Key Points
The level of MRD quantified by flow cytometry is more informative than a 0.01% threshold and independently predicts OS.
There was approximately 1 year survival benefit per log depletion. A lower cut point for predicting improved outcome was not reached.
Abstract
The detection of minimal residual disease (MRD) in myeloma using a 0.01% threshold (10−4) after treatment is an independent predictor of progression-free survival (PFS), but not always of overall survival (OS). However, MRD level is a continuous variable, and the predictive value of the depth of tumor depletion was evaluated in 397 patients treated intensively in the Medical Research Council Myeloma IX study. There was a significant improvement in OS for each log depletion in MRD level (median OS was 1 year for ≥10%, 4 years for 1% to <10%, 5.9 years for 0.1% to <1%, 6.8 years for 0.01% to <0.1%, and more than 7.5 years for <0.01% MRD). MRD level as a continuous variable determined by flow cytometry independently predicts both PFS and OS, with approximately 1 year median OS benefit per log depletion. The trial was registered at www.isrctn.com as #68454111.
Introduction
Minimal residual disease (MRD) in myeloma is an independent predictor of progression-free survival (PFS)1-3 and is under consideration as a surrogate trial endpoint to improve the identification of effective treatments,4,5 particularly for frontline trials, which now require 5 to 10 years of follow-up to identify survival differences.6 Conventional response assessment is unsuitable in this setting because of insufficient sensitivity and/or because changes in serum M-protein concentration may only occur several months after tumor depletion because of a long immunoglobulin half-life. In addition, MRD-negative patients in a partial response may have a similar outcome to MRD-negative patients in a complete response (CR) and better outcomes than patients with an MRD-positive CR.2,3
Acceptance of MRD as a surrogate endpoint requires correlation with PFS and overall survival (OS) benefit in multiple trials. Although most studies confirm longer PFS for MRD-negative patients, OS benefits have been more difficult to demonstrate, at least in multivariate models. This may reflect the development of more effective treatments for relapsed disease,7 as well as also the fact that optimal timing for MRD assessment is not known. MRD assays are quantitative and can assess a large range of disease levels,8 and the degree of tumor depletion may be more informative than a positive-negative analysis.9 Quantitative analysis may also improve understanding of the kinetics of response to specific treatments in multicomponent sequential strategies typical of current myeloma trials.
Methods
The Medical Research Council Myeloma IX study was a multicenter, randomized phase 3 trial, with protocol, clinical results, and cytogenetic characterization all having been previously reported.6,10 The protocol was approved by the relevant institutional review boards, and all patients provided written informed consent. This analysis involves 397 patients randomly assigned to receive CTD (cyclophosphamide, thalidomide, and dexamethasone) or CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) for 4 to 6 cycles, and then high-dose melphalan (200 mg/m2) and autologous stem cell transplant (ASCT). Bone marrow aspirates for MRD assessment were obtained at the end of induction and day 100 after ASCT. Patients were then randomly assigned to maintenance thalidomide (50-100 mg daily) or no further therapy at day 100 after ASCT. Flow cytometry for MRD detection was performed as reported previously, briefly assessing 500 000 cells incubated with 6-color antibody combinations including CD138/CD38/CD45/CD19 with CD56/CD27 in all cases and CD81/CD117 in some cases, as required.3 Statistical analysis was also performed as reported previously, with PFS and OS data landmarked to the date of the MRD assessment, with a median follow-up of 71 months.3 For the Cox proportional hazards model, analyses of PFS and OS variables were considered continuous unless otherwise stated. Simple transformations were also considered, and beta-2-microglobulin (B2M) and MRD were subsequently log-transformed. Hemoglobin and platelets were used as stratification factors, and these stratification codings were used in the model. Cytogenetic groups were classified as unfavorable for patients with gain(1q), del(1p32), t(4;14), t(14;20), t(14;16), and del(17p), or favorable for hyperdiploidy, t(11;14), and t(6;14), as previously described,3 or unknown/inevaluable. For the multivariate model, all variables were included to ensure the MRD result allows for all the other variables, whether significant or not.
Results and discussion
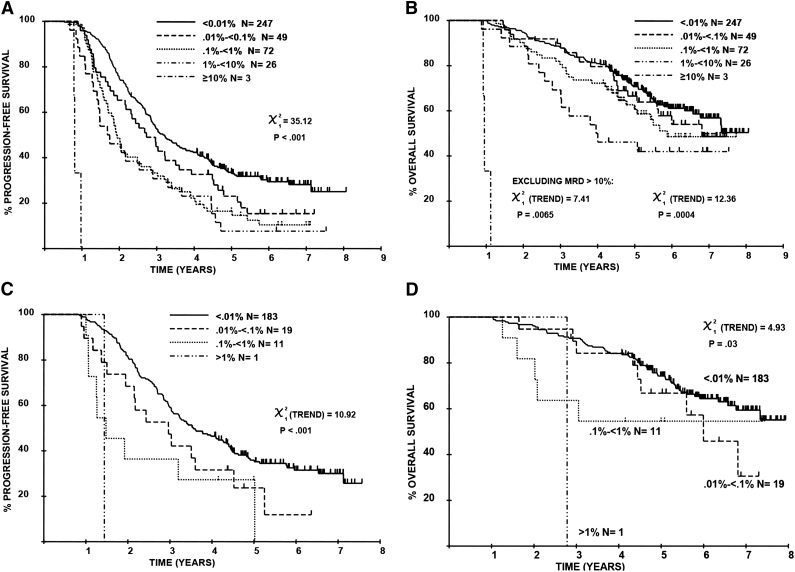
The level of residual disease at day 100 after ASCT varied across 5 logs: less than 0.01% (ie, MRD-negative) in 247/397 (62.2%), 0.01% to less than 0.1% in 49/397 (12.3%), 0.1% to less than 1% in 72/397 (18.1%), 1% to less than 10% in 26/397 (6.5%), and 10% or higher in 3/397 (0.8%; supplemental Table 1, available on the Blood Web site). The PFS and OS for individuals with 1% or more residual disease was comparable to that of individuals with a partial response/minor response/stable disease (supplemental Figure 1), confirming that MRD assessment is most relevant in patients achieving good-quality M-protein responses. The level of MRD correlated with PFS/OS (Figure 1). Across the 5-log MRD range, the median PFS values were 0.8, 1.7, 1.9, 2.7, and 3.1 years, respectively (P < .001); median OS values were 1, 4, 5.9, and 6.8 years and “not reached,” respectively (P < .001), with 5.9 years’ median follow-up. This pattern was also demonstrable when the analysis was restricted to patients achieving a conventional CR (Figure 1C-D).
Figure 1.
Sequential improvements in PFS and OS for each log depletion in MRD level, as assessed by multiparameter flow cytometry. This effect is demonstrable in all patients ([A] PFS; [B] OS) as well as those achieving conventional CR ([C] PFS; [D] OS).
Induction therapy affected MRD. The proportion of patients achieving less than 0.01% MRD was higher with CTD than CVAD (25% vs 13%, respectively, after induction [P = .0039]; 71% vs 54%, respectively, after ASCT [P < .001]).3 Induction treatment also affected the distribution and overall levels of MRD, such that a higher proportion of CR patients were MRD-negative after CTD, and a higher proportion had more than 0.1% MRD after CVAD (supplemental Table 3). We therefore investigated the kinetics of response to induction and ASCT. Bone marrow samples were available both at end of induction and at day 100 after ASCT in 253/397 cases, of whom 47/253 had less than 0.01% MRD at both times, 96/253 had 0.01% or more MRD at both times, and 110/253 achieved less than 0.01% MRD after ASCT only. In those patients with 0.01% or more MRD at the end of induction, the level of disease was approximately 0.5 log lower in those achieving less than 0.01% MRD after ASCT (postinduction median, 0.44%; range, 0.02%-14%) compared with those with 0.01% or higher MRD after ASCT (postinduction median, 1.5% MRD; range, 0.02%-25%; P < .001). For patients with MRD at both times, ASCT resulted in a median 0.67 log tumor depletion (best 2.6 log depletion, worst 1.4 log increase). For patients with 0.01% or higher MRD after induction and less than 0.01% MRD post-ASCT, the minimum log depletion was higher than 1.7 in 50% of cases. The log tumor depletion was similar for patients whether they achieved CR or not (supplemental Table 4). Patients achieving less than 0.01% MRD typically had a 2-log tumor reduction with ASCT, although more sensitive MRD assessments would be required to determine the true tumor depletion.
Given that CR patients had residual disease levels detectable across 4 or more logs, we chose to perform our initial multivariate analysis in all patients regardless of M protein response. This demonstrated that both quantitative MRD assessment and cytogenetic risk profile were the only factors that predicted independently for both PFS and OS (P < .001; Table 1). The benefit of attaining a conventional CR loses independent significance when considered with MRD level, as do International Staging System and B2M. This analysis was also performed in CR patients, and again quantitative MRD and cytogenetics were the only factors that independently predicted for PFS and OS (multivariate Cox model for PFS, P = .0001; for OS, P = .002; supplemental Table 2).
Table 1.
Multivariate Cox model results, including response
| Variable | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | |
| Log (MRD) | 33.0 | <.0001 | 19.4 | <.0001 | 12.4 | .0004 | 11.8 | .0006 |
| Response after ASCT* | 20.5 | <.0001 | 1.3 | .25 | 6.60 | .01 | 0.00 | .99 |
| International Staging System (1-3) | 4.5 | .03 | 0.72 | .40 | 12.9 | .0003 | 1.9 | .16 |
| Cytogenetics† | 39.8 | <.0001 | 41.3 | <.0001 | 36.9 | <.0001 | 35.5 | <.0001 |
| Log (B2M) | 8.3 | .004 | 1.8 | .18 | 11.9 | .0006 | 0.34 | .56 |
| Platelets‡ | 10.6 | .001 | 3.8 | .05 | 2.1 | .14 | 0.00 | .98 |
| Hemoglobin¶ | 15.0 | .0001 | 7.5 | .006 | 8.5 | .003 | 1.7 | .19 |
| Age (continuous) | 0.1 | .75 | 0.01 | .92 | 1.7 | .20 | 1.9 | .17 |
| Sex | 0.3 | .58 | 0.23 | .63 | 0.01 | .93 | 0.04 | .85 |
Univariate and multivariate analysis demonstrating that the level of MRD at day 100 after ASCT, assessed by multiparameter flow cytometry, is an independent predictor of both PFS and OS. Shaded cells indicate significant variables (P < .05).
CRs vs rest.
Favorable vs unfavorable vs unknown/inevaluable.
Stratification factor: <11.5 vs ≥11.5.
Stratification factor: <150 vs ≥150.
The prognostic value of MRD is also independent of treatment type, with patients achieving less than 0.01% residual disease having the same outcome whether they received CVAD or CTD induction.11 Patients with adverse cytogenetics had slightly lower MRD (median log MRD, −1.63 for adverse vs −1.53 for favorable cytogenetics); the difference was not significant (P = .40), and this confirms previous reports that the worse outcome for adverse cytogenetics is not a result of posttreatment disease burden.1,2
There was no evidence for a lower cutpoint to predict survival benefit per log tumor depletion; therefore, more sensitive approaches are likely to provide better prediction of outcome. The 0.01% MRD threshold was originally based on technical capability,12 but quantitative MRD detection is now possible at 10−5 by flow cytometry and 10−6 by high-throughput sequencing, and there is evidence that very deep remissions predict even better survival.13 Current treatment approaches are now sufficiently effective that high-sensitivity quantitative MRD analysis is required for meaningful response measurement, particularly in large multicenter trials. As discussed recently at a US Food and Drug Administration roundtable meeting,5 these data strongly support the role of MRD assessment as surrogate endpoint for survival in clinical trials.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.C.R., W.M.G., G.J.M., J.A.C., and R.G.O. conceived and designed the study; F.E.D., G.C., G.H.J., G.J.M., J.A.C., and R.G.O. provided study materials or patients; A.C.R., W.M.G., R.M.d.T., S.E.B., M.T.D., and R.G.O. collected and assembled the data; A.C.R., W.M.G., R.M.d.T., M.T.D., J.A.C., and R.G.O. analyzed and interpreted the data; all authors participated in the writing of the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: A.C.R. acted as a consultant for Celgene and BD Biosciences; received honoraria from Genzyme, GlaxoSmithKline, and Roche; and received other remuneration from BD Biosciences. F.E.D. acted as a consultant for Celgene, Ortho Biotech, and Novartis and received honoraria and other remuneration from Ortho Biotech and Celgene. W.M.G. acted as a consultant for Celgene and received other remuneration from Celgene and Ortho Biotech. G.C. acted as a consultant for and received honoraria from Celgene and Janssen-Cilag, received research funding from Celgene, and received other remuneration from Celgene and Janssen-Cilag. G.J.M. acted as a consultant for, received honoraria from, and received other remuneration from Celgene, Merck, Novartis, and Johnson & Johnson. J.A.C. received honoraria and other remuneration from Science Agency and Network. G.H.J. received honoraria from Celgene and Janssen-Cilag. R.G.O. received honoraria and other remuneration from Celgene and Ortho Biotech. S.B. received other remuneration from Celgene and Ortho Biotech.
Correspondence: Roger G. Owen, Haematological Malignancy Diagnostic Service Laboratory, Level 3, Bexley Wing, St James's University Hospital, Leeds, United Kingdom, LS9 7TF; e-mail: rogerowen@nhs.net.
References
- 1.Paiva B, Gutiérrez NC, Rosiñol L, et al. PETHEMA/GEM (Programa para el Estudio de la Terapéutica en Hemopatías Malignas/Grupo Español de Mieloma) Cooperative Study Groups. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–691. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 2.Paiva B, Vidriales M-B, Cerveró J, et al. GEM (Grupo Español de MM)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Groups. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017–4023. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540–2547. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 4.Munshi NC, Anderson KC. Minimal residual disease in multiple myeloma. J Clin Oncol. 2013;31(20):2523–2526. doi: 10.1200/JCO.2013.49.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Gormley N, Turley D, et al. Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI roundtable symposium. Am J Hematol. 2014;89(12):1159–1160. doi: 10.1002/ajh.23831. [DOI] [PubMed] [Google Scholar]
- 6.Morgan GJ, Davies FE, Gregory WM, et al. National Cancer Research Institute Haematological Oncology Clinical Studies Group. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97(3):442–450. doi: 10.3324/haematol.2011.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ocio EM, Richardson PG, Rajkumar SV, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia. 2014;28(3):525–542. doi: 10.1038/leu.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawstron AC, Orfao A, Beksac M, et al. European Myeloma Network. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93(3):431–438. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- 9.Böttcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 10.Morgan GJ, Davies FE, Gregory WM, et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19(21):6030–6038. doi: 10.1158/1078-0432.CCR-12-3211. [DOI] [PubMed] [Google Scholar]
- 11.De Tute RM, Rawstron AC, Child JA, et al. Impact of minimal residual disease and induction therapy on outcome post ASCT: insights from the MRD Myeloma IX trial. Clin Lymphoma Myeloma Leuk. 2013;13:S45. [Google Scholar]
- 12.Sarasquete ME, García-Sanz R, González D, et al. Minimal residual disease monitoring in multiple myeloma: a comparison between allelic-specific oligonucleotide real-time quantitative polymerase chain reaction and flow cytometry. Haematologica. 2005;90(10):1365–1372. [PubMed] [Google Scholar]
- 13.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]