Abstract
Neuroanatomical differences in the cerebellum are among the most consistent findings in autism spectrum disorder (ASD), but little is known about the relationship between cerebellar dysfunction and core ASD symptoms. The newly-emerging existence of cerebellar sensorimotor and cognitive subregions provides a new framework for interpreting the functional significance of cerebellar findings in ASD. Here we use two complementary analyses — whole-brain voxel-based morphometry (VBM) and the SUIT cerebellar atlas — to investigate cerebellar regional gray matter (GM) and volumetric lobular measurements in 35 children with ASD and 35 typically-developing (TD) children (mean age 10.4 ± 1.6 years; range 8–13 years). To examine the relationships between cerebellar structure and core ASD symptoms, correlations were calculated between scores on the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview (ADI) and the VBM and volumetric data. Both VBM and the SUIT analyses revealed reduced GM in ASD children in cerebellar lobule VII (Crus I/II). The degree of regional and lobular gray matter reductions in different cerebellar subregions correlated with the severity of symptoms in social interaction, communication, and repetitive behaviors. Structural differences and behavioral correlations converged on right cerebellar Crus I/II, a region which shows structural and functional connectivity with fronto-parietal and default mode networks. These results emphasize the importance of the location within the cerebellum to the potential functional impact of structural differences in ASD, and suggest that GM differences in cerebellar right Crus I/II are associated with the core ASD profile.
Keywords: Autism spectrum disorder, Cerebellum, Voxel based morphometry, SUIT, ADOS, ADI
Highlights
-
•
The cerebellum is one of the most consistent sites of abnormality in autism.
-
•
We investigated cerebellar structure in autism using two independent methods.
-
•
Cerebellar gray matter was reduced in several regions in children with autism.
-
•
The degree of cerebellar gray matter reduction predicted core autism symptom severity.
-
•
Structural differences and behavioral correlations converged on cerebellar Crus I/II.
1. Introduction
Autism spectrum disorders (ASDs) are characterized by impairments in social interaction, communication, and restricted or repetitive behaviors and interests (DSM-IV: American Psychiatric Association, 1994). Neuroimaging research has revealed a broad network of regional brain abnormalities in ASD, including frontal, parietal, and limbic regions, the basal ganglia, and the cerebellum (Amaral et al., 2008). Cerebellar structural and functional differences are consistently reported in ASD, suggesting that cerebellar dysfunction may be important in the etiology of the disorder (Allen et al., 2004; Courchesne, 1997; Fatemi et al., 2012). Supporting this, almost all post-mortem analyses of ASD individuals have reported reduced Purkinje cell size and number regardless of age, sex, or cognitive ability (Bailey et al., 1998; Bauman and Kemper, 2005; Fatemi et al., 2002; Whitney et al., 2009). Although neuroimaging meta-analyses suggest that several different regions of the cerebellum are affected in ASD (Duerden et al., 2012; Stoodley, 2014; Yu et al., 2011), no study has used both voxel-based and lobular region of interest analyses to examine structural differences within the cerebella of autistic individuals, while also assessing the relationship between these cerebellar subregions and core ASD symptoms.
Anatomically, the cerebellum is divided into ten lobules (lobules I–X) and three lobes: the anterior lobe (lobules I–V), the posterior lobe (lobules VI–IX), and the flocculonodular lobe (lobule X; Fig. 1). Anatomical, clinical, and neuroimaging studies support the idea that regions within the cerebellum have functionally distinct roles in movement, cognition and affective processing (Stoodley and Schmahmann, 2010). Somatomotor representations of the body are found in the anterior lobe and lobule VIII, which interconnect with sensorimotor areas of the cerebral cortex and are engaged during sensorimotor tasks (Stoodley and Schmahmann, 2010). The large posterior lobe, including lobules VI and VII (which is subdivided into Crus I, Crus II and VIIB), receives input from prefrontal and parietal association areas and is engaged during cognitive tasks (Strick et al., 2009). Recent functional connectivity data show that the majority of the cerebellum is functionally connected to association networks involved in cognitive and affective processes, rather than somatomotor networks (Buckner et al., 2011). Clinical outcomes also reflect the topography seen in healthy controls, as lesions involving posterior regions of cerebellum can lead to difficulties in executive functioning, language, memory and affect, while damage to the anterior cerebellum can result in motor impairments with minimal cognitive effects (Schmahmann and Sherman, 1998). Based on these data, the putative role of the human cerebellum has been expanded to include higher order cognitive and affective processes (Ito, 2008; Stoodley and Schmahmann, 2009; Strick et al., 2009). The unique patterns of connectivity of different cerebellar subregions result in a functional topography, whereby different regions process different types of information (Stoodley, 2012; Strick et al., 2009). This topography is of importance when considering the localization of cerebellar structural and functional differences in ASD, and may be beneficial in the interpretation of cerebellar findings in ASD.
Fig. 1.

The human cerebellum with lobules I–X color-coded. From the spatially unbiased infratentorial template [SUIT] of the cerebellum and brainstem (Diedrichsen et al., 2009; Diedrichsen, 2006).
Neuroimaging studies comparing ASD with typically developing (TD) individuals reveal differences in several regions of the cerebellum. Structural MRI studies have described hypoplasia of the posterior vermis in ASD (Carper and Courchesne, 2000; Courchesne et al., 1988, 1994, 2011; Murakami et al., 1989), and meta-analyses of voxel-based morphometry (VBM) studies have reported consistent gray matter (GM) decreases in right Crus I, lobule VIII, and lobule IX (Duerden et al., 2012; Stoodley, 2014; Yu et al., 2011). Decreased GM is less commonly reported in regions such as left Crus I, and sometimes overall increased cerebellar GM is noted (Duerden et al., 2012; Yu et al., 2011). Functional MRI studies have revealed reduced activation in the cerebellum in ASD during social, language, and motor tasks. Individuals with ASD underactivate Crus I while processing facial and vocal stimuli (Wang et al., 2007) and during executive functioning paradigms (Solomon et al., 2009). Language tasks elicit abnormal activation in lobule VII in autistic individuals during core aspects of communication such as semantic processing (Harris et al., 2006). Lastly, children with ASD fail to engage the anterior cerebellum (lobule IV/V) during motor tasks when compared to their TD peers (Mostofsky et al., 2009). While convergence across studies exists, there remains significant variation among cerebellar neuroimaging findings in ASD.
Clinical studies also report ASD-like symptomology in patients with cerebellar abnormalities. Malformations of the cerebellar vermis are associated with social and affective disorders, while cerebellar hemisphere malformations are linked to expressive language, gross motor, and executive functioning deficits, symptoms relevant to ASD (Bolduc et al., 2012; Tavano et al., 2007). Premature infants sustaining cerebellar damage have a 40-fold increase in positive ASD screens relative to controls (Limperopoulos et al., 2007). Further, individuals with Tuberous Sclerosis (TSC) have high rates of ASD symptoms (Gillberg et al., 1994; Hunt and Shepherd, 1993; Smalley et al., 1992; Wing and Gould, 1979) which have been specifically related to tubers located within the cerebellum (Weber et al., 2000).
The links between cerebellar dysfunction and ASD symptomology have led some to posit that autism might be a “disease of the cerebellum” (Rogers et al., 2013). However, few studies have examined the role of specific cerebellar subregions in autism, and even fewer have investigated correlations between regional GM and behavioral measures in ASD (Kosaka et al., 2010; Riva et al., 2013; Rojas et al., 2006). Thus far, most studies have examined differences at the hemispheric level, and studies investigating regional differences often have not localized findings to particular cerebellar lobules. Given the emerging functional topography of the cerebellum and the various cerebellar regions implicated in autism pathophysiology, it is important to investigate more discrete subdivisions within the cerebellum and to consider their functional relevance. The present study investigates cerebellar structure in ASD and links the structural findings to the core symptoms of the disorder.
To our knowledge, this is the first study to examine the cerebellum in autism at both a voxel-based and lobular level by using two complementary approaches — voxel-based morphometry (VBM) (Ashburner and Friston, 2000; Good et al., 2001) and the Spatially Unbiased Infratentorial Template (SUIT) (Diedrichsen et al., 2009; Diedrichsen, 2006) for lobular region of interest (ROI) volumetric analysis. Moreover, this is the first study to correlate cerebellar lobular volumes with behavioral measures. Unlike ROI approaches, VBM allows for a precise, voxel-level examination of the cerebellum in the context of the whole brain in an unbiased, operator-independent manner. Complementing this approach, the SUIT template and atlas allow for a ROI-based examination of cerebellar substructure by providing a high-resolution atlas and template of the human cerebellum and brainstem. The more commonly-used MNI template provides little contrast for the cerebellum and cerebellar structures, while the SUIT template preserves the anatomical detail of the cerebellum and allows for better localization of cerebellar findings. As lobules are anatomically (rather than functionally) defined, VBM can elucidate with millimeter resolution how structure is related to ASD symptomology without the confines of lobular boundaries, and might reveal GM differences that span or cross lobules. On the other hand, the SUIT ROI method provides an excellent template for measuring specific cerebellar lobules and may be more statistically powerful than the VBM approach; however, the more gross lobular measures might hide subtle GM differences between groups. Because there can be differences in results when voxel-based vs. ROI approaches are employed, using both of these techniques in the same dataset helps to establish structural differences that converge across analysis methods. Therefore, combining VBM and SUIT methods in the same dataset allows for an examination of the cerebellum at the voxel-level and lobular-level, capitalizing on the strengths of each approach. For both approaches, we examined the relationship between ASD symptoms and cerebellar structure.
2. Materials and methods
2.1. Participants
Seventy children aged 8–13 years participated in this study: 35 children with ASD (30 males; mean age = 10.4 ± 1.6 years; 32 right-handed, 3 left-handed) and 35 TD children (21 males; mean age = 10.4 ± 1.5 years; 32 right-handed, 2 left-handed, 1 mixed dominance). TD participants were age-matched to ASD subjects by closest age. When there were multiple exact or closest-aged TD participants, participants were matched by sex. Participants were recruited as part of an on-going study conducted by the Center for Neurodevelopment and Imaging Research (CNIR) at the Kennedy Krieger Institute. Sources of recruitment included advertisements posted in the community, local pediatricians' and psychologists' offices, local schools, social service organizations, chapters of the Autism Society of America, the Interactive Autism Network (IAN) database, outpatient clinics at Kennedy Krieger Institute, and word of mouth. This study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and assent was obtained from each child.
None of the children had intellectual disability, seizure or other neurological disorder, any severe chronic medical disorder, diagnosed genetic disorder, or psychotic disorder. In the TD group, additional exclusions included any psychiatric disorder (except specific or social phobia), speech and language disorder, broader autism phenotype effects (Piven and Palmer, 1997), and a family history of first-degree relatives with ASD.
Intellectual ability was assessed using the Wechsler Intelligence Scale for Children 4th edition (WISC-IV; Wechsler, 2003). All TD subjects and 33/35 ASD subjects had full scale IQs (FSIQs) above 79. Two ASD subjects with FSIQs below 79 were also included based on Verbal Comprehension Index (VCI) scores of 85 or greater. This is in line with recommendations to individualize measures best suited for estimation of cognitive abilities in children with ASD (Mottron, 2004).
2.2. ASD diagnosis
Participants with a diagnosis of ASD were assessed by a master's level or higher psychologist who was reliable according to research criteria. The Autism Diagnostic Interview — Revised (ADI-R; Lord et al., 1994) and the Autism Diagnostic Observation Schedule — Generic (ADOS-G; Lord et al., 2000) module 3 were administered to confirm ASD diagnosis. All participants met DSM-IV criteria for ASD based on the ADOS-G or ADI-R and the clinical impression of the investigators.
2.3. Image acquisition
For each participant, a high-resolution T1-weighted MP-RAGE was acquired on a Philips 3 T Achieva MRI scanner (Best, Netherlands) using an 8-channel head coil (TR = 7.99 ms, TE = 3.76 ms, flip angle = 8°, voxel size = 1 mm3 isotropic). Scans containing significant motion artifacts or poor gray/white matter differentiation were excluded from a larger sample to produce the current dataset.
2.4. Image processing
2.4.1. Voxel based morphometry
Voxel based morphometry (VBM) was used to identify differences in regional GM volume between the autism and TD groups using SPM8 implemented in MATLAB 2012b. T1 anatomical images were pre-processed using an optimized VBM procedure (Ashburner and Friston, 2000; Good et al., 2001; Mechelli et al., 2005) including: examination of each image for gross anatomical abnormalities and scanner artifacts; setting the origin to the anterior commissure; segmentation into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using “New Segment” in SPM8; creation of a study-specific template by importing parameter files produced during segmentation into DARTEL; affine transformation of segmented tissues into MNI space; and standard smoothing with an 8 mm FWHM Gaussian kernel. The smoothed, modulated, normalized data were used in the statistical analyses. The modulation step was added so that the final VBM statistics reflect “volume” differences rather than “concentration” differences in GM (Good et al., 2001; Mechelli et al., 2005).
2.4.2. Lobular volume analysis
Lobular volumes were calculated using the SUIT toolbox (Diedrichsen et al., 2009; Diedrichsen, 2006) implemented in SPM8. The procedure involved: cropping and isolating the cerebellum from the T1 anatomical images; normalizing each cropped image into SUIT space; reslicing the probabilistic cerebellar atlas into individual subject space using the deformation parameters from normalization; and calculating the number of voxels in each lobule in the resliced images. The SUIT probabilistic atlas is a GM template only and excludes WM. This process therefore resulted in 28 volumetric GM measurements, reflecting the ten bilateral lobules (I–X right and I–X left; lobules I–IV are combined into one measure, and lobule VII is divided into VIIB, Crus I and Crus II; lobule VIII is divided into VIIIA and VIIIB) and vermis lobules VI–X.
2.5. Statistical analysis
2.5.1. Comparisons of total intracranial volume
Total intracranial volume (TIV) was calculated by summing the total GM, WM, and CSF volumes. Two-tailed t-tests were performed to identify any group differences in total GM, total WM, total CSF, and TIV.
2.5.2. Voxel based morphometry
Regional differences in GM between groups were assessed using the general linear model (GLM) in SPM8. Smoothed, modulated, normalized GM images were entered into a voxel-wise two-sample t-test analysis. Results were thresholded at p < 0.001 (uncorrected) with an extent threshold of 50 voxels to control for type I error. An absolute threshold mask of 0.2 was used to avoid edge effects at the borders of GM and WM. Total intracranial volume (TIV = GM + WM + CSF) was entered as a covariate. Gender was also entered as a covariate in the statistical model due to significantly different gender distributions in the ASD and TD groups (χ2 = 4.62, p = 0.032).
2.5.3. Lobular volume analysis
Lobular volumes were corrected for total cerebellar volume (individual lobular volumes / total cerebellar volume) and two-tailed t-tests were performed to identify group differences.
2.6. Behavioral correlations with regional GM
Multiple regression analyses were conducted in SPM8 to identify correlations between the ADI and ADOS scores and regional GM volume. Total intracranial volume (TIV) was entered as a covariate. Results were thresholded at p < 0.005 (uncorrected) with an extent threshold of 50 voxels. Multiple regression analyses were conducted between GM and ADOS scores for 32 subjects and between GM and ADI scores for 33 subjects due to missing data. For the lobular volumes, Spearman rank-order correlations between lobular volumes and ADOS and ADI behavioral scores were calculated in SPSS (IBM SPSS Statistics). Correlations significant at p < 0.01 are reported.
3. Results
3.1. Total intracranial volume (TIV)
No significant effects of diagnosis were observed for TIV (p = 0.077, ASD > TD), total GM (p = 0.114), or total WM (p = 0.090, ASD > TD). A significant effect of diagnosis was observed for total CSF volume (p = 0.037, ASD > TD). Because TIV and total WM approached significance, TIV was entered as a covariate for subsequent analyses.
3.2. Cerebellar analyses
3.2.1. Voxel based morphometry
Children with ASD showed significantly reduced GM in right Crus I/II (Fig. 2). Right Crus I/II was the largest cluster in the brain in which ASD < TD (MNI 24 −70 −39, T = 3.91, k = 170), with the second-highest T-value. There were no cerebellar regions where children with ASD showed greater GM relative to TD children. Whole-brain findings are reported in Supplemental Data Table S1.
Fig. 2.
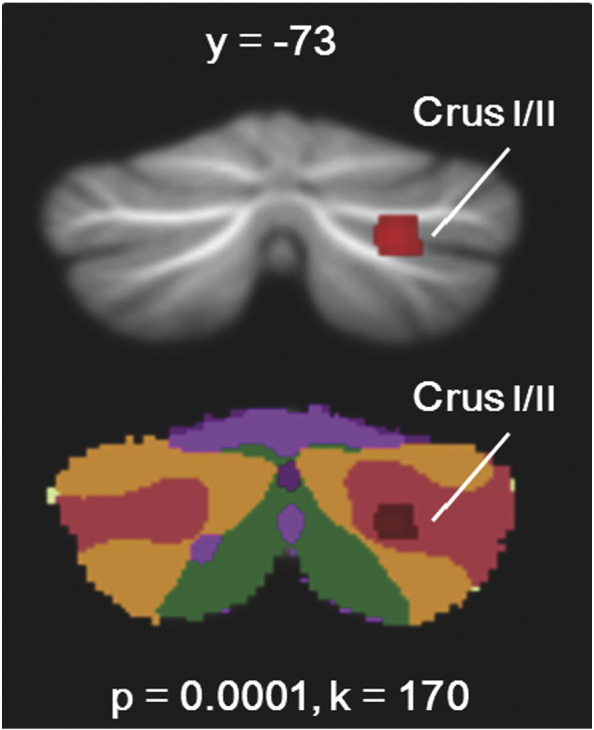
Reduced GM in ASD relative to controls. Children with ASD had reduced GM in right Crus I and II. This cluster falls within the default mode network in the context of Buckner et al.'s (2011) functional connectivity maps. Networks: blue = somatomotor; Violet = ventral attention; Green = dorsal attention; Cream = limbic; Orange = frontoparietal; Red = default mode.
3.2.2. Cerebellar lobular Volumes
Similar to the VBM data, the lobular analysis revealed that right Crus I was significantly smaller in children with ASD (11.16% of cerebellar volume) compared to TD children (11.70% of cerebellar volume, p = 0.033). Vermis VIIIA and vermis VIIIB were significantly larger in children with ASD (vermis VIIIA, 0.90% of cerebellar volume; vermis VIIIB, 0.43%) when compared to TD children (vermis VIIIA, 0.80% of cerebellar volume; vermis VIIIB, 0.40%; p = 0.005 and p = 0.014, respectively).
3.3. Behavioral correlations
3.3.1. VBM: regional GM
Multiple regression analyses revealed significant negative correlations between regional cerebellar GM volume and ADOS and ADI scores, such that smaller GM volumes were associated with higher (more impaired) scores (Table 1). No positive correlations between cerebellar GM and behavioral scores were found.
Table 1.
Cerebellar regions in which GM correlated with behavioral (ADOS and ADI) scores.
| Behavioral Measure | Cerebellar region | Cluster Size (voxels) | Max T | p-Value | MNI coordinates |
|---|---|---|---|---|---|
| ADOS Social + Communication | Right VI/Crus I | 328 | 3.51 | 0.001 | 18 −82 −23 |
| Right VIIIA/VIIIB | 309 | 3.46 | 0.001 | 23 −57 −56 | |
| ADOS Social Interaction | Right VI/Crus I/Crus II | 2173a | 4.61 | 0.00004 | 18 −82 −23 |
| Right VIIIA/VIIIB | 407 | 3.62 | 0.001 | 23 −57 −53 | |
| Left VIIIB/IX | 143 | 3.33 | 0.001 | −14 −45 −51 | |
| Vermis I–IV | 140 | 3.30 | 0.001 | −2 −48 −23 | |
| Vermis V/VI | 64 | 3.15 | 0.002 | 2 −66 −15 | |
| Right I–IV | 71 | 3.03 | 0.003 | 18 −34 −23 | |
| Left VIIIA | 71 | 3.02 | 0.003 | −27 −49 −53 | |
| ADOS Stereotyped Behaviors and Restricted Interests | Right Crus I/II | 49b | 3.16 | 0.002 | 24 −73 −38 |
| ADI Restricted, Repetitive, and Stereotyped Behaviors | Right IV/V | 244 | 3.33 | 0.001 | 29 −31 −30 |
| ADI Social Interaction | Right I–IV | 49b | 2.90 | 0.003 | 21 −30 −27 |
MNI coordinates = x, y, z depict coordinates of cluster peak.
Largest, most significant cluster in the brain.
This cluster was only evident at an extent threshold of k = 49.
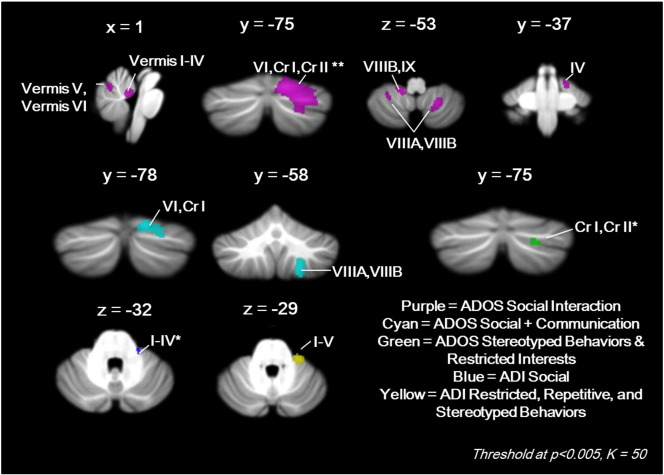
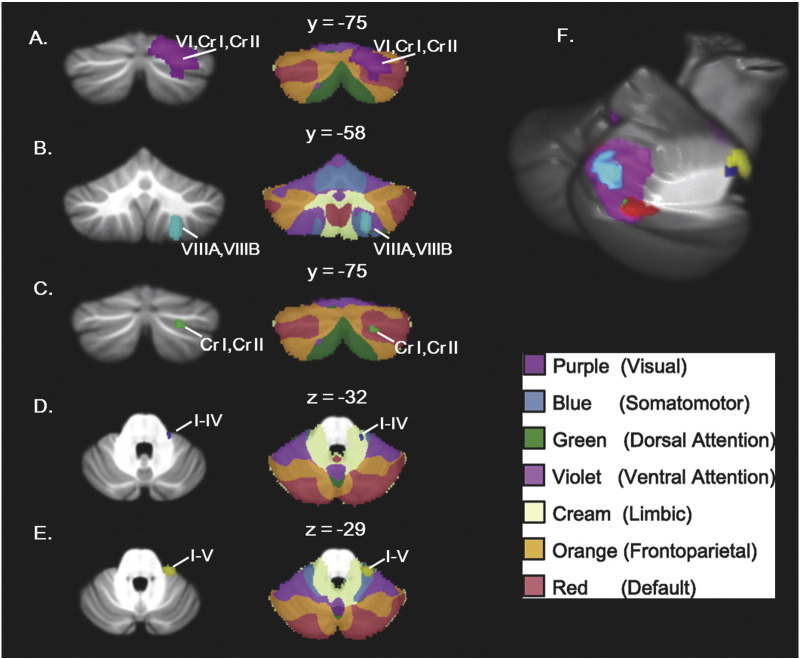
No significant correlations were found between cerebellar GM and ADOS or ADI Communication scores. Higher severity ADOS Social + Communication scores correlated with reduced GM in right lobule VI/Crus I and right lobule VIIIA/VIIIB (cyan, Fig. 3). Poorer ADOS Social Interaction scores correlated with reduced GM in several clusters in vermis I–IV; vermis V–VI; right lobule I–IV; right lobule VI/Crus I/Crus II (largest and most significant cluster in the brain); right lobule VIIIA/VIIIB; left lobule VIIIA/VIIIB; and left lobule VIIIB/IX (purple, Fig. 3). Higher severity ADOS Stereotyped Behaviors and Restricted Interests scores correlated with reduced GM in right Crus I/II (green, Fig. 3), and more severe ADI Restricted, Repetitive, and Stereotyped Behaviors scores correlated with reduced GM in right lobules I–V (yellow, Fig. 3). Higher severity ADI, Social Interaction scores correlated with reduced GM in right lobules I–IV (blue, Fig. 3). Fig. 4 shows these findings overlaid on the 7-network functional connectivity maps established by Buckner et al. (2011). Correlations with core ASD symptoms converged on right Crus I/II, where there was significantly reduced GM in the ASD group in both the VBM and lobular analyses (Fig. 4F).
Fig. 3.
Cerebellar regions in which reduced GM correlated with ADOS and ADI scores. **Largest and most significant cluster in the brain (k = 2173, T = 4.61); *k = 49.
Fig. 4.
Behavioral correlations (A–E, left) shown in the context of Buckner et al.'s (2011) functional connectivity maps (A–E, right) and convergence of findings on right Crus I/II (F). (A) ADOS Social Interaction scores; (B) ADOS communication + Social Interaction scores; (C) ADOS stereotyped behaviors and restricted interests; (D) ADI, Social Interaction; (E) ADI restricted, repetitive, and stereotyped behaviors; (F) convergence of main diagnostic criteria of ASD on right Crus I/II area. Network legend is from Yeo et al. (2011).
3.3.2. SUIT: lobular volumes
Consistent with the VBM results, smaller vermis VI correlated with higher severity ADOS Social scores (Table 2). However, some SUIT findings diverged from the VBM results. Smaller vermis VI was also associated with worse ADI Communication scores. Smaller left and right lobules VIIIB correlated with more severe ADOS Stereotyped Behaviors and Restricted Interest scores. Lastly, larger vermis VIIB and VIIIA correlated with higher severity ADI Restricted, Repetitive and Stereotyped Behavior scores.
Table 2.
Correlations between SUIT lobular volumes and behavioral scores.
| Behavioral subscale | Vermis VI | Vermis VIIB | Vermis VIIIA | Left VIIIB | Right VIIIB |
|---|---|---|---|---|---|
| ADOS Social Interaction |
p = .006, r = −.435a |
– | – | – | – |
| ADOS Stereotyped Behaviors and Restricted Interests | – | – | – |
p = .006, r = −.436 |
p = .007, r = −.432 |
| ADI Restricted, Repetitive, and Stereotyped Behaviors | – |
p = .009, r = .408 |
p = .002, r = .478 |
– | – |
| ADI Communication |
p = .006, r = −.372 |
– | – | – | – |
Consistent with VBM findings.
4. Discussion
We investigated the localization of cerebellar structural differences in ASD and the relationship between cerebellar gray matter, lobular volumes, and core autistic symptoms. To our knowledge, this is the first study to examine structural differences in the cerebellum in children with ASD using two independent methods: whole-brain VBM and lobular volume analysis using the SUIT atlas. Further, in each analysis we examined the correlations between behavioral scores and structural measures. Across analysis methods, right Crus I/II was consistently reduced in ASD participants when compared to TD participants. In the VBM analysis, reductions in the right Crus I/II cluster predicted the severity of core ASD symptoms. In several cerebellar regions, more abnormal GM findings were associated with more severe ASD symptoms, suggesting that these structural differences are functionally significant. These findings support the involvement of the cerebellum in ASD, and further identify specific cerebellar subregions where structural differences are found.
4.1. Right Crus I and II
The most consistent finding was in right Crus I/II, where both whole-brain VBM and lobular analyses revealed smaller GM volumes in ASD relative to the TD group. In particular, the VBM results showed that reduced GM in right Crus I predicted higher severity on all ADOS subscales, suggesting that this region might be a valid potential cerebellar biomarker in ASD. These findings are also consistent with previous studies: meta-analyses of GM volume differences in ASD have reported decreased GM in right Crus I (Duerden et al., 2012; Stoodley, 2014; Yu et al., 2011), and reduced GM in right Crus I/II was found to correlate with increased repetitive behaviors, poorer communication scores, and poorer Social Interaction scores in both children and adults with ASD (Riva et al., 2013; Rojas et al., 2006).
What might be the functional significance of structural differences in right Crus I/II? Crus I and Crus II are anatomically and functionally connected to prefrontal and parietal regions of the cerebral cortex (Buckner et al., 2011; Hoover, and Strick, 1999; Kelly and Strick, 2003; Strick et al., 2009), and resting-state functional connectivity data suggest that Crus I is functionally connected to ventral attention, frontoparietal, and default mode networks (Buckner et al., 2011). Reduced connectivity within these networks, particularly the default mode and frontoparietal networks, has been related to impaired social and communicative abilities in ASD (Assaf et al., 2010; Redcay et al., 2013; Washington et al., 2014). Task-based fMRI studies reveal right Crus I activation during executive function paradigms, language tasks and subtasks supporting language, and emotional processing (Mathiak et al., 2002, 2004; Sen et al., 2011; Stoodley, 2012; Stoodley and Schmahmann, 2009) and a recent meta-analysis found that this region is involved in abstract social cognition (Van Overwalle et al., 2014). In ASD, abnormal activation in right Crus I/II is found during language and executive function measures, and during processing of facial and vocal stimuli (Harris et al., 2006; Solomon et al., 2009; Wang et al., 2007). Differences in right cerebellar development could affect the function of contralateral left language regions. Consistent with this idea, Verly et al. (2014) found a prominent decrease of connectivity in ASD between right Crus I and supratentorial language regions, including the supplementary motor area (SMA), inferior frontal gyrus, and dorsolateral prefrontal cortex. These data, together with the results of our correlation analyses, suggest that structural differences in right Crus I/II could impact the core social and communication deficits in ASD.
Crus I and II are also implicated in imitation and praxis. Healthy individuals engage lobule VI and Crus I during motor imitation and when copying emotional facial expressions (Dapretto et al., 2006; Leslie et al., 2004). The task-specific increase in right Crus II activation during imitation, together with the significant psychophysiological interactions between right Crus I and the superior temporal sulcus (STS), implies connections between right Crus I and cortical regions involved in biological motion processing (Jack et al., 2011). Significant psychophysiological interactions were also found between left Crus II and the superior parietal lobe (SPL), which is implicated in attentional processes underlying imitation (Jack et al., 2011). Others report enhanced synchronization between right lobule VI/Crus I and left superior temporal cortex prior to movement, suggesting that the cerebellum is involved in early motor planning during observation as well as imitation execution (Kessler et al., 2006). Behaviorally, individuals with autism are impaired in imitation and praxis (Edwards, 2014; Mostofsky et al., 2006, 2009; Müller et al., 2003), and GM differences in Crus I and II could result in underconnectivity with multiple cortical areas involved in imitation and praxis. The resulting deficits might underlie some of the social, communication, and motor impairments in autism (Mostofsky et al., 2006; Rogers and Pennington, 1991).
4.2. Anterior cerebellum and the cerebellar vermis
Poorer ADI Social Interaction scores were related to decreased GM in anterior cerebellar lobules I–V, and poorer ADOS Social Interaction scores correlated with decreased GM in vermis I–V and vermis VI–VII. These findings are consistent with previous work showing decreased volume in vermis I–V in language-impaired individuals with autism (Hodge et al., 2010). This region of the cerebellum is typically associated with motor function, although recent functional connectivity mapping of the anterior cerebellum suggests connections with both motor and limbic regions of the cerebral cortex (Buckner et al., 2011). Supporting this, in our study the ADI repetitive behavior cluster was located within the proposed somatomotor region (Fig. 4E), and ADI social scores correlated with a cluster falling within the limbic network (Fig. 4D). Motor symptoms are common in individuals with ASD (Gidley Larson and Mostofsky, 2008), and are among some of the earliest identifiable signs distinguishing children with autism from their TD peers (Landa and Garrett-Mayer, 2006). Consistent with our structural findings, functional imaging studies show that individuals with autism fail to recruit cerebellar lobules IV/V during a motor task (Mostofsky et al., 2009). Importantly, motor impairment in autism often co-occurs with verbal impairment (Noterdaeme et al., 2010) and can be predictive of social, communication, and repetitive behavior impairments (Leonard et al., 2014; Linkenauger et al., 2012; Travers et al., 2013). Altogether, these data support supports our finding that the greater the GM reduction in the anterior cerebellum, the more impaired the ADI and ADOS Social Interaction scores.
ADOS Social Interaction scores also correlated with a cluster in the posterior vermis. Decreased volume of vermis VI–VII is inversely related to the volume of the frontal cortex in ASD (Carper and Courchesne, 2000), suggesting that abnormalities in this posterior midline cerebellar region might have knock-on effects on frontally-mediated cognitive skills involved in social interaction.
The SUIT analyses also revealed autism-associated abnormalities within the vermis. Lobular volumes of vermis VIIIA and vermis VIIIB were increased in children with ASD, and increased lobular volumes in vermis VIIB and VIIIA were associated with increased repetitive behaviors. While hypoplasia of the posterior vermis has been reported in ASD (Courchesne et al., 1988; Levitt et al., 1999), a small subset of studies have noted hyperplasia of the posterior vermis in autism. For example, Salmond et al. (2007) found an increase in GM in posterior vermis VIII in individuals with ASD, and hyperplasia of the posterior vermis has also been noted in Fragile X individuals with autism (Kaufmann et al., 2003). It has been suggested that hyperplasia in vermis VI and VII may only be evident in a subset (~11%) of individuals with ASD (Akshoomoff et al., 2004; Courchesne et al., 1994).
Poor registration, different normalization techniques, and the relatively small size of the vermal lobules may account for differences in localization to specific posterior vermal lobules between studies. In addition, factors such as IQ have been shown to mediate vermis size in ASD (Courchesne et al., 1994). Although Courchesne et al. (1994) observed that all instances of vermal hyperplasia occurred in individuals with IQs less than 70, little is known about the conditions under which hyperplasia occurs. Behaviorally, abnormalities in vermis VI–VII have been shown to correlate with increased repetitive behaviors and decreased exploratory actions (Pierce and Courchesne, 2001), consistent with our findings.
4.3. Lobule VIII
In children with ASD, we found that smaller lobular volumes in bilateral VIIIB were associated with higher ratings of repetitive behaviors and restricted interests, consistent with the proposed role of cerebellar lobule VIII in sensorimotor function. GM reductions in lobule VIII were also associated with increased social/communicative impairment. This finding is not unprecedented, as a previous study reported that decreased GM in lobule VIII was associated with impaired social interaction and communication scores (Rojas et al., 2006). In healthy individuals, lobule VIII is activated during a variety of cognitive tasks, including verb generation and working memory paradigms (Stoodley and Schmahmann, 2009). Specifically, it has been proposed that lobule VIII is involved in cerebro-cerebellar loops important in sustaining the phonological store (Chen and Desmond, 2005), suggesting that reduced GM in lobule VIII in ASD might impair cerebro-cerebellar loops important in working memory. Working memory impairments are thought to exacerbate social and communication deficits in children with ASD (D'Urso et al., 2014), and our finding that reduced GM in lobule VIIIA was associated with impairments in social interaction and social + communication scores support this hypothesis.
4.4. Lobule IX
GM reductions in lobule IX also were associated with increased social/communicative impairments. While the function of lobule IX is not well defined, Buckner et al. (2011) suggest it may form a tertiary representation of cerebral cortical networks based on its functional connectivity patterns (Kelly and Strick, 2003). Lobule IX is functionally connected to areas such as the posterior cingulate, thalamus, and angular gyrus, and is part of the default mode network (Bernard et al., 2012; Buckner et al., 2011). Within the cerebellum, lobule IX is highly functionally connected to Crus I, VIIIA, and the anterior cerebellum (Bernard et al., 2012), regions that are consistently abnormal in ASD (Duerden et al., 2012; Stoodley, 2014; Yu et al., 2011). In healthy individuals, lobule IX is activated in response to conflict experienced when breaking with social norms (Klucharev et al., 2009). Therefore, decreased GM in lobule IX might inhibit proper default mode network connectivity, leading to dysfunction in brain areas involved in social and communication processes.
4.5. What is the cerebellum doing and why is it relevant to ASD?
The combined evidence from the voxel-based and lobular analyses provides further support that autism is associated with structural differences in the cerebellum, and that these differences have functional relevance. It is thought that, due to its stereotyped neuronal architecture, the cerebellum performs the same operation on any input it receives (see Ito, 2006). The computation performed by the cerebellum (which Schmahmann calls the “universal cerebellar transform”; Schmahmann, 1991) can be applied to many types of information involved in motor, cognitive and affective processing (Ito, 2006; Schmahmann, 1991). Cerebellar output, therefore, is determined by the input received via its anatomical connections with different regions of the brain. Damage or developmental abnormalities affecting the cerebellum could impede the basic processing of the cerebellum, with knock-on effects on cerebellar modulation of cerebro-cerebellar loops in a way that is relevant to ASD symptomology. Previous studies have shown this to be the case. For example, preterm infants with injury to the cerebellum had impaired growth of the contralateral cerebral cortex (Limperopoulos et al., 2010), as well as significantly impaired expressive language, delayed receptive language, cognitive deficits, and motor disabilities (Limperopoulos et al., 2007).
In the current study, the degree of GM reduction in discrete regions of the cerebellum was correlated with the severity of social, communication, and repetitive behavior impairments on autism diagnostic scales. This suggests that the processing provided by the cerebellum is relevant to a range of ASD symptoms, and not only motor behaviors. Further, the findings indicate that the degree of cerebellar abnormality predicts the severity of ASD symptoms. Reduced regional cerebellar GM might impair specific cerebro-cerebellar loops, linking the cerebellum to the multiple, distributed neural circuits underlying the disorder. Our data support this concept, as cerebellar regions associated with poorer ADOS and ADI scores included limbic, fronto-parietal, somatomotor and default mode networks. It has been suggested that the cerebellum may be important in setting up distant cortical networks in the brain during development (see Wang et al., 2014), indicating that structural deficits in the cerebellum might have long-term effects on cortical specialization. Disruptions in specific cerebro-cerebellar loops might impair proper specialization of cortical regions involved in language, social interaction, and motor control in ASD, leading to long-term impairments in these domains. Therefore, the GM differences in the cerebellum could potentially impact the cerebral cortical networks that support both motor and non-motor language and social cognitive functions (Van Overwalle et al., 2014).
4.6. Limitations
The results from the VBM and SUIT methods were not always consistent, which may be due to the differences in the resolution of these two approaches. For example, SUIT analyses revealed increased volumes in vermis VIIIA and VIIIB, while no increases were found in these regions in the VBM analysis. Increased vermis VIIIA and VIIIB volumes have been reported in a previous VBM study (Salmond et al., 2007), but this finding is not consistent across studies (e.g., Riva et al., 2013). It might be the case that in the current analysis, increased vermis volume was only apparent at the lobular level because the GM differences did not meet our cluster threshold in the VBM analysis. In fact, when using a more lenient voxel-level threshold without a cluster correction, a small cluster (k = 36; p = 0.026) of increased GM was evident in vermal VIIIA/VIIIB. Therefore, it is possible that inconsistencies between the two approaches reflect the differences in statistical power in voxel-based vs. ROI analyses.
4.7. Conclusion
Of particular interest for future research is the specific contribution of the right Crus I/II region where we found that decreased GM correlated with impaired social interaction, impaired communication, and increased repetitive behaviors — the hallmarks of an ASD diagnosis. Future research will aim to elucidate the specific contribution of this area to ASD symptomology.
The following is the supplementary data related to this article.
Whole-brain results for VBM group comparison between ASD and TD.
Acknowledgments
This work was supported by NIH/NINDS R01 NS048527-08, NIH/NCATS grants UL1 TR 000424-06 and P41 EB015909-13, and the Autism Speaks Foundation grants #2506, #2384, and #1739.
Contributor Information
Anila M. D'Mello, Email: anila.dmello@american.edu.
Deana Crocetti, Email: crocetti@kennedykrieger.org.
Stewart H. Mostofsky, Email: mostofsky@kennedykrieger.org.
Catherine J. Stoodley, Email: stoodley@american.edu.
References
- Akshoomoff N., Lord C., Lincoln A.J., Courchesne R.Y., Carper R.A., Townsend J., Courchesne E. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(3):349–357. doi: 10.1097/00004583-200403000-00018. 15076269 [DOI] [PubMed] [Google Scholar]
- Allen G., Müller R.-A., Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol. Psychiatry. 2004;56(4):269–278. doi: 10.1016/j.biopsych.2004.06.005. 15312815 [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. 18258309 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fourth edition. Psychiatric Press; Arlington, VA, American: 1994. [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry — the methods. NeuroImage. 2000;11(6 1):805–821. doi: 10.1006/nimg.2000.0582. 10860804 [DOI] [PubMed] [Google Scholar]
- Assaf M., Jagannathan K., Calhoun V.D., Miller L., Stevens M.C., Sahl R. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05.067. 20621638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A., Luthert P., Dean A., Harding B., Janota I., Montgomery M. A clinicopathological study of autism. Brain. 1998;121(5):889–905. doi: 10.1093/brain/121.5.889. 9619192 [DOI] [PubMed] [Google Scholar]
- Bauman M.L., Kemper T.L. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. 15749244 [DOI] [PubMed] [Google Scholar]
- Bernard J.A., Seidler R.D., Hassevoort K.M., Benson B.L., Welsh R.C., Wiggins J.L. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. 22907994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc M.-E., du Plessis A.J., Sullivan N., Guizard N., Zhang X., Robertson R.L., Limperopoulos C. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 2012;11(2):531–542. doi: 10.1007/s12311-011-0312-z. 21901523 [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. 21795627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper R.A., Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123(4):836–844. doi: 10.1093/brain/123.4.836. 10734014 [DOI] [PubMed] [Google Scholar]
- Chen S.H., Desmond J.E. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43(9):1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. 15949507 [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr. Opin. Neurobiol. 1997;7(2):269–278. doi: 10.1016/s0959-4388(97)80016-5. 9142760 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Campbell K., Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. 20920490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Saitoh O., Yeung-Courchesne R., Press G.A., Lincoln A.J., Haas R.H., Schreibman L. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. A.J.R. Am. J. Roentgenol. 1994;162(1):123–130. doi: 10.2214/ajr.162.1.8273650. 8273650 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Yeung-Courchesne R., Press G.A., Hesselink J.R., Jernigan T.L. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. 3367935 [DOI] [PubMed] [Google Scholar]
- D'Urso G., Ferrucci R., Bruzzese D., Pascotto A., Priori A., Altamura C.A. Transcranial direct current stimulation for autistic disorder. Biol. Psychiatry. 2014;76(5):e5–e6. doi: 10.1016/j.biopsych.2013.11.009. 24342925 [DOI] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. 16327784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33(1):127–138. doi: 10.1016/j.neuroimage.2006.05.056. 16904911 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. 19457380 [DOI] [PubMed] [Google Scholar]
- Duerden E.G., Mak-Fan K.M., Taylor M.J., Roberts S.W. Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Res. 2012;5(1):49–66. doi: 10.1002/aur.235. 22139976 [DOI] [PubMed] [Google Scholar]
- Edwards L.A. A meta-analysis of imitation abilities in individuals with autism spectrum disorders. Autism Res. 2014;7(3):363–380. doi: 10.1002/aur.1379. 24863681 [DOI] [PubMed] [Google Scholar]
- Fatemi S.H., Aldinger K.A., Ashwood P., Bauman M.L., Blaha C.D., Blatt G.J. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11(3):777–807. doi: 10.1007/s12311-012-0355-9. 22370873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Halt A.R., Realmuto G., Earle J., Kist D.A., Thuras P., Merz A. Purkinje cell Size is reduced in cerebellum of patients with autism. Cell. Mol. Neurobiol. 2002;22(2):171–175. doi: 10.1023/A:1019861721160. 12363198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson J.C., Mostofsky S.H. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Res. 2008;1(6):341–353. doi: 10.1002/aur.54. 19360689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg I.C., Gillberg C., Ahlsén G. Autistic behaviour and attention deficits in tuberous sclerosis: a population-based Study. Dev. Med. Child Neurol. 1994;36(1):50–56. doi: 10.1111/j.1469-8749.1994.tb11765.x. 8132114 [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 1):21–36. doi: 10.1006/nimg.2001.0786. 11525331 [DOI] [PubMed] [Google Scholar]
- Harris G.J., Chabris C.F., Clark J., Urban T., Aharon I., Steele S. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61(1):54–68. doi: 10.1016/j.bandc.2005.12.015. 16473449 [DOI] [PubMed] [Google Scholar]
- Hodge S.M., Makris N., Kennedy D.N., Caviness V.S., Howard J., McGrath L. Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 2010;40(3):300–316. doi: 10.1007/s10803-009-0872-7. 19924522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J.E., Strick P.L. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus Type 1. J. Neurosci. 1999;19(4):1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. 9952421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A., Shepherd C. A prevalence study of autism in tuberous sclerosis. J. Autism Dev. Disord. 1993;23(2):323–339. doi: 10.1007/BF01046223. 8331050 [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006;78(3–5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. 16759785 [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. 18319727 [DOI] [PubMed] [Google Scholar]
- Jack A., Englander Z.A., Morris J.P. Subcortical contributions to effective connectivity in brain networks supporting imitation. Neuropsychologia. 2011;49(13):3689–3698. doi: 10.1016/j.neuropsychologia.2011.09.024. 21958651 [DOI] [PubMed] [Google Scholar]
- Kaufmann W.E., Cooper K.L., Mostofsky S.H., Capone G.T., Kates W.R., Newschaffer C.J. Specificity of cerebellar vermian abnormalities in autism: a quantitative magnetic resonance imaging study. J. Child Neurol. 2003;18(7):463–470. doi: 10.1177/08830738030180070501. 12940651 [DOI] [PubMed] [Google Scholar]
- Kelly R.M., Strick P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. 12968006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler K., Biermann-Ruben K., Jonas M., Siebner H.R., Bäumer T., Münchau A., Schnitzler A. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. NeuroImage. 2006;33(1):227–238. doi: 10.1016/j.neuroimage.2006.06.014. 16876435 [DOI] [PubMed] [Google Scholar]
- Klucharev V., Hytönen K., Rijpkema M., Smidts A., Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61(1):140–151. doi: 10.1016/j.neuron.2008.11.027. 19146819 [DOI] [PubMed] [Google Scholar]
- Kosaka H., Omori M., Munesue T., Ishitobi M., Matsumura Y., Takahashi T. Smaller insula and inferior frontal Volumes in young adults with pervasive developmental disorders. NeuroImage. 2010;50(4):1357–1363. doi: 10.1016/j.neuroimage.2010.01.085. 20123027 [DOI] [PubMed] [Google Scholar]
- Landa R., Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J. Child Psychol. Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. 16712640 [DOI] [PubMed] [Google Scholar]
- Leonard H.C., Bedford R., Charman T., Elsabbagh M., Johnson M.H., Hill E.L., BASIS Team Motor development in children at risk of autism: a follow-up study of infant siblings. Autism. 2014;18(3):281–291. doi: 10.1177/1362361312470037. 24101718 [DOI] [PubMed] [Google Scholar]
- Leslie K.R., Johnson-Frey S.H., Grafton S.T. Functional imaging of face and hand imitation: towards a motor theory of empathy. NeuroImage. 2004;21(2):601–607. doi: 10.1016/j.neuroimage.2003.09.038. 14980562 [DOI] [PubMed] [Google Scholar]
- Levitt J.G., Blanton R., Capetillo-Cunliffe L., Guthrie D., Toga A., McCracken J.T. Cerebellar vermis lobules VIII-X in autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23(4):625–633. doi: 10.1016/s0278-5846(99)00021-4. 10390721 [DOI] [PubMed] [Google Scholar]
- Limperopoulos C., Bassan H., Gauvreau K., Robertson R.L., Sullivan N.R., Benson C.B. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C., Chilingaryan G., Guizard N., Robertson R.L., Du Plessis A.J. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr. Res. 2010;68(2):145–150. doi: 10.1203/PDR.0b013e3181e1d032. 20389260 [DOI] [PubMed] [Google Scholar]
- Linkenauger S.A., Lerner M.D., Ramenzoni V.C., Proffitt D.R. A perceptual-motor deficit predicts social and communicative impairments in individuals with autism spectrum disorders. Autism Res. 2012;5(5):352–362. doi: 10.1002/aur.1248. 22961977 [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr, Leventhal B.L., DiLavore P.C. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. 11055457 [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. 7814313 [DOI] [PubMed] [Google Scholar]
- Mathiak K., Hertrich I., Grodd W., Ackermann H. Cerebellum and speech perception: A Functional Magnetic Resonance Imaging Study. J. Cogn. Neurosci. 2002;14(6):902–912. doi: 10.1162/089892902760191126. 12191457 [DOI] [PubMed] [Google Scholar]
- Mathiak K., Hertrich I., Grodd W., Ackermann H. Discrimination of temporal information at the cerebellum: functional magnetic resonance imaging of nonverbal auditory memory. NeuroImage. 2004;21(1):154–162. doi: 10.1016/j.neuroimage.2003.09.036. 14741652 [DOI] [PubMed] [Google Scholar]
- Mechelli A., Price C., Friston K., Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr. Med. Imaging Rev. 2005;1(2):105–113. [Google Scholar]
- Mostofsky S.H., Dubey P., Jerath V.K., Jansiewicz E.M., Goldberg M.C., Denckla M.B. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J. Int. Neuropsychol. Soc. 2006;12(3):314–326. doi: 10.1017/s1355617706060437. 16903124 [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Powell S.K., Simmonds D.J., Goldberg M.C., Caffo B., Pekar J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(9):2413–2425. doi: 10.1093/brain/awp088. 19389870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L. Matching strategies in cognitive research with individuals with high-functioning autism: current practices, instrument biases, and recommendations. J. Autism Dev. Disord. 2004;34(1):19–27. doi: 10.1023/b:jadd.0000018070.88380.83. 15098953 [DOI] [PubMed] [Google Scholar]
- Müller R.-A., Kleinhans N., Kemmotsu N., Pierce K., Courchesne E. Abnormal variability and distribution of functional maps in autism: an fMRI study of visuomotor learning. Am. J. Psychiatry. 2003;160(10):1847–1862. doi: 10.1176/appi.ajp.160.10.1847. 14514501 [DOI] [PubMed] [Google Scholar]
- Murakami J.W., Courchesne E., Press G.A., Yeung-Courchesne R., Hesselink J.R. Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch. Neurol. 1989;46(6):689–694. doi: 10.1001/archneur.1989.00520420111032. 2730382 [DOI] [PubMed] [Google Scholar]
- Noterdaeme M., Wriedt E., Höhne C. Asperger's syndrome and high-functioning autism: language, motor and cognitive profiles. Eur. Child Adolesc. Psychiatry. 2010;19(6):475–481. doi: 10.1007/s00787-009-0057-0. 19813070 [DOI] [PubMed] [Google Scholar]
- Pierce K., Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry. 2001;49(8):655–664. doi: 10.1016/s0006-3223(00)01008-8. 11313033 [DOI] [PubMed] [Google Scholar]
- Piven J., Palmer P. Cognitive deficits in parents from multiple-incidence autism families. J. Child Psychol. Psychiatry. 1997;38(8):1011–1021. doi: 10.1111/j.1469-7610.1997.tb01618.x. 9413799 [DOI] [PubMed] [Google Scholar]
- Redcay E., Moran J.M., Mavros P.L., Tager-Flusberg H., Gabrieli J.D., Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front. Hum. Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. 24062673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D., Annunziata S., Contarino V., Erbetta A., Aquino D., Bulgheroni S. Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic Spectrum disorders: a VBM-DARTEL study. Cerebellum. 2013;12(5):676–685. doi: 10.1007/s12311-013-0469-8. 23572290 [DOI] [PubMed] [Google Scholar]
- Rogers S.J., Pennington B.F. A theoretical approach to the deficits in infantile autism. Develop. Psychopathol. 1991;3(02):137–162. [Google Scholar]
- Rogers T.D., McKimm E., Dickson P.E., Goldowitz D., Blaha C.D., Mittleman G. Is autism a disease of the cerebellum? An integration of clinical and pre-clinical research. Front. Syst. Neurosci. 2013;7:15. doi: 10.3389/fnsys.2013.00015. 23717269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D.C., Peterson E., Winterrowd E., Reite M.L., Rogers S.J., Tregellas J.R. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. B.M.C. Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. 17166273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond C.H., Vargha-Khadem F., Gadian D.G., de Haan M., Baldeweg T. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex. 2007;43(6):686–699. doi: 10.1016/s0010-9452(08)70498-2. 17710821 [DOI] [PubMed] [Google Scholar]
- Schmahmann J., Sherman J.C. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. An emerging concept. The cerebellar contribution to higher function. Arch. Neurol. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. 1953406 [DOI] [PubMed] [Google Scholar]
- Sens P.M., Almeida C.I., Souza M.M., Gonçalves J.B., Carmo L.C. The role of the cerebellum in auditory processing using the SSI test. Braz. J Otorhinolaryngol. 2011;77(5):584–588. doi: 10.1590/S1808-86942011000500008. 22030965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley S.L., Tanguay P.E., Smith M., Gutierrez G. Autism and tuberous sclerosis. J. Autism Dev. Disord. 1992;22(3):339–355. doi: 10.1007/BF01048239. 1400103 [DOI] [PubMed] [Google Scholar]
- Solomon M., Ozonoff S.J., Ursu S., Ravizza S., Cummings N., Ly S., Carter C.S. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. 19410583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. 21373864 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front. Syst. Neurosci. 2014;8:92. doi: 10.3389/fnsys.2014.00092. 24904314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. 18835452 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. 20152963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. 19555291 [DOI] [PubMed] [Google Scholar]
- Tavano A., Grasso R., Gagliardi C., Triulzi F., Bresolin N., Fabbro F., Borgatti R. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130(10):2646–2660. doi: 10.1093/brain/awm201. 17872929 [DOI] [PubMed] [Google Scholar]
- Travers B.G., Powell P.S., Klinger L.G., Klinger M.R. Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability. J. Autism Dev. Disord. 2013;43(7):1568–1583. doi: 10.1007/s10803-012-1702-x. 23132272 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. NeuroImage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. 24076206 [DOI] [PubMed] [Google Scholar]
- Verly M., Verhoeven J., Zink I., Mantini D., Peeters R., Deprez S. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. Neuroimage Clin. 2014;4:374–382. doi: 10.1016/j.nicl.2014.01.008. 24567909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch. Gen. Psychiatry. 2007;64(6):698–708. doi: 10.1001/archpsyc.64.6.698. 17548751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.S., Kloth A.D., Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518–532. doi: 10.1016/j.neuron.2014.07.016. 25102558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington S.D., Gordon E.M., Brar J., Warburton S., Sawyer A.T., Wolfe A. Dysmaturation of the default mode network in autism. Hum. Brain Mapp. 2014;35(4):1284–1296. doi: 10.1002/hbm.22252. 23334984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A.M., Egelhoff J.C., McKellop J.M., Franz D.N. Autism and the cerebellum: evidence from tuberous sclerosis. J. Autism Dev. Disord. 2000;30(6):511–517. doi: 10.1023/a:1005679108529. 11261463 [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition (WISC-IV) The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Whitney E.R., Kemper T.L., Rosene D.L., Bauman M.L., Blatt G.J. Density of cerebellar basket and stellate cells in autism: evidence for a late developmental loss of Purkinje cells. J. Neurosci. Res. 2009;87(10):2245–2254. doi: 10.1002/jnr.22056. 19301429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L., Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 1979;9(1):11–29. doi: 10.1007/BF01531288. 155684 [DOI] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. 21653723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Cheung C., Chua S.E., McAlonan G.M. Can Asperger syndrome be distinguished from autism? An anatomic likelihood meta-analysis of MRI studies. J Psychiatry Neurosci. 2011;36(6):412–421. doi: 10.1503/jpn.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole-brain results for VBM group comparison between ASD and TD.