Abstract
Expression of the EGF receptors (EGFRs) is abnormally high in many types of cancer, including 25% of lung cancers. Successful treatments target mutations in the EGFR tyrosine kinase domain with EGFR tyrosine kinase inhibitors (TKIs). However, almost all patients develop resistance to this treatment, and acquired resistance to first-generation TKI has prompted the clinical development of a second generation of EGFR TKI. Because of the development of resistance to treatment of TKIs, there is a need to collect genomic information about EGFR levels in non-small-cell lung cancer patients. Herein, we focus on current molecular targets that have therapies available as well as other targets for which therapies will be available in the near future.
Keywords: acquired resistance, afatinib, cetuximab, EGF receptor, gefitinib, neratinib, predictive markers, trastuzumab, tyrosine kinase inhibitors
It is well known that lung cancer is the leading cause of cancer-related deaths in both men and women. Lung cancer causes more deaths annually than the four major cancers – prostate, breast, colon, and pancreatic – combined (American Cancer Society Facts and Figures 2013 [1]). Because they are frequently asymptomatic for a long period of time, lung cancers are associated with poor prognosis. Although smoking is considered the leading cause of lung cancer, in 15–20% of lung cancer patients smoking was not observed [2]. Lung cancer is subdivided into two types based on cell type and pathology: small-cell lung cancers and non-small-cell lung cancers (NSCLCs), of which approximately 85% are NSCLCs [3]. NSCLC is subdivided further based on histological phenotypes to squamous-cell carcinoma, large-cell carcinoma and adenocarcinoma (ADC). ADC will occur mostly in distal airways while squamous-cell carcinoma, which occurs in proximal airways, seems to be associated with smoking and chronic inflammation [4-6]. The prognosis of patients with lung cancer is poor, with a survival rate of around one year, making it one of the least understood cancers. Traditional chemotherapeutic agents such as cisplatin, paclitaxel and docetaxel provide standard therapies for lung cancer. However, clinical response is observed in only 30–40% of patients [7]. The overall 5-year survival rate of NSCLC patients is 16%, and this rate decreases rapidly among patients diagnosed at late stages of the disease [8-10]. The quality of life in advanced lung cancer patients is constantly at risk due to treatment toxicity and disease progression. So, increasing the chances of benefit and avoiding futile treatment are very important in the management of the disease. The introduction of targeted therapy in NSCLC gives hope that this may be achieved. Conventional chemotherapy is known to cause adverse toxic effects due to lack of selectivity for tumor cells. The introduction of targeted drugs for the treatment of NSCLC with EGF receptor (EGFR)-directed small-molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs) has had a significant impact. However, as yet, a relatively small overall improvement in clinical outcome has been observed in unselected patients with advanced disease [11]. The key targets of various agents in NSCLC in clinical phases of development include the EGF family of receptors. Various target proteins that have significance in lung cancer are provided in Table 1 [12,13]. The aim of this paper is to review the literature and provide a broad understanding of the current and future therapies targeting EGFRs. Additionally, we will cover some issues of resistance in these targeted therapies.
Table 1. The key targets of various agents in non-small-cell lung cancer in clinical phases of development.
| Target proteins/receptors | Agents approved/under investigation for NSCLCs |
|---|---|
| EGFRs | Lapatinib†, afatinib (BIBW2992)†, dacomitinib (PF00299804), CO-1686, AZD9291, pertuzumab |
| mTOR | Sirolimus, everolimus, temsirolimus |
| BRAF | Dabrafenib (GSK2118436), vemurafenib (PLX4032), LGX818 |
| MAPK | Trametenib (GSK1120212), selumetinib (AZD6244), GDC-0973 |
| c-MET | EMD1214063, INC280, cabozantinib (XL184), tivantinib (ARQ197), foretinib (GSK1363089), onartuzumab (OAM4558g) |
| FGFR/VEGFR | Cediranib, nintedanib (BIBF 1120), pazopanib†, ponatinib† [14] |
| DDR2/Bcr-Abl | Dasatinib, sorafenib, ponatinib |
| PIK3CA | PF-4989216 |
| AKT | MK2206, GDC0068, AZD5363 |
| ALK | Crizotinib (PF-02341066), LDK378, AP26113, CH5424802 |
| RET | XL-184, sorafenib |
US FDA-approved drugs; for more information see [15].
EGFR: EGF receptor; FGFR: FGF receptor; NSCLC: Non-small-cell lung cancer; VEGFR: VEGF receptor.
The ErbB family includes four members – EGFR, HER2, HER3 and HER4 – all of which are cell-surface receptor tyrosine kinases (RTKs). Typically, an ErbB receptor is a 170-kDa RTK with an extracellular ligand-binding domain, a transmembrane region and an intracellular tyrosine kinase. The RTKs form homodimers and heterodimers after binding to specific ligands (except HER2, which does not have an endogenous ligand), leading to autophosphorylation of tyrosine residues on the intracellular TK domain. This interaction recruits a diverse set of signaling molecules and cascades, including the PI3K/protein kinase B (Akt)/mTOR, STAT, and RAS/RAF/MAPK proliferation pathway. From 18 to 33% of NSCLC tumors show a positive result (2+/3+) with the HercepTest, a test that evaluates the level of HER2 protein expression on tumor tissue. Testing for mutations in the EGFR gene and rearrangements of the ALK gene in ADC of the lung are now in routine clinical use as predictive genomic biomarkers in the management of advanced lung cancer. Patients with lung ADCs that harbor either of these genomic alterations (15–50%, depending on the population studied) are already benefiting from targeted therapy with oral kinase inhibitors such as erlotinib and crizotinib. Other potential predictive genomic biomarkers in known oncogenes such as BRAF, ROS1, mesenchymal–epithelial transition (MET) and PIK3CA have been identified in a systematic fashion, and efforts are underway to target them with novel drug compounds. Because lung cancer is a heterogeneous group of diseases, it must be targeted using multiple drugs rather than drugs specific to a single target. This review will focus on the rationale for the development of targeted therapies in NSCLC, the recent advances in the therapeutic strategies and agents recently approved by the US FDA for EGFR aberrant lung cancers. In addition, we will discuss various strategies employed in preventing or overcoming the inevitable occurrence of resistance during the treatment and new treatment methods that are underway for NSCLC.
EGFRs in NSCLC
EGFR
EGFR overexpression in premalignant and malignant lung tissues is varied showing overexpression in the range of 40–80% of NSCLC patients [16]. In 2004, before EGFR mutation was known to be a predictive biomarker, it was assumed that certain patient populations benefited more from EGFR TKIs, namely, those with lung ADCs, those of Asian ethnicity, females, and never smokers. It is now known that the enhanced efficacy in these populations is due to the EGFR mutations in their tumors. Also, these mutations are found almost exclusively in ADCs of the lung. There is, however, no clinical characteristic that can be used instead of EGFR mutation testing to detect lung cancers. Recently, it has been observed that the role of EGFR signaling is significant in glycolysis, the pentose phosphate pathway and pyrimidine b iosynthesis in EGFR-mutated lung cancer [17].
HER2
ERBB2/HER2/neu is overexpressed in NSCLC and is considered to be a significant and independent prognostic factor in lung cancer. The exact percentage of HER2 overexpression in NSCLC is not clear as reported literature suggests overexpression rates ranging from 4 to 27% [18]. This variation is not due to lung cancer cells, but seems to arise from the methods used to assess HER2 overexpression. The frequency of HER2 positivity depends on the types of tumor tissues, in other words, whether they are ADCs (17–42%), large-cell carcinomas (2–40%) or squamous carcinomas (0–5%). Some NSCLC patients with a chemoresistant phenotype may also show overexpression of HER2. During 2004–2005 clinical trials conducted on NSCLC patients by treating them with the HER2-targeted antibody trastuzumab [19] in addition to gemcitabine-cisplatin or docetaxel, the benefits of HER2-targeted treatment for lung cancer were not demonstrated. However, Capizzo et al. [20] have shown that patients with lung cancer who have HER2 mutation G776L respond to treatment with trastuzumab and paclitaxel therapy. This reinforces the fact that HER2-targeted therapy for HER2-overexpressed lung cancer depends on the method used for assessment of HER2 overexpression. Studies related to a dual kinase inhibitor afatinib (inhibits both EGFR and HER2 kinase activity) clearly demonstrate the importance of HER2 overexpression and mutation and targeted therapy for HER2-positive NSCLC [21]. Recent findings suggest that long-term HER2 overexpression could induce serious lung inflammation and some precancerous lesions by upregulating inflammatory factors such as TNF, IL-1 and IL-6 [22]. De novo mutations in HER2 are present in 2–5% of NSCLC [23] and up to approximately 10% in ADCs with a phenotype similar to tumors with EGFR mutations. The majority (>95%) of these represent small insertions in exon 20, which cause a duplication of the amino acids YVMA that results in constitutive activation of HER2 [24]. Based on cumulative experience to date, HER2 mutations are thought to be more clinically relevant in NSCLC than overexpression of HER2 protein or HER2 gene amplification [12]. Studies regarding HER2 mutations suggest that they occurred in never smokers and, in particular, in the Asian population. Further studies are needed to answer some controversial results observed in HER2-positive NSCLC patients. There is an ongoing Phase II study of the use of the dual inhibitor neratinib in patients with NSCLC [25].
Coexpression of EGFR & HER2
NSCLCs that overexpress both EGFR and HER2 demonstrate aggressive tumor cell growth. HER2 has been identified as the preferred binding partner of the other ERBB receptors, in particular, of EGFR with the formation of HER2/EGFR heterodimers with greater potential for signaling than EGFR homodimers. Some studies have demonstrated that NSCLCs that overexpress both EGFR and HER2 are more sensitive TKIs than a tumor with an increased expression of EGFR alone [16]. Recently, it has been shown that NSCLCs that overexpress both EGFR and HER2 proteins demonstrate aggressive tumor cell growth [16,26] and are associated with a significantly shortened overall survival rate [27]. It is known from a clinical trial that a correlation exists between the overall survival rate in NSCLC and coexpression of EGFR and HER2 than that in patients with tumors with high levels of EGFR or HER2 alone [27]. It is well known that heterodimerization of EGFR and transphosphorylation results in downstream signaling in pathways such as PI3K/Akt. This pathway is initiated by HER3, which lacks a kinase domain. However, HER2 is a major dimerization partner for EGFR and HER3. Since HER2 plays an important role in dimerization of receptors and phosphorylation and is important in driving the MAPK and PI3K/Akt pathways, targeting HER2 and inhibiting EGFR:HER2 and HER2:HER3 dimerization should have a significant impact on HER2-overexpressed lung cancer, in particular, NSCLC.
HER3
Recent evidence suggests that ErbB3/HER3, one of the members of the EGFR receptor family, is upregulated in NSCLC and is involved in drug resistance through increased intracellular phosphorylation, which thereby activates PI3K/Akt signaling [28]. However, the HER3 kinase domain is only weakly active, and HER3 needs a dimerization partner for signaling. HER3 must be phosphorylated for signaling, and the most probable partner is HER2. Hence, studies related to HER3 are in progress at present [29]. The erbB3/PI3K/Akt pathway is a major cause of treatment failure in cancer therapy because of its role in therapeutic resistance [30]. Attempts at treatments targeted to HER3 are concentrated particularly on EGFR TKI-resistant NSCLC [29].
EGFR gene mutations
In 2003, EGFR TKIs were first administered to nonselected patients with advanced NSCLC, but only very few patients showed dramatic response [31]. Subsequent studies revealed that a subset of patients who showed dramatic response to EGFR TKIs was found to harbor gene mutations in the intracellular tyrosine kinase domain that mediates downstream signaling of the EGFR. The mutations exist in exons 18–21 which correspond to N-lobe and part of C-lobe of EGFR kinase domain. The most frequently found EGFR mutations in patients with NSCLC include short in-frame deletions in exon 19 and a specific point mutation in exon 21 at 858; these constitute 80–90% of the mutations detected [31]. In previous studies, NSCLC patients showing effective response to gefitinib were found to have mutations primarily in exons 18, 19 and 21. Most of these activating mutations exist near the ATP binding cleft of EGFR. These mutated EGFRs show lower affinity to ATP compared with the wild-type EGFR. The presence of deletion mutations in EGFR exon 19 in patients receiving gefitinib or erlotinib therapy increased their median survival time by 38 months; in patients with L858R mutation in exon 21, survival time was improved by 17 months [32]. Other less commonly occurring mutations include point mutations in exon 18 (G719C, G719S and G719A) (Figure 1) and exon 20 (V765A and T783A) [15]. On the contrary, mutations in exon 20 are associated with resistance to TKIs such as erlotinib and gefitinib. These mutations could be in-frame duplication and/or insertion, and could also be point mutations. EGFR exon 20 insertion mutations are typically located near the C-helix of the tyrosine kinase domain and only account for up to 4% of all EGFR mutations. Preclinical models have shown that the most prevalent EGFR exon 20 insertion mutated proteins are resistant to gefitinib and erlotinib. Exon 20 mutations were observed in small number of patients and had a shorter duration of gefitinib response than those with other mutations. The significance of this resistance by mutations on exon 20 is not well established. Further studies are needed for a better outcome of treatment of lung cancer patients with EGFR exon 20 mutations [33,34].
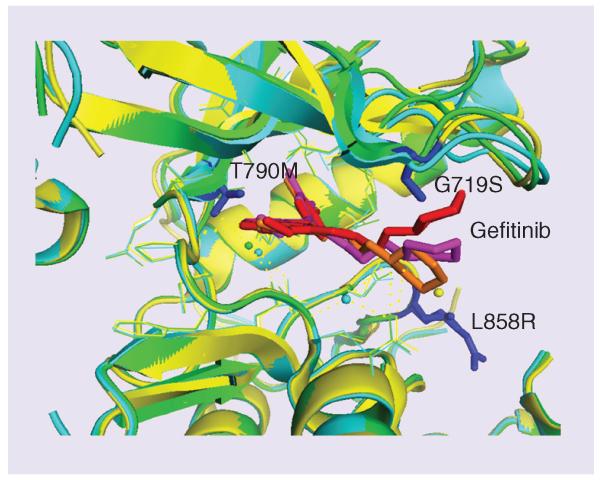
Figure 1. Crystal structures of wild-type and mutant EGF receptor kinase domain showing the ATP binding site occupied by the anticancer drug gefitinib.
Note the mutations G719S and L858R near the binding site shown as blue sticks. The kinase domain of EGF receptor and its mutants are shown in overlapped ribbons: green – wild-type (PDB ID 2ITY), gefitinib is shown as red sticks; yellow – mutant (PDB ID 2ITO), gefitinib is shown as orange sticks; and cyan – mutant (PDB ID 2ITZ), gefitinib is shown as magenta sticks. These mutations affect the binding of the drug to the receptor and cause resistance to cancer treatment. Mutation in the binding site that restores the ATP binding (T790M) is also shown in blue. T790M was suggested to be used as a biomarker for acquired resistance of tyrosine kinase inhibitor therapy.
ADC histology, female gender, nonsmoking history and Asian ethnicity were the clinical predictors for harboring EGFR-activating mutations, and patients with these show promising response to EGFR TKIs [31]. As a result of the investigation, mutations in exons 18, 19 and 21 are now the most reliable predictive biomarkers for the efficacy of EGFR TKIs. At present, patients are tested for EGFR mutations, and it is proposed that those testing positive should receive EGFR TKIs as initial therapy for metastatic lung cancer. Subsequently, erlotinib has been approved by the FDA as the first-line treatment for the patients with metastatic NSCLC harboring mutations in the EGFR in exon 19 and a specific point mutation in exon 21.
After 9–24 months, patients treated with gefitinib or erlotinib will develop resistance [35] due to the secondary mutations in exon 20, which is a substitution of a hydrophilic threonine residue (T) for a bulkier and hydrophobic methionine (M) in codon 790 (T790M) (Figure 1) [36]. Recently, the FDA approved afatinib, an irreversible TKI that is active against EGFR mutations targeted by first-generation TKIs like erlotinib or gefitinib and also against those not sensitive to these standard therapies. About 50% of acquired resistance in ADC cases was associated with T790M. Initially, it was thought that T790M would prevent binding of EGFR TKIs to the ATP cleft of EGFR, but later, based on crystal structure analysis, it was discovered that T790M mutation restores the ATP binding capability of mutated EGFR. Based on these results, it can be suggested that T790M is the best predictive biomarker for acquired resistance of EGFR TKIs. In addition to T790M, insertion mutations in exon 19 and exon 20 also cause acquired resistance to EGFR-TKIs.
EGFR detection methods
Studies suggest that EGFR TKIs produce a promising response in patients with NSCLC harboring EGFR mutations; hence, the detection of somatic EGFR tyrosine kinase domain mutations has gained importance. Various molecular predictive detection methods such as DNA sequencing, immunohistochemistry, PCR, and Therascreen® (QIAGEN, CA, USA) EGFR RGQ PCR mutation kit are commercially available. Some of these methods have limitations in terms of detection of EGFR mutation. For example, the immunohistochemistry method failed to detect an uncommon EGFR mutation, resistance mutation T790M in exon 20, and failed to predict patient response to EGFR TKIs. The EGFR29 mutation kit from Qiagen is one of those commercially available in the market that can detect the 29 most frequently found EGFR mutations against a background of wild type genomic DNA. Cobas® (Roche Molecular Diagnostics, CA, USA) EGFR mutation test is the first FDA-approved test for mutations in NSCLC [37,38].
Lung cancer therapy will involve screening patients for biomarkers [39-42]. However, there are controversies regarding the screening procedures. The National Cancer Institute initiated the National Lung Screening Trial in 2002. This involved randomized clinical trials that screened high-risk individuals with low-dose CT scan or standard chest radiography. The results from this trial produced a 20% reduction in mortality rates [43-45]. Such screening involves questions such as who should be considered as high-risk patients for screening, the effectiveness of screening, the risks associated with radiation, adverse events from additional diagnostic testing and the effects of false-positive results. However, such screenings may be necessary since TKIs and chemotherapeutic agents may do more harm than good if not treated with proper strategies.
Therapies
Traditional chemotherapeutic agents such as cisplatin have provided standard therapies for lung cancer. The treatment for metastatic NSCLC consists of a two-drug combination, including a platinum compound and a non-platinum drug such as pemetrexed, gemcitabine and vinorelbine, or a taxane. However, clinical response is observed in only 30–40% of patients [7]. Chemotherapeutic agents are often met with resistance and, hence, targeted therapy approaches are being pursued to treat lung cancer [46]. Since EGFRs are known to play a major role in lung cancer as well as in breast and ovarian cancers, targeting EGFRs could have a significant impact on several cancer therapies [47]. Several targeted therapies are available, depending on the stage of the cancer diagnosed and the type of mutation. Therapy is given in several rounds, depending on the patient’s condition. Here we have highlighted some of the widely used therapeutic agents that target EGFR in lung cancer.
TKIs
Erlotinib and gefitinib are first-generation EGFR TKIs approved for treatment of NSCLC [48]. These are orally available anilinoquinazolines that bind reversibly to the ATP binding site of the kinase domain of EGFR. In the early trials of the drugs, both erlotinib and gefitinib showed promising results for NSCLC patients [49]. However, use of these TKIs as a second and third line of therapy for advanced NSCLC patients did not yield improved results. But treatment may be further customized by EGFR genotype and pathology subtype or histology [46,50-52]. Despite encouraging responses to EGFR TKIs, most of the patients develop progression of the disease within one year, usually because of secondary or acquired resistance. It may involve, on the one hand, activation of MAPK-dependent pathways by a T790M mutation (constituting 50–60% of acquired resistance), decreasing the binding affinity to the first-generation TKIs, amplification of HER2 or MEK1, or activating of mutations in RAS or BRAF. MAPK-independent pathways, on the other hand, involve acquired PIK3CA mutations, amplification of MET proto-oncogene, which provides a bypass avenue through transactivation of HER3/PI3K signaling, or impairment of cell death mechanisms as seen with certain germline polymorphic variants of the proapoptotic molecule proapoptotic BCL-2-interacting mediator. Other documented phenomena to explain treatment resistance include epithelial-to-mesenchymal transition mediated by either AXL kinase activation or activation of TGF-β pathway through downregulation of mediator of RNA polymerase II transcription, subunit 12 (MED12) and phenotypic transformation to small-cell histology.
Second- and third-generation EGFR TKIs are being developed as part of the strategy to overcome treatment resistance to first-generation EGFR TKIs (Table 2). The second-generation TKIs include the irreversible inhibitors of the ErbB family of receptors: afatinib (also known as BIBW 2992, which targets EGFR, HER2 and HER4), dacomitinib (also known as PF0299804, which targets EGFR, HER2 and HER4) and neratinib (also known as HKI272, which targets EGFR and HER2). These agents are intended to improve the efficacy of treatment in patients with activated mutant EGFR and acquired resistance to first-generation EGFR TKIs. These agents have been or are being evaluated in clinical trials, with afatinib and dacomitinib having progressed the furthest in clinical development. The FDA has recently approved afatinib for metastatic NSCLC patients with tumors harboring exon 19 deletion or exon 21 substitution mutations in EGFR gene as a first-line treatment. Afatinib is an irreversible pan inhibitor that binds to multiple kinases. Dacomitinib is in Phase III clinical studies due to its acceptable safety profile. Further development of neratinib in NSCLC is unlikely because of its low clinical activity as well as dosing limitations arising from diarrhea-related toxicities. Pelitinib (EKB-569) and canertinib (CI-1033) have also been discontinued from further clinical development [48].
Table 2. Different tyrosine kinase inhibitors and monoclonal antibodies that are approved or in clinical trial/discontinued for non-small-cell lung cancer therapy.
| Therapy | Mechanism of action | Stage of development | Adverse effects† |
|---|---|---|---|
| First-generation TKIs | |||
| Gefitinib [53] | Reversible EGFR TKI | US FDA approved in 2003; withdrawal of approval in 2005 due to lack of survival benefit |
Rash, vomiting, stomatitis, dehydration |
| Erlotinib [54] | Reversible EGFR TKI | FDA approved for metastatic NSCLC in 2014 |
Rash, diarrhea, loss of appetite, rarely interstitial pneumonitis |
| Second-generation TKIs | |||
| Afatinib [21] | Irreversible EGFR (including T790M mutant EGFR), HER2 and HER4 TKI |
FDA approved | Diarrhea, stomatitis, dermatitis acne form, decreased appetite, dry skin, nose bleed |
| Dacomitinib [55] | Irreversible TKI targeting EGFR, HER2, HER4 | Phase III, disappointing results in terms of superiority |
Diarrhea, acne form dermatitis, fatigue, stomatitis, rash, dry skin |
| Neratinib [56,57] | Irreversible EGFR and HER2 TKI | Phase II, poor bioavailability, diarrhea related dose limitation |
Severe diarrhea toxicity |
| Third-generation TKIs | |||
| Poziotinib [58] | Irreversible mutant EGFR TKI | Phase II | Diarrhea, stomatitis, rash, dermatitis acneiform, anorexia, dry skin |
| AZD9291 [59] | Irreversible mutant EGFR TKI | Phase I | Diarrhea, rash, nausea |
| CO-1686 [60] | Irreversible mutant EGFR TKI | Phase II | Hyperglycemia, nausea, diarrhea, decreased appetite, vomiting, fatigue, myalgia |
| Monoclonal antibodies | |||
| Cetuximab [61] | Monoclonal antibody against EGFR | Phase III | Breathing difficulty, low blood pressure, acne-like rash |
| Necitumumab [62] |
Monoclonal antibody against EGFR | Phase III in combination with gemcitabine-cisplatin chemotherapy |
|
| Panitumumab [63] |
Human monoclonal antibody against EGFR | Phase II in combination with carboplatin and pemetrexed |
Nausea, fatigue, rash, thrombocytopenia, neutropenia, dehydration |
| Nimotuzumab [64] |
Humanized monoclonal antibody to EGFR | Phase II along with radiation ordocetaxel and cisplatin |
Fatigue, anorexia, chills, pain, hypophosphatemia |
| Matuzumab [65] | Humanized monoclonal antibody to EGFR | Phase II in combination with paclitaxel, development discontinued |
|
| Zalutumumab [66] |
Human monoclonal antibody against domain III of EGFR |
Phase II discontinued | |
| Combination therapies | |||
| Pertuzumab + erlotinib [67,68] |
Pertuzumab–HER2 dimerization inhibitor Erlotinib–EGFR TKI |
Phase II | Pneumatosis intestinalis |
| Cetuximab + erlotinib [69] |
Cetuximab–anti-EGFR antibody that prevents EGFR activation Erlotinib–EGFR TKI |
Phase II | Rash, fatigue, hypomagnesemia |
| Trastuzumab + pertuzumab [70] |
Trastuzumab–antibody-dependent cellular cytotoxicity, blockade of HER2 signals Pertuzumab–HER2 dimerization inhibitor |
Preclinical | |
Adverse effects were cited from clinicaltrials.gov.
EGFR: EGF receptor; NSCLC: Non-small-cell lung cancer; TKI: Tyrosine kinase inhibitor.
The dominant resistance mechanism developed to EGFR-directed therapy is a kinase-activating mutation T790M. Third-generation EGFR inhibitors designed to inhibit the EGFR T790M mutant include HM781–36B, WZ4002, CO-1686 and AZD9291. Poziotinib (HM781–36B) is a new potent irreversible inhibitor of EGFR, HER2, HER4 and the TEC family of kinase inhibitors (Bruton’s tyrosine kinase, B lymphocyte kinase and BMX). In pre-clinical studies, it demonstrated efficacy against T790M mutant at a dose eightfold lower than that for afatinib, and is presently being evaluated in Phase II clinical studies. Another new agent, AZD9291, showed promising results in clinical Phase I trials – tumor shrinkage was observed in 64% of 205 patients with mutant EGFR. Similarly, treatment with CO-1686, a covalent inhibitor of mutant EGFR, was well-tolerated and showed tumor shrinkage in 58% of 72 patients with T790M mutations. Currently, both of these agents are subjects of Phase II as well as Phase III clinical studies and have received a special ‘breakthrough status’ from the FDA for expediting the approval process. Icotinib is another candidate with an acceptable adverse effect profile that is under investigation for activity in NSCLC patients harboring EGFR mutations [48,71].
Possible mechanisms of acquired resistance to these next-generation TKIs are increased extracellular signal-regulated kinase activation by increased MEK1 signaling or downregulation of negative regulators of extracellular signal-regulated kinase signaling, which may be overcome by the use of MEK inhibitors. Findings from recent studies suggest that the combination of WZ4002 and an MEK inhibitor appears to be preclinically effective in treating drug-resistant tumors as well as in delaying the emergence of tertiary drug-resistant clones. The multitargeted EGFR/HER2/VEGFR/EphB4 inhibitor XL547 and the dual reversible ALK/EGFR inhibitor AP26113 also demonstrated preclinical activity against EGFR T790M mutant tumors. Early clinical phase data on XL647 and AP26113 suggested a preliminary hint of modest clinical activity in patients with resistance to other EGFR TKIs [11]. The FDA recently approved crizotinib, which is an ALK and c-ros oncogene 1 (ROS1) inhibitor, for lung cancer treatment.
Monoclonal antibodies
Cetuximab (erbitux) is an immunoglobulin G chimeric mAb against EGFR. It competitively inhibits ligand binding and has been investigated in combination with chemotherapy in Phase III trials (ErbituX in lung cancer [FLEX] study) of molecularly unselected NSCLC patients [72]. A FISH assay to determine EGFR gene copy number had demonstrated potential promise as a predictive marker of response to cetuximab in a small study [15,73]. However, no biomarker was found to consistently correlate with the benefit from cetuximab in the concluded Phase III clinical studies for NSCLC, including EGFR, FISH or KRAS mutation status. Other mAbs against EGFR under investigation in trials for NSCLC include necitumumab, panitumumab, nimotuzumab, matuzumab and zalutumumab (Table 2).
A different approach in addressing EGFR TKI resistance involves the use of combination regimens. For the first time, a combination of afatinib and cetuximab was reported to be very promising in terms of an overall response rate of 29% even in mutant T790M cases. Other combinations of erlotinib with cetuximab and erlotinib with MM-121, a fully human mAb that targets HER3, in patients with acquired resistance to EGFR TKI did not show sufficient clinical activity for further investigation. Also, the combination of anti-ErbB3 antibodies with EGFR TKIs synergistically affected cell proliferation in vitro, caused cell cycle arrest, upregulated p21 expression and inhibited tumor growth in mouse xenografts [28]. An approach using chemotherapies, antibodies and immunotherapy seems to show shrinkage of tumors with a T790M mutation. Such methods of blocking at both intracellular and extracellular levels, called ‘vertical blockade’, could improve the therapeutic outcomes for EGFR-TKI resistant tumors [74,75].
Novel agents like dual-targeting antibodies that bind to two antigens or tumor marker proteins are also under development [76]. Encouraging results were observed with a combination of the HER2 dimerization inhibitor pertuzumab and EGFR TKI erlotinib in NSCLC patients in Phase Ib clinical trials [67]. However, the tolerance levels were poor, with adverse effects such as pneumatosis intestinalis [68]. Scheuer et al. studied the effectiveness of a combination of trastuzumab and pertuzumab in the inhibition of HER2-overexpressed tumor growth. They concluded that this combination has a synergistic antitumor effect on NSCLC (Calu-3) and breast cancer xenograft models. Moreover, it was shown to reduce the metastasis of breast cancer to lung tissues. Initially, clinical trials in HER2-positive NSCLC patients undergoing chemotherapy failed to show any benefit from adjuvant therapy with the anti-HER2 antibody trastuzumab. However, in later studies HER2 mutations were shown to play a more significant role in lung carcinogenesis than overexpression of HER2 protein in patients with HER2-mutant NSCLC [20,70].
Conclusion & future perspective
Lung cancer has always been viewed as one of the most difficult cancers to treat. To make treatment more complicated, the incidence of lung cancer rises substantially with age. Only about one-third of cases develop below the age of 65 years; the majorities of patients are 65 years and over, with the median age of onset around 70 years. The general strategy of cancer therapy that involves surgery to remove as much of the tumor as possible and then treat with chemotherapy or radiation therapy to remove the remaining cancer tissue does not apply to lung cancer treatment [77,78]. This is because, in the case of lung cancers, tumors are diagnosed after the cancer has already spread past the lungs. As a result, surgery alone very rarely leads to a cure; pulmonary function and age complicate the option of surgery and, thus, most patients are unfortunately not even candidates for surgery. The opportunity to detect the cancer early means that more individuals could be cured by surgical excision or radiation therapy. Either of these can be followed by adjuvant chemotherapy for those with a high likelihood of microscopic disease spread. Since smoking is related to lung cancer, one of the ways to reduce the number of lung cancer patients is to reduce smoking. The result of reduced smoking has not been clear as yet, since a high percentage of lung cancer is seen in individuals who are only casual smokers or former smokers and the risk of lung cancer does not seem to decline for many years following smoking cessation (American Cancer Society Facts and Figures 2013 [79]).
In the past ten years, the EGFR family has gained significance in NSCLC biology and has become a key focus of targeted therapies. There is no doubt that personalized therapy for advanced NSCLC has been improved by the introduction of the TKIs gefitinib and erlotinib. However, TKI therapy is limited by the development of resistance in most of the patients that are treated. Hence, several efforts are being undertaken to understand the mechanisms of resistance in order to develop combination treatments capable of sensitizing cells resistant to EGFR TKIs. Despite the number of possible drugs and treatments available for lung cancer therapy, lung cancer still results in the largest number of cancer-related deaths worldwide of which more than 85% are from NSCLC [80]. In 2001, the overall 5-year survival rate was 14% for lung cancer [81]. After nearly a decade, the predicted overall 5-year survival rate was 15.9%. Although saving each patient is considered remarkable in cancer treatment, a decade of improvement in science and technology has only marginally improved the therapeutic effects in lung cancer. The major reasons for this are the development of resistance to most of the TKI or antibody treatments and the heterogeneity of the disease [6]. In addition, lung cancer treatment is complicated by the fact that most patients do not exhibit any symptoms until the cancer has spread too far to be cured. In addition to therapy for lung cancer tumors, patients need improved quality of life as they suffer from airway obstruction. In many cases, bulky endobronchial disease or extrinsic compression of the major airways results in significant difficulty in maintaining a good quality of life during treatment [82].
There has been significant progress in recent years in the area of lung cancer diagnosis and treatment. Further, there has been progress in treatment with combination drug therapies after radiation therapy and with new compounds targeted at driver mutations [83]. Based on the literature reports and the development of resistance, it is clear that lung cancer treatment, in particular NSCLC treatment, cannot be viewed as ‘one-size fits-all’ type of therapy. Future treatments will involve individualized therapies based on extensive knowledge about the type of pathological condition the patient exhibits in NSCLC [84]. The pretreatment detection of responsive predictor markers and individualized effective treatments will help to maximize the therapeutic index of lung cancer treatment. The last decade has seen advances in screening procedures using next-generation sequencing [85,86], large databases of genomics and proteomics for tumor types [87], and molecular markers and biochemical knowledge of the molecular basis of the development of resistance in terms of mutation. All of these will help physicians and scientists decide how a particular subset of NSCLC can be treated. Future therapies will be based on the mutations found in patients’ tumors. In the USA, there is a lung cancer mutation consortium, and in Europe, the International Association for the Study of Lung Cancer, European Thoracic Oncology Platform, and European Respiratory Society have been established; these involve many centers across the USA and Europe [88]. The data generated by these centers can be shared so that any new mutation can easily be screened and an effective therapy can be proposed. The majority of the NSCLC mutations have been observed in EGFR [89]. Several combination therapies proposed are already in clinical trials. One example is EML4-ALK/crizotinib [90,91]. The lung cancer mutation consortium is also conducting a study in which lung cancer tissue is assessed for ten known driver mutations in EGFR, ALK, KRAS, HER2, BRAF, PIK3CA, AKTI, MEKI, NRAS and MET using a multiplex assay. New drug-like molecules targeting these mutations are under development. In the case of HER2 and EGFR overexpression, targeted drugs that are already on the market (such as trastuzumab and lapatinib, which are used in breast cancer) are being evaluated for lung cancer therapy. New TKIs such as afatinib, which was approved in July 2013 for EGFR with mutations, have shown positive results [21,92]. Such pan inhibitors and combination therapies with these pan inhibitors are the future drugs of choice [55,93]. However, all of these treatments require testing of EGFR mutations in patients with NSCLC. In the coming decade, lung cancer therapy will involve screening patients for biomarkers [94,95].
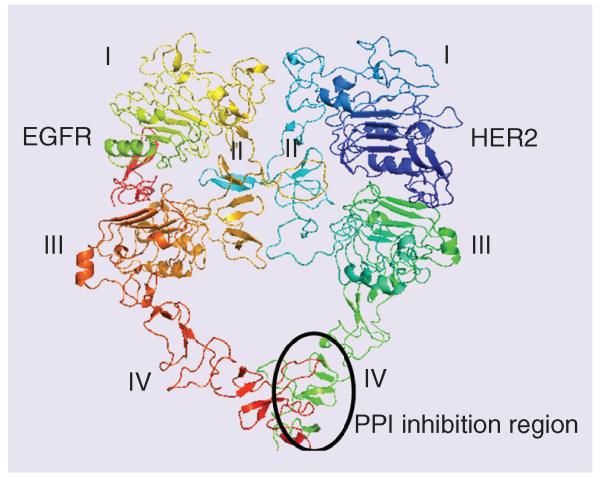
Possible treatments for lung cancer in the near future include next-generation therapies such as therapeutic cancer vaccines [96-100] and stem-cell treatments [101,102] that will play a major role. Therapeutic cancer vaccines, also known as immunotherapy treatments, are drugs that attempt to teach a patient’s immune system to recognize cancer cells so that they can be naturally destroyed. Stem-cell targeting in NSCLS is still in the infancy stage, and much more detail about cancer stem cells has to be studied before drugs can be targeted to stem cells. Protein–protein interactions have also been targeted for lung cancer [103]. These could be interfaces of EGFR proteins or other oncogenic proteins such as BCL1-Beclin 1 [104,105]. The targeting molecules could be antibodies, peptides, peptidomimetics or small molecules. Many of these molecules are in the preclinical stages. Our research group has developed new and novel peptidomimetics that target the extracellular domain of HER2 protein, in particular, domain IV of HER2 protein, and inhibit protein–protein interactions of EGFR (Figure 2) [106-108]. These are dual inhibitors that inhibit EGFR:HER2 and HER2:HER3. Such dual inhibitors block phosphorylation of EGFR and HER2 and also block the downstream signaling of the MAPK and PI3K pathways. HER2 plays an important role in the dimerization of receptors and phosphorylation and is important in the MAPK and PI3K/Akt pathways. Targeting HER2 and inhibiting EGFR:HER2 and HER2:HER3 dimerization may have a significant impact on HER2-overexpressed lung cancer, in particular, NSCLC. These compounds bind to the extracellular domain and, hence, do not need to be transported into the cells. Furthermore, since these molecules target protein–protein interfaces but not the kinase domain, the probability of developing mutation is less and hence less resistance to the treatment. The development of such molecules as therapeutic agents is yet to be seen.
Figure 2. Protein–protein interaction inhibition method for the development of drugs for different types of EGF receptor-overexpressed cancer.
The extracellular domains of EGFR and HER2 are shown as heterodimers. Note that domain IV is involved in the interaction of two proteins. Domain IV can be targeted with small molecules, peptides or peptidomimetics for the inhibition of dimerization. The region of PPI and its inhibition is marked by an oval shape. The model of EGFR:HER2 heterodimer was generated [108,109] using the crystal structures of EGFR (PDB ID 3NJP) [110] and HER2 (PDB ID) [111] with a homodimer of EGFR as a template (PDB ID 3NJP). EGFR: EGF receptor; PPI: Protein–protein interaction.
Systematic genotypic testing in NSCLC patients for the detection of HER2 and EGFR mutations is crucial for treatment with targeted therapies. The co-overexpression of EGFR and HER2 in NSCLC and the poor survival rate of patients with coexpression of these receptors suggest that EGFR and HER2 should be simultaneously targeted for treatment. Most of the next-generation drugs, including afatinib and neratinib, target both EGFR and HER2. Although there is much research being conducted in this area, there are very few EGFR-targeted therapies available to patients with advanced lung cancer. This may be attributed to challenges encountered in the development process – cost, quick development of resistance, lack of novelty, efficacy versus toxicity and barriers in the late clinical trials. Addressing all of these issues is important for the development of more targeted therapies in the future. The future of lung cancer therapy is a long road compared with that of breast and prostate cancer and depends heavily on genetic screening.
EXECUTIVE SUMMARY.
Background
Lung cancer causes more deaths annually than the four major cancers: prostate, breast, colon and pancreatic cancers combined. Among different types of lung cancer, approximately 85% of them are non-small-cell lung cancers (NSCLCs) making it the most common type of lung cancer.
EGF receptors in NSCLC
Mutations in EGFR are thought to be more clinically relevant in NSCLC than overexpression of HER2 protein or gene amplification. Currently, patients are routinely tested for EGFR/HER2 mutations. Lung cancer patients harboring activating mutations for EGFR receive an EGF receptor (EGFR)–tyrosine kinase inhibitor (TKI) as initial treatment.
TKIs & resistance
Patients harboring EGFR mutations initially respond well to EGFR-TKIs, but acquired mutations render the patients resistant to the available drugs. These mutations include EGFR T790M point mutation.
The strategies currently under development to overcome resistance include the use of oral irreversible, small molecules or human EGFR and pan-HER inhibitors. These drugs include afatinib (approved by the US FDA), neratinib, pelitinib, AZD8931, canertinib and PF299.
Monoclonal antibodies
Cetuximab and other monoclonal antibodies against EGFR under investigation in trials for NSCLC include necitumumab, panitumumab, nimotuzumab, matuzumab and zalutumumab.
Future perspective
NSCLC treatment cannot be viewed as ‘one-size-fits-all’ type of therapy. Future treatments will involve individualized therapies based on extensive knowledge of the type of pathological condition the NSCLC patient exhibits. Screening procedures using proteomics and genomics will play a major role in the therapeutic effect of lung cancer.
Apart from combination therapies from TKIs, new novel molecules based on protein–protein interaction and peptide or protein vaccines will dominate lung cancer therapy.
Acknowledgments
Financial & competing interests disclosure
This project was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the NIH under grant number 8P20GM103424. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2••.Ferketich AK, Niland JC, Mamet R, et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer. 2013;119(4):847–853. doi: 10.1002/cncr.27824. Provides information about population with lung cancer that never smoked.
- 3.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl. 7):vii56–vii64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 4.Davidson MR, Gazdar AF, Clarke BE. The pivotal role of pathology in the management of lung cancer. J. Thorac. Dis. 2013;5(Suppl. 5):S463–S478. doi: 10.3978/j.issn.2072-1439.2013.08.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28(36):5311–5320. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat. Rev. Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calikusu Z, Yildirim Y, Akcali Z, et al. The effect of HER2 expression on cisplatin-based chemotherapy in advanced non-small cell lung cancer patients. J. Exp. Clin. Cancer Res. 2009;28:97. doi: 10.1186/1756-9966-28-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med. 2011;32(4):605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schabath MB, Thompson ZJ, Gray JE. Temporal trends in demographics and overall survival of non-small-cell lung cancer patients at Moffitt Cancer Center from 1986 to 2008. Cancer Control. 2014;21(1):51–56. doi: 10.1177/107327481402100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McErlean A, Ginsberg MS. Epidemiology of lung cancer. Semin. Roentgenol. 2011;46(3):173–177. doi: 10.1053/j.ro.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Savas P, Hughes B, Solomon B. Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer. J. Thorac. Dis. 2013;5(Suppl. 5):S579–592. doi: 10.3978/j.issn.2072-1439.2013.08.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen KS, Neal JW, Wakelee H. Review of the current targeted therapies for non-small-cell lung cancer. World J. Clin. Oncol. 2014;5(4):576–587. doi: 10.5306/wjco.v5.i4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stinchcombe TE. Novel agents in development for advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2014;6(5):240–253. doi: 10.1177/1758834014532510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salgia R. Fibroblast growth factor signaling and inhibition in non-small cell lung cancer and their role in squamous cell tumors. Cancer Med. 2014;3(3):681–692. doi: 10.1002/cam4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reungwetwattana T, Dy GK. Targeted therapies in development for non-small cell lung cancer. J. Carcinog. 2013;12:22. doi: 10.4103/1477-3163.123972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch FR, Varella-Garcia M, Cappuzzo F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene. 2009;28(Suppl. 1):S32–S37. doi: 10.1038/onc.2009.199. [DOI] [PubMed] [Google Scholar]
- 17.Makinoshima H, Takita M, Matsumoto S, et al. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J. Biol. Chem. 2014;289(30):20813–20823. doi: 10.1074/jbc.M114.575464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro ACG, Felip E. HER2 driven non-small lung cancer (NSCLC): potential therapeutic approaches. Transl. Lung Cancer Res. 2013;2(2):122–127. doi: 10.3978/j.issn.2218-6751.2013.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug LM, Miller VA, Patel J, et al. Randomized Phase II study of weekly docetaxel plus trastuzumab versus weekly paclitaxel plus trastuzumab in patients with previously untreated advanced nonsmall cell lung carcinoma. Cancer. 2005;104(10):2149–2155. doi: 10.1002/cncr.21428. [DOI] [PubMed] [Google Scholar]
- 20.Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N. Engl. J. Med. 2006;354(24):2619–2621. doi: 10.1056/NEJMc060020. [DOI] [PubMed] [Google Scholar]
- 21.De Greve J, Teugels E, Geers C, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer. 2012;76(1):123–127. doi: 10.1016/j.lungcan.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Zeng S, Yang Y, Tan Y, et al. ERBB2-induced inflammation in lung carcinogenesis. Mol. Biol. Rep. 2012;39(8):7911–7917. doi: 10.1007/s11033-012-1635-7. [DOI] [PubMed] [Google Scholar]
- 23•.Mazieres J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 2013;31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095. Details of HER2 mutation in treatment of lung cancer.
- 24.Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 2012;18(18):4910–4918. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat. Rev. 2014;40(6):770–780. doi: 10.1016/j.ctrv.2014.02.008. Discusses importance of HER2 and treatment option based on mutation.
- 26.Menju T, Hashimoto S, Hashimoto A, et al. Engagement of overexpressed Her2 with GEP100 induces autonomous invasive activities and provides a biomarker for metastases of lung adenocarcinoma. PLoS ONE. 2011;6(9):e25301. doi: 10.1371/journal.pone.0025301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brabender J, Danenberg KD, Metzger R, et al. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin. Cancer Res. 2001;7(7):1850–1855. [PubMed] [Google Scholar]
- 28.Noto A, De Vitis C, Roscilli G, et al. Combination therapy with anti-ErbB3 monoclonal antibodies and EGFR TKIs potently inhibits non-small cell lung cancer. Oncotarget. 2013;4(8):1253–1265. doi: 10.18632/oncotarget.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Ma J, Lyu H, Huang J, Liu B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol. Cancer. 2014;13:105. doi: 10.1186/1476-4598-13-105. Novel method that uses HER3 for EGFR-mutated lung cancer.
- 30.Amin DN, Campbell MR, Moasser MM. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin. Cell Dev. Biol. 2010;21(9):944–950. doi: 10.1016/j.semcdb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat. Rev. 2013;39(8):839–850. doi: 10.1016/j.ctrv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Villaflor VM, Salgia R. Targeted agents in non-small cell lung cancer therapy: What is there on the horizon? J. Carcinog. 2013;12:7. doi: 10.4103/1477-3163.109253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin. Cancer Res. 2008;14(15):4877–4882. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 35.Lee CC, Shiao HY, Wang WC, Hsieh HP. Small-molecule EGFR tyrosine kinase inhibitors for the treatment of cancer. Expert Opin. Investig. Drugs. 2014;23(10):1333–1348. doi: 10.1517/13543784.2014.928283. [DOI] [PubMed] [Google Scholar]
- 36.Yang SH. Molecular basis of drug resistance: epidermal growth factor receptor tyrosine kinase inhibitors and anaplastic lymphoma kinase inhibitors. Tuberc. Respir. Dis. (Seoul) 2013;75(5):188–198. doi: 10.4046/trd.2013.75.5.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber B, Meldgaard P, Hager H, et al. Detection of EGFR mutations in plasma and biopsies from non-small cell lung cancer patients by allele-specific PCR assays. BMC Cancer. 2014;14:294. doi: 10.1186/1471-2407-14-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J. Mol. Diagn. 2010;12(2):169–176. doi: 10.2353/jmoldx.2010.090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nanavaty P, Alvarez MS, Alberts WM. Lung cancer screening: advantages, controversies, and applications. Cancer Control. 2014;21(1):9–14. doi: 10.1177/107327481402100102. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J. Clin. Oncol. 2013;31(8):1039–1049. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanvetyanon T, Creelan BC, Chiappori AA. Current clinical application of genomic and proteomic profiling in non-small-cell lung cancer. Cancer Control. 2014;21(1):32–39. doi: 10.1177/107327481402100105. [DOI] [PubMed] [Google Scholar]
- 42.Huber RM, Reck M, Thomas M. Current status of and future strategies for multimodality treatment of unresectable stage III nonsmall cell lung cancer. Eur. Respir. J. 2013;42(4):1119–1133. doi: 10.1183/09031936.00143112. [DOI] [PubMed] [Google Scholar]
- 43.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horeweg N, Nackaerts K, Oudkerk M, De Koning HJ. Low-dose computed tomography screening for lung cancer: results of the first screening round. J. Comp. Eff. Res. 2013;2(5):433–436. doi: 10.2217/cer.13.57. [DOI] [PubMed] [Google Scholar]
- 45.Church TR, Black WC, Aberle DR, et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013;368(21):1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 47.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol. Ther. 2004;102(1):37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Tsao AS, Papadimitrakopoulou V. The importance of molecular profiling in predicting response to epidermal growth factor receptor family inhibitors in non-small-cell lung cancer: focus on clinical trial results. Clin. Lung Cancer. 2013;14(4):311–321. doi: 10.1016/j.cllc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Asami K, Atagi S. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small cell lung cancer. World J. Clin. Oncol. 2014;5(4):646–659. doi: 10.5306/wjco.v5.i4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scagliotti GV, Parikh P, Von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 51.Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three Phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 2011;6(1):64–70. doi: 10.1097/JTO.0b013e3181f7c6d4. [DOI] [PubMed] [Google Scholar]
- 52.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 53.Velcheti V, Morgensztern D, Govindan R. Management of patients with advanced non-small cell lung cancer: role of gefitinib. Biologics. 2010;4:83–90. doi: 10.2147/btt.s4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khozin S, Blumenthal GM, Jiang X, et al. U.S. Food and Drug Administration approval summary. Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774–779. doi: 10.1634/theoncologist.2014-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reckamp KL, Giaccone G, Camidge DR, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120(8):1145–1154. doi: 10.1002/cncr.28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J. Clin. Oncol. 2014;32(2):68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 57.Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor. results of a Phase II trial in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28(18):3076–3083. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 58.Cha MY, Lee KO, Kim M, et al. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int. J. Cancer. 2012;130(10):2445–2454. doi: 10.1002/ijc.26276. [DOI] [PubMed] [Google Scholar]
- 59.AZD9291 Could Be an Option for NSCLC. Cancer Discov. 2014;4(8):OF10. doi: 10.1158/2159-8290.CD-NB2014-077. [DOI] [PubMed] [Google Scholar]
- 60.Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: Comparative pharmacokinetics and drug-drug interactions. Cancer Treat. Rev. 2014;40(8):917–926. doi: 10.1016/j.ctrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 61.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised Phase III trial. Lancet. 2009;373(9674):1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 62.Dienstmann R, Felip E. Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development. Expert Opin. Biol. Ther. 2011;11(9):1223–1231. doi: 10.1517/14712598.2011.595709. [DOI] [PubMed] [Google Scholar]
- 63.Crawford J, Swanson P, Schwarzenberger P, et al. A phase 2 randomized trial of paclitaxel and carboplatin with or without panitumumab for first-line treatment of advanced non-small-cell lung cancer. J. Thorac. Oncol. 2013;8(12):1510–1518. doi: 10.1097/JTO.0b013e3182a7d1da. [DOI] [PubMed] [Google Scholar]
- 64.Babu KG, Prabhash K, Vaid AK, et al. Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced non-small-cell lung cancer. a multicenter, randomized, open-label Phase I study. Onco Targets Ther. 2014;7:1051–1060. doi: 10.2147/OTT.S63168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiller JH, Von Pawel J, Schutt P, et al. Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer. J. Thorac. Oncol. 2010;5(12):1977–1985. doi: 10.1097/JTO.0b013e3181f4a5c9. [DOI] [PubMed] [Google Scholar]
- 66.Saloura V, Cohen EE, Licitra L, et al. An open-label single-arm, Phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother. Pharmacol. 2014;73(6):1227–1239. doi: 10.1007/s00280-014-2459-z. [DOI] [PubMed] [Google Scholar]
- 67.Barthelemy P, Leblanc J, Goldbarg V, Wendling F, Kurtz JE. Pertuzumab: development beyond breast cancer. Anticancer Res. 2014;34(4):1483–1491. [PubMed] [Google Scholar]
- 68.Hughes B, Mileshkin L, Townley P, et al. Pertuzumab and erlotinib in patients with relapsed non-small cell lung cancer. a Phase II study using 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging. Oncologist. 2014;19(2):175–176. doi: 10.1634/theoncologist.2013-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janjigian YY, Azzoli CG, Krug LM, et al. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clin. Cancer Res. 2011;17(8):2521–2527. doi: 10.1158/1078-0432.CCR-10-2662. [DOI] [PubMed] [Google Scholar]
- 70.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Zhu Q, Liu Y, et al. Icotinib is an active treatment of non-small-cell lung cancer. a retrospective study. PLoS ONE. 2014;9(5):e95897. doi: 10.1371/journal.pone.0095897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pirker R, Pereira JR, Von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer. analysis of data from the Phase 3 FLEX study. Lancet Oncol. 2012;13(1):33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 73.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J. Clin. Oncol. 2008;26(20):3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shien K, Yamamoto H, Soh J, Miyoshi S, Toyooka S. Drug resistance to EGFR tyrosine kinase inhibitors for non-small cell lung cancer. Acta Medica Okayama. 2014;68(4):191–200. doi: 10.18926/AMO/52785. [DOI] [PubMed] [Google Scholar]
- 75.Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J. Clin. Invest. 2009;119(10):3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, Yun S, Batuwangala TD, et al. A dual-targeting antibody against EGFR-VEGF for lung and head and neck cancer treatment. Int. J. Cancer. 2012;131(4):956–969. doi: 10.1002/ijc.26427. [DOI] [PubMed] [Google Scholar]
- 77.Cardenal F, Nadal E, Jove M, Faivre-Finn C. Concurrent systemic therapy with radiotherapy for the treatment of poor-risk patients with unresectable stage III non-small-cell lung cancer: a review of the literature. Ann. Oncol. 2015;26(2):278–288. doi: 10.1093/annonc/mdu229. [DOI] [PubMed] [Google Scholar]
- 78.Ottlakan A, Martucci N, Rocco G. Is surgery still the best management option for early stage NSCLC? Transl. Lung Cancer Res. 2014;3(3):159–163. doi: 10.3978/j.issn.2218-6751.2014.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cancer facts and figures. 2013 www.cancer.org.
- 80.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 81.Alberts WM. The future and lung cancer: room for optimism? Cancer Control. 2000;7(1):13–14. doi: 10.1177/107327480000700107. [DOI] [PubMed] [Google Scholar]
- 82.Hirsch FR, Jotte RM, Berry CA, Mencia WA, Stowell SA, Gardner AJ. Quality of care of patients with non-small-cell lung cancer: a report of a performance improvement initiative. Cancer Control. 2014;21(1):90–97. doi: 10.1177/107327481402100113. [DOI] [PubMed] [Google Scholar]
- 83.Minuti G, D’incecco A, Cappuzzo F. Targeted therapy for NSCLC with driver mutations. Expert Opin. Biol. Ther. 2013;13(10):1401–1412. doi: 10.1517/14712598.2013.827657. [DOI] [PubMed] [Google Scholar]
- 84••.Perez-Soler R. Individualized therapy in non-small-cell lung cancer: future versus current clinical practice. Oncogene. 2009;28(Suppl. 1):S38–45. doi: 10.1038/onc.2009.200. Provides good future perspectives of lung cancer therapy.
- 85.Han JY, Kim SH, Lee YS, et al. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in never-smokers with lung adenocarcinoma. Lung Cancer. 2014;85(2):161–167. doi: 10.1016/j.lungcan.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Ma J, Mannoor K, Gao L, et al. Characterization of microRNA transcriptome in lung cancer by next-generation deep sequencing. Mol. Oncol. 2014;8(7):1208–1219. doi: 10.1016/j.molonc.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu H, Yuan J, Xiao C, Qin Y. Integrative genomic analyses of recepteur d’origine nantais and its prognostic value in cancer. Int. J. Mol. Med. 2013;31(5):1248–1254. doi: 10.3892/ijmm.2013.1296. [DOI] [PubMed] [Google Scholar]
- 88.Scoccianti C, Vesin A, Martel G, et al. Prognostic value of TP53, KRAS and EGFR mutations in nonsmall cell lung cancer: the EUELC cohort. Eur. Respir. J. 2012;40(1):177–184. doi: 10.1183/09031936.00097311. [DOI] [PubMed] [Google Scholar]
- 89.Kuykendall A, Chiappori A. Advanced EGFR mutation-positive non-small-cell lung cancer: case report, literature review, and treatment recommendations. Cancer. 2014;21(1):67–73. doi: 10.1177/107327481402100110. [DOI] [PubMed] [Google Scholar]
- 90.Wigle DA. Biologic approaches to drug selection and targeted therapy: hype or clinical reality? Thorac. Surg. Clin. 2013;23(3):421–428. doi: 10.1016/j.thorsurg.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Morán T, Quiroga V, Gil MDL, et al. Targeting EML4-ALK driven non-small cell lung cancer (NSCLC) Transl. Lung Cancer Res. 2013;2(2):128–141. doi: 10.3978/j.issn.2218-6751.2013.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93••.Kohler J, Schuler M. LUX-Lung 3: redundancy, toxicity or a major step forward? Afatinib as front-line therapy for patients with metastatic EGFR-mutated lung cancer. Future Oncol. 2014;10(4):533–540. doi: 10.2217/fon.14.9. Interesting recent clinical study report of EGFR mutated lung cancer treatment.
- 94.Sozzi G, Boeri M. Potential biomarkers for lung cancer screening. Transl. Lung Cancer Res. 2014;3(3):139–148. doi: 10.3978/j.issn.2218-6751.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu CQ, Tsao MS. Prognostic markers in lung cancer: is it ready for prime time? Transl. Lung Cancer Res. 2014;3(3):149–158. doi: 10.3978/j.issn.2218-6751.2014.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96••.Ruiz R, Hunis B, Raez LE. Immunotherapeutic Agents in Non-small-cell Lung Cancer Finally Coming to the Front Lines. Curr. Oncol. Rep. 2014;16(9):400. doi: 10.1007/s11912-014-0400-6. Discusses new and promising immunotherapy and possible agents that are in pipeline for lung cancer treatment.
- 97•.Szyszka-Barth K, Ramlau K, Gozdzik-Spychalska J, et al. Actual status of therapeutic vaccination in non-small cell lung cancer. Contemp. Oncol. (Pozn.) 2014;18(2):77–84. doi: 10.5114/wo.2014.42724. Discusses the limitation of vaccine/immunotherapy for lung cancer and how it is useful for only small set of population.
- 98.Seetharamu N. The state of the art in non-small cell lung cancer immunotherapy. Semin. Thorac. Cardiovasc. Surg. 2014;26(1):26–35. doi: 10.1053/j.semtcvs.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Thomas A, Jakopovic M. Immunotherapy for non-small-cell lung cancer. Expert Opin. Biol. Ther. 2014;14(8):1061–1064. doi: 10.1517/14712598.2014.925874. [DOI] [PubMed] [Google Scholar]
- 100.Herrera ZM, Ramos TC. Pilot study of a novel combination of two therapeutic vaccines in advanced non-small-cell lung cancer patients. Cancer Immunol. Immunother. 2014;63(7):737–747. doi: 10.1007/s00262-014-1552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alison MR, Lebrenne AC, Islam S. Stem cells and lung cancer: future therapeutic targets? Expert Opin. Biol. Ther. 2009;9(9):1127–1141. doi: 10.1517/14712590903103803. [DOI] [PubMed] [Google Scholar]
- 102.Hsu HS, Huang PI, Chang YL, et al. Cucurbitacin I inhibits tumorigenic ability and enhances radiochemosensitivity in nonsmall cell lung cancer-derived CD133-positive cells. Cancer. 2011;117(13):2970–2985. doi: 10.1002/cncr.25869. [DOI] [PubMed] [Google Scholar]
- 103.Nero TL, Morton CJ, Holien JK, Wielens J, Parker MW. Oncogenic protein interfaces: small molecules, big challenges. Nat. Rev. Cancer. 2014;14(4):248–262. doi: 10.1038/nrc3690. [DOI] [PubMed] [Google Scholar]
- 104.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, Phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin. Cancer Res. 2009;15(9):3172–3176. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin. Cancer Res. 2009;15(2):723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 106.Kanthala S, Gauthier T, Satyanarayanajois S. Structure-activity relationships of peptidomimetics that inhibit PPI of HER2–HER3. Biopolymers. 2014;101(6):693–702. doi: 10.1002/bip.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Banappagari S, Ronald S, Satyanarayanajois SD. Structure-activity relationship of conformationally constrained peptidomimetics for antiproliferative activity in HER2-overexpressing breast cancer cell lines. MedChemComm. 2011;2(8):752–759. doi: 10.1039/C1MD00126D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banappagari S, Corti M, Pincus S, Satyanarayanajois S. Inhibition of protein-protein interaction of HER2–EGFR and HER2–HER3 by a rationally designed peptidomimetic. J. Biomol. Str. Dyn. 2012;30(5):594–606. doi: 10.1080/07391102.2012.687525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Banappagari S, Ronald S, Satyanarayanajois SD. A conformationally constrained peptidomimetic binds to the extracellular region of HER2 protein. J. Biomol. Str. Dyn. 2010;28(3):289–308. doi: 10.1080/07391102.2010.10507360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu C, Mi LZ, Grey MJ, et al. Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Mol. Cell Biol. 2010;30(22):5432–5443. doi: 10.1128/MCB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fisher RD, Ultsch M, Lingel A, et al. Structure of the complex between HER2 and an antibody paratope formed by side chains from tryptophan and serine. J. Mol. Biol. 2010;402(1):217–229. doi: 10.1016/j.jmb.2010.07.027. [DOI] [PubMed] [Google Scholar]