Abstract
Periodontal disease (PD) is among the most common infectious diseases affecting humans. While the burden of periodontal disease on oral health has been extensively investigated, a possible specific relationship between the disease and systemic health is a relatively new area of interest. More recently it has been suggested that PD has an etiological role in the development of atherosclerotic cardiovascular disease, diabetes mellitus, and preterm low-birth weight, among others. In this review, we critically evaluate the current knowledge on the relation between PD and systemic diseases overall, and specifically with cardiovascular diseases. The best available evidence today suggests that the infection and inflammatory reaction associated with PD may contribute toward systemic disease. It is critical that dentists and physicians are well informed of the potential general health impact of periodontal disease so that they are in a position to knowledgeably counsel patients.
Periodontal disease (PD) is among the most common infectious diseases afflicts humans. Its presence has been documented for thousands of years, and it has been the subject of intense study. Progressive periodontal disease is characterized by the destruction of the alveolar bones of the jaws and the other supporting structures of the teeth. As a consequence, PD has been identified as one of the 2 major causes of tooth loss, in addition to dental caries.1 In addition to its effect on oral tissues, PD has been linked to a multiplicity of systemic diseases including cardiovascular disease (CVD), diabetes mellitus (DM), and preterm low-birth weight, and many more including cancer. Although this putative relationship has been reported in many publications, the nature of such a linkage remains to be fully vetted and understood. The concept that oral disease could impact systemic health is not novel. Hippocrates suggested that tooth extraction might cure arthritis. More recently, in 1891, Miller2 reported that bacteria from mouth infections could seed distant sites in the body leading to pathology. As recently as the 1970’s, full mouth extraction for children with leukemia was advocated as a way to prevent sepsis.3
Interest in the subject continued and has led to multiple case-based reports and investigations.2,4,5 In the late 80’s, dental journals published multiple observational studies to identify systemic diseases in PD patients, and in the 1990’s the term “periodontal medicine” was introduced.6,7 At the beginning of the 21st century, dentists started to warn their patients of the potential relationship between PD and a number of systemic diseases. The association was often presented in a one directional way in which PD was suggested to be a contributing factor in the development of the systemic disease, namely, PD and CVD. Alternatively, the relationship between PD and the systemic disease was seen as being bi-directional with each condition capable of modifying the other, namely PD and DM. The literature now contains a myriad of studies documenting and discussing the direct and indirect association between PD and CVD, DM, and preterm low-birth weight, among other systemic conditions. While there is a robust literature noting the coincident occurrence of PD and systemic diseases, critical questions around the etiological role of PD in these conditions remains to be aggressively studied. The purpose of this review is to critically evaluate the current knowledge on the relation between PD and systemic diseases, analyzing the literature and to generate a statement based on the available evidence.
Periodontitis
Periodontal diseas is a heterogeneous, multifactorial, chronic inflammatory process affecting 10-15% of the general population.8,9 It is defined as the bacterial (microbial biofilm) and inflammatory destruction of gingiva and teeth supporting structure including bone and periodontium (Figure 1).10 Periodontal disease affects quality of life by reducing chewing function, impairing aesthetics, and inducing tooth loss. Plaque bacteria are the major drivers of PD pathogenesis, specifically gram negative bacteria such as Porphyromonas gingivalis (Pg) and Fusibacterium nucleatum (Fn).11
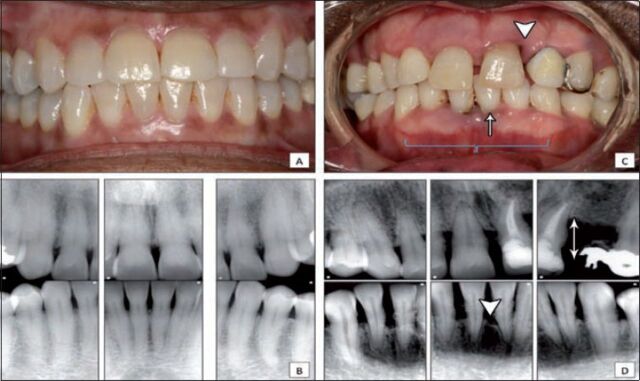
Figure 1.
Clinical comparison between healthy and periodontitis patients A) frontal intra-oral view for a patient with gingival health and stable periodontium demonstrating normal gingival color, marginal gingival level and intact inter-dental papillae; B) periapical radiographs for the patient in A, showing stable periodontium, normal bone level and absence of vertical bone defect; C) frontal intra-oral view of a patient with moderate to severe periodontitis presenting as loss of attachment (triangle), recession (arrow) and gingival edema (brace); D) periapical radiographs for the patient in C, showing calculus accumulation, horizontal bone loss (arrow) and vertical bone defect (triangle).
The PD starts with the accumulation of bacteria within tooth surface dental plaque, which release cell wall products (lipopolysaccharides; LPS and endotoxins), which activate the body immune cells, including monocytes, to produce pro-inflammatory mediators (interleukin 1α [IL-1α], IL-6, and tumor necrosis factor-α [TNF-α]).12,13 Initial gingival inflammation is considered to be a protective mechanism against bacterial invasion. When inflammation, in susceptible individuals, fails to resolve, it transforms into a chronic pathological periodontal process 14,15 in which, bacterial antigens are presented to and processed by cells of the adaptive immune system including macrophages and dendritic cells. This results in the release of a significant amount of inflammatory mediators including C-reactive protein (CRP), fibrinogen, and various cytokines that contribute to the chronic nature of the disease and destruction of periodontal tissues.14,16
Early PD may be managed by non-surgical therapy that includes scaling, root planning, and local or systemic antibiotics aimed to control periodontal inflammation.17 Failure to resolve periodontal inflammation, or relapse of PD may require surgical intervention. The surgical phase of periodontal therapy focuses on periodontal pocket elimination, modifying alveolar bone architecture and creating better accessibility for home oral care.
Assessment and diagnosis of PD is based on multiple parameters, but most importantly probing depth and clinical attachment loss.18 The severity of PD can be staged as mild, moderate, or severe, in addition to being localized or generalized. Considering the chronic and episodic nature of PD, most of the available assessment tools measure the cumulative effect of the disease rather than the current disease activity. Thus, longitudinal studies are of particular value to asses PD, and eventually any causality relation with systemic disease.
Biological plausibility
As already noted, a connection between PD and systemic disease has been discussed extensively in the literature.19 The association was based on a holistic concept in which the body was viewed as an inclusive system, with multiple and intense inter-organ biological processes including the mouth. The initial mechanism proposed under the guise of the “focal infection theory” suggested that oral bacteria would infiltrate into the blood stream and then migrate to different body organs where their presence might contribute to multiple maladies such as arthritis or endocarditis.2 Interventional strategies were developed including the introduction of antibiotic prophylaxis for the prevention of sub-acute bacterial endocarditis. Overall, however, the failure of systemic antibiotics in effectively mitigating systemic infections created significant doubt as to the merits of the hypothesis.20,21
There are currently 2 postulated mechanisms to support a link between PD and systemic disease: 1) metastatic infection and dissemination of bacterial toxins; and 2) immunological injuries and inflammation.22 Metastatic infection seems, in some ways, remarkably similar to the focal infection theory. It is characterized by the systemic spread of bacteria and/or bacterial products from an oral source.23 One way for oral bacteria to gain access into blood stream is via diseased and ulcerated gingival pockets as a result of PD. Oral bacteria survives in the blood stream, and had the ability to adhere to non-oral sites, resulting in a systemic disease event.3
The second mechanistic theory suggests that systemic damage is the consequence of an inflammatory cascade, which is initiated in the mouth. When leukocytes and endothelial cells encounter bacterial virulence antigens in the blood stream, they secrete pro-inflammatory mediators including CRP and prostaglandin (PG). With continuous exposure, bacterial antigens form and deposit immune complexes with the help of circulating antibodies to amplify systemic inflammation.24 Immune cells including TNF-α, IL-6, and IL-1β produce more pro-inflammatory cytokines.25 These cytokines are generally associated with tissue destruction and osteoclastogenesis.25,26 Under the influence of inflammatory cytokines, PG are also released from diseased gingiva and periodontium to stimulate local bone resorption, and play a role in systemic inflammation, platelet aggregation, and endothelial cell activation.27,28 The presence of a general inflammatory state in which, high levels of pro-inflammatory cytokines abound, has been associated with a number of systemic diseases including atherosclerotic vessel diseases, gastrointestinal ulcer formation, and cancer.
Periodontitis and systemic diseases
While PD has been linked to enumerable systemic diseases, this review focuses on the link between PD and CVD as being representative of its potential impact on a leading cause of mortality and morbidity.29-31 Cardiovascular disease is responsible for 50% of deaths in developed countries. Atherosclerosis of vessels (ASD) resulting in coronary heart disease manifests clinically as angina, myocardial infarction, and stroke. Atherosclerotic plaque formation triggered by endothelium damage and inflammation results in blood vessel narrowing with consequent reduced cardiac blood supply.32 Traditional established risk factors for CVD and atherosclerosis include hypertension, dyslipidemia, diabetes, and smoking.33 However, these factors may not fully account for CVD risk, suggesting a role for other contributing factor to initiate an inflammatory response.34 The nature and source of this inflammation is unclear, but possibly could be related to periodontal pathogen.
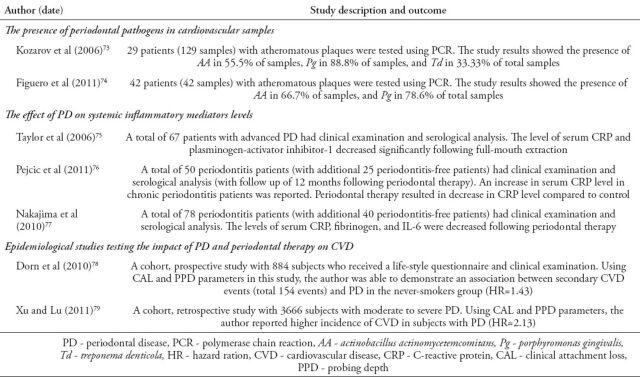
The observation that eliminating risk factors resulted in a 40% reduction of chronic, non communicable disease-related deaths, encouraged scientist to focus on assessing a PD role in ASD and CVD.17,35-38 Results of animal studies suggested that PD might contribute to ASD.39 Subsequent human studies showed that pro-inflammatory markers produced in PD were linked to CVD.40 Arbes et al41 reported data from the Third National Health and Nutrition study (NHANES III) linking clinical attachment loss with a relative risk (RR) of 3.8 in self-reported heart attacks. This report was then supported by multiple clinical studies. Humphrey et al37 reported PD as an independent risk factor for CVD in which the risk ranged between 1.24 to 1.34 depending on a range of PD parameters including periodontitis status, tooth loss, gingivitis, and bone loss. In a meta-analysis of prospective studies, Bahekar et al35 reported RR of 1.14 for developing CVD for patients with PD. Similar results were reported by Beck et al42 with odds ratio (OR) of 2, and by DeStefano et al43 with 25% risk to develop CVS in the setting of PD. A recent longitudinal study has also indicated PD to be a risk factor for future ASD in patients with CVD with OR of 3.6.44 A list of recent studies discussing the impact of PD on CVD can be found in Table 1.
Table 1.
Recent studies reporting on the impact of periodontal disease on cardiovascular disease.
Among the numerous hypotheses to describe the pathogenesis of a possible PD/ASD link is the comprehensive one proposed by Reyes et al.45 They suggest that oral bacteria, or their products which have gained access into blood stream stimulate the release of pro-inflammatory mediators46 with consequent endothelial activation or dysfunction and atheroma formation.47,48 Subsequently, the formed atheroma mature, host bacterial products, which leads to the production of antibodies that may cross-react with endothelial cells and promote helper-T cells (Th1) response. As a result, macrophages are activated, inflammation is exaggerated and an ASD event results.
However, it has been very challenging to differentiate the role of oral bacteria from the inflammatory response as a contributing factor in this process. The observation that, in animal models, the uses of anti-inflammatory blockers mitigate the development of atheromas related to PD seems consistent with the hypothesis.39 Furthermore, recent human studies demonstrated that patients with elevated serum Pg-antibodies (IgG) have higher blood pressure (systolic and diastolic), as well as periodontal bacteria in atheroma plaques.49,50
The impact of periodontal therapy on systemic condition outcome, including CVD, has been tested in few interventional studies. Non-surgical periodontal therapy has shown to elicit an initial, transient elevation in inflammatory and pro-thrombotic mediators and overall decrease of endothelial function as a result of bacteremia.51 This was followed by a reduction of inflammatory markers on both, a local and systemic level.52 A recent systematic review by D’Aiuto et al51 on the effect of periodontal therapy on CVD biomarkers have concluded that there is no or limited evidence for periodontal therapy to reduce subclinical markers including CD40, serum amyloid A, leukocyte count, fibrinogen, arterial blood pressure, and CVD events.51 In addition, there is moderate evidence that periodontal therapy helps in reducing CRP levels and improves endothelial dysfunction. This finding was supported by The American Academy of Periodontology (AAP) workshop statement on the effect of periodontal therapy, which concluded that: “there is moderate evidence for reduction of systemic inflammation as evidenced by reduction in CRP and improvement of both clinical and surrogate measures of endothelial function”.53 The group also indicated that there is moderate evidence that periodontal therapy doesn’t affect lipid profile, and there is limited evidence for a beneficial effect on blood pressure and subclinical CVD.53 On the other hand, recent data from insurance claims presented at the last International Association of Dental Research (IADR) meeting (Jeffcoat M, Jeffcoat R, Gladowski P, Bramson J, Blum J. Periodontal therapy improves outcomes in systemic conditions: insurance claims evidence. Oral abstract presented at IADR meeting, Charlotte, NC, 2014. Unpublished data), indicated that periodontal therapy can lead to a reduction in total cost and hospital admission for patients with diabetes, CVD, cerebral-vascular disease, and pregnancy. The study looked at data from 338,891 subscribers to both medical and dental insurance plans.
To date, there are no trials that have studied the efficacy of periodontal therapy as a primary preventive intervention for CVD. A somewhat contradictory conclusion supporting a PD/ASD association was reached by Johansson et al54 who followed patients established histories of ASD and PD for up to 8 years. At the study endpoint, while there was no difference in significant cardiac events between ASD and control patients, at the final periodontal examination, the PD prevalence and severity in ASD patients was significantly higher compared with healthy individuals. This finding is consistent with a conclusion that there is no association between PD and CVD, based on disease events. In addition, this may support the possibility that CVD patients are generally at risk for PD due to life style trending that may be common to the etiology of both diseases. There is currently no evidence showing that treating PD results in the prevention or progression CVD. This was supported recently (2012) by an American Heart Association (AHA) statement based on current evidence where oral bacteria does not cause or exacerbate acute CVD. In addition, the promotion of dental/periodontal therapy to prevent CVD was not recommended.55,56
Evaluation of the current evidence
Since first broached in 1891, a causal relationship between PD and systemic disease has been the subject of numerous studies.2 The potential appeal of such an association may have resulted in results of these trials receiving a less rigorous assessment as to their meaningfulness than might have been expected. In fact, in 2009, a joint AAP and AHA consensus was published in with recommendation on the link between PD and CVD.17 This consensus advised that: 1) patients with moderate to severe periodontitis should be informed of the risk of CVD associated with PD; 2) patients with moderate to severe periodontitis, and have one major risk factor for CVD, should consider medical evaluation every year; 3) patients with periodontitis and 2 major risk factors for CVD should be referred for medical evaluation if one had not been performed within the last 12 months.17 This statement seems less realistic as contradictory evidence is now emerging, which has revealed multiple compounding factors that could impact study outcomes.
Despite the fact that the term “association” should not imply causality, the terms “association” and “causation” have often been used interchangeably in the literature. On the other hand, causality is defined as the connection between 2 events, in this case PD and systemic disease, in which one event leads to or brings about the other. The current literature broadly assesses epidemiological analyses and interventional, cross-sectional and retrospective studies which address the association or causality relation between PD and systemic diseases. Retrospective and cross-sectional studies have limited value in identifying an association and cannot confirm causality. On the other hands, interventional and prospective longitudinal studies are more likely to provide a more rigorous platform to objectively evaluate PD/systemic disease relationships and are no more critical then ever.
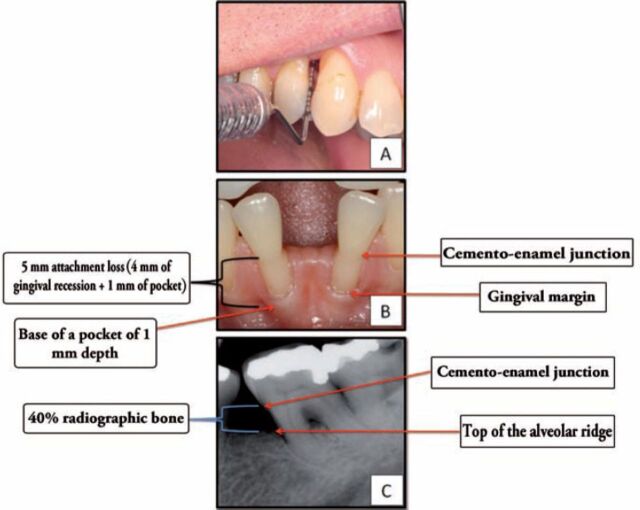
One variable that was largely under-addressed prior to 2002 was the lack of uniformity in the criteria that have been used to define the presence or absence of PD in study populations. Tooth loss, as a surrogate measure for PD, and probing depth and clinical attachment loss, and radiographic outcomes were often randomly assigned as defining PD severity. In an attempt to standardize PD severity in the context of epidemiological studies, especially for the purpose of identifying an “association” or “causality”, a threshold for PD diagnosis was set and unified. The universal agreement on PD criteria, developed by the Centers for Disease Control and Prevention in partnership with the AAP for research purposes, included the presence of ≥2 interproximal sites with clinical attachment loss (CAL) of ≥4 mm OR ≥2 interproximal sites with probing depth (PPD) of ≥5 mm (Figure 2).57,58 For studies using radiographic assessment, alveolar bone loss of ≥40% is accepted for PD case inclusion criteria. This definition however, captures moderate to severe PD only. As a result, the definition has been updated in 2012 to include a definition for “mild” PD for better description of overall prevalence of PD.58 The mild form of PD was defined as ≥ 2 interproximal sites with CAL ≥ 3 mm and ≥ 2 interproximal sites with PPD ≥ 4 mm (not on the same tooth) or one site with PD ≥5 mm. The heterogeneous definition of PD severity in clinical trials is a major confounding factor that explains the diversity in studies findings. Most conducted studies have used “total periodontitis” allocating all cases to one entity, rather than identifying PD severity as mild, moderate, and severe. Dangerously, such study designs could result in the dilution of study outcome, as more mild cases are included rather than moderate or severe ones.
Figure 2.
Diagnostic criteria for mild periodontal disease; A) probing depth of 4 mm; B) attachment loss of 4 mm; C) radiographic bone loss of more than 40%.57,58
The complexity of oral flora should also be considered as an additional confounding factor. The oral cavity has more than 700 species of bacteria, and only 5% are considered strongly associated with PD.59-61 In addition, the intermittent and episodic nature of disease activity may hamper the identification of the causative agent. Previous studies detected periodontal bacteria in carotid atheromas, and distal to the source using PCR and DNA testing.62 Eight studies indicated a correlation between subgingival microbiota and pathogens detected in atheromas. If the bacteria survive the elimination by immune system, it may disseminate virulence factors to act as antigens and initiate systemic inflammation.3 However, the role of these bacterial components is still to be confirmed as part of systemic disease pathogenesis. It is possible that atheromic bacteria are initiating an inflammatory response leading to tissue damage, as previous evidence indicated the presence of viable bacteria in atheromas, or simply being inactive while in blood vessels. The patient infectious disease history and total pathogen burden could be another confounding factor. Infectious and inflammatory stimuli from other infections can induce a chronic systemic impact similar to PD.63 The impact of PD on systemic diseases may be confounded by other multiple factors. Factors that are commonly overlooked and considered co-morbidities are diabetes and smoking. Few studies controlled for smoking, or conducted the study in never-smokers, and demonstrated excess risk for CVD. However, excess risk could be related to unknown confounding factors.
Biomarkers have become popular surrogates for assessing the presence and severity of many diseases, including those under discussion. Typically these measure plasma levels of proteins thought to correlate with the presence or severity of a targeted illness. Their ease of attainment, the relatively low cost of assays, and their utility in some very specific diseases, have contributed to biomarker assay attractiveness. However, biomarkers often fail to critically evaluate or define the presence or progression of disease. A more accurate pathway will be investigating the disease events and outcomes. C-reactive protein (CRP) provides an example. As an inflammatory marker, CRP is well documented in the literature, where studies have been cross-sectional in nature, and show a positive association between CRP and inflammatory disease.64-66 The CRP is a non-specific, type-1 acute phase protein that has been associated with trauma, inflammation, and infection.67 It plays an important role in the innate immune response via opsonization and complement activation. The CRP levels can be detected in the serum, which are more accurate, in addition to gingival crevicular fluid.68 Interventional PD studies have demonstrated conflicting results in relation to CRP level change. Few studies have indicated a significant increase in CRP level, immediately following non-surgical periodontal therapy, while others demonstrated a reduction in CRP level.69,70 Meta-analysis conducted by Loannidou et al71 concluded that the available body of evidence does not support the hypothesis that periodontal therapy can reduce serum CRP level. Contrastingly, a recent, well-designed interventional study, which included 3 groups of healthy, gingivitis and mild periodontitis patients demonstrated a decrease in CRP serum level at the 3 months time point after periodontal therapy.70 No further follow up was carried out for these patients. This observation was noted in most CRP-level studies, where the follow up data is limited to 6 months following periodontal therapy. The question remains as to what will happen beyond the study end point, what happens after one year or 5 years? More importantly, if CRP is increased in PD patients, does it act as a predictor for systemic disease, or is it merely a coincident finding in patients who also develop other inflammatory diseases? The current evidence fails to support a hypothesis suggesting that increased CRP levels in the face of PD are predictive of subsequent systemic disease.72
Conclusions and clinical implications
Systematic reviews suggest that the presence of PD may be independently associated with a risk of multiple systemic diseases. Retrospective epidemiological studies and animal studies indicate a connection with biological plausibility. However, the clinical proof of causality will be extremely difficult in this setting due to multiple reasons. First, the initiating factor for systemic disease could be overlooked, as disease in the early phase is usually asymptomatic. Second, the proposed infection and inflammation role in causing systemic disease could be originating from sites other than the oral cavity. Lastly, most of the conducted studies are generating mixed results, which requires deeper evaluation of study design method.
The available evidence supporting the association between PD and systemic diseases is relatively immature at this point. The best available evidence today indicates that infection and inflammatory reaction associated with PD may contribute toward systemic disease. Further studies are needed for confirmation. To summarize, this is what is known so far: 1) Strong evidence suggests that PD is associated with systemic diseases; 2) The available epidemiological and animal studies indicate PD increases the risk for systemic disease; 3) No causality between PD and systemic is confirmed so far, although it may exists based on one of the proposed mechanisms mentioned earlier; 4) Periodontal therapy overall has a favorable effect on systemic disease subclinical markers, despite heterogeneous response; 5) Interventional studies are needed to confirm the biological plausibility of PD to directly or indirectly cause systemic disease.
The current gap in knowledge, and the relation between PD and systemic disease may impact the provided dental care to patients overall. However, decision making for the type and necessity of periodontal therapy should be based solely on the patient’s periodontal needs. Until then, it is crucial to have a consensus by the dental and medical community on the best statement to discuss with patients based on the available evidence.
Future research should focus on understating the exact role of oral bacterial and inflammation in systemic pathogenesis. More interventional, periodontal, longitudinal studies are needed to investigate the effect of PD exposure on systemic disease development and outcome. Factors to consider while designing any clinical trial are standardization of the criteria for PD diagnosis, periodontal therapy outcome, and systemic disease risk markers.
Acknowledgment
The authors gratefully acknowledge Dr. Dalia Salem, Harvard School of Dental Medicine, Boston, MA, United States of America for the clinical photograph.
Footnotes
References
- 1.Di Benedetto A, Gigante I, Colucci S, Grano M. Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol. 2013;2013:503754. doi: 10.1155/2013/503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller W. The human mouth as a focus of infection. The Dental Cosmos. 1891;33:689–713. [Google Scholar]
- 3.Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Periodontol. 2013;84:S1–S7. doi: 10.1902/jop.2013.1340018. [DOI] [PubMed] [Google Scholar]
- 4.Hunter W. Oral sepsis as a cause of disease. Br Med J. 1900;2:215–216. doi: 10.1136/bmj.2.2065.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billings FH, Young CC. A New Shipping Outfit for Iced Water Samples. Am J Public Health (N Y) 1914;4:450–454. doi: 10.2105/ajph.4.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nery EB, Meister F, Jr, Ellinger RF, Eslami A, McNamara TJ. Prevalence of medical problems in periodontal patients obtained from three different populations. J Periodontol. 1987;58:564–568. doi: 10.1902/jop.1987.58.8.564. [DOI] [PubMed] [Google Scholar]
- 7.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 8.Murray Thomson W. Epidemiology of oral health conditions in older people. Gerodontology. 2014;31:9–16. doi: 10.1111/ger.12085. [DOI] [PubMed] [Google Scholar]
- 9.Baelum V, Lopez R. Periodontal disease epidemiology - learned and unlearned? Periodontol 2000. 2013;62:37–58. doi: 10.1111/j.1600-0757.2012.00449.x. [DOI] [PubMed] [Google Scholar]
- 10.Loe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 11.Genco RJ. Pathogenesis of periodontal disease: new concepts. J Can Dent Assoc. 1984;50:391–395. [PubMed] [Google Scholar]
- 12.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deo V, Bhongade ML. Pathogenesis of periodontitis: role of cytokines in host response. Dent Today. 2010;29:60–62. 64–66. quiz 68-69. [PubMed] [Google Scholar]
- 14.Van Dyke TE. The etiology and pathogenesis of periodontitis revisited. J Appl Oral Sci. 2009:17. doi: 10.1590/S1678-77572009000100001. pii: S1678-77572009000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yucel-Lindberg T, Bage T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med. 2013;15:e7. doi: 10.1017/erm.2013.8. [DOI] [PubMed] [Google Scholar]
- 16.Black RA. TIMP3 checks inflammation. Nat Genet. 2004;36:934–935. doi: 10.1038/ng0904-934. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, et al. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Armitage GC Research, Science and Therapy Committee of the American Academy of Periodontology. Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–1247. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 19.Pussinen PJ, Nyyssonen K, Alfthan G, Salonen R, Laukkanen JA, Salonen JT. Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler Thromb Vasc Biol. 2005;25:833–838. doi: 10.1161/01.ATV.0000157982.69663.59. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459–1466. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- 21.Gamonal J, Sanz M, O’Connor A, Acevedo A, Suarez I, Sanz A, et al. Delayed neutrophil apoptosis in chronic periodontitis patients. J Clin Periodontol. 2003;30:616–623. doi: 10.1034/j.1600-051x.2003.00350.x. [DOI] [PubMed] [Google Scholar]
- 22.Thoden van Velzen SK, Abraham-Inpijn L, Moorer WR. Plaque and systemic disease: a reappraisal of the focal infection concept. J Clin Periodontol. 1984;11:209–220. doi: 10.1111/j.1600-051x.1984.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 23.Kinane DF, Attstrom R. Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J Clin Periodontol. 2005;32(Suppl 6):130–131. doi: 10.1111/j.1600-051X.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 24.Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000. 2013;62:271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 25.Garlet GP, Cardoso CR, Mariano FS, Claudino M, de Assis GF, Campanelli AP, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;37:591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 26.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 27.Mendieta CF, Reeve CM, Romero JC. Biosynthesis of prostaglandins in gingiva of patients with chronic periodontitis. J Periodontol. 1985;56:44–47. doi: 10.1902/jop.1985.56.1.44. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Rosol TJ, Saito M, Ngan PW, Shanfeld J, Davidovitch Z. Bone-resorbing activity and prostaglandin E produced by human periodontal ligament cells in vitro. J Bone Miner Res. 1990;5:1013–1018. doi: 10.1002/jbmr.5650051004. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Periodontol. 2013;84:S70–S84. doi: 10.1902/jop.2013.134008. [DOI] [PubMed] [Google Scholar]
- 30.Borgnakke WS, Ylostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84:S135–S152. doi: 10.1902/jop.2013.1340013. [DOI] [PubMed] [Google Scholar]
- 31.Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes--systematic review. J Periodontol. 2013;84:S181–S194. doi: 10.1902/jop.2013.134009. [DOI] [PubMed] [Google Scholar]
- 32.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 34.Vita JA, Loscalzo J. Shouldering the risk factor burden: infection, atherosclerosis, and the vascular endothelium. Circulation. 2002;106:164–166. doi: 10.1161/01.cir.0000023452.26135.34. [DOI] [PubMed] [Google Scholar]
- 35.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 36.Buhlin K, Mäntylä P, Paju S, Peltola JS, Nieminen MS, Sinisalo J, et al. Periodontitis is associated with angiographically verified coronary artery disease. J Clin Periodontol. 2011;38:1007–1014. doi: 10.1111/j.1600-051X.2011.01775.x. [DOI] [PubMed] [Google Scholar]
- 37.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!”--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain A, Batista EL, Jr, Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infect Immun. 2003;71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arbes SJ, Jr, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78:1777–1782. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- 42.Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol. 1998;3:127–141. doi: 10.1902/annals.1998.3.1.127. [DOI] [PubMed] [Google Scholar]
- 43.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renvert S, Ohlsson O, Pettersson T, Persson GR. Periodontitis: a future risk of acute coronary syndrome? A follow-up study over 3 years. J Periodontol. 2010;81:992–1000. doi: 10.1902/jop.2010.090105. [DOI] [PubMed] [Google Scholar]
- 45.Reyes L, Herrera D, Kozarov E, Rolda S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Periodontol. 2013;84:S30–S50. doi: 10.1902/jop.2013.1340012. [DOI] [PubMed] [Google Scholar]
- 46.Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol. 2013;84:S51–S69. doi: 10.1902/jop.2013.134006. [DOI] [PubMed] [Google Scholar]
- 47.Higashi Y, Goto C, Hidaka T, Soga J, Nakamura S, Fujii Y, et al. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis. 2009;206:604–610. doi: 10.1016/j.atherosclerosis.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Holtfreter B, Empen K, Gläser S, Lorbeer R, Völzke H, Ewert R, et al. Periodontitis is associated with endothelial dysfunction in a general population: a cross-sectional study. PLoS One. 2013;8:e84603. doi: 10.1371/journal.pone.0084603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanaoka Y, Soejima H, Yasuda O, Nakayama H, Nagata M, Matsuo K, et al. Level of serum antibody against a periodontal pathogen is associated with atherosclerosis and hypertension. Hypertens Res. 2013;36:829–833. doi: 10.1038/hr.2013.46. [DOI] [PubMed] [Google Scholar]
- 50.Tsakos G, Sabbah W, Hingorani AD, Netuveli G, Donos N, Watt RG, et al. Is periodontal inflammation associated with raised blood pressure? Evidence from a National US survey. J Hypertens. 2010;28:2386–2393. doi: 10.1097/HJH.0b013e32833e0fe1. [DOI] [PubMed] [Google Scholar]
- 51.D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Periodontol. 2013;84:S85–S105. doi: 10.1902/jop.2013.134007. [DOI] [PubMed] [Google Scholar]
- 52.Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol. 2007;78:2289–2302. doi: 10.1902/jop.2007.070140. [DOI] [PubMed] [Google Scholar]
- 53.Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(4 Suppl):S24–S29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 54.Johansson CS, Ravald N, Pagonis C, Richter A. Periodontitis in patients with coronary artery disease: an 8-year follow-up. J Periodontol. 2014;85:417–425. doi: 10.1902/jop.2013.120730. [DOI] [PubMed] [Google Scholar]
- 55.AHA Expert Committee finds no cause and effect relationship between gum disease and heart disease or stroke. J Okla Dent Assoc. 2012;103:30. [PubMed] [Google Scholar]
- 56.AHA statement on periodontal disease and heart disease. J Can Dent Assoc. 2012;77:c54. [PubMed] [Google Scholar]
- 57.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 58.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanner AC, Paster BJ, Lu SC, Kanasi E, Kent R, Jr, Van Dyke T, et al. Subgingival and tongue microbiota during early periodontitis. J Dent Res. 2006;85:318–323. doi: 10.1177/154405910608500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 62.Haraszthy VI, Hariharan G, Tinoco EM, Cortelli JR, Lally ET, Davis E, et al. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J Periodontol. 2000;71:912–922. doi: 10.1902/jop.2000.71.6.912. [DOI] [PubMed] [Google Scholar]
- 63.Kinane D, Bouchard P. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:333–337. doi: 10.1111/j.1600-051X.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 64.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 65.Loos BG. Systemic effects of periodontitis. Ann R Australas Coll Dent Surg. 2006;18:27–29. [PubMed] [Google Scholar]
- 66.Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74:1007–1016. doi: 10.1902/jop.2003.74.7.1007. [DOI] [PubMed] [Google Scholar]
- 67.Ramamoorthy RD, Nallasamy V, Reddy R, Esther N, Maruthappan Y. A review of C-reactive protein: A diagnostic indicator in periodontal medicine. J Pharm Bioallied Sci. 2012;4:S422–S426. doi: 10.4103/0975-7406.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuter G, Kurtis B, Serdar M. Evaluation of gingival crevicular fluid and serum levels of high-sensitivity C-reactive protein in chronic periodontitis patients with or without coronary artery disease. J Periodontol. 2007;78:2319–2324. doi: 10.1902/jop.2007.070150. [DOI] [PubMed] [Google Scholar]
- 69.Behle JH, Sedaghatfar MH, Demmer RT, Wolf DL, Celenti R, Kebschull M, et al. Heterogeneity of systemic inflammatory responses to periodontal therapy. J Clin Periodontol. 2009;36:287–294. doi: 10.1111/j.1600-051X.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patil VA, Desai MH. Effect of periodontal therapy on serum C-reactive protein levels in patients with gingivitis and chronic periodontitis: a clinicobiochemical study. J Contemp Dent Pract. 2013;14:233–237. doi: 10.5005/jp-journals-10024-1305. [DOI] [PubMed] [Google Scholar]
- 71.Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. Effect of periodontal treatment on serum C-reactive protein levels: a systematic review and meta-analysis. J Periodontol. 2006;77:1635–1642. doi: 10.1902/jop.2006.050443. [DOI] [PubMed] [Google Scholar]
- 72.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 73.Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006;8:687–693. doi: 10.1016/j.micinf.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, Tejerina JM, del Castro JA, Gutiérrez JM, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82:1469–1477. doi: 10.1902/jop.2011.100719. [DOI] [PubMed] [Google Scholar]
- 75.Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–78. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 76.Pejcic A, Kesic L, Brkic Z, Pesic Z, Mirkovic D. Effect of periodontal treatment on lipoproteins levels in plasma in patients with periodontitis. South Med J. 2011;104:547–552. doi: 10.1097/SMJ.0b013e3182242eaa. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima T, Honda T, Domon H, Okui T, Kajita K, Ito H, et al. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. J Periodontal Res. 2010;45:116–122. doi: 10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- 78.Dorn JM, Genco RJ, Grossi SG, Falkner KL, Hovey KM, Iacoviello L, et al. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): the Western New York Acute MI Study. J Periodontol. 2010;81:502–511. doi: 10.1902/jop.2009.090499. [DOI] [PubMed] [Google Scholar]
- 79.Xu F, Lu B. Prospective association of periodontal disease with cardiovascular and all-cause mortality: NHANES III follow-up study. Atherosclerosis. 2011;218:536–542. doi: 10.1016/j.atherosclerosis.2011.07.091. [DOI] [PubMed] [Google Scholar]