Abstract
Objectives:
To assess the association between hypoxic inducible factor-2alpha (HIF-2α) and hepatocellular carcinoma (HCC) by meta-analysis.
Methods:
This study was carried out at Anhui Province Hospital, Hefei, Anhui, China in February 2014. We searched various databases for studies published in English or Chinese up to February 28, 2014. The hazard ratio for overall survival and analyzed odds ratio were combined to evaluate the clinicopathological features of HIF-2α expression in HCC.
Results:
A total of 7 eligible studies comprising 1066 patients with HCC were identified after our full assessment according to inclusion criteria. All of the patients came from China. The results indicated that the association between HIF-2α expression and prognostic values in HCC was inconspicuous, while the expression of HIF-2α was significantly associated with capsule infiltration, vein invasion, and histological grade.
Conclusion:
Expression of HIF-2α was associated with invasion and metastasis in HCC, but did not have a distinct significance in prognosis, according to the limited evidence. However, high quality, large sample size, and controlled trials are required.
Hepatocellular carcinoma (HCC) is the sixth most common cancer of humans, and the third leading cause of cancer-relation deaths worldwide.1 In spite of advances in surgical technique and early diagnosis, the prognosis of HCC is still poor due to high recurrence and metastatic rate. The recurrence rate in the first year after curative surgery reaches up to 40%.2 However, the mechanism is complex and unclear. Thus, it is essential to identify new molecules targeted in the development of HCC. Hypoxic inducible factors (HIFs) which include HIF-1, HIF-2, and HIF-3, were reported to play a crucial role in HCC development. The latest evidence indicates that HIF-1a is up-regulated in HCC development, and its expression may be associated with the clinical outcome of HCC.3 Similarly, HIF-2α which is encoded by the endothelial PAS domain protein 1 (EPAS1) gene, shares 48% similarity in amino acid sequence and certain overlapping functions with HIF-1a.4 Under the condition of hypoxia in vitro, the HIF-2α protein is gradually accumulated so that it can persistently activate downstream target genes, while HIF-1a is transiently stabilized and primarily mediates acute responses, which suggests that HIF-2α plays a more essential role in the hypoxia response in tumors.5 Increasing evidence indicates that expression of HIF-2α is deregulated in a variety of solid tumors, and its expression may be associated with clinical progression and outcome of tumors. However, less is known about the expression and roles of HIF-2α in HCC. The existing studies have not provided conclusive evidence that HIF-2α overexpression was relevant to the progression of HCC.
In this study, we reviewed the currently available evidence in medical literature on the relationship between HIF-2α expression and clinical features and prognosis of HCC, and further assessed the strength of association between them to better understand the development and progression of HCC.
Methods
Search strategy and literature search. We searched a range of databases including PubMed (1966-2014), Web of Science (1945-2014), ELSEVIER Science Direct (1823-2014), EMBASE (1990-2014), Chinese Biological Medicine (CBM, 1982-2014) and China National Knowledge Infrastructure (CNKI, 1979-2014) updated to February 28th, 2014 using the terms: “HIF-2α” or “hypoxia-inducible factor 2, alpha subunit” or “hypoxia inducible factor 2α” or “endothelial PAS domain protein 1” or “EPAS1” with “carcinoma, hepatocellular” or “hepatocellular carcinoma” or “liver cell carcinoma” or “HCC”. All eligible studies were retrieved, while the review of reference lists was also conducted. Some of them were ruled out because of insufficient data. When faced with the same study specimens, we filtered the latest and the most informative study, to avoid duplicate data.
Criteria for inclusion and exclusion
1) studies regarding HCC, 2) studies using immunohistochemical staining (IHC) to detect the expression of HIF-2α, 3) samples obtained via surgical resection, 4) studies revealing that the expression of HIF-2α in HCC was relevant to clinical features or the prognostic indexes. We excluded studies complying with the following: 1) studies on cell lines or animals, 2) reviews without any data, case studies, or conference, 3) tumor samples without intratumoral tissues, or just involving the para-carcinoma tissues, 4) the detection method was not IHC.
Data extraction and synthesis
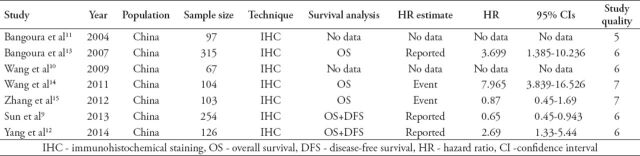
The data was independently extracted from all studies by 2 authors, and the following information obtained: authors, publication year, sample size, index of the prognostic and clinical features (Table 1). They also rated the quality of each eligible study using the Newcastle-Ottawa quality assessment scale (NOS).6 The assessment has 8 items and each of them range from one to 2 points, a total of 10 points. We summed the scores of each study, as shown in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis extracted from different studies and quality rating of each study using the Newcastle-Ottawa quality assessment scale.6
Statistical analysis
We measured the impact of HIF-2α expression on HCC in 2 steps. Firstly, we pooled the data of overall survival (OS) by hazard ratios (HRs) and 95% confidence intervals (CIs) to calculate the effective value to assess the correlation between HIF-2α and prognosis of HCC. If the HR and 95% CI were described in the study, we pooled it directly. When the variables were not given explicitly, they were calculated from available numerical data according to the method described by Parmar.7 Secondly, to assess the importance of HIF-2α expression on clinical features in HCC, we compared the rate of HIF-2α expression and clinicopathological features in HCC by odds ratio (OR) and 95% CI. By convention, an observed HR<1 implies favorable parameters for the test of HIF-2α overexpression on the variable; otherwise, it implies poorer survival in the group with overexpression of HIF-2α in HCC. If the 95% CI >1 or <1 completely, it suggests that HIF-2α expression was statistically significantly different between the negative group on OS or clinicopathological features.
Heterogeneity test was assessed by Chi-square based on Q statistical and I2 statistical test. If the HR of the studies had fine homogeneity, the fixed effect model (Mantel-Haenszel method) was used to pool the data; otherwise, we used the DerSimonian-Laird method as the random-effect model.8 We assessed the possibility of publication bias by performing Begg’s test. Publication bias was indicated when the p-value of Begg’s test was <0.05. All of the calculations were performed by using Stata version 11.0 (Stata Corporation, College Station, TX, USA).
Results
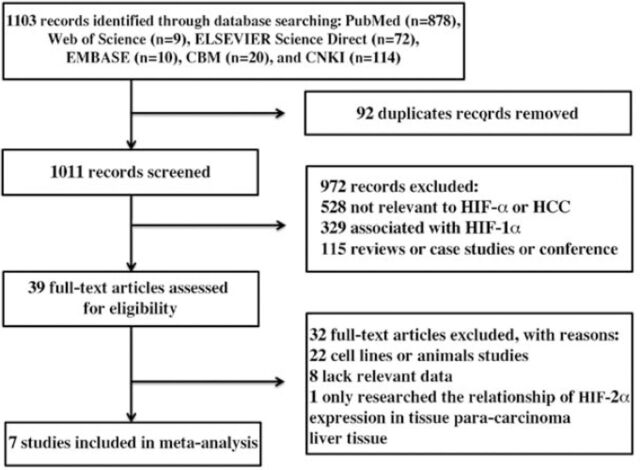
Study identification and quality. We identified 1103 potentially relevant studies, and 1095 of them were excluded from analysis (Figure 1) after careful extraction. As a result, 7 studies9-15 were finally included in the meta-analysis, and coincidentally they all derived from China. From these 7 studies, 1066 HCC patients were retrospectively analyzed, and all the tissue samples were examined using IHC.
Figure 1.
Study flow chart of data selection of 1103 records identified through database selection. HCC - hepatocellular carcinoma. CBM - Chinese Biological Medicine, CNKI - China National Knowledge Infrastructure
The study characteristics and the results of quality assessment are shown in Table 1. By assaying the methods and confounders in these studies, we found that the quality scores ranged from 5 to 7 with a median value of 6.1. Five of them described the items prognostic in OS, only 2 studies contained disease free survival rates, we only analyzed the OS by meta-analysis. We then analyzed the correlation of HIF-2α expression with tumor characteristics, included tumor size,10-14 liver cirrhosis,11-14 capsule infiltration,10-13 vein invasion,11-13 histological grade,10-14 and necrosis.10,11,13
Meta-analysis of HIF-2α for HCC. 1. The prognosis value of HIF-2α expression in HCC
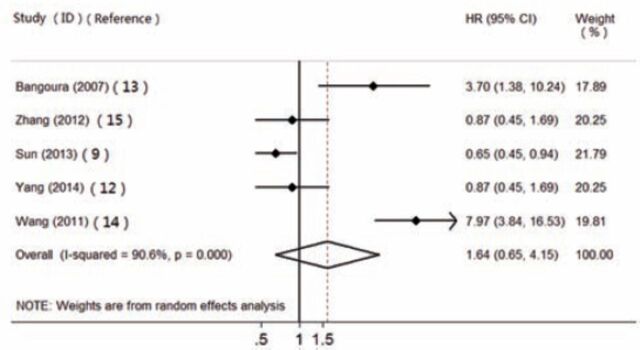
By assuming the heterogeneity between primary studies, we meta-analyzed the data in a random-effects model. Five studies were included in this meta-analysis to assess the association of HIF-2α expression with OS (Figure 2). The pooled HR was 1.640, but 95% CI was 0.648 to 4.151. Then we randomly removed one of the studies and combined the HR. The results showed that the HRs ranged from 1.374 (95% CI: 0.495-3.815) to 2.129 (95% CI: 0.693-6.544), which suggests that the difference between expression of HIF-2α and OS in patients with HCC was not statistically significant. There was no evidence of publication bias on OS using Begg’s test (p=0.881).
Figure 2.
The association of hypoxic inducible factor-2alpha expression with overall survival in hepatocellular carcinoma. HR - hazard ratio
2. Correlation of HIF-2α expression with clinicopathological features
We also assessed the correlation between expression of HIF-2α and clinicopathological features in HCC. Overexpression of HIF-2α was significantly associated with capsule infiltration (OR=2.738, 95% CI: 1.709-4.386) (Figure 3A), vein invasion (OR=2.458, 95% CI: 1.053-5.734) (Figure 3B), and histological grade (OR=0.172, 95% CI: 0.042-0.713) in HCC (Figure 3C). We also found that there was no statistical significance between HIF-2α expression and other tumor features, including tumor size, necrosis, and liver cirrhosis in HCC. All of the results are summarized in Table 2.
Figure 3.
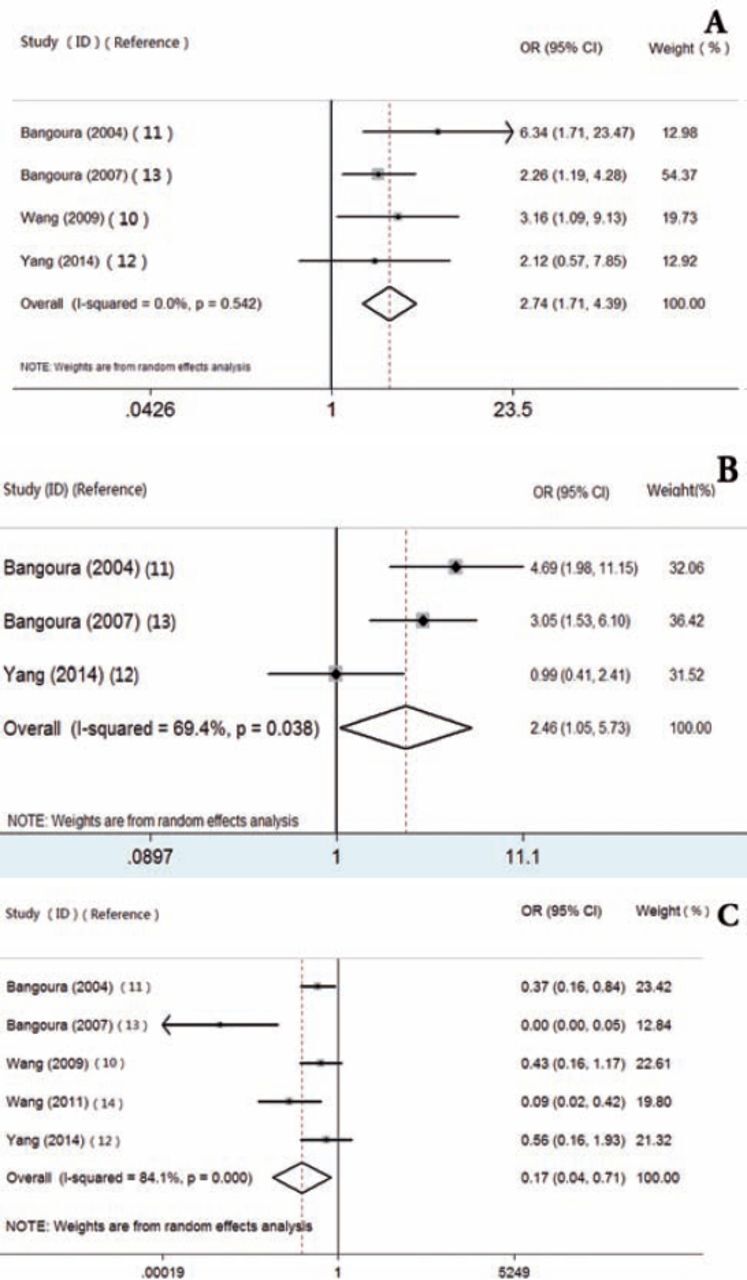
The correlation between expression of hypoxic inducible factor-2alpha and capsule infiltration in HCC (A), vein invasion (B), histological grade (C) in HCC. HCC - Hepatocellular carcinoma
Table 2.
Correlation of hypoxic inducible factor-2alpha expression with clinicopathological features.
Discussion
Due to rapid cell proliferation and the formation of aberrant blood vessels, hypoxia has been proven to be present in solid tumors,16 similarly in HCC, even though it is one of the most hyper-vascularized tumors. Intrahepatic hypoxia has been shown to be correlated with tumor progression, invasiveness, resistance to chemotherapy, and metastasis.17 In addition, HIFs play a central role in hypoxia response in HCC. Recently, numerous immunochemical analyses have demonstrated that overexpression of HIFs was detected in a number of primary and metastatic human cancers. Some of them mentioned that overexpression of HIF-2α was associated with tumor progression, metastasis, and poor outcome in HCC.
Our meta-analysis aimed to examine the association between HIF-2α expression and the prognosis and clinicopathologic features of HCC. We combined the outcome of 902 patients with HCC from the 5 studies, and result showed that the 95% CI overlapped 1, which means no statistically significant, even removed one of the studies. They all suggested that the overexpression of HIF-2α was not relevant to poor outcome in HCC by statistical analysis, but was correlated with capsule infiltration, vein invasion, and histological grade. This indicates that HIF-2α may play an important role in tumor angiogenesis, metastasis, and recurrence in HCC, but not in prognosis.
It was reported17 that HIF-2, which was the heterodimerization of HIF-2α and HIFb (ARNT)can activate TGF-a and cyclinD1 in promoting cell proliferation in numbers of tumors, and can promote metastasis through regulating the factors involved in controlling metastasis such as CXCR4, TWIST, and E-cadherin. As we know, CXCR4 and its ligand SDF1 are highly expressed under hypoxia in a number of cell types.18 It was reported that19 HIF-1 can promote the regulation of TWIST in tumor metastasis and E-cadherin, which were involved in EMT (epithelial-mesenchymal transition), while HIF-2α seemed to repress this expression.20 Moreover, HIF-2 can activate tumor suppressor genes such as SCGB3A1 to repress the growth of a tumor.21
Conditional inactivation of HIF-2α suppressed the development of VHL-associated liver hemangiomas, suggesting that liver angiogenesis was predominantly regulated by HIF-2α.22 The expression of HIF-2α was positively correlated with VEGF in HCC, and it can directly activate the expression of genes encoding the pro-angiogenic factor, erythropoietin and angiopoietin. All of them provide evidence for the role of HIF-2α in tumor angiogenesis in HCC.
The conduction of higher quality, lager sample size, and more controlled trials is required to verify the hypothesis. Limitations of our meta-analysis inlcude small sample size and regional limitations (all the patients of HCC were derived from China). We used a random effects models to analyze the data of OS, and the heterogeneity was detected (I2=90.6% p=0.000). The limitation of restricted exclusion may explain the heterogeneity, and different methods of extracting HRs may also lead to bias in results. Direct analysis of variance may be more reliable than calculating from data or extracting from survival curves.
In summary, HIF-2α was correlated with the progression of HCC but not with poor prognosis. However, the conclusion was restricted by the limitations of the included studies. More studies evaluating the significance of HIF-2α expression in HCC are necessary in the future.
Footnotes
Related Articles
Zhao J, Wang YC, Yang LY, Yu DH, Pan PT, Wang L. Neamine inhibits cell proliferation, migration, and invasion in H7402 human hepatoma cells. Saudi Med J 2010; 31: 1309-1314.
Guo YM, Yu WW, Shen XZ. Tumor necrosis factor rs361525 (-238G>A) polymorphism contributes to hepatocellular carcinoma susceptibility. Saudi Med J 2010; 31: 1101-1105.
Tang L, Sun H, Zhang L, Deng JC, Guo H, Zhang L, Liu Q. Effects of the augmenter of liver regeneration on the biological behavior of hepatocellular carcinoma. Saudi Med J 2009; 30: 1001-1009.
Kashgari AA, Al-Mana HM, Al-Kadhi YA. Intrahepatic splenosis mimicking hepatocellular carcinoma in a cirrhotic liver. Saudi Med J 2009; 30: 429-432.
Yang JX, Luo Y, Qiu HM, Tang WX. Characterization and resistance mechanisms of cisplatin-resistant human hepatocellular carcinoma cell line. Saudi Med J 2009; 30: 35-40.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 3.Zheng SS, Chen XH, Yin X, Zhang BH. Prognostic significance of HIF-1a expression in hepatocellular carcinoma: a meta-analysis. PLoS ONE. 2013;8:e65753. doi: 10.1371/journal.pone.0065753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, Warnecke C, et al. Widespread hypoxia-inducible expression of HIF-2αlpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 5.Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2αlpha to common target genes is differentially regulated in neuroblastoma: HIF-2αlpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 7.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Lu HJ, Na FF, Deng L, Xue JX, Wang JW, et al. Prognostic role of hypoxic inducible factor expression in non-small cell lung cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:3607–3612. doi: 10.7314/apjcp.2013.14.6.3607. [DOI] [PubMed] [Google Scholar]
- 9.Sun HX, Xu Y, Yang XR, Wang WM, Bai H, Shi RY, et al. Hypoxia inducible factor 2 alpha inhibits hepatocellular carcinoma growth through the transcription factor dimerization partner 3/ E2F transcription factor 1-dependent apoptotic pathway. Hepatology. 2013;57:1088–1097. doi: 10.1002/hep.26188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Yi JL, Wang CJ, Wang WY, Xu RH. Expression of HIF-2α in hepatocellular Carcinoma and its clinical significance. Journal of Chinese Physician. 2009;11:1707–1708. [Google Scholar]
- 11.Bangoura G, Yang LY, Huang GW, Wang W. Expression of HIF-2αlpha/EPAS1 in hepatocellular carcinoma. World J Gastroenterol. 2004;10:525–530. doi: 10.3748/wjg.v10.i4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ, Wu C. The correlation of expression levels of HIF-1a and HIF-2α in hepatocellular carcinoma with capsular invasion, portal vein tumor thrombi and patients’ clinical outcome. Jpn J Clin Oncol. 2014;44:159–167. doi: 10.1093/jjco/hyt194. [DOI] [PubMed] [Google Scholar]
- 13.Bangoura G, Liu ZS, Qian Q, Jiang CQ, Yang GF, Jing S. Prognostic significance of HIF-2αlpha/EPAS1 expression in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3176–3182. doi: 10.3748/wjg.v13.i23.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Yuan Z, Li S, Jun S, Zhao T, Song G, et al. Expression of Hypoxia-Inducible Factor-2a in Hepatocellular Carcinoma and its clinical significance. Chinese Journal of Clinical Oncology. 2011;39:560–563. [Google Scholar]
- 15.Zhang JB, Guo K, Zhu XD, Kong LQ, Cai ZT, Wang WQ, et al. High expression of hypoxia inducible factor 2α in peritumoral liver tissue is associated with early recurrence and poor survival after curative resection of hepatocellular carcinoma. Fudan University Journal of Medical Science. 2012;39:449–453. [Google Scholar]
- 16.Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87:227–247. doi: 10.1007/s00204-012-0931-2. [DOI] [PubMed] [Google Scholar]
- 17.Tang CM, Yu J. Hypoxia-inducible factor-1 as a therapeutic target in cancer. J Gastroenterol Hepatol. 2013;28:401–405. doi: 10.1111/jgh.12038. [DOI] [PubMed] [Google Scholar]
- 18.Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am J Transl Res. 2014;6:340–352. [PMC free article] [PubMed] [Google Scholar]
- 19.Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J, et al. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575–583. doi: 10.1016/j.ygyno.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2αlpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, et al. HIF-2αlpha deletion promotes Kras-driven lung tumor development. Proc Natl Acad Sci USA. 2010;107:14182–14187. doi: 10.1073/pnas.1001296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, et al. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]