Abstract
Objective
The purpose of this study was to determine the role of the endothelial glucocorticoid receptor in the pathogenesis of atherosclerosis.
Approach and Results
Control mice and mice lacking the endothelial glucocorticoid receptor were bred onto an Apoe knockout background and subjected to high-fat diet feeding for 12 weeks. Assessment of body weight and total cholesterol and triglycerides before and after the diet revealed no differences between the two groups of mice. However, mice lacking the endothelial glucocorticoid receptor developed more severe atherosclerotic lesions in the aorta, brachiocephalic artery and aortic sinus as well as a heightened inflammatory milieu as evidence by increased macrophage recruitment in the lesions.
Conclusions
These data suggest the endothelial glucocorticoid receptor is important for tonic inhibition of inflammation and limitation of atherosclerosis progression in this model.
Keywords: endothelium, glucocorticoid, mouse model, atherosclerosis
INTRODUCTION
The glucocorticoid receptor (GR) is a nuclear hormone receptor that is expressed ubiquitously in most cell types and is important in many states of health and disease. Recent work has demonstrated that tissue-specific loss of this receptor can produce profound phenotypes. 1-4 The role of glucocorticoids in cardiovascular disease is complex. For example the stress response, which is elevated in chronic conditions such as atherosclerosis, hypertension and the metabolic syndrome, has been implicated in the heightened vulnerability to disease found in these conditions by activating the hypothalamic-pituitary-adrenal axis and increasing production of circulating endogenous steroid.5, 6 Conversely, acute high-dose exogenous corticosteroids have been shown to be cardioprotective under some conditions,7 and have been used as potential inhibitors of atherosclerosis and coronary restenosis after coronary intervention.8
To try to discern more clearly the role of endogenous glucocorticoids during the progression of atherosclerosis and the cell types regulated by endogenous corticosterone, we created a mouse model with an endothelial cell specific deletion of GR bred onto an Apoe knockout background. Here we show that loss of the endothelial GR worsens the atherosclerotic phenotype suggesting that endogenous corticosterone acting via endothelial GR tonically suppresses vascular inflammation and plays a role in limiting the progression of atherosclerosis.
MATERIALS AND METHODS
Material and Methods are available in the online-only Data Supplement.
RESULTS
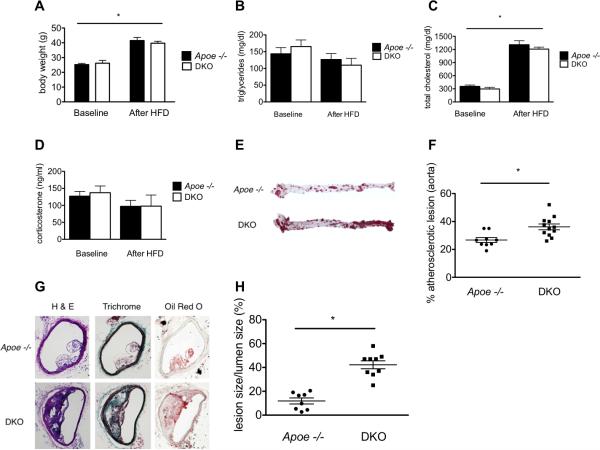
Littermate Apoe −/− GR fl/fl (Apoe−/−) and Apoe −/− GR fl/fl Tie1-Cre (DKO) mice were fed a high fat diet (HFD) for 12 weeks as described.9 Previously, we have documented the endothelial specificity of the deleted GR in several studies.1, 3 Both groups of mice had similar weights (Fig 1A), triglycerides (Fig 1B), cholesterol levels (Fig 1C) and corticosterone levels (Fig 1D) at baseline and after feeding mice with HFD. Similar corticosterone levels were indicative that there was no derangement in the hypothalamic-pituitary-adrenal axis in DKO animals nor evidence of heightened stress in these animals. These results are in agreement with previously published work.1
Figure 1.
Loss of the endothelial glucocorticoid receptor accelerates atherosclerosis in Apo E −/− mice (A) Weight is significantly increased in both groups following 12 weeks of HFD feeding. (B) No significant difference in triglycerides before or after HFD. (C) Both groups show a statistically significant increase in cholesterol after HFD as expected. (D) No difference in corticosterone levels before or after HFD. (E) Representative examples of aortic Oil Red O staining with (F) quantification of aortic lesions. (G) Representative staining of brachiocephalic arteries with hematoxylin and eosin, Trichrome and Oil Red O staining with (H) quantification of lesion size. Data are mean ± SEM. n=5-8 mice/group. *p<0.05.
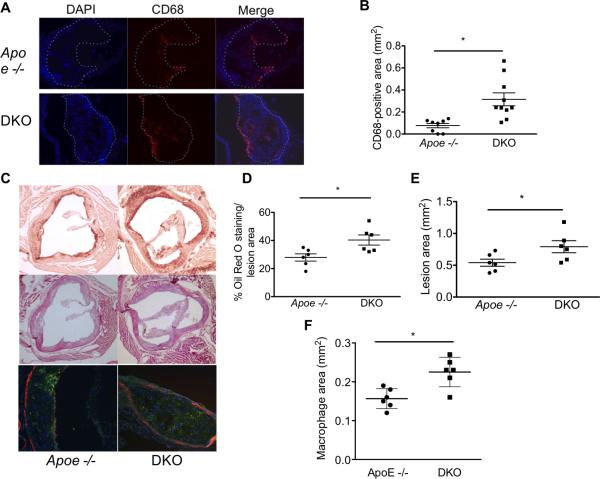
At the completion of the feeding period, mice were sacrificed, perfused and the extent of atherosclerosis in multiple vessels was examined. Aortas were stained en face with Oil Red O and the percentage of total aortic neutral lipids deposition quantified. As shown in Figure 1E and quantified in 1F, DKO mice showed significantly greater lesion areas than did Apoe -/- mice. There were no differences noted in the anatomic distribution of the lesions when suprarenal and infrarenal aortas were analyzed separately (Figure I-online only Data Supplement). Similar results were obtained in cross sections of brachiocephalic arteries (Figs 1G and H). To determine if the increased lesion size in the DKO mice was associated with heightened inflammation, tissue macrophages (via CD68 labeling) were assessed immunohistochemically. As shown in Figure 2A and quantified in Figure 2B, DKO animals showed greater macrophage accumulation in the atherosclerotic lesions of brachiocephalic arteries indicating a heightened inflammatory state. As additional evidence that inflammation was increased in the DKO animals, staining for VCAM was also performed in these vessels. DKO mice also showed increased VCAM-positive staining in the atherosclerotic lesions (Figure II-online only Data Supplement).
Figure 2.
More inflammation and larger cardiac lesions in Apo E −/− mice lacking the endothelial glucocorticoid receptor. (A) Representative immunofluorescent sections of brachiocephalic lesions stained with DAPI and CD68. Dotted line indicates plaque area. (B) Quantification of CD68 staining in each group. (C) Representative sections of the aortic sinus stained with Oil Red O (top, 4x), hematoxylin and eosin (middle, 4x) and CD68 (bottom, 10x). CD68 is stained in green, DAPI in blue and α-SMA in red. Quantification of (D) Oil Red O staining area, (E) total lesion area and (F) CD68 positive lesion area in the aortic root. Data represent mean ± SEM. N=6-10/group. * p<0.05
We also examined aortic root lesions in hearts from Apoe -/- and DKO mice. Hearts were prepared and sectioned as described above to allow visualization of the aortic sinus lesions and representative sections are shown in Figure 2C. Quantification of Oil Red O staining/lesion area (Figure 2D) and total lesion area (Figure 2E) showed more extensive lesions in DKO mice compared to Apoe -/- mice. Macrophage infiltration was also assessed in aortic root lesions via CD68 staining. As shown in Figure 2C and quantified in 2F, DKO mice had increased absolute macrophage area. In addition, lesions in the coronary ostiae were identified in 3/6 DKO mice but in none of the Apoe -/- mice (Figure III- online only Data Supplement).
In a separate series of experiments, mice were fed the Paigen diet to examine the effect of diet-induced, TLR4-dependent inflammation on lesion progression.10, 11 Though this diet has been studied in this context previously, it is important to note that it may have other effects which were not assessed in our study. Importantly, only 6 out of 16 DKO mice survived this diet whereas 12 out of 13 Apoe-/- mice survived (Figure IV online-only Data Supplement). There were no differences in body weight between the groups, however DKO mice exhibited higher corticosterone levels (Figure VA and VB, online-only Data Supplement). Atherosclerotic lesions in the aorta and brachiocephalic artery were also significantly more diseased compared to Apoe-/- mice. DKO mice had statistically more infrarenal lesion compared to Apoe -/- mice, though no differences were observed in the distribution of suprarenal lesions (Figures VI and VII, online-only Data Supplement). Collectively, these data imply that endothelial GR is critical for the atheroprotective actions of endogenous corticosterone.
DISCUSSION
The major finding of this study is that loss of the endothelial GR results in a more severe atherosclerotic phenotype in Apoe−/− mice. These results are striking since they demonstrate that elimination of the receptor for the endogenous ligand, corticosterone, in a single cell type, namely the endothelium, is sufficient to produce this dramatic phenotype. These data support the importance of the permissive actions of endogenous corticosterone via endothelial GR in reducing vascular inflammation and highlights the possibility of tissue-specific manipulation of local glucocorticoid metabolism as a potential therapy for cardiovascular disease. Isozymes of 11 beta hydroxysteroid dehydrogenase are responsible for local regulation of the access of glucocorticoids to their receptors, with the enzyme 11βHSD1 responsible for the conversion of the inactive cortisone to cortisol (or corticosterone in mouse) and 11βHSD2 converting the active cortisol (corticosterone) to cortisone.12 Both enzymes are known to exist in the endothelium. Previous studies have shown that 11βHSD1 knockout attenuates atherosclerosis13 while 11βHSD2 deficiency accelerates atherosclerosis14 indeed suggesting that local glucocorticoid metabolism is the key to understanding endogenous steroid effects in the context of cardiovascular disease. It is interesting to note that both of these studies used mice globally deficient for the enzymes whereas we achieved a similar magnitude of enhanced atherosclerosis by altering the endogenous steroid milieu in the endothelium only. Our data in mice fed the Paigen diet are somewhat unexpected given that most of the DKO animals die during the feeding period, a phenomenon that is not often seen in mouse models of atherosclerosis. However the cause of death was not directly assessed. We suspect the higher corticosterone levels observed in the DKO animals fed the Paigen diet are a marker of increased stress and may have contributed to mortality. These results suggest that that the endothelial glucocorticoid receptor is profoundly important for maintaining vascular homeostasis and that its loss in combination with a ‘second hit,’ such as the inflammatory diet used here, accelerates disease. Collectively, these data indicate that both local glucocorticoid metabolism and tissue-specific glucocorticoid interactions are likely important for mediating the complex interplay between endogenous steroids and cardiovascular disease.
Supplementary Material
SIGNIFICANCE.
It is well appreciated that endothelial dysfunction is one of the first steps in the development of many cardiovascular diseases, including atherosclerosis. The role of steroids in the pathophysiology of cardiovascular disease is not as clear-cut and published studies exist which demonstrate evidence for both beneficial and detrimental effects of exogenous steroids. Our study directly assesses the role of the endothelial glucocorticoid receptor (GR) in the pathogenesis of atherosclerosis. Here we show that loss of endothelial GR results in markedly more severe atherosclerotic lesions both in mice fed a standard Western diet as well as mice fed the more inflammatory Paigen diet. These data support an important role for endothelial GR in suppressing inflammation and also highlight the importance of tissue-specific glucocorticoid metabolism in the pathogenesis of cardiovascular disease. This concept is novel, and provides an explanation for the well-documented permissive role of endogenous cortisol.
Acknowledgments
SOURCES OF FUNDING
This work was supported by a grant from the Charles H. Hood Foundation, Inc, Boston, MA to J.E.G. N.R. was supported by a postdoctoral fellowship from the Spanish Ministry (Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+i 2008-2011). J.Y. was supported by NIH HL117064 and an award from the American Heart Association. WCS was supported by NIH HL64793, HL61371, HL081190 from the National Institutes of Health .
Abbreviations
- GR
glucocorticoid receptor
- HFD
high fat diet
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Goodwin JE, Feng Y, Velazquez H, Sessa WC. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci U S A. 2013;110:306–311. doi: 10.1073/pnas.1210200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin JE, Zhang J, Geller DS. A critical role for vascular smooth muscle in acute glucocorticoid-induced hypertension. J Am Soc Nephrol. 2008;19:1291–1299. doi: 10.1681/ASN.2007080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodwin JE, Zhang J, Gonzalez D, Albinsson S, Geller DS. Knockout of the vascular endothelial glucocorticoid receptor abrogates dexamethasone-induced hypertension. J Hypertens. 2011;29:1347–1356. doi: 10.1097/HJH.0b013e328347da54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tronche F, Kellendonk C, Reichardt HM, Schutz G. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev. 1998;8:532–538. doi: 10.1016/s0959-437x(98)80007-5. [DOI] [PubMed] [Google Scholar]
- 5.Martocchia A, Stefanelli M, Falaschi GM, Toussan L, Rocchietti March M, Raja S, Romano G, Falaschi P. Targets of anti-glucocorticoid therapy for stress-related diseases. Recent patents on CNS drug discovery. 2013;8:79–87. doi: 10.2174/1574889811308010007. [DOI] [PubMed] [Google Scholar]
- 6.Fantidis P. The role of the stress-related anti-inflammatory hormones acth and cortisol in atherosclerosis. Current vascular pharmacology. 2010;8:517–525. doi: 10.2174/157016110791330889. [DOI] [PubMed] [Google Scholar]
- 7.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadoke PW, Macdonald L, Logie JJ, Small GR, Dover AR, Walker BR. Intravascular glucocorticoid metabolism as a modulator of vascular structure and function. Cellular and molecular life sciences : CMLS. 2006;63:565–578. doi: 10.1007/s00018-005-5427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell metabolism. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samokhin AO, Wilson S, Nho B, Lizame ML, Musenden OE, Bromme D. Cholate-containing high-fat diet induces the formation of multinucleated giant cells in atherosclerotic plaques of apolipoprotein e−/− mice. Arterioscler Thromb Vasc Biol. 2010;30:1166–1173. doi: 10.1161/ATVBAHA.110.203976. [DOI] [PubMed] [Google Scholar]
- 11.Desai MS, Mariscalco MM, Tawil A, Vallejo JG, Smith CW. Atherogenic diet-induced hepatitis is partially dependent on murine tlr4. Journal of leukocyte biology. 2008;83:1336–1344. doi: 10.1189/jlb.0607390. [DOI] [PubMed] [Google Scholar]
- 12.Hadoke PW, Iqbal J, Walker BR. Therapeutic manipulation of glucocorticoid metabolism in cardiovascular disease. Br J Pharmacol. 2009;156:689–712. doi: 10.1111/j.1476-5381.2008.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia RA, Search DJ, Lupisella JA, et al. 11beta-hydroxysteroid dehydrogenase type 1 gene knockout attenuates atherosclerosis and in vivo foam cell formation in hyperlipidemic apoe(−)/(−) mice. PloS one. 2013;8:e53192. doi: 10.1371/journal.pone.0053192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deuchar GA, McLean D, Hadoke PW, Brownstein DG, Webb DJ, Mullins JJ, Chapman K, Seckl JR, Kotelevtsev YV. 11beta-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe−/− mice. Endocrinology. 2011;152:236–246. doi: 10.1210/en.2010-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.