Abstract
Background
Women with signs and symptoms of ischemia and no obstructive coronary artery disease often have coronary microvascular dysfunction (CMD), diagnosed by invasive coronary reactivity testing (CRT). While traditional noninvasive stress imaging is often normal in CMD, cardiac magnetic resonance imaging (CMRI) may be able to detect CMD in this population.
Methods and Results
Vasodilator stress CMRI was performed in 118 women with suspected CMD who had undergone CRT and 21 asymptomatic reference subjects. Semi quantitative evaluation of the first-pass perfusion images was completed to determine myocardial perfusion reserve index (MPRI). The relationship between CRT findings and MPRI was examined by Pearson correlations, logistic regression and sensitivity/specificity. Symptomatic women had lower mean pharmacologic stress MPRI compared to reference subjects (1.71±0.43 vs. 2.23±0.37, p<0.0001). Lower MPRI was predictive of one or more abnormal CRT variables (OR = 0.78 [0.70, 0.88], p<0.0001, c-statistic 0.78 [0.68, 0.88]). An MPRI threshold of 1.84 predicted CRT abnormality with sensitivity 73% and specificity 74%.
Conclusions
Noninvasive CMRI MPRI can detect CMD defined by invasive CRT. Further work is aimed to optimize the non-invasive identification and management of CMD patients.
Keywords: coronary microvascular dysfunction, cardiac magnetic resonance imaging, myocardial perfusion, women
More than 40% of women with signs and symptoms of ischemia undergoing coronary angiography have no obstructive coronary artery disease (CAD). Evidence from the Women's Ischemia Syndrome Evaluation (WISE) study indicates that many have coronary microvascular dysfunction (CMD) defined as abnormal responses to invasive testing of endothelial and non-endothelial macro- and microvascular pathways1. These women are at higher risk of major adverse cardiac events compared to similar women with normal responses to such invasive testing and asymptomatic women 2, 3. At 5.4-year follow-up, adverse events, including cardiac death, stroke, and new onset heart failure, in particular among women with reduced invasive coronary flow reserve (CFR) to adenosine4. Noninvasive methods for defining the presence of CMD could be of importance in guiding symptom management and potentially prevention of subsequent cardiac events.
The reference standard for diagnosis of CMD is invasive coronary reactivity testing (CRT) using vasoactive substances to test endothelial and non-endothelial dependent coronary function. CMD measured by CRT predicts adverse events in populations of men and women with and without obstructive CAD 2, 4. Standard non-invasive imaging (stress echo and myocardial perfusion SPECT) is often normal in CMD 5, 6, so it has been suggested that advanced imaging techniques may be useful for non-invasive detection of CMD.
Cardiac magnetic resonance imaging (CMRI) stress perfusion can detect myocardial perfusion abnormalities due to obstructive CAD with advantages of high spatial resolution without ionizing radiation7. Semi-quantitative evaluation of the first-pass perfusion images can be used to calculate an indexed ratio of perfusion-time intensity curve upslopes as a measure of myocardial perfusion reserve index (MPRI) in response to vasodilator stress. We, and others, have reported in prior pilot studies differences between symptomatic subjects and reference subjects with open coronary arteries 8, 9 and women with systemic lupus erythematosus and chest pain 10. CMRI can be readily performed in a clinical setting with current 1.5T systems and commercially available software.
We studied a new large cohort of women with symptoms and signs of myocardial ischemia in the absence of obstructive epicardial CAD and determined noninvasive cardiac MPRI in subjects and asymptomatic controls with comparison to invasive CRT.
Methods
This investigation was a part of the National Heart, Lung, and Blood Institute-sponsored prospective multicenter WISE-Coronary Vascular Dysfunction (WISE-CVD) study. All studies were performed at Cedars-Sinai Medical Center or the University of Florida, Gainesville between January 2009 and June 2012 where institutional review boards approved the project and all participants provided written informed consent.
The WISE subjects were women with signs and symptoms of ischemia, had clinically indicated invasive CRT, and underwent CMRI. Exclusion criteria: acute myocardial infarction within 30 days, percutaneous coronary intervention, coronary artery bypass grafting or valve surgery subsequent to baseline qualifying coronary angiogram and any conditions that precluded accurate or safe testing or follow up. Specifically: obstructive CAD ≥50% luminal diameter stenosis in ≥1 epicardial coronary artery, acute coronary syndrome, primary valvular heart disease with need for valve repair or replacement, concurrent cardiogenic shock, prior noncardiac illness with estimated life expectancy <4 years, chest pain with known non-ischemic etiology (e.g. pericarditis, pneumonia, esophageal spasm), contraindications to CMRI (pacemaker, other electronic device, severe claustrophobia); severe asthma (vasodilator stress contraindicated), severe renal impairment (gadolinium contrast contraindicated). Long-acting nitrates, short-acting calcium-channel blockers, alpha-blockers, beta-blockers, and ACE-I/angiotensin-II-receptor antagonists were withdrawn 24 hours and long acting calcium-channel blockers were held for 48 hours prior to CRT and CMRI testing. Sublingual nitroglycerin was not taken within 4 hours prior to testing and participants were caffeine-free and nicotine-free for 24 hours prior to vasodilator stress. Reference subjects were age-matched women without symptoms or cardiac risk factors who had a normal maximal Bruce-protocol exercise treadmill stress test.
Coronary reactivity testing (CRT)
CRT was performed using a standardized protocol 11. Briefly, a Doppler guide wire was placed in the proximal left anterior descending and coronary endothelial and non-endothelial pathways were tested. Microvascular coronary function was assessed from coronary flow with intracoronary adenosine (18 and 36 μg) used to achieve hyperemia. CFR was derived from the ratio of the average peak velocity (APV) of blood flow at maximal hyperemia and APV at rest as previously described 4. We have previously shown that this ratio closely approximates volumetric CFR in similar women enrolled in WISE 2, 12. Coronary endothelial function was assessed by graded intracoronary infusions of acetylcholine (0.182 and 18.2 μg/mL), 2 mL infused over 3 minutes. These concentrations of 10-6 and 10-4 M were infused to obtain effective coronary concentrations of 10-8 and 10-6 M respectively. Coronary macrovascular non-endothelial function was tested using 200 μg of intracoronary nitroglycerin. Hemodynamic data, Doppler velocities and coronary cine angiography were obtained after each infusion.
CMRI protocol
A standardized CMRI protocol and equipment were used (1.5 T Magnetom Avanto, Siemens Healthcare, Erlangen, Germany). First-pass contrast perfusion imaging was performed using gadolinium contrast of 0.05 mM/kg (Gadodiamide, Omniscan, Amersham, Piscataway, NJ) infused at 4 ml/sec, followed by 20 ml saline at 4 ml/sec. Vasodilator stress was adenosine 140 μg/kg/min infused for two minutes into the arm contralateral to the contrast injection, prior to first-pass perfusion imaging, and continued until completion of the perfusion imaging data acquisition. Resting first-pass perfusion was done 10 minutes later.
Perfusion images were obtained in three left ventricular (LV) short-axis imaging slices (basal, mid and distal LV slice positions) with the following parameters: Gradient echo–EPI hybrid sequence, TR per slice: 148 msec, TE: 1.1 msec, BW: 1420 Hz/pixel, echo train length: 4, readout flip angle: 20°, slice thickness: 8 mm, image matrix: 160 × 70 pixels, in-plane resolution: 2.7 × 2.2 mm2, parallel imaging (GRAPPA) factor: 2, imaging 3 slices every heartbeat. In the event of a peak stress heart rate of >120 bpm, two slices were obtained during stress first-pass imaging with exclusion of the distal LV slice position.
LV function and delayed enhancement imaging were performed using a standardized approach, as previously described13.
Data analysis
CRT was interpreted blinded to clinical data by an expert reader experienced in performance and interpretation of CRT (JP) in a dedicated core laboratory using our published methods 2. Coronary artery diameter was measured 5 mm distal to the tip of the Doppler wire. Four CRT measures were assessed: 1) abnormal endothelial function defined as a change in epicardial coronary artery diameter ≤ 0% in response to a maximum dose of acetylcholine (ΔACH)14; 2) abnormal CFR, defined as CFR<2.5 in response to adenosine15; 3) abnormal microvascular endothelial dysfunction, defined as an increase in coronary blood flow (CBF)≤50% in response to acetylcholine (ΔCBF); 4) abnormal non-endothelial function defined as a change in epicardial coronary artery diameter≤20% in response to nitroglycerin (ΔNTG). CBF was determined as π(coronary artery diameter/2)2 × (APV/2). An abnormal CRT was defined as one or more abnormal measures. Reference subjects were considered to have normal CRT.
CMRI data were interpreted by computer-based analysis by expert readers experienced in performance and interpretation of CMRI (LT, MA, AH, JW) blinded to clinical and CRT data in a dedicated core laboratory. CAAS MRV 3.4 (PIE Medical Imaging) was used for analysis of the MPRI. The endocardial and epicardial contours were manually defined and adjusted to sample data from LV myocardium alone. Care was taken to exclude blood pool activity and to exclude any linear dark rim artifact at the LV cavity/endocardial border. The LV cavity region of interest was manually adjusted to include the region of maximal signal intensity within the cavity and to exclude papillary muscle. MPRI was defined as: MPRI = RUstress/RUrest.. RU is defined as the ratio between the maximum upslope of the first-pass myocardial perfusion time-intensity curve divided by the maximum upslope of the first-pass LV cavity time-intensity curve. An AHA 16-segment model was used (true apex not imaged); mean MPRI was the average of 16 segments. In the case of two-slice image data being acquired due to high stress heart rate, data were recorded for 12 segments and mean MPRI was the average of 12 segments. Subendocardial MPRI, subepicardial MPRI and “whole” (transmural) MPRI were calculated.
LV mass and volumes were assessed by manually tracing the epicardial and endocardial borders of short-axis cine images13. Stroke volume was calculated as end-diastolic volume minus end-systolic volume. Ejection fraction was calculated as stroke volume divided by end-diastolic volume. Stress and rest perfusion were scored visually by consensus of two blinded readers, using a 4-point 16-segment system. Summed stress score was the sum of visually abnormal segments. Delayed enhancement images were read by an experienced investigator to identify and describe areas of late enhancement.
Statistical Analysis
Values are expressed as mean ± standard deviation or percentages as indicated, and the t-test or chi-square statistic was used to evaluate differences in WISE versus reference control women. For diagnostic measures, medians and interquartile ranges (IQR) are also reported, and Pearson correlation coefficients (p-values) were used to report associations among these measures. The Jonckheere-Terpstra test for continuous variables was used to test whether the distribution of MPRI differs across increasing numbers of abnormal CRT variables or increasing numbers of cardiac risk factors.
CRT was considered normal if all four CRT measures were normal. CRT was considered abnormal if one or more measures were abnormal. Logistic regression was used to estimate the probability of abnormal CRT given MPRI; due to the small range of MPRI values such that a unit change represents a large relative change, MPRI was multiplied by 10 for this analysis. We used ROC curve analysis to identify the optimal threshold in the MPRI for predicting an abnormal CRT test. The threshold was chosen that gave the largest area under the ROC curve (AUC), which represented a trade-off between sensitivity and specificity. The AUC and 95% confidence intervals are reported to indicate the probability that a randomly selected diseased case (woman with abnormal CRT) will have a lower MPRI measurement than a randomly selected control. A p-value < 0.05 was considered statistically significant. SAS version 9.3 was used for all analyses.
Results
Pertinent demographic characteristics are summarized in Table 1. All WISE subjects were symptomatic, most (88%) with angina and the remainder with dyspnea. Hypertension, family history and prior smoking were frequent, and relatively few were diabetic (9%) or currently smoking (6%). WISE subjects had a greater BMI than reference subjects.
Table 1. Baseline Characteristics.
| Baseline Characteristics | WISE Subjects (n=118) Mean±SD or n(%) |
Reference Subjects (n=21) Mean±SD or n(%) |
p*value |
|---|---|---|---|
| Age | 53.9±11.4 | 53.6±9.1 | 0.90 |
| Body Mass Index | 30.3±8.9 | 25.3±3.6 | <0.0001 |
| Medications: | |||
| Beta blockers | 28(25) | 0 | 0.007 |
| Ca channel blockers | 17(15) | 0 | 0.07 |
| Nitrates | 32(28) | 0 | 0.004 |
| Aspirin | 81(69) | 4(19) | <0.0001 |
| Statins | 46(40) | 0 | <0.0001 |
| Hormone Replacement Therapy | 53(45) | 7(33) | 0.35 |
| Oral Contraceptive (current or past) | 84(71) | 18(86) | 0.19 |
| Risk Factors | |||
| Hypertension | 39(35) | 0 | 0.0004 |
| Dyslipidemia | 18(20) | 0 | 0.020 |
| Diabetes+ | 10(9) | 0 | 0.36 |
| Current smoker | 7(6) | 1(5) | >0.99 |
| Ever smoker | 55(47) | 7(33) | 0.26 |
| Family history | 44(41) | 5(24) | 0.14 |
| Symptoms | |||
| Chest pain | 104(88) | 0 | <0.0001 |
| Dyspnea | 75(64) | 0 | <0.0001 |
p values by t-test for continuous variables and Fisher's Exact for frequencies
9 subjects with type 2 diabetes, 1 subject with type 1 diabetes
WISE= Women's Ischemia Syndrome Evaluation
Results of CRT are summarized in Table 2 and demonstrate abnormal coronary diameter changes in response to acetylcholine and nitroglycerin. Overall among the WISE subjects 95% had CMD defined as at least one CRT abnormality. The median time (IQR) between CRT and CMRI was 28 days (14, 46).
Table 2. CRT Measures (n=118).
| Measure (Definition of Normal) | N | Mean±SD | Median(IQR) | Range |
|---|---|---|---|---|
| ΔACH (>0% increase) | 96 | - 2.4±14.6 | - 1.0(-10.4,6.6) | -43.4—47.3 |
| CFR (≥2.5) | 110 | 2.62±0.61 | 2.60(2.20,2.80) | 1.3—4.7 |
| ΔCBF (>50% increase) | 86 | 68±86 | 46(10,101) | -68—456 |
| ΔNTG (>20% increase) | 98 | 12.4±12.1 | 10.2(3.8,20.2) | -14.6—52.1 |
ΔACH= % diameter change in response to acetylcholine, CFR=coronary flow reserve in response to adenosine, ΔCBF= % coronary blood flow change in response to acetylcholine, IQR=interquartile range, ΔNTG= % diameter change in response to nitroglycerin
CMRI stress testing was completed without complications. All CMRI scans were included in analysis. There were no differences between WISE subjects and reference subjects in terms of baseline rest or vasodilator stress heart rate and blood pressure (Table 3).
Table 3. CMRI Hemodynamic Variables (WISE=118, Reference=21).
| Measure | Mean±SD | Median(IQR) | Range | p |
|---|---|---|---|---|
| Rest HR: | 0.09 | |||
| WISE subjects | 68±10 | 68(60,74) | 40—103 | |
| Reference subjects | 64±8 | 62(57,69) | 54—82 | |
| Peak Stress HR: | 0.52 | |||
| WISE subjects | 94±17 | 96(81,106) | 51—127 | |
| Reference subjects | 97±13 | 97(87,106) | 72—120 | |
| Rest SBP: | 0.76 | |||
| WISE subjects | 129±22 | 129(113,140) | 81—193 | |
| Reference subjects | 128±20 | 129(116,138) | 96—176 | |
| Peak Stress SBP: | 0.29 | |||
| WISE subjects | 134±27 | 130(118,148) | 50—241 | |
| Reference subjects | 129±17 | 127(117,141) | 106—163 | |
| Rest RPP: | 0.17 | |||
| WISE subjects | 8749±1870 | 8578(7424,9880) | 5040—15440 | |
| Reference subjects | 8145±1667 | 7840(6820,9204) | 5454—12144 |
HR=heart rate, IQR=interquartile range, RPP= rate pressure product, SBP=systolic blood pressure, WISE= Women's Ischemia Syndrome Evaluation
The mean transmural MPRI was lower in WISE subjects compared to reference subjects (1.71±0.43 vs. 2.23±0.37, p <0.0001). Differences between WISE subjects and reference subjects remained significant by examining mid-ventricular short-axis segments alone (1.75±0.48 vs. 2.23±0.45, p <0.0001), and by examining subendocardial and subepicardial MPRI (Table 4). There was no difference between the 9 subjects with 12-segment MPRI data (peak heart rate precluded three-slice imaging during pharmacologic stress) and 130 subjects with 16-segment data (mean MPRI 1.88±0.45 vs. 1.78±0.46, p=0.54). Visual evaluation of images did not reveal any significant difference between groups. The ratio of mass/volume was greater in WISE subjects; all subjects had normal LV ejection fraction. There were 6/118 WISE subjects with myocardial fibrosis on delayed enhancement imaging, mean 6.16±3.38 g, range 3.2-11.2g. Fibrosis was subendocardial in four and a nonischemic-type distribution of late enhancement was present in two WISE subjects. No reference subject had late enhancement.
Table 4. CMRI Measures.
| Measure | WISE Subjects (n=118) Mean±SD |
Reference Subjects (n=21) Mean±SD |
p |
|---|---|---|---|
| Whole MPRI | 1.71±0.43 | 2.23±0.37 | <0.0001 |
| Subendocardial MPRI | 1.55±0.39 | 2.01±0.35 | <0.0001 |
| Subepicardial MPRI | 1.79±0.45 | 2.38±0.41 | <0.0001 |
| Summed Stress Score | 6.66±5.62 | 4.45±4.97 | 0.09 |
| Ejection Fraction (%) | 67.19±7.05 | 69.4±4.26 | 0.17 |
| LV EDV (mL) | 121.96±25.54 | 131.71±28.16 | 0.11 |
| LV Mass (g) | 91.94±17.07 | 85.69±12.04 | 0.11 |
| Mass/Volume (g/mL) | 0.77±0.14 | 0.68±0.12 | 0.0064 |
EDV= end-diastolic volume; LV= left ventricle; MPRI=myocardial perfusion reserve index; WISE= Women's Ischemia Syndrome Evaluation;
Relationships between CRT variables and mean MPRI are summarized in Table 5 and demonstrate modest statistically significant positive correlations between individual CRT variables and MPRI. MPRI did not significantly differ between subjects with and without CRT abnormality (mean MPRI 1.71±0.42 vs. 1.76 ±0.55, p=0.76).
Table 5. Correlation between MPRI and CRT Measures.
| MPRI | ΔACH R (p value) |
CFR R (p value) |
ΔCBF R (p value) |
ΔNTG R (p value) |
|---|---|---|---|---|
| Whole | 0.22(0.029) | 0.16(0.084) | 0.29(0.005) | 0.24(0.016) |
| Subendocardial | 0.20(0.046) | 0.15(0.11) | 0.30(0.004) | 0.22(0.031) |
| Mid-ventricular short axis | 0.24(0.016) | 0.12(0.19) | 0.32(0.002) | 0.22(0.03) |
ΔACH= % diameter change in response to acetylcholine, CFR=coronary flow reserve in response to adenosine, ΔCBF= % coronary blood flow change in response to acetylcholine, MPRI=myocardial perfusion reserve index, ΔNTG= % diameter change in response to nitroglycerin
There was a trend towards decreasing MPRI as the number of abnormal CRT variables increased (Figure 1). This trend was examined in women who had all CRT variables for analysis, and included the reference subjects (assumed to have normal CRT). A similar trend towards decreasing MPRI was also present as the number of CAD risk factors increased among WISE subjects (Figure 2).
Figure 1. Relationship between MPRI and CRT measures.
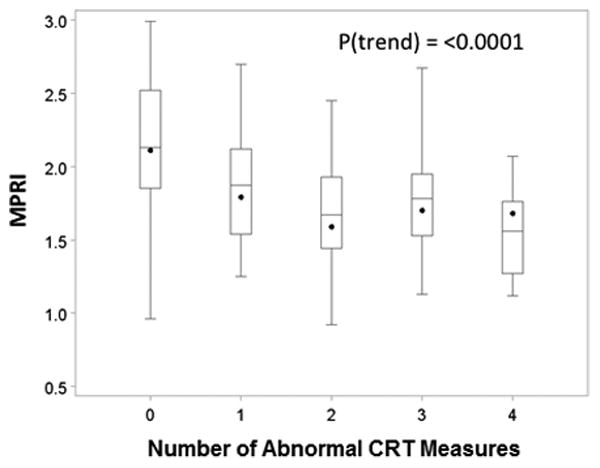
Boxplot demonstrating trend for decreasing MPRI with increasing numbers of abnormal CRT measures. Data are for CRT in 82 WISE subjects who had all four CRT measures and 21 reference subjects. (MPRI=myocardial perfusion reserve index; CRT=coronary reactivity testing; WISE=Women's Ischemia Syndrome Evaluation)
Figure 2. Relationship between MPRI and increasing number of cardiac risk factors.
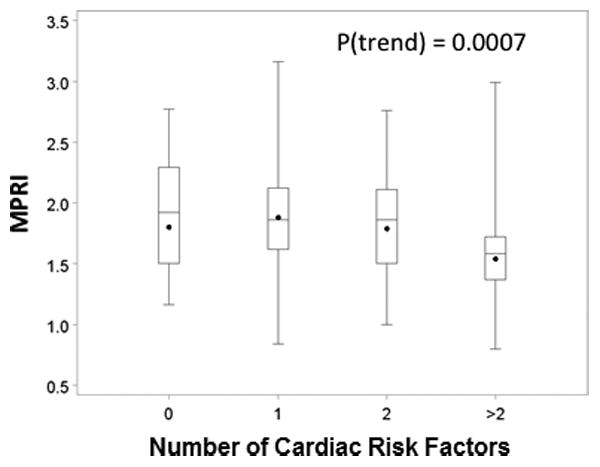
Boxplot demonstrating trend for decreasing MPRI with increasing numbers of cardiac risk factors. Data are for WISE subjects and reference subjects, excluding one woman with incomplete data. (MPRI=myocardial perfusion reserve index; WISE=Women's Ischemia Syndrome Evaluation)
ROC analysis
This analysis included a subgroup 82 WISE subjects who had all four CRT variables available for analysis as well as reference subjects for whom normal CRT was assumed in all. Logistic regression to estimate the ability of the whole mean MPRI to predict a normal CRT yielded an odds ratio of 0.78 (95% confidence interval = 0.70, 0.88), p<0.0001. This means that there is a 22% relative increase in the probability of an abnormal CRT (any pathway abnormality) for each 0.1 decrease in the MPRI. The AUC of the ROC was 0.78 (95% confidence interval 0.68, 0.88) (Figure 3). An MPRI threshold of 1.84 in this analysis predicted CMD status with sensitivity 73% (95% confidence interval= 64, 82) and specificity 74% (95% confidence interval= 58, 90).
Figure 3. Relationship between CRT abnormalities and MPRI.
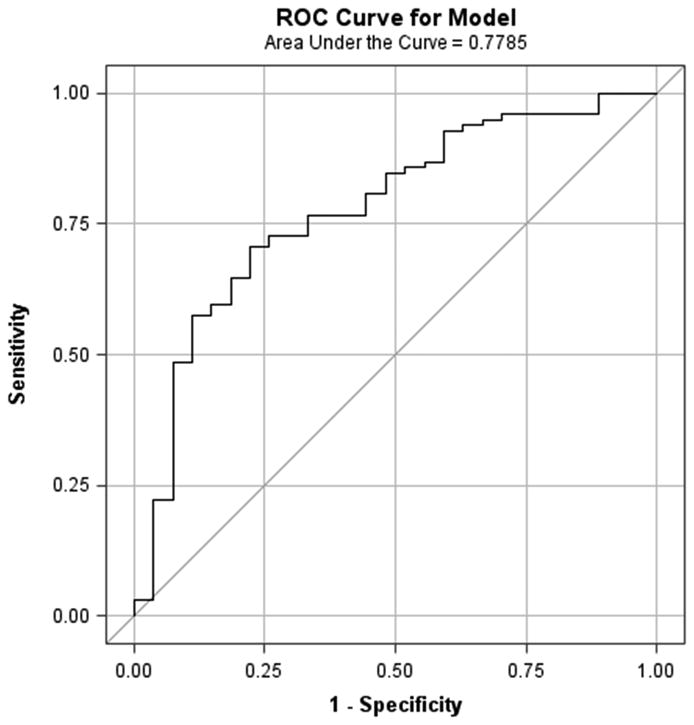
ROC curve for the presence of at least one CRT pathway abnormality vs mean MPRI for segments 1-16. Area under the curve= 0.78 (0.68, 0.88), OR= 0.78 (0.70, 0.88), p<0.0001. (CRT=coronary reactivity testing; MPRI=myocardial perfusion reserve index; ROC=receiver operating characteristic)
Discussion
Our results show that noninvasive CMRI MPRI is useful for detection of CMD determined by invasive CRT in women. Women with CMD defined by presence of abnormal invasive CRT have reduced MPRI with vasodilator stress first-pass perfusion CMRI, compared to an age-matched reference group. The differences between groups were observed in all LV regions, with a gradient between subepicardial and subendocardial MPRI in both WISE and reference subjects. There is a trend toward lower MPRI among women with extensive CRT abnormalities. In this selected population, a threshold MPRI value of 1.84 predicted CMD status with moderate sensitivity (73%) and specificity (74%).
A growing body of evidence supports the presence of CMD among women with symptoms and/or signs of ischemia in the absence of obstructive epicardial CAD. It is likely that CMD encompasses a spectrum of disorders of coronary endothelial and non-endothelial dependent vascular reactivity. A multitude of factors have been suggested as causative factors in CMD, including risk factors for atherosclerotic disease, hormonal and structural factors.
CMD is hypothesized to be a generalized process that may result in patchy but more or less generalized subendocardial myocardial ischemia as opposed to the highly regional location of ischemia resulting from obstructive epicardial CAD 8, 16. Standard non-invasive imaging (echo/nuclear stress testing) is often normal in patients with CMD without obstructive CAD, due to a lack of regional vascular territory ischemia that is characteristic of obstructive CAD 5, 6. The reference standard for diagnosis is CRT that is an invasive procedure for patients with persisting symptoms of ischemia in the absence of obstructive CAD. CMRI is potentially a noninvasive tool for diagnosis of CMD in this population.
CMRI perfusion abnormalities have been described in patients with angina and in the absence of obstructive CAD. Impairment of myocardial perfusion reserve has also been documented in some asymptomatic individuals with multiple cardiac risk factors in the Multi-Ethnic Study of Atherosclerosis, in asymptomatic patients with hypertrophic cardiomyopathy and in women with chest pain and systemic lupus erythematosus 10, 17. A variety of CMRI techniques have been reported in single center studies of small groups of patients and these vary widely in terms of technical complexity, clinical applicability and system field strength (3.0T vs. 1.5T). Relatively simple approaches include visual detection of subendocardial first-pass hypoperfusion 18, measurement of perfusion upslope curve steepness 8, and calculation of a ratio of stress to rest upslope 9 in response to vasodilator, dobutamine or cold pressorstress. More complex approaches involve calculation of absolute myocardial blood flow reserve 19, detection of diffuse myocardial fibrosis 20, noninvasive measurement of cellular oxygenation 21 or the detection of earliest abnormalities in diastolic filling. Studies also vary widely in the method of selection of subjects; many do not have a control group.
These results extend our previously published pilot data 9 that showed the same difference in MPRI between a different group of women with CMD defined by invasive CRT compared to control subjects. The current study extends those results to a new and larger cohort of women with CMD from two sites supporting the scalability of these CMR examinations. Study inclusion was on the basis of symptoms and signs of myocardial ischemia in the absence of obstructive epicardial CAD, with entry criteria being broader than prior pilot data. Again, we documented a lower MPRI compared to reference women. Additionally, in this much larger cohort we observed a relationship between presence of CRT abnormalities and MPRI measured noninvasively. Interestingly, by simple visual analysis no significant differences were noted between groups. It is likely that variable human light-level sensory thresholds as well as possible artifacts on images contributed to inability to discriminate between WISE subjects and reference subjects using unaided visual scoring.
Our study builds on previous work from Panting et al 8 who first emphasized the importance of a lack of increment in subendocardial perfusion index during adenosine stress in symptomatic subjects without obstructive CAD, not observed in controls. They were unable to show a difference between groups in terms of the ratio of stress to rest perfusion (MPRI); however they noted differences of borderline significance in both subendocardial and subepicardial MPRI in their small sample of 20 patients with 10 controls. Our study had greater power to detect differences between groups, and used somewhat different criteria for selection of both subjects and the reference group. Nevertheless, we observed a difference in response to adenosine vasodilator stress between the subjects and reference groups, expressed in terms of MPRI.
Our findings and methods are similar to those of Vermeltfoort et al 22 who reported on 20 subjects with clinically defined syndrome X using adenosine first-pass perfusion at 1.5T and noted a mean transmural myocardial MPRI 1.83±0.5, subendocardial MPRI 1.67±0.38 and subepicardial MPRI 1.98±0.64. They noted an increment in first-pass perfusion upslope in response to adenosine that could be measured in endocardial and epicardial regions, and concluded that they had failed to replicate the observations of Panting et al who had emphasized the importance of the lack of a subendocardial stress perfusion response in subjects with syndrome X. However, Vermeltfoort et al did not have a control group for comparison.
A previous 3T CMRI study of 18 patients with clinically defined syndrome X and 14 controls by Karamitsos et al 21 did not find differences in either absolute myocardial flow or oxygenation between groups. We had a different and larger population of women who were younger and heavier (mean age 55, BMI 30 vs. mean age 62, BMI 26). Our inclusion criteria required invasive CRT for characterization of CMD and our reference group was selected based on absence of physical limitation and ischemia during exercise testing.
The importance of an appropriate control group should be reemphasized. There is a paucity of published data defining what is “normal” MPRI for stress CMRI using this technique in middle-aged women. Regional heterogeneity in myocardial perfusion has been observed in 3T studies of absolute myocardial blood flow in healthy human myocardium 23 and it has been observed that the hyperemic myocardial blood flow response to vasodilator stress is reduced in older vs. younger populations 24. If we had selected a younger, more fit female population as a control group, there would potentially have been a greater difference between groups in terms of MPRI, but study results would have had less applicability to clinical management of a “typical” symptomatic middle-aged woman.
Limitations
There are several potential limitations to the study. Our WISE population was selected with the absence of obstructive CAD defined by coronary angiography. Obstructive CAD is expected to be associated with lower mean MPRI, vascular territory regional variation in MPRI and segmental visual perfusion abnormalities. Our results cannot be extrapolated to unselected populations, and the ROC data are not representative of the ability of MPRI to define the presence of obstructive CAD. In this CRT analysis, we used a sensitive threshold for the CBF variable (%ΔCBF≤50), understanding that this may reduce specificity for this initial analysis. In addition, the threshold value is data derived, so the sensitivity and specificity may be over-estimated in the absence of validation. We compared subjects from the WISE population to an age-matched group of healthy women because there is a paucity of data defining normal MPRI using this technique in middle-aged overweight women. A merged sample of cases and reference controls was used to extend the range of MPRI values for analyses of the relationships between MPRI and the number of abnormal CRT measures (Figure 1) and number of atherosclerosis risk factors (Figure 2); further work will evaluate this with respect to the proportion of cases in the population. The size of our reference group was relatively small compared to the WISE group, thus inadvertent inclusion of subjects with CMD within the reference group could decrease the ability of CMRI to discriminate between WISE and reference subjects. Our reference group did not have coronary angiography or CRT due to the unacceptable risk of invasive testing without clinical indication. The reference group had lower BMI than WISE subjects, potentially contributing to the observed difference in MPRI between groups. However, DiBella et al reported a similar “normal” mean MPRI of 2.25±0.59 in a group of asymptomatic volunteers undergoing adenosine stress at 3T, with mean BMI 30.3±6.5 25. We chose to use a clinical 1.5T CMRI scanner and standard commercially available image data analysis software to test the scalability of the method to another center. The imaging used an accelerated pulse sequence with inherent image artifacts, and thus care was taken to avoid incorporation of dark rim artifact within the myocardial region used for semi-quantitative analysis. It is possible that inadvertent incorporation of artifact contributed to apparent reduction in subendocardial vs subepicardial MPRI; however the MPRI was reduced in WISE population compared to reference subjects in both subendocardial and subepicardial regions. Finally, we did not measure absolute myocardial flow, which is most accurately performed at 3T and requires specialized post-processing that was not available for routine clinical use. Additionally, it has been demonstrated that semi-quantitative measurement of MPRI is more reproducible than measurement of absolute flow26.
Implications
Our results demonstrate that noninvasive CMRI MPRI can detect CMD defined by invasive CRT. Analysis of the relationship between non-invasive and invasive CRT suggests that there may be a threshold value for MPRI that will predict likelihood of abnormal invasive CRT in this population. These findings have potential implications for management of women who have persisting chest pain in the absence of obstructive epicardial disease in whom CMD has previously only been identifiable by invasive means. Importantly, the methodology utilized standard equipment and protocol that is available in most tertiary institutions. Further work is required to define the relationship between non-invasive and invasive measures of myocardial perfusion reserve in this population in order to optimally identify patients with abnormalities of endothelial and non-endothelial coronary microvascular function. Additional long-term follow-up is needed to determine whether CMRI-derived MPRI testing leads to improved cardiovascular outcomes.
Acknowledgments
Sources of Funding: This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, K23HL105787, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, T32HL116273, R01 HL090957, 1R03AG032631 from the National Institute on Aging, General Clinical Research Center grant MO1-RR00425 from the National Center for Research Resources, UL1TR000124 and UL1TR000064, grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women's Health Research (SWHR), Washington, D.C., The Linda Joy Pollin Women's Heart Health Program, and the Erika Glazer Women's Heart Health Project, Cedars-Sinai Medical Center, Los Angeles.
Footnotes
Disclosures: Thomson: NHLBI, Wei: none; Agarwal: none; Haft-Baradaran: none; Shufelt: Gilead; Mehta: Gilead; Gill: none; Johnson: none; Kenkre: none; Handberg: Gilead, AstraZeneca, Daiiehi Sankyo, Amarin, Daiichi, Mesoblast, ISIS Pharmaceuticals, Esperion Therapeudics, Vessex, Genentech, Cytori, Daiichi-Sankyo, Medtronic, Baxter, United Therapeudics, Sanofi/Aventis, Amgen, Catabasis; Li: none; Sharif: none; Berman: Spectrum Dynamics, Cedars Sinai Medical Center -software royalties, Lantheus, Siemens, Astellas, GE/Amersham, Cardium Therapeutics Inc; Petersen: none; Pepine: NIH Study Section of Cardiovascular Sciences Small Business Activities 2RG1 CVS-K-10, Lilly/Cleveland Clinic DSMB Member for a Phase 2 Efficacy and Safety Study of Ly2484595, Medtelligence, NHLBI Study Section for Progenitor Cell Biology Consortium, NHLBI DSMB Chair for Freedom Trial; Gilead Sciences, Inc, Pfizer, Park-Davis, Sanofi-Aventis, Fujisawa HealthCare Inc, Baxter, Brigham & Women's Hospital, AstraZeneca, NIH/NHLBI, Amorcyte/Neostem, Cytori, InfraReDx, NHLBI/NCRR CTSA grant 1UL1RR029890, AHA; Bairey Merz: Research Triangle Institute International, UCSF, Kaiser, Gilead (grant review committee), Garden State AHA, Allegheny General Hospital, PCNA, Mayo Foundation (lectures; symposiums), Bryn Mawr Hospital, Victor Chang Cardiac Research Institute (Australia, Duke (Consulting, Japanese Circ Society, U of New Mexico, Emory, Practice Pont Communications (lectures), Vox Media (lectures), WISE CVD, FAMRI, RWISE, Normal Control, Microvascular, NIH-SEP.
References
- 1.Doyle M, Weinberg N, Pohost GM, Bairey Merz CN, Shaw LJ, Sopko G, Fuisz A, Rogers WJ, Walsh EG, Johnson BD, Sharaf BL, Pepine CJ, Mankad S, Reis SE, Vido DA, Rayarao G, Bittner V, Tauxe L, Olson MB, Kelsey SF, Biederman RW. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovascular imaging. 2010;3:1030–6. doi: 10.1016/j.jcmg.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart L, Blood I. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 3.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine. 2009;169:843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. Journal of the American College of Cardiology. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nihoyannopoulos P, Kaski JC, Crake T, Maseri A. Absence of myocardial dysfunction during stress in patients with syndrome X. Journal of the American College of Cardiology. 1991;18:1463–70. doi: 10.1016/0735-1097(91)90676-z. [DOI] [PubMed] [Google Scholar]
- 6.Saghari M, Assadi M, Eftekhari M, Yaghoubi M, Fard-Esfahani A, Malekzadeh JM, Sichani BF, Beiki D, Takavar A. Frequency and severity of myocardial perfusion abnormalities using Tc-99m MIBI SPECT in cardiac syndrome X. BMC nuclear medicine. 2006;6:1. doi: 10.1186/1471-2385-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, Fleck E. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–7. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 8.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. The New England journal of medicine. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 9.Shufelt CL, Thomson LE, Goykhman P, Agarwal M, Mehta PK, Sedlak T, Li N, Gill E, Samuels B, Azabal B, Kar S, Kothawade K, Minissian M, Slomka P, Berman DS, Bairey Merz CN. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovascular diagnosis and therapy. 2013;3:153–60. doi: 10.3978/j.issn.2223-3652.2013.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y, Wallace DJ, Weisman MH, Bairey Merz CN. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovascular imaging. 2011;4:27–33. doi: 10.1016/j.jcmg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovascular interventions. 2012;5:646–53. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. Journal of the American College of Cardiology. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 13.Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, Mehta P, Zhang X, Thomson LE, Berman DS, Li D, Bairey Merz CN. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circulation Cardiovascular imaging. 2014;7:510–6. doi: 10.1161/CIRCIMAGING.114.001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 15.Kern MJ. Curriculum in interventional cardiology: coronary pressure and flow measurements in the cardiac catheterization laboratory. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2001;54:378–400. doi: 10.1002/ccd.1303. [DOI] [PubMed] [Google Scholar]
- 16.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. The American journal of cardiology. 1987;59:23C–30C. doi: 10.1016/0002-9149(87)90192-5. [DOI] [PubMed] [Google Scholar]
- 17.Tyan CC, Armstrong S, Scholl D, Stirrat J, Blackwood K, El-Sherif O, Thompson T, Wisenberg G, Prato FS, So A, Lee TY, Drangova M, White JA. Stress hypoperfusion and tissue injury in hypertrophic cardiomyopathy: spatial characterization using high-resolution 3-tesla magnetic resonance imaging. Circulation Cardiovascular imaging. 2013;6:229–38. doi: 10.1161/CIRCIMAGING.112.000170. [DOI] [PubMed] [Google Scholar]
- 18.Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. Journal of the American College of Cardiology. 2008;51:466–72. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 19.Fritz-Hansen T, Hove JD, Kofoed KF, Kelbaek H, Larsson HB. Quantification of MRI measured myocardial perfusion reserve in healthy humans: a comparison with positron emission tomography. Journal of magnetic resonance imaging : JMRI. 2008;27:818–24. doi: 10.1002/jmri.21306. [DOI] [PubMed] [Google Scholar]
- 20.Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circulation Cardiovascular imaging. 2011;4:693–702. doi: 10.1161/CIRCIMAGING.111.963587. [DOI] [PubMed] [Google Scholar]
- 21.Karamitsos TD, Arnold JR, Pegg TJ, Francis JM, Birks J, Jerosch-Herold M, Neubauer S, Selvanayagam JB. Patients with syndrome X have normal transmural myocardial perfusion and oxygenation: a 3-T cardiovascular magnetic resonance imaging study. Circulation Cardiovascular imaging. 2012;5:194–200. doi: 10.1161/CIRCIMAGING.111.969667. [DOI] [PubMed] [Google Scholar]
- 22.Vermeltfoort IA, Bondarenko O, Raijmakers PG, Odekerken DA, Kuijper AF, Zwijnenburg A, van der Vis-Melsen MJ, Twisk JW, Beek AM, Teule GJ, van Rossum AC. Is subendocardial ischaemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. European heart journal. 2007;28:1554–8. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 23.Muehling OM, Jerosch-Herold M, Panse P, Zenovich A, Wilson BV, Wilson RF, Wilke N. Regional heterogeneity of myocardial perfusion in healthy human myocardium: assessment with magnetic resonance perfusion imaging. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2004;6:499–507. doi: 10.1081/jcmr-120030570. [DOI] [PubMed] [Google Scholar]
- 24.Rosen BD, Lima JA, Nasir K, Edvardsen T, Folsom AR, Lai S, Bluemke DA, Jerosch-Herold M. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the multi-ethnic study of atherosclerosis. Circulation. 2006;114:289–97. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 25.DiBella EV, Fluckiger JU, Chen L, Kim TH, Pack NA, Matthews B, Adluru G, Priester T, Kuppahally S, Jiji R, McGann C, Litwin SE. The effect of obesity on regadenoson-induced myocardial hyperemia: a quantitative magnetic resonance imaging study. The international journal of cardiovascular imaging. 2012;28:1435–44. doi: 10.1007/s10554-011-9949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larghat AM, Maredia N, Biglands J, Greenwood JP, Ball SG, Jerosch-Herold M, Radjenovic A, Plein S. Reproducibility of first-pass cardiovascular magnetic resonance myocardial perfusion. Journal of magnetic resonance imaging : JMRI. 2013;37:865–74. doi: 10.1002/jmri.23889. [DOI] [PubMed] [Google Scholar]


