Abstract
Herein we present the case of a 58 year old woman with porocarcinoma of the left forehead with perineural invasion, diagnosed after recurrence of previously excised benign poroma. This case serves as a reminder of the potential of malignant degeneration within long-standing benign adnexal tumors as well as the spectrum of histological features that may be seen in porocarcinoma.
Keywords: Perineural invasion, porocarcinoma, sweat gland neoplasm
INTRODUCTION
Porocarcinoma is a malignant sweat gland tumor, first described under the name “epidermotropic eccrine carcinoma” by Pinkus and Mehregan in 1963.[1] Porocarcinoma may be considered a single entity or subdivided into apocrine and eccrine types. Some of the dermatopathologists consider this distinction redundant, as differentiating between them is often histologically impossible and of no clinical significance. Porocarcinoma may arise de novo or from a pre-existing benign poroma. Herein we present the case of a 58-year-old woman with porocarcinoma of the left forehead with perineural invasion, diagnosed after recurrence of previously excised benign poroma.
CASE REPORT
This was a case report of a 58-year-old woman was referred to the dermatologist with an approximately four years history of an asymptomatic blue papule, approximately 6 mm in diameter, on the left forehead [Figure 1a]. The lesion had been biopsied twice previously with histology consistent with benign sweat gland neoplasm on both occasions. Due to recurrence, a further punch biopsy was performed. This demonstrated a well-circumscribed dermal neoplasm; some scattered cells of the neoplasm were enlarged and had eosinophilic cytoplasm [Figures 2–4].
Figure 1.
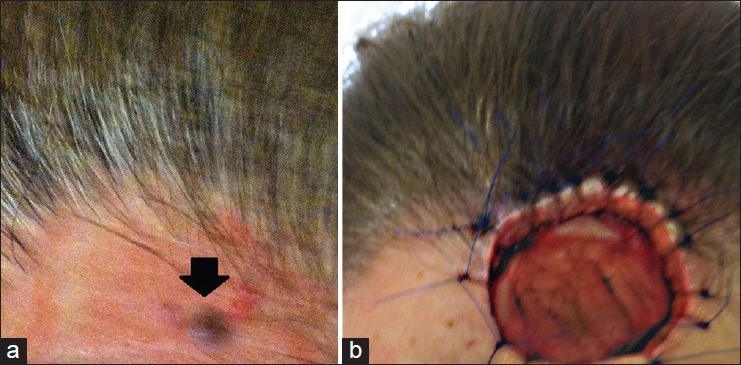
(a) Patient during her first presentation, bluish papule on the forehead approximately 6 mm in diameter, (b) Recurrence of the lesion as a much larger nodule in June 2012
Figure 2.
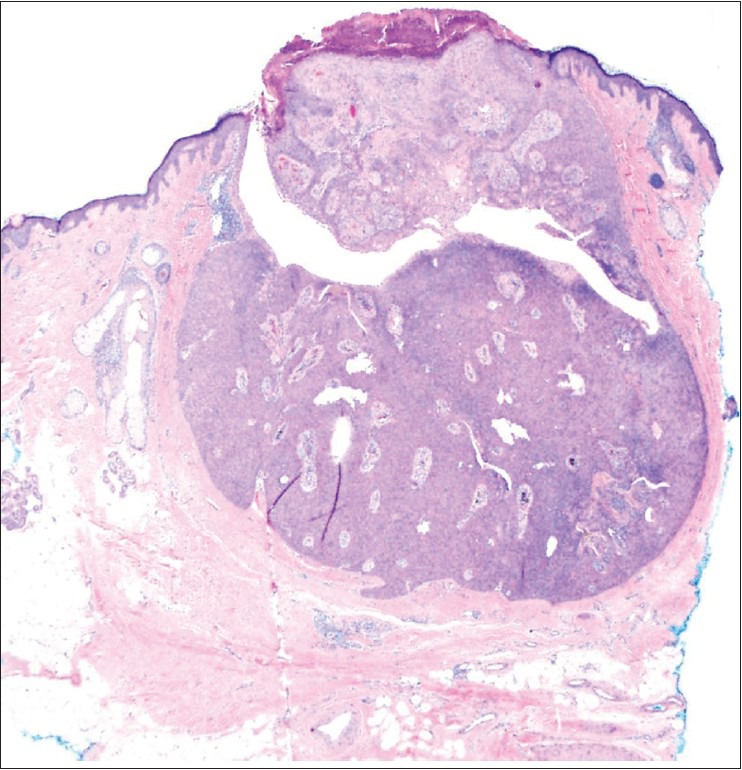
Small, well-circumscribed, slightly eroded adnexal neoplasm connected with the epidermis. H and E, ×40
Figure 4.
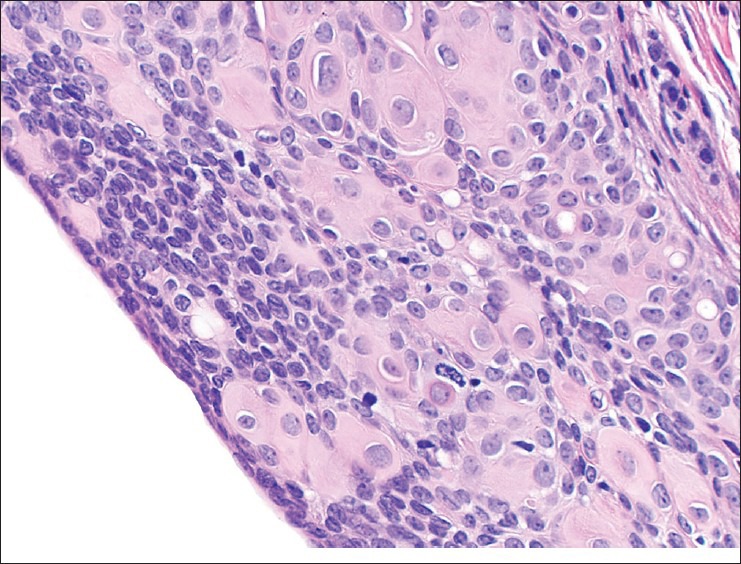
Ductal cells, some with abundant pink cytoplasm. H and E, ×400
Figure 3.
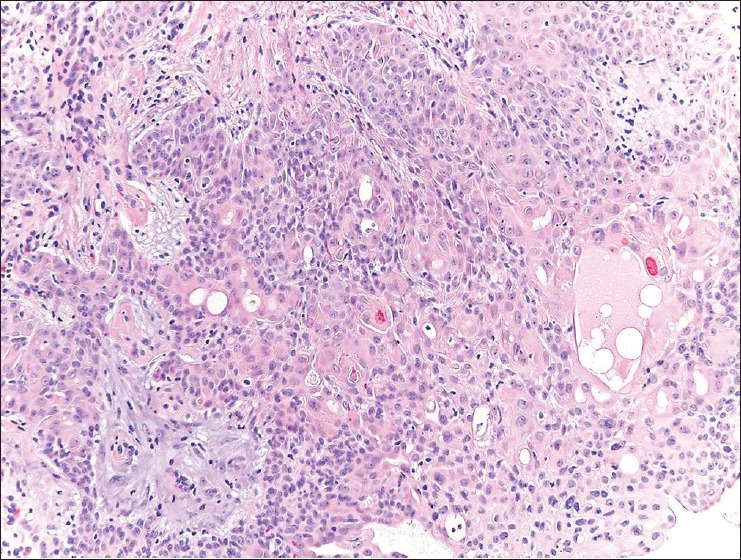
Neoplasm consisting of poroid and cuticular cells, with ductal differentiation. H and E, ×200
The diagnosis of “benign poroma with unusual features” was rendered and complete re-excision of the lesion was recommended. Within 3 months the lesion had recurred once again as a much larger nodule [Figure 1b]. Excisional biopsy exhibited a neoplasm characterized by a circumscribed epithelial proliferation with ductal differentiation [Figure 5].
Figure 5.
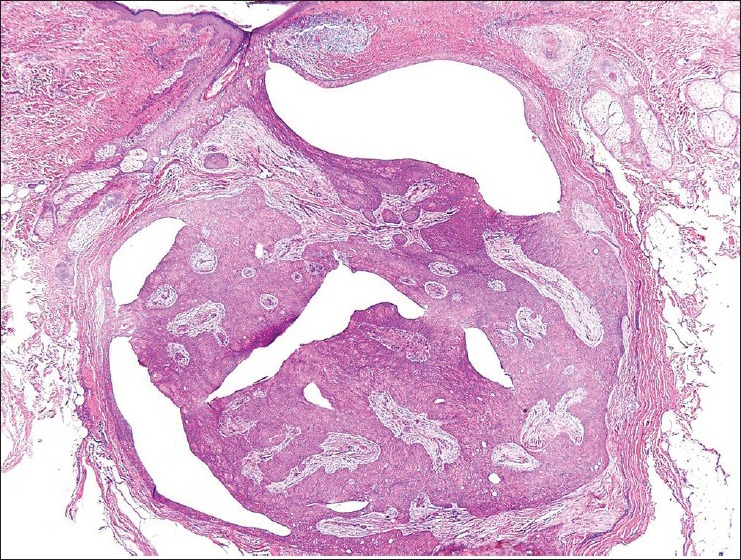
Mostly circumscribed epithelial proliferation with ductal differentiation. H and E, ×40
In one of the subsequent sections the shape of the neoplasm showed a focus suspicious for malignancy, with irregular islands of proliferating cells [Figures 6 and 7].
Figure 6.
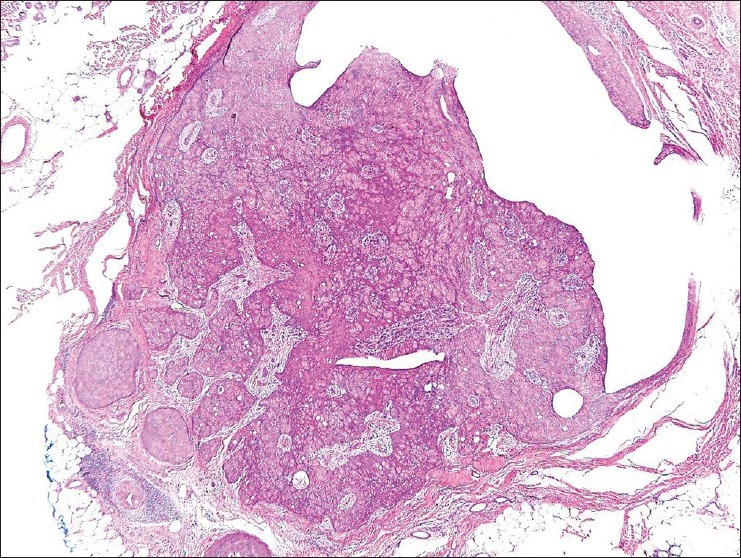
Irregular focus with separate islands of cellular proliferation. H and E, ×40
Figure 7.
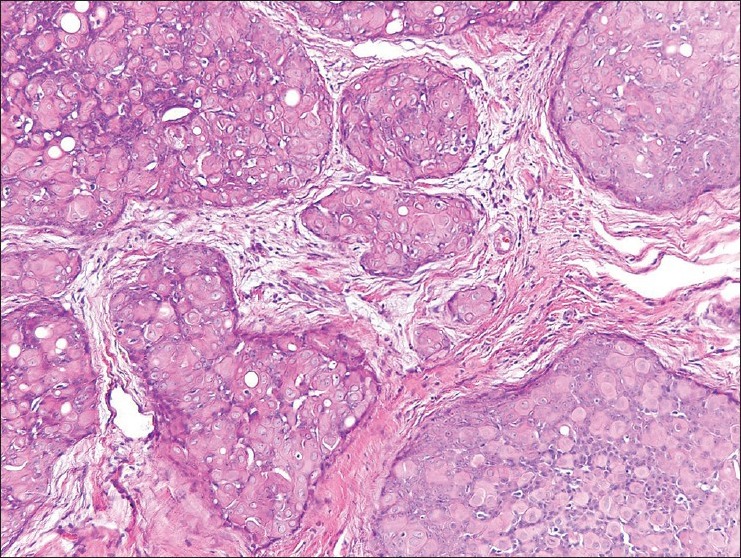
Separated islands of cellular proliferation with similar ductal differentiation. H and E, ×200
Compared with the previous excision specimen, a greater number of cells had abundant eosinophilic cytoplasm. The presence of abnormal mitotic figures were also noted [Figure 8].
Figure 8.
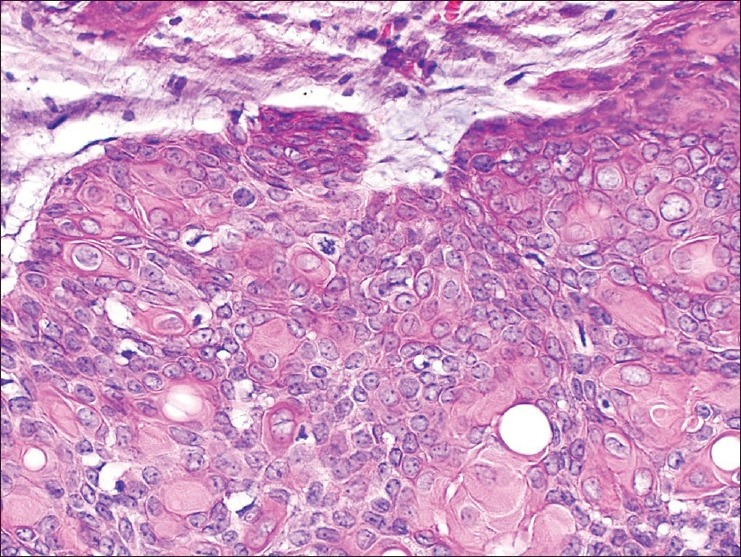
Cells of the proliferation with abundant pink cytoplasm and nuclear atypia and numerous mitotic figures. H and E, ×200
Perineural infiltration of these atypical cells with abundant eosinophilic cytoplasm was seen at the edge of the specimen [Figure 9].
Figure 9.
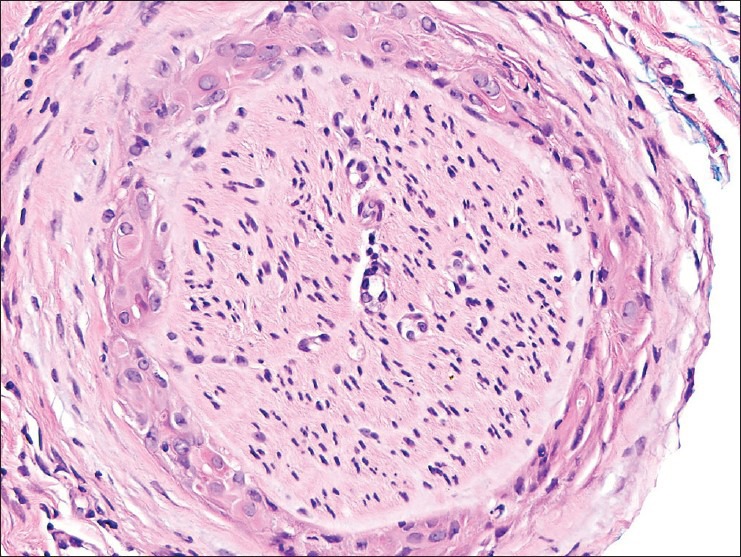
Perineural invasion by atypical cells with abundant pink cytoplasm. H and E, ×400
Clinically, there were no neuropathic symptoms reported, nor evidence of local or distant metastatic spread. This was confirmed by a negative positron emission tomography scan. Definitive management following this diagnosis was wide excision at the biopsy site, given that the lesion extended to the peripheral margin on histology.
DISCUSSION
Porocarcinoma affects both sexes equally, most often in the 6th to 7th decade, and all races may be affected. It may develop de novo or arise with in a pre-existing poroma. In the case of the latter a history of a longstanding lesion with recent rapid growth may be elicited. Porocarcinoma has also been reported as occurring in patients with other malignancies (chronic lymphocytic leukemia, Hodgkin's disease) and autoimmune conditions (pernicious anemia, human immunodeficiency virus), as well as extramammary Paget's disease, sarcoidosis, nevus sebaceous, xeroderma pigmentosum, trauma and chronic radiation exposure.
Histopathological features of porocarcinoma are highly variable, and when the tumor occurs in conjunction with a benign poroma, the diagnosis can be missed by sampling error. Usually, the intraepidermal part of the tumor is made up of nests and islands of mid-sized polygonal cells forming broad connecting cords and large aggregates of cells extending into the dermis. Clear cell differentiation and spindle cells may be present and ductal structures with a periodic acid-Schiff positive cuticle are seen.
The criteria to distinguish between benign and malignant neoplasms include the presence of an invasive growth pattern, increased nuclear to cytoplasmic ratio, mitoses, atypical mitotic figures and perineural invasion. Ackerman emphasized that the well-circumscribed shape of the neoplasm is the single most important distinguishing feature of malignant versus benign neoplasm, as varying degrees of cellular atypia occur in benign poromas.[2] Both necrosis en masse and mitotic figures may be present in both benign and malignant counterparts. Robson et al. in the largest series of 69 porocarcinomas reported to date described a number of histopathological features of porocarcinoma which they suggested may be predictive of poorer clinical outcome and death. These included the presence of more than 14 mitoses per high power field, lymphovascular invasion, tumor depth >7 mm and tumors with an “infiltrating” advancing margin (small irregular groups and strands of cells), the latter which is postulated to make local recurrence more likely.[3] However some reports confirm that tumors with a minimal degree of atypia have the potential to behave aggressively.[4] Therefore, a careful assessment of the cytology, growth pattern and nuclear/cytoplasmic ratio of any ductal neoplasm is needed and complete excision may be prudent in lesions with atypical features. Lymphovascular invasion and perineural invasion are seen in only 15% and 1% of lesions respectively.[3]
The clinical differential diagnosis is broad and often includes basal cell carcinoma, squamous cell carcinoma, Paget's disease and metastatic cancer. Special stains are rarely necessary, but may include: Carcinoembryonic antigen, cytokeratin (pancytokeratin and CK5/6), epithelial membrane antigen and p53. D2-40 staining has been reported as useful to help to distinguish it from the cutaneous metastases of adenocarcinoma. P63 positivity also favors a primary cutaneous neoplasm.
The change in behavior of the lesion, with rapid growth, a more irregular outline, and the presence of atypical mitotic figures and perineural involvement indicated malignant degeneration in our patient's lesions. However, other histopathological features suggestive of malignancy were scant. The tumor had a preponderance of cells with abundant pink cytoplasm, with a relatively low nuclear/cytoplasmic ratio. Invasive growth pattern and mitotic figures were absent in the previous biopsies, suggesting the development of porocarcinoma in a long-standing benign poroma.
Although porocarcinoma is rare, it is important to identify it early to avoid patient morbidity. In early lesions, cure is possible by primary excision in 70-80%, although a recurrence rate of up to 20% has been observed.[5] Regional lymph nodes should be assessed where malignancy is clinically suspected, as porocarcinoma has a propensity to invade the dermal lymphatics which may lead to local (20%) and distant (10% to solid organ) metastatic spread.[5] This case serves as a reminder of the potential of malignant degeneration within long-standing benign adnexal tumors as well as the spectrum of histological features that may be seen in porocarcinoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pinkus H, Mehregan AH. Epidermotropic eccrine carcinoma. A case combining features of eccrine poroma and paget's dermatosis. Arch Dermatol. 1963;88:597–606. doi: 10.1001/archderm.1963.01590230105015. [DOI] [PubMed] [Google Scholar]
- 2.Requena L, Kiryu H, Ackerman AB. Philadelphia: Lippincott Williams and Wilkins; 1998. Porocarcinoma. Neoplasms with Apocrine Differentiation; pp. 785–94. [Google Scholar]
- 3.Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, et al. Eccrine porocarcinoma (malignant eccrine poroma): A clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710–20. doi: 10.1097/00000478-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Elston DM, Ferringer T. Sweat gland neoplasms. In: Elston DM, editor. Requisites in Dermatology. Philadelphia: Elsevier; 2009. 100 pp. [Google Scholar]
- 5.Brown CW, Jr, Dy LC. Eccrine porocarcinoma. Dermatol Ther. 2008;21:433–8. doi: 10.1111/j.1529-8019.2008.00243.x. [DOI] [PubMed] [Google Scholar]


