Abstract
Despite the overwhelming evidence justifying the use of non-invasive ventilation (NIV) for providing ventilatory support in chronic obstructive pulmonary disease (COPD) exacerbations, recent studies demonstrated that its application in real-life settings remains suboptimal. European clinical audits have shown that 1) NIV is not invariably available, 2) its availability depends on countries and hospital sizes, and 3) numerous centers declare their inability to provide NIV to all of the eligible patients presenting throughout the year. Even with an established indication, the use of NIV in acute respiratory failure due to COPD exacerbations faces important challenges. First, the location and personnel using NIV should be carefully selected. Second, the use of NIV is not straightforward despite the availability of technologically advanced ventilators. Third, NIV therapy of critically ill patients requires a thorough knowledge of both respiratory physiology and existing ventilatory devices. Accordingly, an optimal team-training experience, the careful selection of patients, and special attention to the selection of devices are critical for optimizing NIV outcomes. Additionally, when applied, NIV should be closely monitored, and endotracheal intubation should be promptly available in the case of failure. Another topic that merits careful consideration is the use of NIV in the elderly. This patient population is particularly fragile, with several physiological and social characteristics requiring specific attention in relation to NIV. Several other novel indications should also be critically examined, including the use of NIV during fiberoptic bronchoscopy or transesophageal echocardiography, as well as in interventional cardiology and pulmonology. The present narrative review aims to provide updated information on the use of NIV in acute settings to improve the clinical outcomes of patients hospitalized for COPD exacerbations.
Keywords: COPD, exacerbations, non-invasive ventilation
Chronic obstructive pulmonary disease (COPD) represents a global health issue. Recently updated data on disease prevalence[1,2,3,4] and mortality[5,6] have shown that it is a major public health problem worldwide, causing substantial morbidity and mortality. Despite recent advances in the understanding of COPD and its management, there are still several critical areas of COPD management in need of improvement, including 1) the potential issue of under-diagnosis (particularly in female patients),[7,8] 2) the variability in the perception of symptoms,[9,10] 3) the presence of different disease guidelines and approaches (e.g., multidimensional evaluations[11,12,13] versus phenotype-based medicine[14]), 4) the impact of risk factors other than tobacco,[15,16] and 5) the role of concomitant comorbidities.[17,18,19,20,21,22]
One area of special interest is the use of non-invasive ventilation (NIV) during COPD exacerbations. Despite the overwhelming evidence justifying the use of non-invasive mechanical ventilatory support in COPD exacerbations, recent studies demonstrated that its application in real-life settings remains suboptimal.[23] Moreover, over the last few decades the indications for NIV have expanded in both acute and chronic care.[24] In this context, a critical update on NIV for COPD exacerbations is needed to examine the indications and potential impact of ventilatory support in this clinical setting.
In this narrative review, we sought to provide updated information on the use of NIV in the respiratory ward to improve the clinical outcomes of patients hospitalized for COPD exacerbations. In addition, the current limitations and future research goals will be discussed. We attempt to provide a balanced view of the current use of NIV in COPD patients, identifying the lights and shadows of this treatment in the acute setting.
NIV for COPD in the Acute Setting
The history of NIV extends back more than 100 years, but it was not until 1987 that what is considered “modern” NIV was developed.[25] The term NIV defines any ventilatory support that does not require tracheal intubation of the patient. NIV was popularized during polio epidemics in the fifties through application of an extrathoracic negative pressure ventilator (i.e., the “iron lung”). At that time, this type of ventilation was successful and it was the only such system in existence, other than positive pressure through a permanent tracheostomy, which was used in a few cases. However, negative pressure ventilation was complex and not without side effects, thus requiring a highly specialized management team. Hence, its use was not generalized, and it was only used in specialized centers. NIV advanced in the late eighties with the advent of positive pressure non-invasive ventilation via a nasal mask to treat respiratory failure in patients with advanced neuromuscular disease, respiratory restrictive conditions, or sleep apnea. Since then, its use has increased. Notably, NIV is currently considered the initial treatment of choice for acute respiratory failure.[26,27]
The use of NIV in the acute setting for COPD first occurred in the 1990s, when the results of major clinical trials became available. Despite the initial negative results,[28] subsequent trials clearly demonstrated the clinical usefulness of NIV for treating acute hypercapnic respiratory failure due to COPD. The primary milestone was most likely the study by Plant et al.[29] This study included 236 patients, half of whom received standard therapy plus additional NIV. The authors concluded that the early use of NIV for mildly and moderately acidotic COPD patients in the general ward results in a rapid improvement of physiological variables, reducing both the need for invasive mechanical ventilation and in-hospital mortality. Although the study participants were not spirometrically confirmed, the authors included patients who were admitted with a diagnosis of acute exacerbations of COPD and their results were consistent with those obtained in other trials.[30,31] Moreover, the authors demonstrated that NIV was cost-effective.[32] Several subsequent investigations and meta-analyses confirmed that NIV decreases both the need for intubation and mortality in COPD patients presenting with acute respiratory failure.[26] Of note, this effect was consistently observed in both intensive care units (ICUs) and respiratory wards.[33] However, the impact on mortality varied from 10%[29] in the context of a clinical trial to 33% in a real-life setting.[34] Furthermore, other physiological advantages have been reported for patients undergoing NIV, including the resolution of obstructive atelectasis.[35] Currently, even with an established indication, the use of NIV in acute respiratory failure caused by COPD exacerbations faces important challenges. In this regard, several issues remain to be addressed, including implementation of NIV, locations at which NIV should be utilized, the predictors of success, the use of NIV in elderly subjects, and novel indications for NIV in acute settings.
Implementation of NIV
Despite evidence regarding its efficacy, the use and implementation of non-invasive mechanical ventilation in COPD remain suboptimal. A clinical audit performed in the United Kingdom reported that COPD admissions treated with NIV in routine clinical practice involved severely ill patients. However, this audit raised concerns that challenged the respiratory community to develop appropriate clinical improvements. Specifically, numerous ventilated patients had mixed metabolic acidosis, some eligible subjects failed to receive NIV, whereas others received it inappropriately. Moreover, in several cases, NIV appeared to be used as a last-ditch treatment in patients for whom its efficacy remains uncertain.[34]
In the European COPD audit (focusing on the clinical performance of hospitals that treated patients with exacerbated COPD in 13 European countries), the investigators assessed the degree of fulfillment of clinical guidelines based on blood gas data. Among patients who were candidates to receive NIV according to arterial blood gas parameters (i.e., moderate to severe acidosis and hypercapnia), only 51.0% underwent NIV accordingly. In turn, 28.6% of patients who were treated with NIV did not meet the arterial blood gas criteria.[23] In the same audit, the authors found that 89.6% of centers provided NIV during admission.[36] However, the variability was considerable (ranging between 60 and 100%), depending on the country and the size of the hospital. The percentages were 84.2% for small hospitals, 87.5% for medium-size hospitals, and 97.1% for large hospitals. Additionally, when the investigators asked whether they had the capacity to noninvasively ventilate all of the eligible patients, 32.5% of the participating centers declared that they did not to have the resources to ventilate all of the eligible subjects presenting throughout the year. This response was independent of the size of the hospital, suggesting a similar effect across all types of centers.[36] In this scenario, clinical managers should ensure that the proper resources required to ventilate all of the eligible patients throughout the year are provided.
One potential factor that might influence the correct application of NIV throughout the year might be related to the learning curve. In a recent investigation, the authors performed a time trend analysis of a retrospective observational study based on the minimum basic hospital discharge data aimed at evaluating the introduction of NIV in patients hospitalized for COPD at all public hospitals in the region of Murcia (Spain) between 1997 and 2010.[37] Although the observed improvements in terms of global mortality or length of stay did not reach statistical significance, the introduction of NIV in these hospitals reduced the number of patients not receiving assisted ventilation. Additionally, by using a join point regression analysis, the authors demonstrated an upward trend in the use of NIV in the participating hospitals.
Locations for NIV Use
Another issue that merits consideration is the optimal location at which NIV should be delivered. Because patients requiring NIV are critically ill, the progression of gas exchange abnormalities and the clinical conditions in the first few hours are paramount for determining the clinical outcomes after the initial acute episode. Accordingly, the use of NIV is not straightforward despite the availability of technologically advanced ventilators. Importantly, NIV therapy of critically ill patients requires a thorough knowledge of both respiratory physiology (including respiratory mechanics and gas exchange abnormalities) and existing ventilatory devices (e.g., interfaces and valves). Moreover, a minimum monitoring level is needed for its use. In a prospective observational cohort study performed in conventional wards, a small group of consecutive patients requiring NIV due to acute hypercapnic respiratory failure were investigated.[38] Besides traditional prognostic variables, an inadequate use of NIV due to a lack of personnel training was detected in all of the patients presenting with NIV failure (relative risk = 3.5; 95% confidence interval = 1.08-11.2; P = 0.007). In this study, the most common caveats included:
The lack of knowledge on how to operate the ventilator by the staff,
An improper mask fitting leading to excessive leaks, and
The inability of the personnel to control oxygen therapy or to address the ventilator alarms.
Subsequently, Sumner et al.[39] demonstrated that the improper use of NIV in non-designated areas resulted in an increased patient mortality. However, it has been also argued that NIV can be safely administered in an adequately staffed and monitored ward when used to prevent intubation in otherwise stable patients.[40] Therefore, a trained team, careful patient selection, and an optimal choice of the device can optimize NIV outcomes. Accordingly, the debate on where to apply NIV is mainly related to the available hospital resources and the knowledge and experience of the staff rather than to the actual location of NIV use. In this regard, it would be desirable to establish a consensus document to help managers decide where to locate NIV facilities and to provide them with adequate resources. Accordingly, a substantial variability in both the availability and resources has been reported.[41] In the study by Plant et al.,[29] the nursing staff received eight hours of training on a monthly basis during the three months preceding the study. However, no information was available on the minimum number of hours used for training physicians to ensure proper implementation of ventilation. Based on these results,[23] we can assume that such a training may be sufficient. However, given the acute situation and the complexity of the management process (particularly in the first few hours), consensus guidelines on minimum requirements or conditions to ensure proper training are eagerly awaited.
Appropriate staff training is guaranteed in ICUs. However, ICU cares are complex and expensive and are not invariably needed for all of the patients with exacerbated COPD requiring NIV. In this regard, several specific intermediate locations (i.e., between the ICU and the respiratory ward) have been recently implemented for the application of NIV in respiratory patients. Such so-called semi-critical, intermediate, or high-dependency units have recently emerged in industrialized countries as an alternative to ICUs, with the specific goal of providing non-invasive respiratory support without the complex environment and the costs of an ICU.[42] The characteristics of the three levels of respiratory units according to the Spanish National Society of Pulmonology and Thoracic Surgery (SEPAR) is summarized in [Table 1].[43] The efficacy and cost-effectiveness of such units have been repeatedly demonstrated[44,45] as they provide an ideal location for ventilatory support (equipped with adequate resources and staff) and a more comfortable environment for patients. Several models of intermediate care units have been proposed.[46] However, the implementation of such units continues to remain suboptimal. The European COPD audit reported that 49.3% of European hospitals were equipped with such units.[36] However, their availability varies widely regardless of the hospital size. In Europe, the average number of available beds is 7.1 (ranging from 5.9 in small hospitals to 8.2 in large hospitals).
Table 1.
Characteristics of the three levels of respiratory units
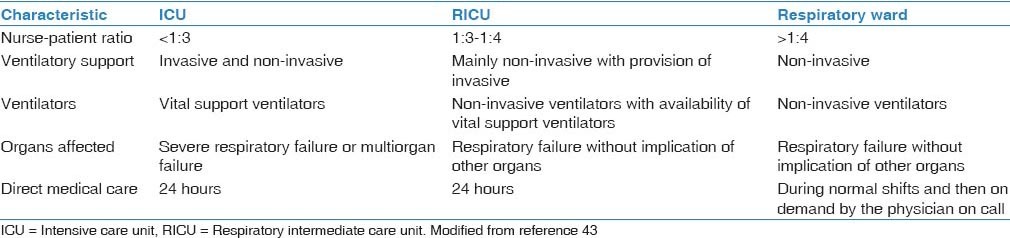
Predictors of Success
Although the use of NIV is currently considered the gold standard for managing respiratory failure during COPD exacerbations, not all patients respond equally well. Consequently, significant efforts have been made to identify the main predictors of successful NIV. Specifically, it would be important to distinguish successful responders from patients requiring endotracheal intubation. This research topic has been recently reviewed in detail.[47] Treatment failure is defined by the British Thoracic Society consensus guidelines as 1) a deterioration in the patient's clinical conditions, 2) lack of improvement or deterioration in arterial blood gas parameters, 3) development of new symptoms or complications that require endotracheal intubation or ICU admission, or 4) a decrease in the level of consciousness.[48] Failures can be divided into early and late. A failure is defined as early when it occurs within 1-48 h of NIV use (either with or without an initial success), whereas late failures occur 48 h after initiation of NIV, following an initial successful response. According to several observational studies,[49,50] the main factors associated with NIV failure include a poor nutritional status, a reduced level of consciousness, and an impaired general condition (as reflected by a high APACHE score, low pH, and/or high partial pressure of carbon dioxide). Such variables might in turn be influenced by a patient's tolerance to NIV, which is directly related to the training and experience of the staff with this technique.[51] Notably, a significant predictor of success in COPD patients treated for acute hypercapnic respiratory failure is the patient's response to the initial NIV treatment.[52] Because NIV is an effective treatment, patients should experience improvement within a few hours after the initiation of the ventilation. Consequently, international guidelines recommend a second complete evaluation of the patient after a few hours of NIV use.[52] When no improvements occur, the prognosis is uncertain. In presence of NIV failure, a decision concerning intubation should be promptly made taking into account the severity of the underlying disease and the previous level of disability.[48,53] In the [Table 2], a summary of the main causes for non-invasive respiratory support failure and the actions to overcome these limitation is provided.[43]
Table 2.
Main causes for non-invasive respiratory support failure and the actions to overcome these limitations
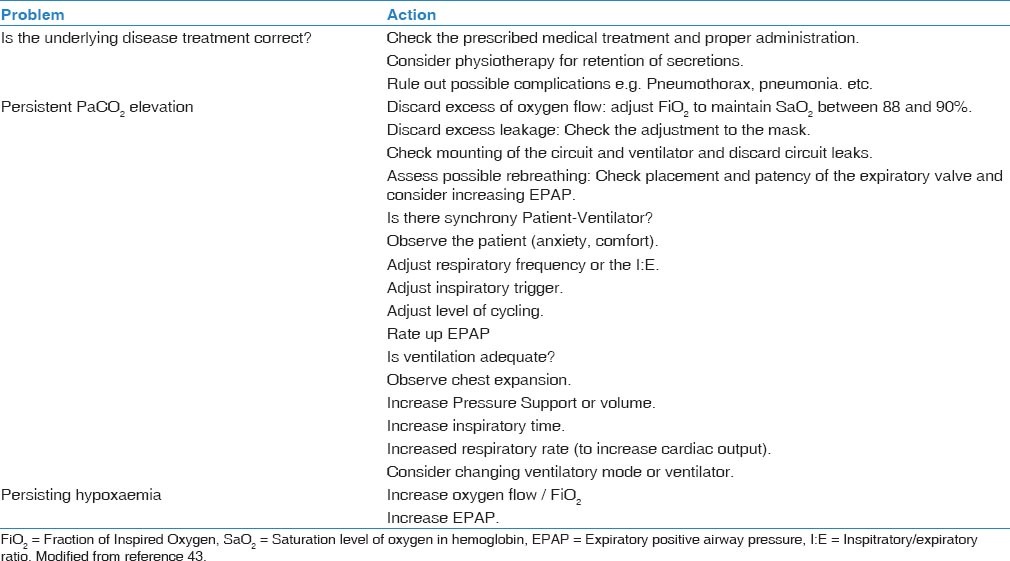
NIV in the Elderly
In the elderly, the use of NIV has specific considerations. This patient population is particularly fragile, with several physiological and social characteristics that require specific health care. In this population, NIV can be considered as a good alternative for treating respiratory acidosis even for those with a do-not-intubate order.[54] A recent study performed in Spain analyzed the outcomes of NIV in elderly patients (aged 75 years or older) who were admitted to a respiratory monitoring unit during hospitalization and one year later.[43] Specifically, the results were compared with those obtained in a younger patient group.[55] The authors did not detect any significant difference in terms of in-hospital mortality between the two groups. However, elderly patients were more frequently re-admitted within 6-12 months after hospital discharge than the younger group. In Spain, the re-admission rate is 37% for all causes and 28% for COPD-related admissions.[56] Although the reasons for this high COPD readmission rate (specifically for elderly patients) are not entirely understood, several arguments have been proposed. Most explanations have involved the poorer functional states and the frailty of such patients following hospitalization.[57] Notably, some authors have suggested adapting or tailoring current guidelines to the needs of elderly patients presenting with frailty and/or cognitive impairment.[58] Overall, the available evidence demonstrates that elderly patients with acute hypercapnic respiratory failure can be successfully treated with NIV, even following a do-not-intubate order, with satisfactory long-term survival rates.[54] We believe that it would be interesting to gather further evidence on the effectiveness of NIV in elderly patients, particularly after their first hospital admission for respiratory failure.[59]
Other Indications for NIV
NIV has been utilized to treat conditions other than COPD, including acute hypercapnic respiratory failure, cardiogenic pulmonary edema, acute lung injury, acute respiratory distress syndrome, community-acquired pneumonia, and weaning/post-extubation failure. These applications are common clinical practice. A novel indication for non-invasive mechanical ventilation is the use of NIV during invasive procedures, such as bronchoscopy. Evidence supports the use of NIV during fiberoptic bronchoscopy, particularly in subjects at high risk of endotracheal intubation (e.g., immunocompromised patients).[60] NIV treats acute respiratory failure by addressing gas exchange abnormalities and reducing the signs of respiratory effort and dyspnea, as well as the activity of the accessory respiratory muscles. Bronchoscopy is a key technique in studying respiratory diseases, and it is necessary for acute and critical patients, often only after endotracheal intubation due to the possible complications of the technique. The use of NIV during bronchoscopy should be considered as an alternative to avoid the complications related to intubation and mechanical ventilation in patients in severe conditions, particularly in subjects with COPD with a tendency to develop hypercapnia.[60] Other indications include transesophageal echocardiography, interventional cardiology, and pulmonology. In these circumstances, NIV can reduce the need for deep sedation or general anesthesia and may prevent the respiratory depression that results from deep sedation.[61,62,63] NIV may also be useful after surgery,[47] including cardiac surgery, and to a lesser extent, in patients with pulmonary contusions.[64] However, NIV should not be considered an alternative to endotracheal intubation for severe communicable airborne infections that are likely to progress to acute respiratory distress syndrome.[65] Finally, NIV is being increasingly used as an alternative to endotracheal intubation in end-stage symptomatic patients, particularly to relieve dyspnea.[66]
Conclusions
The use of NIV in the acute setting has a positive impact in patients with COPD exacerbations and hypercapnic respiratory failure. Moreover, its use can be considered in elderly patients with do-not-intubate orders and subjects who have exhausted all other treatment options. Notably, NIV may be clinically useful for improving outcomes in clinical situations other than COPD exacerbations. Despite its efficacy, the implementation of NIV remains suboptimal, and managers should ensure the availability of trained staff and sufficient resources to guarantee its availability throughout the year. NIV should be applied with close monitoring, and endotracheal intubation should be promptly available in cases of failure. An optimal team-training experience, the careful selection of patients, and special attention to the selection of devices are critical for optimizing NIV outcomes in critically ill patients presenting with COPD exacerbations.
Acknowledgements
The authors gratefully acknowledge Enzo Emanuele (Living Research s.a.s., Robbio, Italy) for his editorial assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cabrera Lopez C, Julia Serda G, Cabrera Lacalzada C, Martin Medina A, Gullon Blanco JA, Garcia Bello MA, et al. Prevalence of chronic obstructive pulmonary disease in the canary islands. Arch Bronconeumol. 2014;50:272–7. doi: 10.1016/j.arbres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Menezes AM, Muino A, Lopez-Varela MV, Valdivia G, Lisboa C, Jardim JR, et al. A population-based cohort study on chronic obstructive pulmonary disease in Latin America: Methods and preliminary results. The PLATINO Study Phase II. Arch Bronconeumol. 2014;50:10–7. doi: 10.1016/j.arbres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landis SH, Muellerova H, Mannino DM, Menezes AM, Han MK, van der Molen T, et al. Continuing to Confront COPD International Patient Survey: Methods, COPD prevalence, and disease burden in 2012-2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597–611. doi: 10.2147/COPD.S61854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Campos JL, Ruiz-Ramos M, Soriano JB. COPD mortality rates in Andalusia, Spain, 1975-2010: A joinpoint regression analysis. Int J Tuberc Lung Dis. 2013;17:131–6. doi: 10.5588/ijtld.12.0419. [DOI] [PubMed] [Google Scholar]
- 6.Represas Represas C, Ruano Ravina A, Fernandez Villar A. Changes in chronic obstructive pulmonary disease mortality trends: Fact or fiction? Arch Bronconeumol. 2014;50:311–2. doi: 10.1016/j.arbres.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Ancochea J, Miravitlles M, Garcia-Rio F, Munoz L, Sanchez G, Sobradillo V, et al. Underdiagnosis of chronic obstructive pulmonary disease in women: Quantification of the problem, determinants and proposed actions. Arch Bronconeumol. 2013;49:223–9. doi: 10.1016/j.arbres.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Cote CG, Chapman KR. Diagnosis and treatment considerations for women with COPD. Int J Clin Pract. 2009;63:486–93. doi: 10.1111/j.1742-1241.2008.01987.x. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: A narrative review. Int J Chron Obstruct Pulmon Dis. 2013;8:231–8. doi: 10.2147/COPD.S42866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miravitlles M, Ferrer J, Baro E, Lleonart M, Galera J. Differences between physician and patient in the perception of symptoms and their severity in COPD. Respir Med. 2013;107:1977–85. doi: 10.1016/j.rmed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Lopez Campos JL, Calero C. Questionnaires in multidimensional assessment of chronic obstructive pulmonary disease: Two sides of the same coin. Arch Bronconeumol. 2014;50:265–6. doi: 10.1016/j.arbres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 13.Rieger-Reyes C, Garcia-Tirado FJ, Rubio-Galan FJ, Marin-Trigo JM. Classification of chronic obstructive pulmonary disease severity according to the new global initiative for chronic obstructive lung disease 2011 guidelines: COPD assessment test versus modified medical research council scale. Arch Bronconeumol. 2014;50:129–34. doi: 10.1016/j.arbres.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Miravitlles M, Soler-Cataluna JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(Suppl 1):1–16. doi: 10.1016/S0300-2896(14)70070-5. [DOI] [PubMed] [Google Scholar]
- 15.Golpe R, Sanjuan Lopez P, Cano Jimenez E, Castro Anon O, Perez de Llano LA. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Arch Bronconeumol. 2014;50:318–24. doi: 10.1016/j.arbres.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Camp PG, Ramirez-Venegas A, Sansores RH, Alva LF, McDougall JE, Sin DD, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43:725–34. doi: 10.1183/09031936.00206112. [DOI] [PubMed] [Google Scholar]
- 17.Gea J, Martinez-Llorens J, Barreiro E. Nutritional abnormalities in chronic obstructive pulmonary disease. Med Clin (Barc) 2014;143:78–84. doi: 10.1016/j.medcli.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Lopez Varela MV, Montes de Oca M, Halbert R, Muino A, Talamo C, Perez-Padilla R, et al. PLATINO team. Comorbidities and health status in individuals with and without COPD in five latin american cities: The PLATINO study. Arch Bronconeumol. 2013;49:468–74. doi: 10.1016/j.arbres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Garcia de Tena J, El Hachem Debek A, Hernandez Gutierrez C, Izquierdo Alonso JL. The role of vitamin D in chronic obstructive pulmonary disease, asthma and other respiratory diseases. Arch Bronconeumol. 2014;50:179–84. doi: 10.1016/j.arbres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Comeche Casanova L, Echave-Sustaeta JM, Garcia Lujan R, Albarran Lozano I, Alonso Gonzalez P, Llorente Alonso MJ. Prevalence of anaemia associated with chronic obstructive pulmonary disease. Study of associated variables. Arch Bronconeumol. 2013;49:383–7. doi: 10.1016/j.arbres.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: Systematic literature review. Chest. 2013;144:1163–78. doi: 10.1378/chest.12-2847. [DOI] [PubMed] [Google Scholar]
- 22.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: A comprehensive analysis using data from primary care. Thorax. 2010;65:956–62. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 23.Roberts CM, Lopez-Campos JL, Pozo-Rodriguez F, Hartl S. European COPD Audit team. European hospital adherence to GOLD recommendations for chronic obstructive pulmonary disease (COPD) exacerbation admissions. Thorax. 2013;68:1169–71. doi: 10.1136/thoraxjnl-2013-203465. [DOI] [PubMed] [Google Scholar]
- 24.Kohnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014 doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 25.Diaz Lobato S, Mayoralas Alises S. Modern non-invasive mechanical ventilation turns 25. Arch Bronconeumol. 2013;49:475–9. doi: 10.1016/j.arbres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 26.McCurdy BR. Noninvasive positive pressure ventilation for acute respiratory failure patients with chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser. 2012;12:1–102. [PMC free article] [PubMed] [Google Scholar]
- 27.Sinuff T, Keenan SP. Department of Medicine, McMaster University. Clinical practice guideline for the use of noninvasive positive pressure ventilation in COPD patients with acute respiratory failure. J Crit Care. 2004;19:82–91. doi: 10.1016/j.jcrc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Barbe F, Togores B, Rubi M, Pons S, Maimo A, Agusti AG. Noninvasive ventilatory support does not facilitate recovery from acute respiratory failure in chronic obstructive pulmonary disease. Eur Respir J. 1996;9:1240–5. doi: 10.1183/09031936.96.09061240. [DOI] [PubMed] [Google Scholar]
- 29.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: A multicentre randomised controlled trial. Lancet. 2000;355:1931–5. doi: 10.1016/s0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- 30.Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–13. doi: 10.1164/ajrccm.161.3.9808143. [DOI] [PubMed] [Google Scholar]
- 31.Bardi G, Pierotello R, Desideri M, Valdisserri L, Bottai M, Palla A. Nasal ventilation in COPD exacerbations: Early and late results of a prospective, controlled study. Eur Respir J. 2000;15:98–104. doi: 10.1183/09031936.00.15109800. [DOI] [PubMed] [Google Scholar]
- 32.Plant PK, Owen JL, Parrott S, Elliott MW. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: Economic analysis of randomised controlled trial. BMJ. 2003;326:956. doi: 10.1136/bmj.326.7396.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez Guerra J, Lopez-Campos Bodineau JL, Perea-Milla Lopez E, Pons Pellicer J, Rivera Irigoin R, Moreno Arrastio LF. Non invasive ventilation for acute exacerbation of chronic obstructive pulmonary disease: A meta-analysis. Med Clin. 2003;120:281–6. [PubMed] [Google Scholar]
- 34.Roberts CM, Stone RA, Buckingham RJ, Pursey NA, Lowe D. National Chronic Obstructive Pulmonary Disease Resources and Outcomes Project implementation group. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–8. doi: 10.1136/thx.2010.153114. [DOI] [PubMed] [Google Scholar]
- 35.Mirambeaux Villalona R, Mayoralas Alises S, Diaz Lobato S. Resolution of obstructive atelectasis with non-invasive mechanical ventilation. Arch Bronconeumol. 2014;50:452–3. doi: 10.1016/j.arbres.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. European COPD Audit team. Variability of hospital resources for acute care of COPD patients: The European COPD Audit. Eur Respir J. 2014;43:754–62. doi: 10.1183/09031936.00074413. [DOI] [PubMed] [Google Scholar]
- 37.Carpe-Carpe B, Hernando-Arizaleta L, Ibanez-Perez MC, Palomar-Rodriguez JA, Esquinas-Rodriguez AM. Evolution of the use of noninvasive mechanical ventilation in chronic obstructive pulmonary disease in a Spanish region, 1997-2010. Arch Bronconeumol. 2013;49:330–6. doi: 10.1016/j.arbres.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Campos JL, Garcia Polo C, Leon Jimenez A, Arnedillo A, Gonzalez-Moya E, Fenandez Berni JJ. Staff training influence on non-invasive ventilation outcome for acute hypercapnic respiratory failure. Monaldi Arch Chest Dis. 2006;65:145–51. doi: 10.4081/monaldi.2006.560. [DOI] [PubMed] [Google Scholar]
- 39.Sumner K, Yadegafar G. The utility and futility of non-invasive ventilation in non-designated areas: Can critical care outreach nurses influence practice? Intensive Crit Care Nurs. 2011;27:211–7. doi: 10.1016/j.iccn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. 2009;374:250–9. doi: 10.1016/S0140-6736(09)60496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doherty MJ, Greenstone MA. Survey of non-invasive ventilation (NIPPV) in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) in the UK. Thorax. 1998;53:863–6. doi: 10.1136/thx.53.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Confalonieri M, Kodric M, Garuti G. Respiratory intermediate (high dependency) unit care in Europe: Models of service provision. Monaldi Arch Chest Dis. 2001;56:248–53. [PubMed] [Google Scholar]
- 43.Manual SEPAR de Procedimientos. 16. Ventilación mecánica no invasiva. Barcelona: Fundación Respira; 2008. Procedimientos en VMNI de pacientes agudos o crónicos agudizados; pp. 25–43. [Google Scholar]
- 44.Torres A, Ferrer M, Blanquer JB, Calle M, Casolive V, Echave JM, et al. Intermediate respiratory intensive care units: Definitions and characteristics. Arch Bronconeumol. 2005;41:505–12. doi: 10.1016/s1579-2129(06)60271-1. [DOI] [PubMed] [Google Scholar]
- 45.Bertolini G, Confalonieri M, Rossi C, Rossi G, Simini B, Gorini M, et al. GiViTI (Gruppo italiano per la Valutazione degli interventi in Terapia Intensiva) Group; Aipo (Associazione Italiana Pneumologi Ospedalieri) Group. Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit. Respir Med. 2005;99:894–900. doi: 10.1016/j.rmed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Cheng DC, Byrick RJ, Knobel E. Structural models for intermediate care areas. Crit Care Med. 1999;27:2266–71. doi: 10.1097/00003246-199910000-00034. [DOI] [PubMed] [Google Scholar]
- 47.Murphy PB, Zoumot Z, Polkey MI. Noninvasive ventilation and lung volume reduction. Clin Chest Med. 2014;35:251–69. doi: 10.1016/j.ccm.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 48.British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moretti M, Cilione C, Tampieri A, Fracchia C, Marchioni A, Nava S. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–25. doi: 10.1136/thorax.55.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ambrosino N, Vagheggini G. Non-invasive ventilation in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:471–6. [PMC free article] [PubMed] [Google Scholar]
- 51.Nava S, Ceriana P. Causes of failure of noninvasive mechanical ventilation. Respir Care. 2004;49:295–303. [PubMed] [Google Scholar]
- 52.Miller D, Fraser K, Murray I, Thain G, Currie GP. Predicting survival following non-invasive ventilation for hypercapnic exacerbations of chronic obstructive pulmonary disease. Int J Clin Pract. 2012;66:434–7. doi: 10.1111/j.1742-1241.2012.02904.x. [DOI] [PubMed] [Google Scholar]
- 53.Hore CT. Non-invasive positive pressure ventilation in patients with acute respiratory failure. Emerg Med (Fremantle) 2002;14:281–95. doi: 10.1046/j.1442-2026.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 54.Scarpazza P, Incorvaia C, Amboni P, di Franco G, Raschi S, Usai P, et al. Long-term survival in elderly patients with a do-not-intubate order treated with noninvasive mechanical ventilation. Int J Chron Obstruct Pulmon Dis. 2011;6:253–7. doi: 10.2147/COPD.S18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segrelles Calvo G, Zamora Garcia E, Giron Moreno R, Vazquez Espinosa E, Gomez Punter RM, Fernandes Vasconcelos G, et al. Non-invasive ventilation in an elderly population admitted to a respiratory monitoring unit: Causes, complications and one-year evolution. Arch Bronconeumol. 2012;48:349–54. doi: 10.1016/j.arbres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Pozo-Rodriguez F, Lopez-Campos JL, Alvarez-Martinez CJ, Castro-Acosta A, Aguero R, Hueto J, et al. AUDIPOC Study Group. Clinical audit of COPD patients requiring hospital admissions in Spain: AUDIPOC study. PloS One. 2012;7:e42156. doi: 10.1371/journal.pone.0042156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006) Heart Lung. 2013;42:163–70. doi: 10.1016/j.hrtlng.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlitzer J, Haubaum S, Frohnhofen H. Treatment of chronic obstructive pulmonary disease in hospitalized geriatric patients. Z Gerontol Geriatr. 2014;47:288–92. doi: 10.1007/s00391-014-0645-6. [DOI] [PubMed] [Google Scholar]
- 59.Esquinas Rodriguez AM, Zamarro Garcia C. Non-invasive mechanical ventilation in elderly patients: Moving towards a new strategy for hospital organization? Arch Bronconeumol. 2013;49:275–6. doi: 10.1016/j.arbres.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Esquinas A, Zuil M, Scala R, Chiner E. Bronchoscopy during non-invasive mechanical ventilation: A review of techniques and procedures. Arch Bronconeumol. 2013;49:105–12. doi: 10.1016/j.arbres.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Pisano A, Angelone M, Iovino T, Gargiulo S, Manduca S, De Pietro A. Transesophageal echocardiography through a non-invasive ventilation helmet. J Cardiothoracic Vasc Anesth. 2013;27:e78–81. doi: 10.1053/j.jvca.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Hilbert G, Clouzeau B, Nam Bui H, Vargas F. Sedation during non-invasive ventilation. Minerva Anestesiol. 2012;78:842–6. [PubMed] [Google Scholar]
- 63.Guarracino F, Cabrini L, Baldassarri R, Cariello C, Covello RD, Landoni G, et al. Non-invasive ventilation-aided transoesophageal echocardiography in high-risk patients: A pilot study. Eur J Echocardiogr. 2010;11:554–6. doi: 10.1093/ejechocard/jeq019. [DOI] [PubMed] [Google Scholar]
- 64.Vidhani K, Kause J, Parr M. Should we follow ATLS guidelines for the management of traumatic pulmonary contusion: The role of non-invasive ventilatory support. Resuscitation. 2002;52:265–8. doi: 10.1016/s0300-9572(01)00475-0. [DOI] [PubMed] [Google Scholar]
- 65.Mal S, McLeod S, Iansavichene A, Dukelow A, Lewell M. Effect of out-of-hospital noninvasive positive-pressure support ventilation in adult patients with severe respiratory distress: A systematic review and meta-analysis. Ann Emerg Med. 2014;63:600–7. doi: 10.1016/j.annemergmed.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Ambrosino N, Guarracino F. Unusual applications of noninvasive ventilation. Eur Respir J. 2011;38:440–9. doi: 10.1183/09031936.00192810. [DOI] [PubMed] [Google Scholar]


