, the cognitive analysis depends on the patient's behavior which could fluctuate with time. A test retest study can be performed to evaluate covariance in the measurement of the MMSE score. On the other hand CSF biomarker analysis reflects the biochemical changes in the CSF not in the actual pathological tissues. The direct measurement of the biochemical changes in the brain tissues associated with the cognitive performance might be better correlated with the MMSE score.
Recently, Watts et al., have evaluated the age dependence changes in GM and WM T1ρ relaxation times in brain of normal human volunteers on a 3-T MRI scanner (Watts et al., 2014). They have observed a positive correlation between WM T1ρ and age while the GM T1ρ showed negative correlation with age. In the current study, both WM T1ρ and GM T1ρ showed no significant correlation with age. This may be due to the fact that in previous study normal subjects with larger range of age have been studied compared to the current study. However, in this study, both WM T1ρ and GM T1ρ in AD and WM T1ρ in MCI showed an increasing trend with age. This may be due to the higher AD related pathology changes with increasing age. In addition, Watts et al. have found ~7–10% lower T1ρ value both in GM and WM compared to the current study. This is due to the reason that previous study has used 3 T MRI, while the current study used 1.5 T MRI, and it is well known fact that with higher field strength T1ρ decreases (Singh et al., 2014).
One of the limitation of the current study is the cross-sectional design, in which the values of two different diagnostic methods were compared in subject with the clinical diagnosis as gold standard. Further, longitudinal study with larger sample size is needed to properly assess the relative value of these markers in tracking changes along the clinical continuum of AD. In conclusion, both CSF biomarkers and T1ρ MRI seem to have incremental value in diagnosis of AD. By applying them together diagnostic accuracy might be increased.
Acknowledgments
This work was performed at a NIH supported resource center (NIH RR02305) and from a grant from the Pennsylvania State Tobacco Settlement grant (SAP4100027296).
References
- Andreasen N., Minthon L., Davidsson P., Vanmechelen E., Vanderstichele H. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch. Neurol. 2001;58(3):373–379. doi: 10.1001/archneur.58.3.373. 11255440 [DOI] [PubMed] [Google Scholar]
- Andreasen N., Sjögren M., Blennow K. CSF markers for Alzheimer's disease: total tau, phospho-tau and Abeta42. World J. Biol. Psychiatry. 2003;4(4):147–155. doi: 10.1080/15622970310029912. 14608585 [DOI] [PubMed] [Google Scholar]
- Arai H., Morikawa Y., Higuchi M., Matsui T., Clark C.M. Cerebrospinal fluid tau levels in neurodegenerative diseases with distinct tau-related pathology. Biochem. Biophys. Res. Commun. 1997;236(2):262–264. doi: 10.1006/bbrc.1997.6908. 9240421 [DOI] [PubMed] [Google Scholar]
- Aronen H.J., Ramadan U.A., Peltonen T.K., Markkola A.T., Tanttu J.I. 3D spin-lock imaging of human gliomas. Magn. Reson. Imaging. 1999;17(7):1001–1010. doi: 10.1016/s0730-725x(99)00041-7. 10463651 [DOI] [PubMed] [Google Scholar]
- Barber R., Gholkar A., Scheltens P., Ballard C., McKeith I.G. Medial temporal lobe atrophy on MRI in dementia with Lewy bodies. Neurology. 1999;52(6):1153–1158. doi: 10.1212/wnl.52.6.1153. 10214736 [DOI] [PubMed] [Google Scholar]
- Blennow K. CSF biomarkers for mild cognitive impairment. J. Intern. Med. 2004;256(3):224–234. doi: 10.1111/j.1365-2796.2004.01368.x. 15324365 [DOI] [PubMed] [Google Scholar]
- Borthakur A., Gur T., Wheaton A.J., Corbo M., Trojanowski J.Q. In vivo measurement of plaque burden in a mouse model of Alzheimer's disease. J. Magn. Reson. Imaging. 2006;24(5):1011–1017. doi: 10.1002/jmri.20751. 17036339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A., Mellon E., Niyogi S., Witschey W., Kneeland J.B. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. N.M.R. Biomed. 2006;19(7):781–821. doi: 10.1002/nbm.1102. 17075961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A., Sochor M., Davatzikos C., Trojanowski J.Q., Clark C.M. T1rho MRI of Alzheimer's disease. Neuroimage. 2008;41(4):1199–1205. doi: 10.1016/j.neuroimage.2008.03.030. 18479942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A., Wheaton A.J., Gougoutas A.J., Akella S.V., Regatte R.R. In vivo measurement of T1rho dispersion in the human brain at 1.5 Tesla. J. Magn. Reson. Imaging. 2004;19(4):403–409. doi: 10.1002/jmri.20016. 15065163 [DOI] [PubMed] [Google Scholar]
- Chin J. Selecting a mouse model of Alzheimer's disease. Methods Mol. Biol. 2011;670:169–189. doi: 10.1007/978-1-60761-744-0_13. 20967591 [DOI] [PubMed] [Google Scholar]
- Chincarini A., Bosco P., Calvini P., Gemme G., Esposito M. Local MRI analysis approach in the diagnosis of early and prodromal Alzheimer's disease. Neuroimage. 2011;58(2):469–480. doi: 10.1016/j.neuroimage.2011.05.083. 21718788 [DOI] [PubMed] [Google Scholar]
- Clerx L., van Rossum I.A., Burns L., Knol D.L., Scheltens P. Measurements of medial temporal lobe atrophy for prediction of Alzheimer's disease in subjects with mild cognitive impairment. Neurobiol. Aging. 2013;34(8):2003–2013. doi: 10.1016/j.neurobiolaging.2013.02.002. 23540941 [DOI] [PubMed] [Google Scholar]
- Cummings J.L. Cognitive and behavioral heterogeneity in Alzheimer's disease: seeking the neurobiological basis. Neurobiol. Aging. 2000;21(6):845–861. doi: 10.1016/s0197-4580(00)00183-4. 11124429 [DOI] [PubMed] [Google Scholar]
- Davatzikos C., Fan Y., Wu X., Shen D., Resnick S.M. Detection of prodromal Alzheimer's disease via pattern classification of magnetic resonance imaging. Neurobiol. Aging. 2008;29(4):514–523. doi: 10.1016/j.neurobiolaging.2006.11.010. 17174012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon M.J., DeSanti S., Zinkowski R., Mehta P.D., Pratico D. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J. Intern. Med. 2004;256(3):205–223. doi: 10.1111/j.1365-2796.2004.01381.x. 15324364 [DOI] [PubMed] [Google Scholar]
- Duara R., Loewenstein D.A., Potter E., Appel J. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology. 2008;71(24):1986–1992. doi: 10.1212/01.wnl.0000336925.79704.9f. 19064880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G.A., Gama Sosa M.A., De Gasperi R. Transgenic mouse models of Alzheimer's disease. Mt Sinai J. Med. 2010;77(1):69–81. doi: 10.1002/msj.20159. 20101721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N., Modrego P.J., Salinas G.R., Gazulla J. Magnetic resonance imaging based clinical research in Alzheimer's disease. J. Alzheimers Dis. 2012;31(Suppl. 3):S5–S18. doi: 10.3233/JAD-2011-111292. 22233763 [DOI] [PubMed] [Google Scholar]
- Frisoni G.B., Fox N.C., Jack C.R., Jr., Scheltens P., Thompson P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. 20139996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H., Blennow K. CSF tau and beta-amyloid as biomarkers for mild cognitive impairment. Dialogues Clin. Neurosci. 2004;6(4):379–390. doi: 10.31887/DCNS.2004.6.4/hhampel. 22034251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H., Bürger K., Pruessner J.C., Zinkowski R., DeBernardis J. Correlation of cerebrospinal fluid levels of Tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch. Neurol. 2005;62(5):770–773. doi: 10.1001/archneur.62.5.770. 15883264 [DOI] [PubMed] [Google Scholar]
- Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. 16488378 [DOI] [PubMed] [Google Scholar]
- Haris M., Singh A., Cai K., McArdle E., Fenty M. T(1ρ) MRI in Alzheimer's disease: detection of pathological changes in medial temporal lobe. J. Neuroimaging. 2011;21(2):e86–ee90. doi: 10.1111/j.1552-6569.2010.00467.x. 20331502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood D.G., Sultzer D.L., Wheatley M.V. Impaired insight in Alzheimer disease: association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry Neuropsychol. Behav. Neurol. 2000;13(2):83–88. 10780626 [PubMed] [Google Scholar]
- Herukka S.K., Pennanen C., Soininen H., Pirttilä T. CSF Abeta42, tau and phosphorylated tau correlate with medial temporal lobe atrophy. J. Alzheimers Dis. 2008;14(1):51–57. doi: 10.3233/jad-2008-14105. 18525127 [DOI] [PubMed] [Google Scholar]
- Hulstaert F., Blennow K., Ivanoiu A., Schoonderwaldt H.C., Riemenschneider M. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555–1562. doi: 10.1212/wnl.52.8.1555. 10331678 [DOI] [PubMed] [Google Scholar]
- Jack C.R., Jr., Petersen R.C., Xu Y.C., Waring S.C., O'Brien P.C. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurol. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. 9305341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir A., Almkvist O., Forsberg A., Wall A., Engler H. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer's disease. Neurobiol. Aging. 2012;33(1) doi: 10.1016/j.neurobiolaging.2010.06.015. 20688420 [DOI] [PubMed] [Google Scholar]
- Koppel J., Goldberg T.E., Gordon M.L., Huey E., Davies P. Relationships between behavioral syndromes and cognitive domains in Alzheimer disease: the impact of mood and psychosis. Am. J. Geriatr. Psychiatry. 2012;20(11):994–1000. doi: 10.1097/JGP.0b013e3182358921. 22048323 [DOI] [PubMed] [Google Scholar]
- Li L.Z., Xu H.N., Reddy R. Characterizing breast cancer mouse xenografts with T(1)rho -MRI: a preliminary study. Adv. Exp. Med. Biol. 2011;701:137–142. doi: 10.1007/978-1-4419-7756-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–393. doi: 10.1001/jama.2009.1064. 19622817 [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurol. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. 6610841 [DOI] [PubMed] [Google Scholar]
- Otto M., Esselmann H., Schulz-Shaeffer W., Neumann M., Schröter A. Decreased beta-amyloid1-42 in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Neurology. 2000;54(5):1099–1102. doi: 10.1212/wnl.54.5.1099. 10720281 [DOI] [PubMed] [Google Scholar]
- Parnetti L., Lanari A., Amici S., Gallai V., Vanmechelen E. CSF phosphorylated tau is a possible marker for discriminating Alzheimer's disease from dementia with Lewy bodies. Phospho-Tau International Study Group. Neurol. Sci. 2001;22(1):77–78. doi: 10.1007/s100720170055. 11487210 [DOI] [PubMed] [Google Scholar]
- Pearson S.D., Ollendorf D.A., Colby J.A. Amyloid-beta positron emission tomography in the diagnostic evaluation of Alzheimer disease: summary of primary findings and conclusions. JAMA Intern. Med. 2013 doi: 10.1001/jamainternmed.2013.11711. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. 11735772 [DOI] [PubMed] [Google Scholar]
- Regatte R.R., Akella S.V., Wheaton A.J., Lech G., Borthakur A. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad. Radiol. 2004;11(7):741–749. doi: 10.1016/j.acra.2004.03.051. 15217591 [DOI] [PubMed] [Google Scholar]
- Reijn T.S., Rikkert M.O., van Geel W.J., de Jong D., Verbeek M.M. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid beta(42) and tau proteins. Clin. Chem. 2007;53(5):859–865. doi: 10.1373/clinchem.2006.081679. 17395712 [DOI] [PubMed] [Google Scholar]
- Richard E., Schmand B.A., Eikelenboom P., Van Gool W.A., Alzheimer's Disease Neuroimaging Initiative MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open. 2013;3(6) doi: 10.1136/bmjopen-2012-002541. 23794572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N., Woerner N., Boreta L., Kornfield T., Shaw L.M. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(4):1067–1077. doi: 10.1093/brain/awp007. 19251758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Haris M., Cai K., Kogan F., Hariharan H. High resolution T1rho mapping of in vivo human knee cartilage at 7T. PLOS One. 2014;9(5):e97486. doi: 10.1371/journal.pone.0097486. 24830386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluimer J.D., Bouwman F.H., Vrenken H., Blankenstein M.A., Barkhof F. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol. Aging. 2010;31(5):758–764. doi: 10.1016/j.neurobiolaging.2008.06.016. 18692273 [DOI] [PubMed] [Google Scholar]
- Tam C.W., Burton E.J., McKeith I.G., Burn D.J., O'Brien J.T. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. 15753423 [DOI] [PubMed] [Google Scholar]
- Urakami K., Wada K., Arai H., Sasaki H., Kanai M. Diagnostic significance of Tau protein in cerebrospinal fluid from patients with corticobasal degeneration or progressive supranuclear palsy. J. Neurol. Sci. 2001;183(1):95–98. doi: 10.1016/s0022-510x(00)00480-9. 11166802 [DOI] [PubMed] [Google Scholar]
- Vanderstichele H., Bibl M., Engelborghs S., Le Bastard N., Lewczuk P. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's biomarkers Standardization Initiative. Alzheimers Dement. 2012;8(1):65–73. doi: 10.1016/j.jalz.2011.07.004. 22047631 [DOI] [PubMed] [Google Scholar]
- Vemuri P., Wiste H.J., Weigand S.D., Knopman D.S., Trojanowski J.Q. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75(2):143–151. doi: 10.1212/WNL.0b013e3181e7ca82. 20625167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R., Andrews T., Hipko S., Gonyea J.V., Filippi C.G. In vivo whole-brain T1-rho mapping across adulthood: normative values and age dependence. J. Magn. Reson. Imaging. 2014;40:376–382. doi: 10.1002/jmri.24358. 24227659 [DOI] [PubMed] [Google Scholar]
- Wheaton A.J., Dodge G.R., Elliott D.M., Nicoll S.B., Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn. Reson. Med. 2005;54(5):1087–1093. doi: 10.1002/mrm.20678. 16200568 [DOI] [PubMed] [Google Scholar]
- Wiltfang J., Esselmann H., Smirnov A., Bibl M., Cepek L. Beta-amyloid peptides in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Ann. Neurol. 2003;54(2):263–267. doi: 10.1002/ana.10661. 12891683 [DOI] [PubMed] [Google Scholar]
- Witschey W.R., Borthakur A., Fenty M., Kneeland B.J., Lonner J.H. T1rho MRI quantification of arthroscopically confirmed cartilage degeneration. Magn. Reson. Med. 2010;63(5):1376–1382. doi: 10.1002/mrm.22272. 20432308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Han D., Tan X., Feng J., Guo Y. Diagnostic accuracy of 18 F-FDG and 11 C-PIB-PET for prediction of short-term conversion to Alzheimer's disease in subjects with mild cognitive impairment. Int. J. Clin. Pract. 2012;66(2):185–198. doi: 10.1111/j.1742-1241.2011.02845.x. 22257044 [DOI] [PubMed] [Google Scholar]