Abstract
Autism spectrum disorders (ASD) are characterized by impairments in social communication and restrictive, repetitive behaviors. While behavioral symptoms are well-documented, investigations into the neurobiological underpinnings of ASD have not resulted in firm biomarkers. Variability in findings across structural neuroimaging studies has contributed to difficulty in reliably characterizing the brain morphology of individuals with ASD. These inconsistencies may also arise from the heterogeneity of ASD, and wider age-range of participants included in MRI studies and in previous meta-analyses. To address this, the current study used coordinate-based anatomical likelihood estimation (ALE) analysis of 21 voxel-based morphometry (VBM) studies examining high-functioning individuals with ASD, resulting in a meta-analysis of 1055 participants (506 ASD, and 549 typically developing individuals). Results consisted of grey, white, and global differences in cortical matter between the groups. Modeled anatomical maps consisting of concentration, thickness, and volume metrics of grey and white matter revealed clusters suggesting age-related decreases in grey and white matter in parietal and inferior temporal regions of the brain in ASD, and age-related increases in grey matter in frontal and anterior-temporal regions. White matter alterations included fiber tracts thought to play key roles in information processing and sensory integration. Many current theories of pathobiology ASD suggest that the brains of individuals with ASD may have less-functional long-range (anterior-to-posterior) connections. Our findings of decreased cortical matter in parietal–temporal and occipital regions, and thickening in frontal cortices in older adults with ASD may entail altered cortical anatomy, and neurodevelopmental adaptations.
Keywords: Autism spectrum disorder, Voxel-based morphometry, Anatomical likelihood estimation, Grey matter, White matter
Highlights
-
•
This is a meta-analysis of voxel-based morphometry studies of individuals with autism.
-
•
Different comparisons are made for global cortical matter, grey matter, and white matter.
-
•
Thinning was present in posterior brain regions and frontal white matter paths.
-
•
Age-related thickening of frontal grey matter was seen in participants with autism.
-
•
Results fit with existing theories of frontal-posterior disconnect in autism.
1. Introduction
Autism spectrum disorders (ASD) are characterized by impairments in social communication as well as the presence of restricted interests/repetitive behaviors (American Psychiatric Association, 2013). While the etiology of ASD is still unclear, many current theories suggest alterations in genetic and neurobiological mechanisms as key underlying factors of this disorder (Amaral et al., 2008; Geschwind and Levitt, 2007). Neuroimaging studies have revealed abnormalities in brain functioning and brain connectivity as critical in defining the phenotype of ASD, and such differences may underlie neuroanatomical alterations in this population. For example, voxel based morphometry (VBM), a technique that measures regional grey and white matter volume using probabilistic mapping (Ashburner and Friston, 2000, 2001), has been used extensively to study the neuroanatomy of ASD in: children and adolescents (Bonilha et al., 2008; Brieber et al., 2007; McAlonan et al., 2005; McAlonan et al., 2008; McAlonan et al., 2009), and adults (Beacher et al., 2012; Ecker et al., 2012; Kosaka et al., 2010, 2010; Schmitz et al., 2006; Schmitz et al., 2008; Toal et al., 2010). These studies have regularly reported differential anatomical measures (i.e. concentration, density, volume) of grey and white matter in brain regions associated with processing cognitive and social functions in individuals with ASD. These include the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), prefrontal cortex, amygdala, caudate nucleus, putamen, and somatosensory cortex (Hollander et al., 2005; Just et al., 2007; Kennedy and Adolphs, 2012; Langen et al., 2007; Oblak et al., 2011; Schumann et al., 2004; Schumann et al., 2010). Many of these anatomical differences also strongly correlate with ASD symptom severity (Hollander et al., 2005; Rojas et al., 2006; Wolff et al., 2013) suggesting a brain–behavior relationship. Some neuroanatomical studies have applied their findings to pattern classification analyses in order to identify potential diagnostic markers of ASD (Ecker et al., 2010, 2010; Uddin et al., 2011).
Despite the promising directions in morphometric investigations of ASD neuroanatomy, the findings have been relatively inconsistent across studies. This inconsistency likely emerges from the differences in approaches to morphometry, specifically VBM, across studies. Methodological differences, type of participants included (high-functioning versus low-functioning, classic ASD versus ASD with comorbid conditions), and age and developmental level of the participants can significantly affect the findings of these studies. Methodological variations are related to the use of different software and toolboxes available for image processing, (i.e. Brain Activation and Morphological Mapping [BAMM], CIVET, “Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra [DARTEL, Ashburner, 2007]), each of which may use different algorithms (Klein et al., 2009; Senjem et al., 2005). While difference in participant selection across studies is another source of variability in findings, perhaps the biggest contributor to this heterogeneity is the developmental stage of participants targeted. Many studies have reported that individuals with ASD show aberrant growth and developmental trajectories from early childhood into adulthood, but relatively few studies are longitudinal, limiting the analyses to cross-sectional designs with smaller numbers of participants and relatively narrow age ranges. This is potentially confounding given the findings of strong age-related effects on cortical development in both typically developing (TD) (Giedd et al., 1999; Gogtay et al., 2004) and ASD (Courchesne et al., 2007; Courchesne et al., 2011; Raznahan et al., 2010; Schumann et al., 2010) individuals. Thus, region-specific morphometric findings in ASD have largely been inconsistent, making it difficult to identify emerging consensus on neuroanatomical differences in this population.
These inconsistencies have made meta-analytic approaches such as anatomical likelihood estimation (ALE) an attractive approach to identifying trends and convergence across a large number of studies. ALE is a coordinate-based meta-analysis technique that uses statistically significant foci reported from different studies to create probability distribution maps for voxels of interest across experiments or foci, which are then used to generate structural or functional maps across groups of datasets or experiments (Eickhoff et al., 2009; Eickhoff et al., 2012; Turkeltaub et al., 2002). This method and other similar techniques (such as Signed Differential Mapping: SDM) have been used to conduct meta-analyses of VBM studies of ASD, and have been useful in identifying areas of consistent differences in cortical matter (CM, which refers to either grey or white matter depending on specific analyses) across studies (Cauda et al., 2011; Duerden et al., 2012; Nickl-Jockschat et al., 2012; Radua et al., 2011; Via et al., 2011). However, many of these meta-analyses have included studies comparing ASD participants with IQ scores below the cutoff for intellectual delay (IQ > 70), to TD controls with average IQ scores (Cauda et al., 2011; Duerden et al., 2012; Nickl-Jockschat et al., 2012). Additionally, some meta-analyses also included region of interest (ROI)-based analyses in their study pool (Via et al., 2011), which may alter statistical outcomes due to the differential statistical models and differences in anatomical boundaries of ROIs across studies (Glahn et al., 2008; Laird et al., 2005).
Limiting these potential sources of variability, the current study included 21 structural neuroimaging studies using conservative inclusion criteria for an ALE analysis of VBM results reported in ASD literature. The participants in these 21 studies largely include individuals with high functioning autism (HFA) and Asperger's syndrome (AS). There are 3 main points that guided our decision to group the participants from each study in this manner. The first is the difficulty in identifying morphometric differences between HFA and AS participants using a meta-analytic approach (as illustrated by Yu et al., 2011) since HFA and AS are rarely separated in morphometry studies. The second is that as per the DSM-V classification, AS is no longer considered a separate diagnosis, with the new criteria focusing on a spectrum diagnosis using 1 of 3 functional levels (dependent on the amount of support needed for the individual) (American Psychiatric Association, 2013). Our decision to group what was previously AS with what was previously HFA is meant to reflect this change. The third is that by combining the two high-functioning diagnostic groups, we are hoping to limit the contribution of intellectual impairment or developmental delay on cortical morphometry without sacrificing power to detect any potential effect. A thorough quality control process was followed to verify the quality of all spatial transformations for regional accuracy, matter type, and limit within-study and within-group contributions that may arise from the inclusion of multiple studies from the same authors (Turkeltaub et al., 2012). Rather than conducting individual ALE maps for age ranges, effectively “blocking” participants by age groups and limiting power due to the strict selection criteria followed, we report the studies contributing to each significant cluster and the age range under which each study falls. By doing so, we hope to identify clusters of anatomical regions that are atypical at younger stages of development, older stages of development, and regions that are consistently atypical in the brains of individuals with HFA/AS. Thus, the focus of this study is to identify the emerging themes from the structural neuroimaging literature in ASD with an emphasis on the neurodevelopmental trajectory of this disorder. The findings of this study will provide important insights into the characterization of the morphology of the brain in ASD, and further illuminate its developmental significance.
2. Methods and materials
2.1. Publication selection (inclusion/exclusion criteria)
The selection criteria for this meta-analysis consisted of studies involving HFA and AS participants with reported IQ measures greater than 70, and methodologies limited to whole-brain VBM. A comprehensive literature search was conducted using Pubmed, Google Scholar, and the Brainmap.org, and the VBM database within the Sleuth 2.2 software (Fox et al., 2005; Fox and Lancaster, 2002; Laird et al., 2005) in order to identify peer reviewed articles investigating neuroanatomical differences in ASD. Keywords used in the search parameters for the databases included: ASD, VBM, morphometry, grey matter volume, white matter volume, autism spectrum disorders, autism, and Asperger's syndrome. Additional papers were located by reviewing the references of studies selected for the meta-analysis, in addition to studies used or reported in previous meta-analyses (Cauda et al., 2011; Duerden et al., 2012; Nickl-Jockschat et al., 2012; Radua et al., 2011; Via et al., 2011). Our search criteria yielded a total of 36 peer-reviewed published articles. Of the 36, two articles were excluded as they used non-VBM modalities (i.e. DTI, Freesurfer), another two were excluded due to region of interest (ROI) analysis as opposed to whole brain analyses, and another eleven were excluded as those studies had subjects with comorbid conditions in the comparison group (i.e. Intellectual disability, ADHD, Fragile X), or in which participants had IQs < 70. The exception to these was Brieber et al. (2007), which included comparisons between individuals with ADHD and autism, and participants from Salmond et al. (2007), and Toal et al. (2010) that had IQs less than 70. In the former, only the significant results comparing individuals with ASD to TD controls were used, in the latter, only results from comparisons of HFA (IQ > 70) to TD controls were used. This conservative selection process yielded a total of 21 articles containing a total of 1055 participants reporting 478 separate neuroanatomical foci. The selected publications had a total of 506 ASD participants (mean age = 19.56 ± 9.15) and 549 (mean age 19.14 ± 9.26) TD controls. The age range was 6–59 years for the ASD group, and 6–58 years for TD controls (see Table 1 for demographic information by study).
Table 1.
Studies and participant demographics.
| ASD participants |
TD participants |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Participants | M/F | Age range | Mean age | Mean IQ | Participants | M/F | Age range | Mean age | Mean IQ |
| Abell et al. (1999) | 15 | 12/3 | NR | 29 | Verbal = 42.5/50, matrix = 48.9/50 | 15 | 12/3 | NR | 25 | Verbal = 45.2/50, matrix = 52.2/50 |
| Brieber et al. (2007) | 15 | 15/0 | 10–16 | 14.2 | 106.8 | 15 | 15/0 | 10–16 | 13.3 | 107.7 |
| Ecker et al. (2010) | 22 | 22/0 | 18–42 | 27 | 104 | 22 | 22/0 | 18–42 | 28 | 111 |
| Ecker et al. (2012) | 89 | 89/0 | 18–43 | 26 | 110 | 89 | 89/0 | 18–43 | 28 | 113 |
| Freitag et al. (2008) | 15 | 13/2 | NR | 17.5 | 101.2 | 15 | 13/2 | NR | 18.6 | 112.1 |
| Greimel et al. (2013) | 47 | 47/0 | 10–50 | 21.4 | 107.5 | 51 | 51/0 | 8–47 | 18.3 | 112.5 |
| Hyde et al. (2010) | 15 | 15/0 | 14–33 | 22.7 | 100.4 | 13 | 13/0 | 14–34 | 19.2 | 106.6 |
| Ke et al. (2008) | 17 | 14/3 | 6–14 | 8.88 | 108.76 | 15 | 12/3 | 6–14 | 9.73 | 108.76 |
| Ke et al. (2009) | 12 | 12/0 | 6–14 | 8.75 | 100.6 | 10 | 10/0 | 6–14 | 9.4 | 99.83 |
| Kosaka et al. (2010) | 32 | 32/0 | 17–32 | 23.8 | 101.6 | 40 | 40/0 | 18–34 | 22.5 | 109.7 |
| McAlonan et al. (2005) | 17 | 16/1 | 8–14 | 12 | 101(VIQ) | 17 | 16/1 | 8–14 | 11 | 114(VIQ) |
| McAlonan et al. (2008) | 33 | 27/6 | 7–16 | 11.6 | 113.2(VIQ) | 55 | 47/8 | 7–16 | 10.7 | 117.1(VIQ) |
| McAlonan et al. (2009) | 36 | 30/6 | 6–16 | 11.4 | 112.3(VIQ) | 55 | 47/8 | 6–16 | 10.7 | 117.1(VIQ) |
| Salmond et al. (2005) | 14 | 13/1 | 8–18 | 12.9 | 102(VIQ) | 13 | 13/0 | 8–18 | 12.1 | 111(VIQ) |
| Salmond et al. (2007) | 13 | 13/0 | 8–18 | 12 | 102(VIQ) | 13 | 13/0 | 8–18 | 12 | 111(VIQ) |
| Schmitz et al. (2006) | 10 | 10/0 | 18–52 | 38 | 105 | 12 | 12/0 | 18–52 | 39 | 106 |
| Schmitz et al. (2008) | 10 | 10/0 | 20–50 | 37.8 | 107 | 10 | 10/0 | 20–50 | 38.2 | 106 |
| Toal et al. (2010) | 39 | 35/4 | 16–59 | 30 | 106 | 33 | 30/3 | 19–58 | 32 | 105 |
| Uddin et al. (2011) | 24 | 22/2 | 8–18 | 13.23 | 105.67 | 24 | 22/2 | 8–18 | 13.25 | 106 |
| Waiter et al. (2004) | 16 | 16/0 | 12–20 | 15.4 | 100.4 | 16 | 16/0 | 12–20 | 15.5 | 99.7 |
| Waiter et al. (2005) | 15 | 15/0 | 12–20 | 15.2 | 100.5 | 16 | 16/0 | 12–20 | 15.5 | 99.7 |
NR, not reported; VIQ, verbal IQ.
MNI coordinates of significantly different grey and white matter measures were extracted from each study meeting the selection criteria. Studies that reported their results in Talairach coordinates were converted to MNI space using the tal2icbm script in Matlab (R2012b) (Laird et al., 2010; Lancaster et al., 2007), and articles that reported Talairach coordinates converted from MNI space using the Brett Transform were converted back to MNI using the tal2mni script (Brett et al., 2001). Articles reporting MNI coordinates converted from Talairach coordinates using the Brett Transform were converted back to Talairach space using the mni2tal function, and then transformed to MNI space using the tal2icbm transformation. The parameters, templates, smoothing kernels, and transformations applied to studies included in our analysis are described in Table 2.
Table 2.
Neuroimaging parameters and statistical thresholds.
| Study | Field strength | Sequence | Software | Resolution | Template | Smoothing | Correction | p-Value | Transform |
|---|---|---|---|---|---|---|---|---|---|
| Abell et al. (1999) | 2 T | NR | SPM96 | 1 × 1 × 1.5 | MNI305 | 12 mm | (unc.) | p < 0.001 | tal2mni |
| Brieber et al. (2007) | 1.5 T | MPRAGE | SPM2 (OVBM) | NR | Custom | 12 mm | (unc.) | p < 0.001 | NA |
| Ecker et al. (2010) | 3 T | NR | SPM5 | 1.09 × 1.09 × 1.09 | Talairach | 8 mm | (unc.) | p < 0.1 | tal2icbm |
| Ecker et al. (2012) | 3 T | SPGR | FSL (FNIRT) | NR | FNIRT | 3 mm | Permutation n = 500 | p < 0.004 (GM) p < 0.005(WM) | NA |
| Freitag et al. (2008) | 1.5 T | MPRAGE | SPM2 (OVBM) | NR | Custom | 12 mm | (unc.) | p < 0.001 | NA |
| Greimel et al. (2013) | 1.5 T/3 T | MPRAGE | SPM5 | 1 × 1 × 1 | SPM5 | 8 mm | Bayesian | > 99% Confidence | NA |
| Hyde et al. (2010) | 3 T | MPRAGE | CIVET | 1 × 1 × 1 | ICBM152 | 12 mm | FDR | p < 0.05 | NA |
| Ke et al. (2008) | 1.5 T | NR | SPM5 (OVBM) | . 94x.94 | Custom | 8 mm | (unc.) | p < 0.001 | tal2mni |
| Ke et al. (2009) | 1.5 T | SPGR | SPM5 (OVBM) | Custom | Custom | 8 mm | (unc.) | p < 0.001 | tal2mni |
| Kosaka et al. (2010) | 3 T | 3DIRSPGR | SPM5/DARTEL | 0.75 × 1.25 × 1.6 | Custom | 8 mm | FDR | p < 0.05 | NA |
| McAlonan et al. (2005) | 1.5 T | DEFSE | BAMM | Bullmore (1999) | Brammer et al. (1997) | 4.4 mm | FWE | < 1 fpc under null | tal2icbm |
| McAlonan et al. (2008) | 1.5 T | DEFSE | Talairach (1988) | . 859x.859x3 | Brammer et al. (1997) | 4.4 mm | FWE | < 1 fpc under null | tal2icbm |
| McAlonan et al. (2009) | 1.5 T | DEFSE | BAMM | . 859x.859x3mm | Brammer et al. (1997) | 4.4 mm | FWE | < 1 fpc under null | tal2icbm |
| Salmond et al. (2005) | 1.5 T | 3DFLASH | SPM99 | 0.8 × 0.8 × 1 | SPM99(averaged) | 12 mm | FDR | p < 0.05 | NA |
| Salmond et al. (2007) | 1.5 T | 3DFLASH | SPM99 | 0.8 × 0.8 × 1 | MNI | 12 mm | FDR | p < 0.05 | NA |
| Schmitz et al. (2006) | 1.5 T | SPGR | SPM99 | . 89x.89x1.5 | MNI | 10 mm | (unc.) | p < 0.001 | tal2mni |
| Schmitz et al. (2008) | 1.5 T | SPGR | SPM2 | . 89x.89x1.5 | Custom | 10 mm | FWE | p < 0.05 | tal2icbm |
| Toal et al. (2010) | 1.5 T | SPGR | SPM2 | 0.859 × 0.859 × 1.5 | Custom | 8 mm | FWE | < 1 fpc under null | tal2icbm |
| Uddin | 1.5 T | SPGR | SPM5 | 1 × 1 × 1 | MNI | 10 mm | FWE; Monte-Carlo | p < .01 | NA |
| Waiter et al. (2004) | 1.5 T | NR | SPM2 | 1 × 1 × 1 | SPM + T1 averages | 8 mm | (unc.) | p < 0.001 | tal2mni |
| Waiter et al. (2005) | 1.5 T | SPGR | SPM2 | NR | SPM + T1 averages | 12 mm | FDR | p < 0.05 | tal2mni |
NR, not reported; NA, not applicable; MP-RAGE, magnetization-prepared rapid acquisition gradient echo; SPGR, spoiled gradient recalled echo; 3DIRSPGR, three-dimensional inversion recovery-prepared fast spoiled gradient recalled; DEFSE, dual-echo fast spin echo; 3DFLASH, 3D Fast Low Angle SHot; MNI, Montreal Neurological Institute; SPM, Statistical Parametric Mapping; FSL, FMRIB Software Library; CIVET, a brain segmentation pipeline; BAMM, brain analysis morphological mapping; VBM, voxel-based morphometry; OVBM, optimized voxel-based morphometry; DARTEL, Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra; FNIRT, FSL non-linear registration tool; TOM, Template-o-matic toolbox; FDR, False discovery rate; FWE, family-wise error; fpc, false-positive cluster; unc, uncorrected statistical threshold.
2.2. ALE parameters
ALE analyses were performed using the Turkeltaub (2012) correction to the Eickhoff (2009) method, in order to minimize within-subject effects of subject groups with multiple experiments and the contribution of multiple-foci to modeled activation maps. Separate analyses were performed comparing clusters of voxel-based increases in CM (grey and white) and decreases in CM (grey and white) between individuals with ASD and TD controls. The analyses were performed at a cluster forming threshold (CFT; reported with each p value and ALE thresholds in the results, ALE values greater than this threshold are statistically significant) computed using an FDR of p < 0.05 (with no assumptions to correlations within the dataset) and a conservative minimum cluster volume of 200 mm3 using GingerALE (v2.3.1) (Eickhoff et al., 2009; Turkeltaub et al., 2012). The weighted centre of the anatomically modelled cluster convergences was then verified using FSLView 5.0.6 (Jenkinson et al., 2012) atlases with the MNI 152 brain template (1 mm) (Jenkinson et al., 2012; Smith et al., 2004; Woolrich et al., 2009). Individual ALE maps were computed for areas of increased and decreased CM in order to minimize directionality-based overlaps caused by the proximity of ROIs. The results of these analyses were followed up by 4 subsequent analyses on CM types (one with studies reporting more grey matter in individuals with ASDs, and others reporting less grey matter in individuals with ASDs, with two similar comparisons for white matter). These were also computed with CFTs using a minimum cluster of 200 mm3 and FDR of p < 0.05. These were used as a comparison to the global CM findings in order to both verify the results of the global CM analyses, as well as to identify differences unique to grey and white matter.
2.3. Assessing the effects of age on computed results
The developmental level of participants for all the studies included in our analyses ranged from children and adolescents (6–16 years of age), to younger and older adults (18–58 years of age), to heterogeneous samples across multiple age ranges (i.e. 8–47 years of age). A challenge for meta-analytic VBM studies in ASD is that few studies assess age-related changes in the cortex, instead employing univariate approaches comparing groups at different developmental windows (i.e. 8–16, 18–40, etc.). Considering this, some previous studies have grouped participants from different age ranges into windows of children and adolescents, and adults (Duerden et al., 2012). There is evidence however, that cortical maturation of many areas peaks at pre-adolescence (11–13), with steady declines in late adolescence (Giedd et al., 1999; Gogtay et al., 2004; Redcay and Courchesne, 2005). As such, a dichotomous grouping based on univariate analyses may not present the complete picture of development in ASD. To address this concern, the age range of significant foci from our analysis will be reported in order to evaluate whether certain structures are more atypical in individuals with ASD across development or if they are confined to an isolated developmental window (i.e. less than 16 years of age or greater than 16 years of age).
3. Results
3.1. Significant clusters of the ALE analysis
The analysis of global decreases in CM found localized decreases in temporal and occipital brain regions in individuals with ASD, with left lateralized decreases in parietal, frontal limbic regions, and right lateralized decreases in limbic regions. Global increases in CM in ASD were primarily localized to the frontal lobe; however, increases in CM were also found in ASD in left anterior temporal and right cerebellar regions. When the analyses were narrowed by CM type, grey matter decreases in ASD were localized to smaller clusters of parietal, cerebellar, and hippocampal regions and all grey matter increases in ASD were predominantly in prefrontal and temporal grey matter. White matter decreases were lateralized to the left hemisphere within the parietal cortex, ACC, and thalamic white matter connections.
3.2. Significant decreases in cortical matter
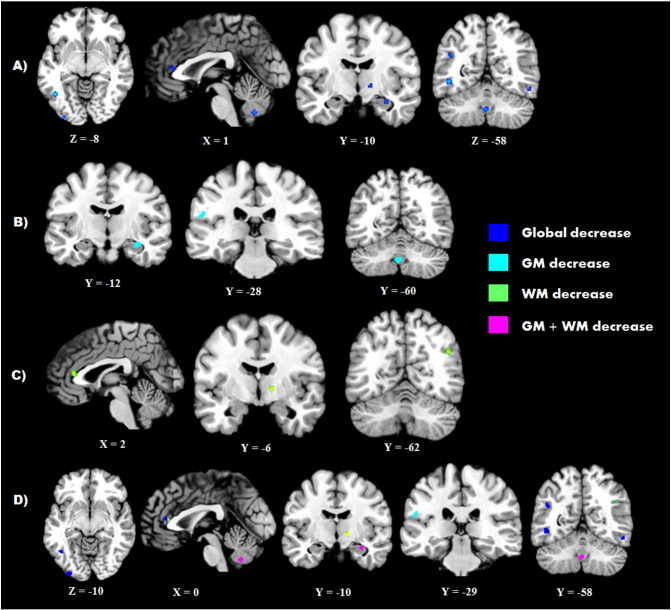
Significant ALE clusters of less CM in ASD (FDR computed CFT; p < 0.002, ALE threshold > 0.008) were found bilaterally within the inferior temporal gyrus (ITG) (LITG volume = 416 mm3; RITG volume = 296 mm3), the left occipital pole (volume = 400 mm3), and right lateral occipital cortex (volume = 200 mm3). Additional decreases in CM were found in the left parietal lobe in the postcentral area (volume = 256 mm3), and angular gyrus (volume = 240 mm3). Frontal limbic and subcortical decreases in CM were identified in the ACC (volume = 376 mm3), hippocampus (volume = 256 mm3), and the posterior limb of the internal capsule (volume = 352 mm3). Finally, a cluster of decrease was found overlapping vermis IX and VIIIb of the left cerebellum. When these foci were divided into separate analyses on grey and white matter, grey matter decreases in ASD (FDR computed CFT; p < 0.0013, ALE threshold > 0.0077) were localized to the right hippocampus (volume = 280 mm3) and cerebellar IX/VIIIb vermis (volume = 344 mm3) which overlapped with clusters in the global analysis, but an additional cluster was found in the left parietal operculum (volume = 200 mm3). Focused ALE on white matter decreases in ASD (FDR computed CFT; p < 0.0011, ALE threshold > 0.007) found clusters within the right internal capsule (volume = 376 mm3), ACC (volume = 352 mm3), and lateral occipital cortex (volume = 336 mm3). The results of the global and focused CM decrease analyses, significant at an FDR correction of p < .05, are displayed in Table 3 and Fig. 1.
Table 3.
ALE maps for matter decreases in ASD participants.
| Region | Hem | x | Y | z | Volume | ALE value |
|---|---|---|---|---|---|---|
| TD > ASD | ||||||
| Inferior temporal | L | −46 | −60 | −8 | 416 | 0.014264904** |
| Occipital pole | L | −32 | −94 | −10 | 400 | 0.01464336** |
| Anterior cingulate | R | 2 | 34 | 10 | 376 | 0.011359522** |
| Internal capsule | L | 14 | −6 | 0 | 352 | 0.01167158** |
| Cerebellum IX/VIIIb vermis | L | −2 | −60 | −40 | 320 | 0.01216303** |
| Inferior temporal | R | 52 | −60 | −16 | 296 | 0.011550702** |
| Hippocampus | R | 32 | −10 | −20 | 256 | 0.010775952** |
| Postcentral gyrus | L | −32 | −38 | 60 | 256 | 0.011418079** |
| Angular gyrus | L | −44 | −56 | 26 | 240 | 0.010977356** |
| Lateral occipital cortex | R | 46 | −64 | 28 | 200 | 0.010175092** |
| TD > ASD grey matter | ||||||
| Cerebellum IX vermis | L | −2 | −60 | −40 | 344 | 0.012163029* |
| Hippocampus | R | 32 | −10 | −20 | 280 | 0.010775952* |
| Parietal operculum | L | −54 | −30 | 22 | 208 | 0.009828495* |
| TD > ASD white matter | ||||||
| Internal capsule | R | 14 | −6 | 0 | 376 | 0.0110779265* |
| Anterior cingulate | R | 4 | 34 | 10 | 352 | 0.010652142* |
| Lateral occipital cortex | R | 46 | −64 | 28 | 336 | 0.010174975* |
Hem: hemisphere; L: left; R: right.
Significant at p < .001.
Significant at p < .002.
Fig. 1.
Significant clusters of decreased CM in individuals with ASD. A) Clusters of global decreases in CM (blue), B) clusters of decreased grey matter in ASD (teal), C) clusters of decreased white matter in ASD (green), D) overlap between comparisons (magenta; global/GM overlap, yellow; global/WM overlap).
3.3. Significant increases in cortical matter
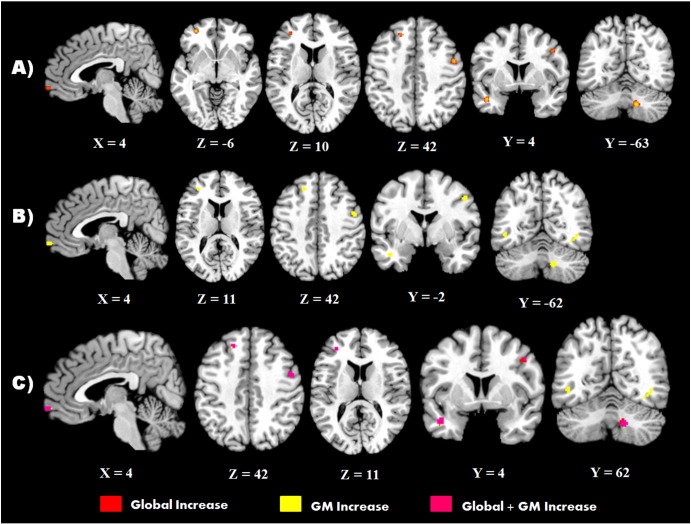
The analysis examining increased CM in individuals with ASD (FDR computed CFT; p < 0.002, ALE threshold > 0.008) revealed a number of significant clusters within the frontal lobe bilaterally. These included a large cluster within the left superior frontal gyrus (LSFG) (volume = 592 mm3), the right precentral gyrus (volume = 520 mm3), and three clusters within the frontal pole (FP) bilaterally (LFP volume = 376 mm3 and volume = 224 mm3; RFP volume = 232 mm3). A large cluster was also found in the left temporal pole (particularly the middle temporal gyrus, volume = 528 mm3), and right area VIIIb of the cerebellar vermis (volume = 616 mm3). When these foci were analysed separately for grey (FDR computed CFT; p < 0.0015, ALE threshold > 0.008) and white matter (FDR computed CFT; p < 0.0004, ALE threshold > 0.007), clusters within the LSFG (volume = 656 mm3), right precentral gyrus (volume = 384 mm3), left temporal pole (volume = 584 mm3), and area VIIIb of the cerebellum (volume = 384 mm3) remained significant, as did two of the three clusters within the FP (right: volume = 248 mm3; left: volume = 232 mm3) for grey matter foci. The focused analysis on grey matter increase also yielded two more significant clusters that were not found in the global CM map. One cluster in the ITG (volume = 240 mm3), and another cluster overlapping the lateral occipital cortex and fusiform (volume = 216 mm3). The results of the global CM increases and grey matter specific increases are displayed in Table 4 and Fig. 2. Separate analysis of increased white matter initially suggested that there was an increase in white matter volume within the left putamen, however, the foci contributing to this maximum were found to be from a single study (McAlonan et al., 2009). When either the HFA or AS group from this study was removed, or the foci from both were grouped, no areas of increased white matter were found.
Table 4.
ALE maps for matter increased in ASD participants.
| Region | Hem | x | Y | z | Volume | ALE value |
|---|---|---|---|---|---|---|
| ASD > TD | ||||||
| Cerebellum area VIIIb | R | 14 | −62 | −42 | 616 | 0.013753387** |
| Superior frontal | L | −18 | 32 | 44 | 592 | 0.013148831** |
| Superior frontal | L | −22 | 22 | 48 | 0.010468714** | |
| Temporal Pole | L | −42 | 6 | −28 | 528 | 0.013823562** |
| Temporal Pole | L | −42 | −2 | −28 | 0.010931179** | |
| Precentral | R | 48 | 0 | 40 | 520 | 0.013296759** |
| Frontal Pole | L | −32 | 50 | −6 | 376 | 0.013463693** |
| Frontal Pole | R | 4 | 64 | −12 | 232 | 0.01040588** |
| Frontal Pole | L | −32 | 42 | 12 | 224 | 0.01076785** |
| ASD > TD grey matter | ||||||
| Superior frontal | L | −18 | 32 | 44 | 656 | 0.013148831* |
| Superior frontal | L | −22 | 22 | 48 | 0.010468714* | |
| Temporal Pole | L | −42 | 6 | −28 | 584 | 0.013823563* |
| Temporal Pole | L | −42 | −2 | −28 | 0.010931179* | |
| Cerebellum area VIIIb | R | 14 | −62 | −42 | 384 | 0.013332463* |
| Precentral | R | 48 | 0 | 40 | 384 | 0.0125858905* |
| Frontal Pole | R | 4 | 64 | −12 | 248 | 0.01040588* |
| Inferior temporal | R | 42 | −60 | −8 | 240 | 0.009480287* |
| Frontal Pole | L | −32 | 42 | 12 | 232 | 0.010767846* |
| Lateral occipital/fusiform | L | −46 | −64 | −6 | 216 | 0.010389547* |
Hem: hemisphere; L: left; R: right.
Significant at p < .001.
Significant at p < .002.
Fig. 2.
Significant clusters of increased CM in individuals with ASD. A) Clusters of global increases in matter (red), B) clusters of increased grey matter in ASD (yellow), C) overlap between comparisons (magenta; global/GM overlap).
3.4. Age-related contributions to reported maxima
An important aspect of characterizing the neurobiology of a pervasive developmental disorder like ASD is to understand its neurodevelopmental trajectory. Due to the variability in participant selection criteria across different studies, isolating specific developmental windows is challenging. Nevertheless, our analysis revealed some interesting results. In the global analysis (analysis of all matter), decreases in the ACC and cerebellar vermis IX/VIIIb emerged from studies on children and adolescents with ASD (6–16 years of age). These results were also seen in the decreased grey matter (along with a decrease in the parietal operculum) and white matter analyses. However, we found increases in volume in the superior frontal and precentral areas in the global increase analysis specific to studies of older individuals with ASD (18–52 years of age), which also persisted within the grey matter analysis.
The rest of the significant clusters are formed across one of three categories based on the age range of foci forming the MA map: child to young adult (6–20 years), adolescent to adult (12–59 years), and throughout development (6–59 years). Clusters for studies of child to young adult participants included thinning of the postcentral gyrus in the global CM decrease and grey-matter specific increases in RITG and area VIIIb of the cerebellar vermis. A large majority of the significant foci stemmed from the adolescent to adult age group. This included global decrease of the occipital pole, angular, temporal pole, frontal pole, and lateral occipital gyri, grey matter decrease in the temporal and frontal poles, and white matter decrease in the internal capsule and superior parietal clusters. Clusters that formed from foci with large age ranges (6–59 years) included the internal capsule and hippocampus in the global CM decrease analysis, with the hippocampus persisting into the grey matter decrease analysis, area VIIIb of the cerebellum vermis in the global increase analysis, and the fusiform gyrus in the grey matter increase analysis. A comprehensive list of which studies contributed to each focus and colour-labelled maps of each group outlined above are displayed in Table S1 and Figs. S1 and S2 of the supplementary material.
4. Discussion
The results of this coordinate-based meta-analysis suggest alterations in grey and white matter volume (increase and decrease) in individuals with ASD, relative to TD control participants. Clusters reflecting increases in grey matter were found in frontal, temporal, and cerebellar areas, and clusters indicating decreases in grey matter were found in parietal–temporal, right hippocampal/amygdala, and cerebellar regions. This is consistent with previous structural MRI findings of increased CM (Carper and Courchesne, 2000; Carper et al., 2002; Duerden et al., 2012; Hazlett et al., 2005; Hazlett et al., 2006; Schumann et al., 2010; Via et al., 2011) and decreased CM (see Chen et al., 2011 for review) in individuals with ASD. Some of these findings are also consistent with previous meta-analyses of brain volumetric differences in the ASD population which survived leave-one-out tests of validity (Cauda et al., 2011; Duerden et al., 2012; Nickl-Jockschat et al., 2012; Via et al., 2011). White matter decrease in ASD was seen primarily within the ACC and internal capsule of the basal ganglia, and lateral–occipital cortex. This may reflect underlying microstructural organization, as alterations in fractional anisotropy (FA) in these areas have been reported in diffusion tensor imaging (DTI) studies of ASD (Alexander et al., 2007; Brito et al., 2009; Ingalhalikar et al., 2011; Keary et al., 2009; Keller et al., 2007; Shukla et al., 2010; Shukla et al., 2011; Travers et al., 2012).
4.1. Increased prefrontal cortex volume in autism
One of the key areas of volumetric alterations found in this study is the prefrontal cortex which, in general, has been considered as the epicenter of neurobiological abnormalities in ASD. Significant clusters of grey matter increase were located within the left superior, middle, and inferior frontal gyri, and right medial prefrontal cortex (SFG, MFG, IFG, and MPFC respectively). MRI and lesion studies have suggested that these structures contribute to a number of cognitive processes, including inhibition (MPFC, IFG; Talati and Hirsch, 2005; Aron et al., 2014), working memory (MFG, SFG; Leung et al., 2002; du Boisgueheneuc et al., 2006), motor sequencing and imitation (IFG, SFG; Toni et al., 1999; Rushworth et al., 2004), and language (IFG; Hischorn and Thompson-Schill, 2006), all of which have been reported to be atypical in ASD (Bennetto et al., 1996; Dziuk et al., 2007; Kana et al., 2007; Kleinhans et al., 2005; Minshew and Goldstein, 2001; Ozonoff et al., 1991; Sinzig et al., 2008). Clusters of increased frontal grey matter are especially interesting considering recent post-mortem findings of significantly increased number of neurons in the frontal cortex, especially in MPFC and dorsolateral prefrontal cortex in ASD (Courchesne et al., 2011). Increased levels of microglia and astroglia in active states in the frontal cortex have also been reported in ASD (Vargas et al., 2005). Cell-structure studies in the frontal and temporal lobes of individuals with ASD have found that cortical minicolumns (basic functional unit of the cerebral cortex) are both smaller and more numerous (Casanova et al., 2002). Numerous neurons packed in smaller columns can lead to altered patterns of connectivity favoring local overconnectivity and long-distance underconnectivity (Cherkassky et al., 2006; Courchesne and Pierce, 2005; Kana et al., 2009). Thus, it is possible that the complex neurobiology of ASD may entail such widespread (anatomical, functional, microstructural, and cellular) abnormalities. The increase in prefrontal cortex grey matter in ASD found in our meta-analysis could reflect a noticeable structural alteration in individuals with ASD, which could contribute to or be the result of cytoarchitectural differences in the brain.
4.2. Frontal-posterior differences
The findings of the present study also suggest distinct morphometric differences between frontal and posterior brain regions in ASD, with increases in grey matter primarily localized to frontal regions, and decreases in grey and white matter localized to parietal–occipital–temporal, limbic, and cingulate areas. Such differences have been reported by previous structural neuroimaging studies (Hazlett et al., 2006). A similar pattern was also seen in terms of altered asymmetry of the IFG and posterior superior temporal cortex in autism (Herbert et al., 2005). Additionally, functional neuroimaging studies of visual and visuospatial processing have reported increased parietal and occipital activation and intact or enhanced connectivity among relatively posterior areas of the brain (Kana et al., 2013). A recent review of the relationship between development and functional connectivity suggested that children with ASD may display increased connectivity, and adolescents and adults display decreased to normal functional and structural connections between frontal–parietal and default mode regions of the brain (Uddin et al., 2013). It should be noted that the increases in temporal and frontal regions, and decreases in parietal regions found in our study are specific to adolescents and adults with ASD, and may support a developmentally shifted pattern of cortical development reflecting less communication between frontal and parietal–temporal regions. Alternatively this could arise from posterior autonomy, which entails a more parietal and occipital reliant method of information processing. However, it is difficult to find direct support for either account, as VBM studies rely on the assumption that MR signal intensity correlates with white matter fiber integrity This does not always seem to be the case in normal or clinical populations (Buchel et al., 2004; Filippi et al., 2001). Despite this limitation, some support for the frontal-posterior differences has come from DTI literature, which report reduced FA and increased mean and radial diffusivity values within temporal, and frontal regions in ASD (Barnea-Goraly et al., 2004; Shukla et al., 2011; Travers et al., 2012). One of the most consistently reported region of white matter alteration (including our meta-analysis) is in the ACC, which serves as an integration hub for many different types of executive and social processes, and has been associated with cytoarchitectural abnormalities in children with ASD (Simms et al., 2009). What these structural differences contribute to the ASD phenotype and how they develop is an important topic of debate and should be addressed by future studies.
4.3. Cerebellar volumetric differences
Consistent with some previous meta-analyses of VBM in ASD (Cauda et al., 2011; Duerden et al., 2012), we found statistically significant clusters of grey matter alterations (less GM in area VIIIb/IX on the left, more GM on area VIIIb on the right) within the vermi of the cerebellum. Grey matter abnormalities within the cerebellum are widely reported in ASD literature (Carper and Courchesne, 2000; Courchesne et al., 1994; Courchesne et al., 2001; Courchesne et al., 2007; Courchesne, 1997; Courchesne et al., 1988; Fatemi et al., 2002; Fatemi et al., 2001; Hashimoto et al., 1995; Levitt et al., 1999; Piven et al., 1992; Vargas et al., 2005). A consistently reported neuroanatomical abnormality in cerebellum in ASD is a significant reduction in the number of Purkinje cells (see Baumann and Kemper, 2005 for review), which can have a significant impact on connectivity (Kern, 2003). The cerebellum has reciprocal connections with the basal ganglia and premotor cortex (Doyon et al., 2003; Middleton and Strick, 2000; Terry and Rosenberg, 1995), the latter of which was found to be enlarged in the right hemisphere in individuals with ASD in the present study. Reduced connectivity between cerebellum and motor cortex was reported in fMRI studies of sequential finger-tapping (Mostofsky et al., 2009). This is interesting not only due to the relationship between the cerebellum and motor cortex, but also because our results suggest increases in grey-matter along the precentral gyrus. This could reflect possible communication deficits between these two regions. Additionally, studies on children with cranial fossa tumours (whom have regions of the cerebellum near the vermis removed), frequently report communication and social deficits such as mutism, which overlap with some communication and social deficits present in autism (Poggi et al., 2005; Pollack et al., 1995).
4.4. Implications for social cognition
Our findings of increased grey matter in the angular gyrus, frontal and temporal pole, and middle temporal gyrus have strong implications for social cognition, especially Theory-of-Mind (ToM) in individuals with ASD as these three regions are routinely activated during ToM tasks (Castelli et al., 2000; Frith and Frith, 2003; Gallagher et al., 2000; Gallagher and Frith, 2003; Ohnishi et al., 2004). In addition, the FP shares functional and white matter connections with the angular gyrus, temporal pole, and ACC (Barnea-Goraly et al., 2004; Kelly et al., 2009; Koski and Paus, 2000; Liu et al., 2013). Atypical connections between these regions have been found in ASD during mentalizing tasks (Castelli et al., 2002; Frith, 2001; Kana et al., 2009; Zilbovicius et al., 2006).
Fusiform activation has consistently been reported as decreased in ASD when viewing faces (and some have argued increased compensatory ITG activation) (Schultz et al., 2000). A recent study by Nickl-Jockschat et al. (2014) used a combination of meta-analytic connectivity modeling (MACM) of co-activating ROIs, and resting state-connectivity analysis using the fusiform (which was an area of significant grey matter increase in our analysis) as a seed. They found bilateral decreases in connectivity with the IFG, temporal–occipital cortex, middle temporal gyrus, SMA, SPL, thalamus, and left medial temporal lobe in both face processing and resting state data, in addition to decreased connections with the cerebellum in ASD. Bilateral alterations in ITG (including the fusiform gyrus), left middle temporal gyrus and angular gyrus volume are also important considering the role of these regions in face, object, and word processing (Koyama et al., 2011; Pierce et al., 2001; Samson et al., 2012). Meta-analyses of activation for faces, objects, and words have suggested that middle and superior frontal, middle and superior temporal, and fusiform regions show altered, predominantly left lateralized activation in ASD (Samson et al., 2012), which overlaps with some of the anatomical regions in the results of our meta-analysis. The connectivity results from this meta-analysis overlap relatively well with our study, as well as previous meta-analyses of VBM, in addition to surface based morphometric approaches (Libero et al., 2014) in ASD. These findings highlight disruptions in discrete, task-dependent networks that may contribute to the ASD phenotype (Caspers et al., 2014; Mishkin and Ungerleider, 1982; Nickl-Jockschat et al., 2014; Ungerleider and Haxby, 1994).
4.5. Sensory and motor circuitry
Consistent with other meta-analyses and DTI, we report a decrease in white matter integrity in ASD in the posterior limb of the internal capsule of the right hemisphere (Duerden et al., 2012; Radua et al., 2011). This particular region consists of many cortical and spinal white matter tracts, including the thalamic radiation, superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), inferior frontal–occipital fasciculus (IFOF), and spinal-thalamic tracts (Lebel et al., 2008; Mori et al., 2008). Due to the widespread targets of these tracts, many have argued that white matter compromises in this area may contribute to problems with sensory and motor integration within individuals with ASD, and it is posited as a culprit for decreased longitudinal connectivity within the brains of ASD individuals (Keller et al., 2007; Shukla et al., 2010; Shukla et al., 2011).
5. Strengths and limitations
Any meta-analytic approach in neuroimaging may be inherently constrained by variables, such as differences in scanner strength, method of data analyses, data quality control measures used, coregistration techniques used, and differences in statistical threshold at which results are reported by individual studies. The studies used in this meta-analysis represent almost 15 years of VBM data on ASD, with significant advances in techniques over the years. As such, a coordinate-based approach was preferred due to the availability of reported results and established methods of anatomical likelihood (Salimi-Khorshidi et al., 2009). Studies by Salimi-Khorshidi et al. (2009) reported that ALE results were closest in concordance with image-based meta-analysis when compared to other coordinate based approaches. While scanner resolution, inter-program registration techniques, and threshold criteria are difficult to control for in a coordinate-based meta-analytic approach, strict precautions were taken in our study to control for experimenter bias and coordinate transformation errors. For the former, we used the Turkeltaub et al. (2012) correction, which reduces potential inflations from multiple foci in close proximity within a single experiment by calculating modelled activation (MA) maps across groups of subjects, rather than by experiment and uses the union/overlap of these maps to report significant results. This is done by using single foci from each experiment or subject group with the shortest Euclidian distance from the voxel of interest as the maximum probability of activation for that voxel (Turkeltaub et al., 2012). As an additional precaution against artefacts, the coordinate space from each study was evaluated and individual transformation into MNI space was applied instead of automated transformations into MNI space.
There are some universal caveats to anatomy-based imaging of individuals on the ASD spectrum. Avino and Hutsler (2010) suggested that individuals with ASD display less defined grey/white matter boundaries than TD individuals. As such, intensity-based imaging techniques such as VBM could artificially inflate or deflate measures of cortical morphometry in this population. Surface-based studies registering to sulcal landmarks have found abnormalities in frontal and temporal cortices (Levitt et al., 2003), along the angular/supramarginal gyrus (Hardan et al., 2004; Libero et al., 2014). Since VBM does not register based on sulcal and gyral landmarks, morphometric alterations in these regions could be a result of these shifts that have not been detected by the technique (Bookstein, 2001; Davatzikos, 2004).
Theories relating meta-analytic assessments of morphometric changes in ASD relative to activation, neurodevelopment, or connectivity has some conjectural limitations. Regarding neurodevelopment, many of the VBM studies included in this study, with the exception of one (Greimel et al., 2013), analysed age-dependent changes in VBM results in favour of univariate approaches controlling for age and IQ. While this is an adequate approach for establishing main effects of group at discrete developmental windows, it does not address the fact that cortical measures differ as a function of age and development, which many studies of the ASD brain have been shown to be longitudinally different from TD individuals especially at younger ages (Courchesne et al., 2007; Redcay and Courchesne, 2005; Schumann et al., 2010). As such, researchers are limited to discrete developmental windows, which may not fully explain the course of ASD. Regarding connectivity, as stated previously in the discussion, a limitation of using VBM to infer white matter integrity is that MR signal does not necessarily correlate with white matter fiber integrity, making it a less optimal approach than diffusion tensor or weighted fiber tractography. Meta-analytic approaches to co-activation of brain regions such as MACM can provide some information with regard to inter-cortical communication across large database samples (Eickhoff et al., 2011). Such approaches to white-matter tractography would benefit ASD research by potentially validating activation or morphometric differences associated with brain connections.
6. Conclusions
To our knowledge, this ALE meta-analysis is the first to conduct a focused analysis within a high functioning subgroup of individuals with ASD. Many of the cluster results suggesting atypical cortical volumes are specific to regions associated with social cognition, which is frequently found to be atypical in individuals with ASD. Additionally, these differences seem to favor CM increases within the frontal cortex of older individuals with ASD, and white matter abnormalities in fiber integrity (such as the cingulate and internal capsule) relatively early in development. These results point to the difference in structural integrity of frontal and posterior brain areas in individuals with ASD which may have an impact on the functions and connections of these regions. Additionally, improvements in meta-analytic approaches for fiber-tractography would greatly aid in substantiating the argument that disrupted connectivity contributes to or is influenced by morphometric alterations in ASD. Furthermore, the localization of frontal grey matter increases in older adults illustrates a need for studying brain development in ASD as a longitudinal process, rather than across developmental windows.
The following are the supplementary data related to this article.
Significant clusters across analyses. A) Global CM decrease, B) grey matter decrease. C) White matter decrease, D) global matter increase, E) grey matter increase, mapped by age group contributing to the cluster's significance (green; 6–16 years, yellow; 6–20 years, purple; 6–59 years, magenta; 12–59 years of age, orange; 18–52 years of age).
ALE map information for each study with foci and cluster information.
Conflicts of interest
Both authors had full access to all of publications used in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Source of funding
The funding for this study was provided by the UAB Department Faculty Funds and the McNulty-Civitan Scientist Award. The funding source was not involved in study design, the collection, analysis and interpretation of the presented data as well as writing of the report or in the decision to submit the article for publication. Further, the authors of this article declare no financial or other conflicts of interest.
Acknowledgement
We thank Rishi Deshpande for providing computational support, and all the administrators and users of the Brainmap.org forums for their valuable input for this study.
References
- Abell F., Krams M., Ashburner J., Passingham R., Friston K., Frackowiak R., Happé F. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10(8):1647–1651. doi: 10.1097/00001756-199906030-00005. 10501551 [DOI] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Boudos R., DuBray M.B., Oakes T.R., Miller J.N. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. 17023185 [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. 18258309 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fifth edition. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. 17761438 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry — the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. 10860804 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Why voxel-based morphometry should be used. Neuroimage. 2001;14(6):1238–1243. doi: 10.1006/nimg.2001.0961. 11707080 [DOI] [PubMed] [Google Scholar]
- Avino T.A., Hutsler J.J. Abnormal cell patterning at the cortical gray-white matter boundary in autism spectrum disorders. Brain Res. 2010;1360:138–146. doi: 10.1016/j.brainres.2010.08.091. 20816758 [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss A.L. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. 14744477 [DOI] [PubMed] [Google Scholar]
- Bauman M.L., Kemper T.L. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. 15749244 [DOI] [PubMed] [Google Scholar]
- Beacher F.D., Minati L., Baron-Cohen S., Lombardo M.V., Lai M.C., Gray M.A., Harrison N.A. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am J Neuroradiol. 2012;33(1):83–89. doi: 10.3174/ajnr.A2880. 22173769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L., Pennington B.F., Rogers S.J. Intact and impaired memory functions in autism. Child Dev. 1996;67(4):1816–1835. 8890510 [PubMed] [Google Scholar]
- Bonilha L., Cendes F., Rorden C., Eckert M., Dalgalarrondo P., Li L.M., Steiner C.E. Gray and white matter imbalance — typical structural abnormality underlying classic autism? Brain Dev. 2008;30(6):396–401. doi: 10.1016/j.braindev.2007.11.006. 18362056 [DOI] [PubMed] [Google Scholar]
- Bookstein F.L. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. 11707101 [DOI] [PubMed] [Google Scholar]
- Brammer M.J., Bullmore E.T., Simmons A., Williams S.C., Grasby P.M., Howard R.J., Rabe-Hesketh S. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging. 1997;15(7):763–770. doi: 10.1016/s0730-725x(97)00135-5. 9309607 [DOI] [PubMed] [Google Scholar]
- Brett M., Christoff K., Cusack R., Lancaster J. Using the Talairach atlas with the MNI template. Neuroimage. 2001;13(6):S85. [Google Scholar]
- Brieber S., Neufang S., Bruning N., Kamp-Becker I., Remschmidt H., Herpertz-Dahlmann B., Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48(12):1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. 18093031 [DOI] [PubMed] [Google Scholar]
- Brito A.R., Vasconcelos M.M., Domingues R.C., Hygino da Cruz L.C., Jr., Rodrigues S., Gasparetto E.L., Calçada C.A. Diffusion tensor imaging findings in school-aged autistic children. J. Neuroimaging. 2009;19(4):337–343. doi: 10.1111/j.1552-6569.2009.00366.x. 19490374 [DOI] [PubMed] [Google Scholar]
- Büchel C., Raedler T., Sommer M., Sach M., Weiller C., Koch M.A. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb. Cortex. 2004;14(9):945–951. doi: 10.1093/cercor/bhh055. 15115737 [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Brammer M.J., Rabe-Hesketh S., Curtis V.A., Morris R.G., Williams S.C.R., Sharma T., McGuire P.K. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp. 1999;7(1):38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper R.A., Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123(4):836–844. doi: 10.1093/brain/123.4.836. 10734014 [DOI] [PubMed] [Google Scholar]
- Carper R.A., Moses P., Tigue Z.D., Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16(4):1038–1051. doi: 10.1006/nimg.2002.1099. 12202091 [DOI] [PubMed] [Google Scholar]
- Casanova M.F., Buxhoeveden D.P., Switala A.E., Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–432. doi: 10.1212/wnl.58.3.428. 11839843 [DOI] [PubMed] [Google Scholar]
- Caspers J., Zilles K., Amunts K., Laird A.R., Fox P.T., Eickhoff S.B. Functional characterization and differential coactivation patterns of two cytoarchitectonic visual areas on the human posterior fusiform gyrus. Hum Brain Mapp. 2014;35(6):2754–2767. doi: 10.1002/hbm.22364. 24038902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Frith C., Happé F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(8):1839–1849. doi: 10.1093/brain/awf189. 12135974 [DOI] [PubMed] [Google Scholar]
- Castelli F., Happé F., Frith U., Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. 10944414 [DOI] [PubMed] [Google Scholar]
- Cauda F., Geda E., Sacco K., D'Agata F., Duca S., Geminiani G., Keller R. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatr. 2011;82(12):1304–1313. doi: 10.1136/jnnp.2010.239111. 21693631 [DOI] [PubMed] [Google Scholar]
- Chen R., Jiao Y., Herskovits E.H. Structural MRI in autism spectrum disorder. Pediatr. Res. 2011;69(5 Pt 2):63R–68R. doi: 10.1203/PDR.0b013e318212c2b3. 21289538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky V.L., Kana R.K., Keller T.A., Just M.A. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. 17047454 [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr. Opin. Neurobiol. 1997;7(2):269–278. doi: 10.1016/s0959-4388(97)80016-5. 9142760 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Campbell K., Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. 20920490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Karns C.M., Davis H.R., Ziccardi R., Carper R.A., Tigue Z.D., Courchesne R.Y. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. 11468308 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. 15831407 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K., Schumann C.M., Redcay E., Buckwalter J.A., Kennedy D.P., Morgan J. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi: 10.1016/j.neuron.2007.10.016. 17964254 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Townsend J., Akshoomoff N.A., Saitoh O., Yeung-Courchesne R., Lincoln A.J., Lau L. Impairment in shifting attention in autistic and cerebellar patients. Behav. Neurosci. 1994;108(5):848–865. doi: 10.1037//0735-7044.108.5.848. 7826509 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Yeung-Courchesne R., Press G.A., Hesselink J.R., Jernigan T.L. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. 3367935 [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23(1):17–20. doi: 10.1016/j.neuroimage.2004.05.010. 15325347 [DOI] [PubMed] [Google Scholar]
- Doyon J., Penhune V., Ungerleider L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. 12457751 [DOI] [PubMed] [Google Scholar]
- Du Boisgueheneuc F., Levy R., Volle E., Seassau M., Duffau H., Kinkingnehun S., Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129(12):3315–3328. doi: 10.1093/brain/awl244. 16984899 [DOI] [PubMed] [Google Scholar]
- Duerden E.G., Mak-Fan K.M., Taylor M.J., Roberts S.W. Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Res. 2012;5(1):49–66. doi: 10.1002/aur.235. 22139976 [DOI] [PubMed] [Google Scholar]
- Dziuk M.A., Gidley Larson J.C., Apostu A., Mahone E.M., Denckla M.B., Mostofsky S.H. Dyspraxia in autism: association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. 17880641 [DOI] [PubMed] [Google Scholar]
- Ecker C., Rocha-Rego V., Johnston P., Mourao-Miranda J., Marquand A., Daly E.M. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage. 2010;49(1):44–56. doi: 10.1016/j.neuroimage.2009.08.024. 19683584 [DOI] [PubMed] [Google Scholar]
- Ecker C., Suckling J., Deoni S.C., Lombardo M.V., Bullmore E.T., Baron-Cohen S. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch. Gen. Psychiatry. 2012;69(2):195–209. doi: 10.1001/archgenpsychiatry.2011.1251. 22310506 [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. 21963913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Roski C., Caspers S., Zilles K., Fox P.T. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57(3):938–949. doi: 10.1016/j.neuroimage.2011.05.021. 21609770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. 19172646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Halt A.R., Realmuto G., Earle J., Kist D.A., Thuras P., Merz A. Purkinje cell size is reduced in cerebellum of patients with autism. Cell. Mol. Neurobiol. 2002;22(2):171–175. doi: 10.1023/A:1019861721160. 12363198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Stary J.M., Halt A.R., Realmuto G.R. Dysregulation of reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord. 2001;31(6):529–535. doi: 10.1023/a:1013234708757. 11814262 [DOI] [PubMed] [Google Scholar]
- Filippi M., Cercignani M., Inglese M., Horsfield M.A., Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56(3):304–311. doi: 10.1212/wnl.56.3.304. 11171893 [DOI] [PubMed] [Google Scholar]
- Fox P.T., Laird A.R., Fox S.P., Fox P.M., Uecker A.M., Crank M., Lancaster J.L. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp. 2005;25(1):185–198. doi: 10.1002/hbm.20141. 15846810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P.T., Lancaster J.L. Opinion: mapping context and content: the BrainMap model. Nat. Rev. Neurosci. 2002;3(4):319–321. doi: 10.1038/nrn789. 11967563 [DOI] [PubMed] [Google Scholar]
- Freitag C.M., Konrad C., Häberlen M., Kleser C., von Gontard A., Reith W., Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46(5):1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. 18262208 [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32(6):969–979. doi: 10.1016/s0896-6273(01)00552-9. 11754830 [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. 12689373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. Functional imaging of ‘theory of mind’. Trends Cogn. Sci. (Regul. Ed.) 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. 12584026 [DOI] [PubMed] [Google Scholar]
- Gallagher H.L., Happé F., Brunswick N., Fletcher P.C., Frith U., Frith C.D. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. 10617288 [DOI] [PubMed] [Google Scholar]
- Geschwind D.H., Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. 17275283 [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. 10491603 [DOI] [PubMed] [Google Scholar]
- Glahn D.C., Laird A.R., Ellison-Wright I., Thelen S.M., Robinson J.L., Lancaster J.L., Fox P.T. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. 18486104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. 15148381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E., Nehrkorn B., Schulte-Rüther M., Fink G.R., Nickl-Jockschat T., Herpertz-Dahlmann B., Eickhoff S.B. Changes in grey matter development in autism spectrum disorder. Brain Struct Funct. 2013;218(4):929–942. doi: 10.1007/s00429-012-0439-9. 22777602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan A.Y., Jou R.J., Keshavan M.S., Varma R., Minshew N.J. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 2004;131(3):263–268. doi: 10.1016/j.pscychresns.2004.06.001. 15465295 [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Tayama M., Murakawa K., Yoshimoto T., Miyazaki M., Harada M., Kuroda Y. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25(1):1–18. doi: 10.1007/BF02178163. 7608030 [DOI] [PubMed] [Google Scholar]
- Hazlett H.C., Poe M., Gerig G., Smith R.G., Provenzale J., Ross A., Gilmore J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. 16330725 [DOI] [PubMed] [Google Scholar]
- Hazlett H.C., Poe M.D., Gerig G., Smith R.G., Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol. Psychiatry. 2006;59(1):1–6. doi: 10.1016/j.biopsych.2005.06.015. 16139816 [DOI] [PubMed] [Google Scholar]
- Herbert M.R., Ziegler D.A., Deutsch C.K., O'Brien L.M., Kennedy D.N., Filipek P.A., Caviness V.S., Jr. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128(1):213–226. doi: 10.1093/brain/awh330. 15563515 [DOI] [PubMed] [Google Scholar]
- Hirshorn E.A., Thompson-Schill S.L. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. 16725162 [DOI] [PubMed] [Google Scholar]
- Hollander E., Anagnostou E., Chaplin W., Esposito K., Haznedar M.M., Licalzi E., Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol. Psychiatry. 2005;58(3):226–232. doi: 10.1016/j.biopsych.2005.03.040. 15939406 [DOI] [PubMed] [Google Scholar]
- Hyde K.L., Samson F., Evans A.C., Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;31(4):556–566. doi: 10.1002/hbm.20887. 19790171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M., Parker D., Bloy L., Roberts T.P., Verma R. Diffusion based abnormality markers of pathology: toward learned diagnostic prediction of ASD. Neuroimage. 2011;57(3):918–927. doi: 10.1016/j.neuroimage.2011.05.023. 21609768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Kana R.K., Minshew N.J. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. 16772313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Soc Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. 18633829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Minshew N.J., Just M.A. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol. Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. 17137558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Liu Y., Williams D.L., Keller T.A., Schipul S.E., Minshew N.J., Just M.A. The local, global, and neural aspects of visuospatial processing in autism spectrum disorders. Neuropsychologia. 2013;51(14):2995–3003. doi: 10.1016/j.neuropsychologia.2013.10.013. 24184351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X., Hong S., Tang T., Zou B., Li H., Hang Y., Liu Y. Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport. 2008;19(9):921–925. doi: 10.1097/WNR.0b013e328300edf3. 18520994 [DOI] [PubMed] [Google Scholar]
- Ke X., Tang T., Hong S., Hang Y., Zou B., Li H., Liu Y. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. 19233148 [DOI] [PubMed] [Google Scholar]
- Keary C.J., Minshew N.J., Bansal R., Goradia D., Fedorov S., Keshavan M.S., Hardan A.Y. Corpus callosum volume and neurocognition in autism. J Autism Dev Disord. 2009;39(6):834–841. doi: 10.1007/s10803-009-0689-4. 19165587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T.A., Kana R.K., Just M.A. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18(1):23–27. doi: 10.1097/01.wnr.0000239965.21685.99. 17259855 [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T., Milham M.P. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. 18653667 [DOI] [PubMed] [Google Scholar]
- Kennedy D.P., Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. (Regul. Ed.) 2012;16(11):559–572. doi: 10.1016/j.tics.2012.09.006. 23047070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J.K. Purkinje cell vulnerability and autism: a possible etiological connection. Brain Dev. 2003;25(6):377–382. doi: 10.1016/s0387-7604(03)00056-1. 12907269 [DOI] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., Ashburner J., Avants B., Chiang M.C., Parsey R.V. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. 19195496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N., Akshoomoff N., Delis D.C. Executive functions in autism and Asperger's disorder: flexibility, fluency, and inhibition. Dev Neuropsychol. 2005;27(3):379–401. doi: 10.1207/s15326942dn2703_5. 15843103 [DOI] [PubMed] [Google Scholar]
- Kosaka H., Omori M., Munesue T., Ishitobi M., Matsumura Y., Takahashi T. Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage. 2010;50(4):1357–1363. doi: 10.1016/j.neuroimage.2010.01.085. 20123027 [DOI] [PubMed] [Google Scholar]
- Koski L., Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133(1):55–65. doi: 10.1007/s002210000400. 10933210 [DOI] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Zuo X.N., Kelly C., Mennes M., Jutagir D.R., Milham M.P. Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. 21653865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Lancaster J.L., Fox P.T. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005;3(1):65–78. doi: 10.1385/ni:3:1:065. 15897617 [DOI] [PubMed] [Google Scholar]
- Laird A.R., McMillan K.M., Lancaster J.L., Kochunov P., Turkeltaub P.E., Pardo J.V., Fox P.T. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25(1):6–21. doi: 10.1002/hbm.20129. 15846823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Robinson J.L., McMillan K.M., Tordesillas-Gutiérrez D., Moran S.T., Gonzales S.M., Ray K.L., Franklin C., Glahn D.C., Fox P.T., Lancaster J.L. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51(2):677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M., Durston S., Staal W.G., Palmen S.J., van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol. Psychiatry. 2007;62(3):262–266. doi: 10.1016/j.biopsych.2006.09.040. 17224135 [DOI] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. 18295509 [DOI] [PubMed] [Google Scholar]
- Leung H.C., Gore J., Goldman-Rakic P. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14(4):659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Levitt J.G., Blanton R., Capetillo-Cunliffe L., Guthrie D., Toga A., McCracken J.T. Cerebellar vermis lobules VIII–X in autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23(4):625–633. doi: 10.1016/s0278-5846(99)00021-4. 10390721 [DOI] [PubMed] [Google Scholar]
- Levitt J.G., Blanton R.E., Smalley S., Thompson P.M., Guthrie D., McCracken J.T., Sadoun T., Heinichen L., Toga A.W. Cortical sulcal maps in autism. Cereb Cortex. 2003;13(7):728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Libero L.E., DeRamus T.P., Deshpande H.D., Kana R.K. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 2014;62:1–10. doi: 10.1016/j.neuropsychologia.2014.07.001. 25019362 [DOI] [PubMed] [Google Scholar]
- Liu H., Qin W., Li W., Fan L., Wang J., Jiang T., Yu C. Connectivity-based parcellation of the human frontal pole with diffusion tensor imaging. J. Neurosci. 2013;33(16):6782–6790. doi: 10.1523/JNEUROSCI.4882-12.2013. 23595737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATLAB and Statistics Toolbox Release. T. M., Inc; Natick, Massachusetts, United States: 2012. [Google Scholar]
- McAlonan G.M., Cheung C., Cheung V., Wong N., Suckling J., Chua S.E. Differential effects on white-matter systems in high-functioning autism and Asperger's syndrome. Psychol Med. 2009;39(11):1885–1893. doi: 10.1017/S0033291709005728. 19356262 [DOI] [PubMed] [Google Scholar]
- McAlonan G.M., Cheung V., Cheung C., Suckling J., Lam G.Y., Tai K.S., Chua S.E. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128(2):268–276. doi: 10.1093/brain/awh332. 15548557 [DOI] [PubMed] [Google Scholar]
- McAlonan G.M., Suckling J., Wong N., Cheung V., Lienenkaemper N., Cheung C., Chua S.E. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger's syndrome. J Child Psychol Psychiatry. 2008;49(12):1287–1295. doi: 10.1111/j.1469-7610.2008.01933.x. 18673405 [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. 10719151 [DOI] [PubMed] [Google Scholar]
- Minshew N.J., Goldstein G. The pattern of intact and impaired memory functions in autism. J Child Psychol Psychiatry. 2001;42(8):1095–1101. doi: 10.1111/1469-7610.00808. 11806691 [DOI] [PubMed] [Google Scholar]
- Mishkin M., Ungerleider L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982;6(1):57–77. doi: 10.1016/0166-4328(82)90081-x. 7126325 [DOI] [PubMed] [Google Scholar]
- Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K., Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. 18255316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S.H., Powell S.K., Simmonds D.J., Goldberg M.C., Caffo B., Pekar J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(9):2413–2425. doi: 10.1093/brain/awp088. 19389870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T., Habel U., Michel T.M., Manning J., Laird A.R., Fox P.T., Eickhoff S.B. Brain structure anomalies in autism spectrum disorder — a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33(6):1470–1489. doi: 10.1002/hbm.21299. 21692142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T., Rottschy C., Thommes J., Schneider F., Laird A.R., Fox P.T., Eickhoff S.B. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0791-z. 24869925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak A.L., Rosene D.L., Kemper T.L., Bauman M.L., Blatt G.J. Altered posterior cingulate cortical cyctoarchitecture, but normal density of neurons and interneurons in the posterior cingulate cortex and fusiform gyrus in autism. Autism Res. 2011;4(3):200–211. doi: 10.1002/aur.188. 21360830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Moriguchi Y., Matsuda H., Mori T., Hirakata M., Imabayashi E., Uno A. The neural network for the mirror system and mentalizing in normally developed children: an fMRI study. Neuroreport. 2004;15(9):1483–1487. doi: 10.1097/01.wnr.0000127464.17770.1f. 15194879 [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Pennington B.F., Rogers S.J. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. 1787138 [DOI] [PubMed] [Google Scholar]
- Pierce K., Müller R.A., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. 11571222 [DOI] [PubMed] [Google Scholar]
- Piven J., Nehme E., Simon J., Barta P., Pearlson G., Folstein S.E. Magnetic resonance imaging in autism: measurement of the cerebellum, pons, and fourth ventricle. Biol. Psychiatry. 1992;31(5):491–504. doi: 10.1016/0006-3223(92)90260-7. 1581425 [DOI] [PubMed] [Google Scholar]
- Poggi G., Liscio M., Galbiati S., Adduci A., Massimino M., Gandola L., Castelli E. Brain tumors in children and adolescents: cognitive and psychological disorders at different ages. Psychooncology. 2005;14(5):386–395. doi: 10.1002/pon.855. 15386759 [DOI] [PubMed] [Google Scholar]
- Pollack I.F., Polinko P., Albright A.L., Towbin R., Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37(5):885–893. doi: 10.1227/00006123-199511000-00006. 8559336 [DOI] [PubMed] [Google Scholar]
- Radua J., Via E., Catani M., Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41(7):1539–1550. doi: 10.1017/S0033291710002187. 21078227 [DOI] [PubMed] [Google Scholar]
- Raznahan A., Toro R., Daly E., Robertson D., Murphy C., Deeley Q., Murphy D.G. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb. Cortex. 2010;20(6):1332–1340. doi: 10.1093/cercor/bhp198. 19819933 [DOI] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry. 2005;58(1):1–9. doi: 10.1016/j.biopsych.2005.03.026. 15935993 [DOI] [PubMed] [Google Scholar]
- Rojas D.C., Peterson E., Winterrowd E., Reite M.L., Rogers S.J., Tregellas J.R. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. B.M.C. Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. 17166273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F.S., Walton M.E., Kennerley S.W., Bannerman D.M. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8(9):410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S.M., Keltner J.R., Wager T.D., Nichols T.E. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45(3):810–823. doi: 10.1016/j.neuroimage.2008.12.039. 19166944 [DOI] [PubMed] [Google Scholar]
- Salmond C.H., Ashburner J., Connelly A., Friston K.J., Gadian D.G., Vargha-Khadem F. The role of the medial temporal lobe in autistic spectrum disorders. Eur. J. Neurosci. 2005;22(3):764–772. doi: 10.1111/j.1460-9568.2005.04217.x. 16101758 [DOI] [PubMed] [Google Scholar]
- Salmond C.H., Vargha-Khadem F., Gadian D.G., de Haan M., Baldeweg T. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex. 2007;43(6):686–699. doi: 10.1016/s0010-9452(08)70498-2. 17710821 [DOI] [PubMed] [Google Scholar]
- Samson F., Mottron L., Soulières I., Zeffiro T.A. Enhanced visual functioning in autism: an ALE meta-analysis. Hum Brain Mapp. 2012;33(7):1553–1581. doi: 10.1002/hbm.21307. 21465627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N., Rubia K., Daly E., Smith A., Williams S., Murphy D.G. Neural correlates of executive function in autistic spectrum disorders. Biol. Psychiatry. 2006;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. 16140278 [DOI] [PubMed] [Google Scholar]
- Schmitz N., Rubia K., van Amelsvoort T., Daly E., Smith A., Murphy D.G. Neural correlates of reward in autism. Br J Psychiatry. 2008;192(1):19–24. doi: 10.1192/bjp.bp.107.036921. 18174503 [DOI] [PubMed] [Google Scholar]
- Schultz R.T., Gauthier I., Klin A., Fulbright R.K., Anderson A.W., Volkmar F., Gore J.C. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch. Gen. Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. 10768694 [DOI] [PubMed] [Google Scholar]
- Schumann C.M., Bloss C.S., Barnes C.C., Wideman G.M., Carper R.A., Akshoomoff N., Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. 20335478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann C.M., Hamstra J., Goodlin-Jones B.L., Lotspeich L.J., Kwon H., Buonocore M.H., Amaral D.G. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. 15254095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senjem M.L., Gunter J.L., Shiung M.M., Petersen R.C., Jack C.R., Jr. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600–608. doi: 10.1016/j.neuroimage.2005.02.005. 15907317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Lincoln A.J., Müller R.A. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269–1278. doi: 10.1016/j.jaac.2010.08.018. 21093776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Müller R.A. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. 21073464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms M.L., Kemper T.L., Timbie C.M., Bauman M.L., Blatt G.J. The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathol. 2009;118(5):673–684. doi: 10.1007/s00401-009-0568-2. 19590881 [DOI] [PubMed] [Google Scholar]
- Sinzig J., Morsch D., Bruning N., Schmidt M.H., Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child Adolesc Psychiatry Ment Health. 2008;2(1):4. doi: 10.1186/1753-2000-2-4. 18237439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. 15501092 [DOI] [PubMed] [Google Scholar]
- Talati A., Hirsch J. Functional Specialization within the Medial Frontal Gyrus for Perceptual Go/No-Go Decisions Based on “What,” “When,” and “Where” Related Information: An fMRI Study. J Cogn Neurosci. 2005;17(7):981–993. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Terry J.B., Rosenberg R.N. Frontal lobe ataxia. Surg Neurol. 1995;44(6):583–588. doi: 10.1016/0090-3019(95)00302-9. 8669037 [DOI] [PubMed] [Google Scholar]
- Toal F., Daly E.M., Page L., Deeley Q., Hallahan B., Bloemen O., Murphy D.G. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol Med. 2010;40(7):1171–1181. doi: 10.1017/S0033291709991541. 19891805 [DOI] [PubMed] [Google Scholar]
- Toni I., Schluter N.D., Josephs O., Friston K., Passingham R.E. Signal-, set- and movement-related activity in the human brain: an event-related fMRI study. Cereb. Cortex. 1999;9(1):35–49. doi: 10.1093/cercor/9.1.35. 10022494 [DOI] [PubMed] [Google Scholar]
- Travers B.G., Adluru N., Ennis C., Tromp doP.M., Destiche D., Doran S., Alexander A.L. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5(5):289–313. doi: 10.1002/aur.1243. 22786754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. doi: 10.1006/nimg.2002.1131. 12169260 [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. 21305667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Menon V., Young C.B., Ryali S., Chen T., Khouzam A., Hardan A.Y. Multivariate searchlight classification of structural magnetic resonance imaging in children and adolescents with autism. Biol. Psychiatry. 2011;70(9):833–841. doi: 10.1016/j.biopsych.2011.07.014. 21890111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. 23966925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider L.G., Haxby J.V. ‘What’ and ‘where’ in the human brain. Curr. Opin. Neurobiol. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. 8038571 [DOI] [PubMed] [Google Scholar]
- Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. 15546155 [DOI] [PubMed] [Google Scholar]
- Via E., Radua J., Cardoner N., Happé F., Mataix-Cols D. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Arch. Gen. Psychiatry. 2011;68(4):409–418. doi: 10.1001/archgenpsychiatry.2011.27. 21464365 [DOI] [PubMed] [Google Scholar]
- Waiter G.D., Williams J.H., Murray A.D., Gilchrist A., Perrett D.I., Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22(2):619–625. doi: 10.1016/j.neuroimage.2004.02.029. 15193590 [DOI] [PubMed] [Google Scholar]
- Waiter G.D., Williams J.H., Murray A.D., Gilchrist A., Perrett D.I., Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. Neuroimage. 2005;24(2):455–461. doi: 10.1016/j.neuroimage.2004.08.049. 15627587 [DOI] [PubMed] [Google Scholar]
- Wolff J.J., Hazlett H.C., Lightbody A.A., Reiss A.L., Piven J. Repetitive and self-injurious behaviors: associations with caudate volume in autism and fragile X syndrome. J Neurodev Disord. 2013;5(1):12. doi: 10.1186/1866-1955-5-12. 23639144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Smith S.M. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl.):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. 19059349 [DOI] [PubMed] [Google Scholar]
- Yu K.K., Cheung C., Chua S.E., McAlonan G.M. Can Asperger syndrome be distinguished from autism? An anatomic likelihood meta-analysis of MRI studies. J Psychiatry Neurosci. 2011;36(6):412–421. doi: 10.1503/jpn.100138. 21406158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M., Meresse I., Chabane N., Brunelle F., Samson Y., Boddaert N. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006;29(7):359–366. doi: 10.1016/j.tins.2006.06.004. 16806505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Significant clusters across analyses. A) Global CM decrease, B) grey matter decrease. C) White matter decrease, D) global matter increase, E) grey matter increase, mapped by age group contributing to the cluster's significance (green; 6–16 years, yellow; 6–20 years, purple; 6–59 years, magenta; 12–59 years of age, orange; 18–52 years of age).
ALE map information for each study with foci and cluster information.