Abstract
Objectives:
To investigate characteristics of euthyroid sick syndrome (ESS) in children with diabetic ketoacidosis (DKA).
Methods:
This retrospective study was carried out between May 2010 and April 2013 at the Pediatric Department of Shandong Provincial Hospital, Shandong University, Shandong, China. Diabetic ketoacidosis children were divided into 2 groups: euthyroidism (group one, n=30) and ESS (group 2, n=40). C-peptide, glycosylated hemoglobin (HbA1c), bicarbonate, anion gap (AG), free triiodothyronine (FT3), free thyroxine (FT4), and thyrotropin (TSH) levels were measured before and after 7 days of insulin treatment. Daily blood glucose (BG) profiles were recorded.
Results:
Glycosylated hemoglobin, AG, the mean daily BG, and fasting blood glucose levels were higher, and bicarbonate, FT3, FT4, and TSH levels were lower in group 2 than in group one (all p<0.05). Free triiodothyronine (r=-0.593, p<0.001) and FT4 (r=-0.402, p=0.001) were negatively correlated with HbA1c. Free triiodothyronine (r=-0.438, p<0.001) and FT4 (r=-0.505, p<0.001) were negatively correlated with AG, and FT3 (r=0.503, p<0.001) and FT4 (r=0.448, p<0.001) were positively correlated with bicarbonate.
Conclusion:
Diabetic ketoacidosis children with ESS have poor diabetic control. Free thyroid hormones are associated with the severity of DKA.
The euthyroid sick syndrome (ESS), also known as low triiodothyronine (T3) syndrome or nonthyroidal illness syndrome, reflects abnormalities in thyroid hormones in patients with acute or chronic stress and illnesses.1 In mild illness, the abnormalities include decreased serum T3 and free triiodothyronine (FT3) levels. In severe illness, there are decreased serum T3, FT3, thyroxine (T4), free thyroxine (FT4), and thyrotropin (TSH) levels.
Type 1 diabetes mellitus (T1DM), particularly diabetic ketoacidosis (DKA), is closely associated with these thyroid hormone abnormalities in ESS.2 The presence of ESS as a predictor of poor prognosis is related to the severity of the illness. It has been shown that serum T3 and T4 levels are correlated with poor glycemic control and ketoacidosis in T1DM.3 It is unclear whether free thyroid hormones are correlated with poor diabetic control in DKA children. In the present study, we investigated characteristics of ESS in DKA children. The correlation between free thyroid hormones and diabetic control were evaluated.
Methods
Patients
Related research was searched using the keywords: euthyroid sick syndrome, diabetic ketoacidosis, and type 1 diabetes mellitus in PubMed. This retrospective case-control study was carried out at the Pediatric Department of Shandong Provincial Hospital Affiliated to Shandong University, Shandong, China between May 2010 and April 2013.
We excluded patients with other endocrinological disorders, systemic illness, pituitary and thyroid disease, and a history of diabetes mellitus. Patients who had previously received any medication apart from insulin were also excluded. The diagnosis of T1DM was based on the American Diabetes Association criteria.4 The biochemical criteria of DKA included blood glucose (BG)>11mmol/L, venous pH<7.3, or bicarbonate <15mmol/L.5 Euthyroidism was defined when FT3, FT4, and TSH levels were within reference ranges; ESS was defined when FT3 and/or FT4 levels were decreased, and TSH levels were normal or decreased.6 A total of 70 DKA patients with euthyroidism and ESS were included in the final analysis. Thirty patients were considered euthyroid (group one), and 40 patients were found to have ESS (group 2).
All patients were managed according to standard guidelines for DKA.7 After resolution of DKA, patients received multiple daily insulin injections, aspart (Novo Nordisk, Bagsvaerd, Denmark) immediately before each meal and glargine (Sanofi-Aventis, Paris, France) once-daily at bedtime. The total daily insulin dose ranged from 0.6 to 1.5 IU/kg.
The study was approved by the Ethical Committee of Shandong Provincial Hospital Affiliated to Shandong University, Shandong, China, and was conducted according to the principles of Helsinki Declaration.
Biochemical assays
Fasting blood samples were taken at the time of initial diagnosis of DKA and stored at -70 °C until assayed. Serum C-peptide was measured by chemiluminescent immunometric assays (Cobas E170, Roche Diagnostics, Mannheim, Germany). Plasma glycosylated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (HLC-723G7, Tosoh Corporation, Tokyo, Japan). Autoantibodies to islet cells, insulin, and glutamic acid decarboxylase were assessed by enzyme-linked immunosorbent assay (ELISA) (Biomerica, Newport Beach, California, USA). Serum bicarbonate and anion gap (AG) were measured using an automatic biochemistry analyzer (AU5400, Beckman Coulter, Tokyo, Japan). Blood glucose levels were measured using a glucometer (Accu-Chek Performa, Roche Diagnostics, Penzberg, Germany). Serum FT3, FT4, TSH, anti-thyroid peroxidase antibody, anti-thyroglobulin antibody, and anti-TSH receptor antibody were measured using an automated chemiluminescent immunoassay system (Advia Centaur, Siemens, Munich, Germany). The intra-assay and inter-assay coefficients of variation were <6% for all parameters. Laboratory data except HbA1c were evaluated after 7 days of treatment (when patients had achieved good control of diabetes and normal thyroid function tests). Daily BG profiles (before and 2 hours after each meal, at bedtime, and at 3 AM) were performed and recorded during treatment.
Statistical analysis
Normally distributed data was presented as mean ± standard deviation (SD). Data of skewed distribution was expressed as median (interquartile range). The Chi-squared test was used to compare categorical variables. Two independent-sample t tests were used for normally distributed data, and Mann-Whitney test was used for skew data between 2 groups. Paired data before and after treatment was compared by the parametric test (paired t test) and nonparametric test (Wilcoxon signed ranks test). The Pearson and Spearman correlation tests were applied to evaluate the relationship between parametric data and nonparametric data. All analysis were performed using the Statistical Package for Social Sciences version 17.0 (SPSS Inc. Chicago, IL, USA). A p-value <0.05 was considered statistically significant.
Results
Clinical characteristics at onset of DKA
With respect to gender, age at onset, duration of symptoms before diagnosis, autoimmune antibodies, and C-peptide, there were no significant differences between the 2 groups (all p>0.05). The levels of HbA1c and AG were significantly higher in group 2 than in group one (both p<0.01). The levels of bicarbonate, FT3, FT4, and TSH were significantly lower in group 2 than in group one (all p<0.01) (Table 1).
Table 1.
Baseline characteristics of children with diabetic ketoacidosis.
Correlation analysis
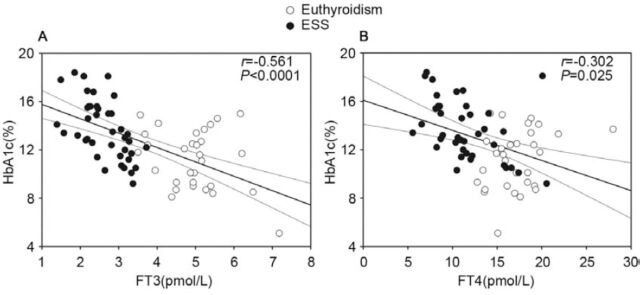
Relationships among baseline laboratory data of all DKA patients were assessed. Free triiodothyronine (r=-0.593, p<0.001) and FT4 (r=-0.402, p=0.001) levels were negatively correlated with the HbA1c level (Figure 1), and FT3 (r=-0.438, p<0.001) and FT4 (r=-0.505, p<0.001) negatively correlated with the AG level. Free triiodothyronine (r=0.503, p<0.001) and FT4 (r=0.448, p<0.001) levels were positively related to the bicarbonate level.
Figure 1.
Baseline correlations of glycosylated hemoglobin (HbA1c) with free triiodothyronine (FT3) levels A) and free thyroxine (FT4) levels B) in children with diabetic ketoacidosis. ESS - euthyroid sick syndrome
Laboratory parameters after treatment
After treatment, in group 2, the mean level of C-peptide increased significantly from a baseline value of 0.2 (0.09, 0.35) (median [25th, 75th percentile]) to 0.31 (0.11, 0.47) ng/mL (p=0.003), bicarbonate increased significantly from 6.6 (3.8, 13.1) to 22.1 (20.8, 23.7) mmol/L (p<0.001), FT3 (2.63±0.58 to 4.77±1.15 pmol/L, p<0.001), and FT4 (11.38±3.58 to 15.57±2.92 pmol/L, p<0.001) also significantly increased. Thyrotropin increased from 1.77±1.19 to 2.17±0.91 mIU/L (p=0.052), AG significantly decreased from 23.7 (21.5, 26.9) to 16.5 (15.0, 17.8) mmol/L (p<0.001). In group one, the mean level of C-peptide increased significantly from a baseline value of 0.27 (0.06, 0.49) to 0.37 (0.11, 0.48) ng/mL (p=0.026), bicarbonate increased significantly from 13.2 (11.3, 14.2) to 22.2 (21.0, 24.8) mmol/L (p<0.001), AG significantly decreased from 20.9 (18.3, 22.6) to 15.9 (14.6, 17.9) mmol/L (p<0.001). There was no significant difference in the C-peptide (p=0.665), bicarbonate (p=0.585), and AG (p=0.399) levels between group one and group 2.
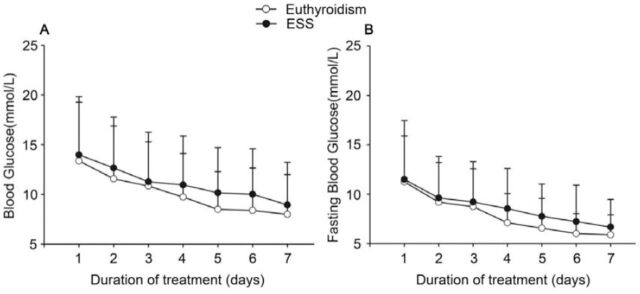
Changes in the mean daily BG and fasting blood glucose (FBG) levels during the 7 days of treatment are shown in Figure 2. The mean daily BG and FBG levels were significantly higher in group 2 (p<0.001) than in group one (p=0.027). The mean daily BG levels and FBG levels of all patients gradually decreased from admission to the end of the study. The mean total daily insulin dose in group one (1.13 [0.97, 1.28] IU/kg) and group 2 (1.08 [0.96, 1.26] IU/kg) showed no significant difference (p=0.674).
Figure 2.
Changes in daily blood glucose and fasting blood glucose levels in children with diabetic ketoacidosis during 7 days of insulin treatment. A) daily blood glucose levels during 7 days of treatment, and B) fasting blood glucose levels during 7 days of treatment. ESS - euthyroid sick syndrome.
Discussion
Type 1 diabetes mellitus is an autoimmune disease. It is associated with the autoimmune thyroid disorders that can affect thyroid hormones.8 Therefore, autoimmune antibodies were measured in our study. The presence of ESS is a predictor of poor prognosis of acute or chronic illnesses. Previous studies have shown that serum T3 and T4 levels are associated with the severity of the disease.9,10 However, studies on the characteristics of ESS, and its associations between free thyroid hormones and other laboratory parameters in children with DKA are rare. In this study, we found that children with ESS had higher HbA1c and AG levels and lower bicarbonate levels compared with children without ESS at the onset of DKA. Serum FT3 and FT4 levels were closely correlated with these changes. Moreover, children with ESS had higher daily BG levels and FBG levels.
The underlying mechanism of ESS is still unclear. Carbohydrate deprivation exists in DKA. Many mechanisms are included:11 downregulated hypothalamic-pituitary-thyroid axis, impaired intracellular uptake of T4 and T3, decreased conversion of T4 to T3 by type 1 deiodinase (D1), increased T4 to reverse T3 (rT3) by D3, reduced thyroid hormone-binding proteins, and diminished expression of nuclear thyroid hormone receptors, are responsible for ESS. In addition, inflammatory cytokines involved in autoimmune T1DM are also attributed to the changes in central and peripheral thyroid hormone metabolism. Hence, there may be low serum levels of T3, FT3, T4, FT4, and TSH, and high serum levels of rT3 in DKA children with ESS. However, serum rT3 levels may be low or elevated in ESS.12 Serum T3 and T4 levels are influenced by alterations in concentrations of thyroid hormone binding proteins. Therefore, serum FT3 and FT4 levels were measured to assess thyroid function in our study, and serum rT3 levels were not measured.
In this study, higher HbA1c, AG, daily BG levels, and FBG levels, and lower bicarbonate levels were found in DKA children with ESS. Euthyroid sick syndrome patients had poor diabetic control. It might be due to attenuated basal insulin levels in hypothyroidism,13 although the difference in serum levels of C-peptide between ESS and euthyroidism was not significant in our children with insulin-deficient T1DM in the acute phase. Free triiodothyronine and FT4 levels were positively correlated with bicarbonate levels and negatively correlated with HbA1c and AG levels. Our results indicate that DKA influences thyroid function and free thyroid hormones reflect the severity of the DKA. Venous pH could not be measured simultaneously with serum bicarbonate in most of our patients. The serum bicarbonate accurately predicts venous pH in the DKA children.14
Thyroid hormone abnormalities revert to normal after appropriate treatment of the underlying disease. Whether ESS patients should be treated with thyroid hormones remains controversial. Our results imply a central defect in the DKA children with ESS by the fact that TSH did not elevate in response to low free thyroid hormones. Serum FT3 and FT4 levels were associated with the severity of the DKA. It might be necessary for future large-scale and long-term research on the effects of T4 treatment on DKA children with ESS. The limitations in our study were limited sample size, one single-center study, and short follow-up.
In conclusion, the presence of ESS in the DKA children is a predictor for poor diabetic control, free thyroid hormones are associated with the severity of the DKA. In the future, large, double-blind, placebo-controlled clinical studies are needed to confirm the findings of this study.
Acknowledgment
The authors are grateful to all parents and their children for participating in this study.
Footnotes
Related Articles
Al-Agha AE, Alafif MM, Abd-Elhameed IA. Glycemic control, complications, and associated autoimmune diseases in children and adolescents with type 1 diabetes in Jeddah, Saudi Arabia. Saudi Med J 2015; 36: 26-31.
Shiva S, Behbahan AG.Autoimmune thyroid disease in children and adolescents with type 1 diabetes mellitus in Northwest Iran. Saudi Med J 2009; 30: 673-676.
Al-Mendalawi MD. Autoimmune thyroid disease in children and adolescents with type 1 diabetes mellitus in Northwest Iran. Saudi Med J 2009; 30: 1489-1490.
Kumar HK, Yadav RK, Prajapati J, Reddy CV, Raghunath M, Modi KD. Association between thyroid hormones, insulin resistance, and metabolic syndrome. Saudi Med J 2009; 30: 907-911.
References
- 1.Pappa TA, Vagenakis AG, Alevizaki M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. 2011;41:212–220. doi: 10.1111/j.1365-2362.2010.02395.x. [DOI] [PubMed] [Google Scholar]
- 2.Joseph J, Saroha V, Payne H, Paul P, Didi M, Isherwood D, et al. Thyroid function at diagnosis of type I diabetes. Arch Dis Child. 2011;96:777–779. doi: 10.1136/adc.2009.168799. [DOI] [PubMed] [Google Scholar]
- 3.Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010;64:1130–1139. doi: 10.1111/j.1742-1241.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 5.Cakan N, Kizilbash S, Kamat D. Changing spectrum of diabetes mellitus in children: challenges with initial classification. Clin Pediatr (Phila) 2012;51:939–944. doi: 10.1177/0009922812441666. [DOI] [PubMed] [Google Scholar]
- 6.Bello G, Pennisi MA, Montini L, Silva S, Maviglia R, Cavallaro F, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest. 2009;135:1448–1454. doi: 10.1378/chest.08-1816. [DOI] [PubMed] [Google Scholar]
- 7.Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee W, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(Suppl 12):S118–S133. doi: 10.1111/j.1399-5448.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 8.Kedari GSR. Estimation of thyroid hormone status and thyroid antibodies in type-1 diabetes mellitus. Indian Journal of Science and Technology. 2010;3:1014–1015. [Google Scholar]
- 9.Song SH, Kwak IS, Lee DW, Kang YH, Seong EY, Park JS. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534–1538. doi: 10.1093/ndt/gfn682. [DOI] [PubMed] [Google Scholar]
- 10.Malekpour B, Mehrafshan A, Saki F, Malekmohammadi Z, Saki N. Effect of posttraumatic serum thyroid hormone levels on severity and mortality of patients with severe traumatic brain injury. Acta Med Iran. 2012;50:113–116. [PubMed] [Google Scholar]
- 11.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 12.Branco RG, Garcia PCR, Piva JP. Thyroid and Growth Hormone Axes Alteration in the Critically Ill Child. In: Wheeler DS, Wong HR, Sharley TP, editors. Pediatric Critical Care Medicine. 2nd ed. London (UK): Springer; 2014. pp. 119–126. [Google Scholar]
- 13.Handisurya A, Pacini G, Tura A, Gessl A, Kautzky-Willer A. Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH) Clin Endocrinol (Oxf) 2008;69:963–969. doi: 10.1111/j.1365-2265.2008.03280.x. [DOI] [PubMed] [Google Scholar]
- 14.Nadler OA, Finkelstein MJ, Reid SR. How well does serum bicarbonate concentration predict the venous pH in children being evaluated for diabetic ketoacidosis? Pediatr Emerg Care. 2011;27:907–910. doi: 10.1097/PEC.0b013e3182302769. [DOI] [PubMed] [Google Scholar]