Abstract
An increasing body of literature indicates that chemotherapy (ChT) for breast cancer (BC) is associated with adverse effects on the brain. Recent research suggests that cognitive and brain function in patients with BC may already be compromised before the start of chemotherapy. This is the first study combining neuropsychological testing, patient-reported outcomes, and multimodal magnetic resonance imaging (MRI) to examine pretreatment cognition and various aspects of brain function and structure in a large sample.
Thirty-two patients with BC scheduled to receive ChT (pre-ChT+), 33 patients with BC not indicated to undergo ChT (pre-ChT−), and 38 no-cancer controls (NCs) were included. The examination consisted of a neuropsychological test battery, self-reported aspects of psychosocial functioning, and multimodal MRI.
Patients with BC reported worse scores on several aspects of quality of life, such as higher levels of fatigue and stress. However, cortisol levels were not elevated in the patient groups compared to the control group. Overall cognitive performance was lower in the pre-ChT+ and the pre-ChT− groups compared to NC. Further, patients demonstrated prefrontal hyperactivation with increasing task difficulty on a planning task compared to NC, but not during a memory task. White matter integrity was lower in both patient groups. No differences in regional brain volume and brain metabolites were found. The cognitive and imaging data converged to show that symptoms of fatigue were associated with the observed abnormalities; the observed differences were no longer significant when fatigue was accounted for.
This study suggests that cancer-related psychological or biological processes may adversely impact cognitive functioning and associated aspects of brain structure and function before the start of adjuvant treatment. Our findings stress the importance to further explore the processes underlying the expression of fatigue and to study whether it has a contributory role in subsequent treatment-related cognitive decline.
Keywords: Breast cancer, Magnetic resonance imaging, Cognition, Fatigue
Highlights
-
•
Breast cancer patients perform worse on cognitive tests than no-cancer controls.
-
•
Patients report lower quality of life and more fatigue and stress than controls.
-
•
DTI indicates lower white matter integrity in patients than controls.
-
•
fMRI shows prefrontal hyperactivation in patients compared to controls.
-
•
Effects occur prior to systemic treatment and are associated with fatigue.
1. Introduction
Neuropsychological and magnetic resonance imaging (MRI) studies show the occurrence of cognitive decline and brain changes following chemotherapy (ChT) in patients with breast cancer (BC) (Pomykala et al., 2013). Preclinical studies support these findings and have demonstrated increased apoptosis in healthy proliferating cells in the central nervous system as well as damage to neural precursor cells (Seigers et al., 2013).
Interestingly, several neuropsychological studies have observed lower than expected cognitive performance in patients with BC already before the start of adjuvant treatment (Lange et al., 2014; Wefel and Schagen, 2012). A small number of MRI studies also explored structural and functional brain differences before exposure to systemic treatment. Of two studies assessing regional brain morphology, one found lower gray matter (GM) density in patients with BC before adjuvant treatment (McDonald and Saykin, 2013). By contrast Deprez et al. (2012) did not find differences in white matter microstructure between patients with BC about to undergo chemotherapy (pre-ChT+), patients with BC who do not require chemotherapy (pre-ChT−), and no-cancer controls (NCs) after correcting for depressive symptoms.
More consistent results on pretreatment cognitive and brain differences between patients with cancer and controls come from functional MRI (fMRI) studies. These studies found a predominant pattern of prefrontal hyperactivation and slightly lower or similar task performance in patients with BC versus NCs before the start of adjuvant treatment, suggestive of compensatory processes (Cimprich et al., 2010; McDonald et al., 2012). One study found that higher levels of worry were associated with lower cognitive performance and lower brain deactivation in patients with BC (Berman et al., 2014). Three fMRI studies assessing various cognitive functions in one sample of pre-ChT+ and NCs showed that group differences in BOLD activation were dependent on the specific cognitive test performed and the inclusion of several covariates, e.g. cortisol or days since surgery (López Zunini et al., 2013; Scherling et al., 2011, 2012). These studies emphasize the potential relevance of psychosocial and biological factors in cognitive and brain function before the start of chemotherapy.
In summary, both neuropsychological and imaging studies point to the potential existence of pretreatment cognitive and brain dysfunction in patients with BC. Psychological and biological mechanisms have been proposed to underlie these impairments including surgical factors and anesthesia, fatigue, comorbidities and cancer staging, but as yet no studies determined and explained this phenomenon convincingly (Ahles et al., 2008; Cimprich et al., 2010; Mandelblatt et al., 2014; Wefel et al., 2004).
The current study sets out to build upon previous findings of cognitive problems and changes in brain function and structure prior to adjuvant treatment by combining neuropsychological testing, MRI, and patient-reported outcomes (PROs). With this set-up, differences in several data types can be related to each other and potential underlying psychological factors can be identified.
2. Materials and methods
2.1. Subjects
Participants were patients with BC who had undergone mastectomy or lumpectomy and age-matched no-cancer controls (NC). Patients were either scheduled to receive adjuvant anthracycline-based chemotherapy (pre-CHT+) or did not require chemotherapy (pre-CHT−). Subjects were eligible if they met the following criteria: female, under 70 years of age, sufficient command of the Dutch language, no previous malignancies. Patients additionally had to have a diagnosis of primary breast cancer, no distant metastases, and no other treatment than surgery at the time of baseline assessment. NCs were recruited through participants, as well as through advertisements in the participating hospitals.
The study was approved by the Institutional Review Board of the Netherlands Cancer Institute, serving as the central ethical committee for all participating institutes. Written informed consent was obtained according to the declaration of Helsinki and following institutional guidelines. The experiment was conducted at the Academic Medical Center of the University of Amsterdam and the Spinoza Centre for Neuroimaging.
Follow-up data collection at approximately 6 months after completion of chemotherapy, or at matched intervals, is ongoing and will be presented elsewhere.
2.2. Procedures
Seven questionnaires were administered to assess PROs, such as health-related quality of life (QOL), anxiety and depression, mood, stress, cognitive problems, and personality dimensions (Supplementary Table 1). Premorbid verbal IQ was estimated with the use of the Dutch Adult Reading Test (NART) (Schmand et al., 1992). A comprehensive neuropsychological test battery was used, consisting of 18 test indices, grouped into the domains of executive function, attention, visual memory, verbal memory, processing speed, and motor speed (Supplementary Table 2). Assignment into domains was determined based on literature and no outcome could be present in more than one domain.
To objectively assess long-term stress, hair samples were collected and consequently analyzed by the Department of Biopsychology of the Technische Universität of Dresden. Cortisol levels were determined in segments of 2 cm, representing a period of 2 months. Wash and steroid extraction procedures are described elsewhere (Kirschbaum et al., 2009).
MRI data were acquired using a 3.0 Tesla Intera full-body MRI scanner (AMC Medical Center) and a 3.0 Tesla Achieva full-body MRI scanner (Spinoza Centre for Neuroimaging) (Philips Medical Systems, Best, The Netherlands). A SENSE 8-channel receiver head coil was used at both locations.
An axial fluid attenuated inversion recovery (FLAIR) scan (TR/TE/TI = 11,000/100/2600 ms, FOV 230 × 230 mm, 27 slices, voxel size 0.9 × 1.4 × 5.0 mm, slice gap 0.5 mm) was acquired to score white matter abnormalities with the visual rating score of Fazekas (range 0–3) (Fazekas et al., 1987). All ratings were performed by a neuroradiologist (L.R.) blind to the clinical data. A T1 weighted three-dimensional magnetization prepared rapid gradient echo (MPRAGE) scan (TR/TE = 6.6/3.0 ms, FOV 270 × 252, 170 slices, voxel size 1.05 × 1.05 × 1.20 mm) was made for anatomical reference and voxel-based morphometry (VBM). Single voxel proton MR spectroscopy (1H-MRS) was acquired in the left semioval center (SC) and the left hippocampus (HC) to assess neurochemical properties of white and gray matter respectively. The semioval center allows for data acquisition in white matter alone and it has been shown to be vulnerable to chemotherapy (De Ruiter et al., 2011). The hippocampus has previously been shown to be affected by cancer treatment and is an interesting brain structure because of its role in memory. The left side of the brain was chosen because of its predominance in cognition. Fully automated point resolved spectroscopy (PRESS) including global shimming (voxel size = 6.0 ml for semioval center and 2.56 ml for hippocampus, TR/TE = 200/35–40 ms, NSA = 64) was obtained. Diffusion Tensor Imaging (DTI) was acquired in 32 directions (TR/TE = 8136/94 ms, FOV 250 × 250 mm, 64 slices, voxel size 2.23 × 2.23 × 2.00 mm, b-value: 1000 s/mm2), covering the entire brain. Functional MRI acquisition was based on T2* weighted gradient echo planar imaging (EPI) of 38 axial slices (voxel size 2.3 × 2.3 × 2.3 mm, interslice gap 0 mm, matrix size 96 × 96, TR = 2.1 s, TE = 25 ms). We acquired 230 volumes during the Tower of London (ToL) task, 170 for memory encoding, and 125 for memory retrieval.
Artrepair was used to detect and repair artifacts (Mazaika et al., 2009). All fMRI preprocessing was performed using SPM8 (Statistical Parametric Mapping; Wellcome Trust Centre for Neuroimaging, London, UK). Slice timing correction was applied to the ToL and Retrieval images. All fMRI images were reoriented and realigned to the first volume. Individual T1 scans were segmented based on gray matter, white matter, and cerebrospinal fluid. Coregistered EPI and T1 scans were normalized to the Montreal Neurologic Institute (MNI) reference brain with the use of the segmentation parameters. Finally, smoothing was applied using an 8-mm full-width half-maximum Gaussian kernel.
An abbreviated version of the Tower of London (ToL) paradigm by van den Heuvel et al. (2003) was used to assess prefrontal function. During planning, five conditions ranging from one to five moves were presented. A starting configuration and a target configuration were displayed. Each consisted of three colored beads placed on three vertical rods, which could accommodate one, two, and three beads respectively. Subjects were instructed to determine the minimum number of steps required to get from the starting to the target configuration by mentally moving the beads one at a time. In the baseline condition, the number of yellow and blue beads had to be counted. The presentation of trials was self-paced with a maximum duration of 1 min per trial. The task lasted 8 min. Participants were instructed to focus on accuracy rather than on speed. The task was practiced outside the scanner.
The Paired Associates memory task was based on a task paradigm by Jager et al. and was shown to reliably activate the parahippocampal region (Jager et al., 2007). During associative learning, subjects were asked to indicate if the person shown in the portrait photo was likely to live in the home interior in the simultaneously presented picture. The baseline condition consisted of three arrowheads pointing to (“<<<” or “>>>”) indicating a left or right button press respectively. The arrows were superimposed on blurred portrait and interior design pictures to match the visual input of the associative learning condition. The learning and baseline trials were presented in a block design. For the learning condition, six blocks were presented with five trials per block. Stimuli were presented for 5 s. The baseline condition consisted of five blocks with five stimuli, which were presented 3 s. Directly after the learning part of the task, a recognition test was administered. The baseline trials were the same as in the learning part. For the recognition part, all pictures from the learning phase were shown and subjects were asked to indicate whether they had seen the same combination of pictures before. Sixty percent of the pairs were the same as in the learning phase. The order of the trial types was pseudo-randomized. All stimuli were presented for 4 s.
2.3. Statistical analysis
Demographic and clinical variables, PROs, neuropsychological data, MR spectra, and fMRI performance data were analyzed with SPSS 20 (IBM, Armonk, NY) by means of ANOVA or chi-squared test, as appropriate. Age and IQ were included in neuropsychological and fMRI analyses. Age and scanner location were included in analyses of all MRI data. Corrections for multiple comparisons were applied and will be specified per data type.
Neuropsychological data were analyzed in line with International Cognition and Cancer Task Force (ICCTF) guidelines (Wefel et al., 2011). Standardized z-scores for all test indices were calculated based on the mean and standard deviation of the NC group. A cut-off for cognitive impairment, based on the 95th percentile of the NCs, was identified as scores of two standard deviations below the mean on at least three test indices (Schagen et al., 2006). The difference in proportion of impaired subjects was tested using logistic regression.
In addition, the Mahalanobis Distance (MHD) was calculated as a summary measure of overall performance (Crawford et al., 2012; Crawford et al., 2012; Crawford et al., 2011; DeCarlo, 1997; Koppelmans et al., 2012). MHD calculations were based on residual scores, the difference between individual scores and the intercept, adjusted for age and IQ. Residual scores that were greater than their respective mean score were assigned a value of zero, so that negative scores could not be compensated (Koppelmans et al., 2012). The residual scores of the control group were used to calculate a variance–covariance matrix, corresponding to the correlation between tests and the variance within the tests in the control group (DeCarlo, 1997; Koppelmans et al., 2012). The variance–covariance matrix was then used to extract the unique variance of each variable for each subject for all groups. Log2 transformation of the resulting MHD was applied because of skewness of its distribution and between group differences were calculated with an ANOVA. By taking into account the correlations between tests, MHD corrects for multiple comparisons. Domain scores and patient-reported outcomes were corrected for multiple comparisons by lowering the critical p-value to 0.01.
Reaction time (RT) for fMRI tasks was calculated for correct trials. For the ToL, all active versus baseline trials as well as a parametric contrast with increasing task load (ToL Load) were modeled. For the Paired Associates task, encoding trials were contrasted to baseline trials, for retrieval, hits were contrasted to baseline. Group differences for contrasts of interest were evaluated with random effects analyses.
MR spectroscopy was analyzed using a standard protocol within LCModel (Provencher, 1993). The standard VBM8 pipeline within SPM8 was used for analysis of MPRAGE images. For fMRI and VBM, Whole-brain and ROI differences were considered statistically significant at a FWE corrected p-value of 0.05. DTI preprocessing and tensor fitting was performed within the FMRIB Diffusion Toolbox (FDT) (part of FMRIBs Software Library (FSL) (Smith et al., 2006)). Diffusion data were ‘skeletonized’ with Tract-based spatial statistics (TBSS, part of FSL (Smith et al., 2006)) and nonparametrically tested (Nichols and Holmes, 2002) (for more detailed information on MRI analyses, see supplemental material).
Correlation analyses were only performed when significant group differences were found or when a strong relation was expected. In line with existing literature, we calculated the following correlations. Correlations between various PROs were examined. Neuropsychological performance (MHD, domain scores) was correlated with PROs. Voxel based analyses for BOLD signal, GM volume and FA and MD were performed within SPM8 and FSL to study associations with specific PROs and neuropsychological performance. Whole-brain FA and MD values were extracted, as well as BOLD signal in significantly different clusters and these were correlated with neuropsychological performance and PROs.
3. Results
3.1. Participants
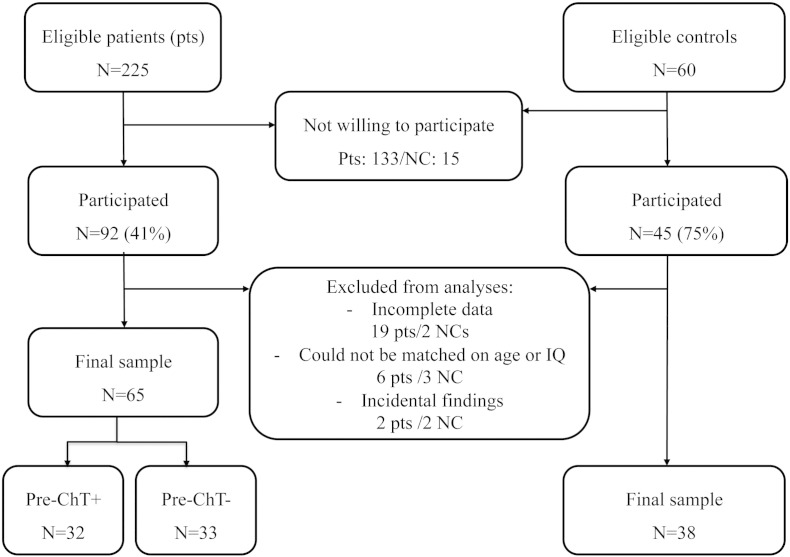
A total of 285 participants were eligible to participate in this study, of which 137 participated in the study. Main reasons given for decline were ‘too burdensome’ and ‘hesitant about MRI’. Four patients were excluded because of incidental findings on MRI scans, 21 patients were excluded because three or more scans of different modalities were missing. The groups were matched on age and IQ leaving 32 pre-ChT+, 33 pre-ChT−, and 38 NC subjects for our final analyses (see Fig. 1).
Fig. 1.
Flow diagram: Selection of participants into the study. Pre-ChT+, patients with BC before chemotherapy; pre-ChT−, patients with BC not scheduled to undergo chemotherapy; NCs, no-cancer controls.
All patient characteristics and PROs are presented in Table 1. As expected, no significant differences were found between groups on age and premorbid IQ. The patient groups also did not differ on time since surgery.
Table 1.
Patient characteristics and self-reported outcomes.
| Pre-ChT+ (n = 32) |
Pre-ChT− (n = 33) |
NC (n = 38) |
p | |
|---|---|---|---|---|
| Age (years) | 50.2 (9.2) | 52.4 (7.3) | 50.1 (8.7) | .442 |
| Estimated IQ (NART) | 100.6 (14.1) | 102.8 (14.7) | 107.0 (11.1) | .120 |
| Education level (n(%))b | ||||
| Middle | 4 (13) | 5 (15) | 1 (3) | |
| High | 28 (88) | 28 (85) | 37 (97) | |
| Time since surgery (days) | 36.1 (20.0) | 31.5 (15.7) | NA | .308 |
| Breast cancer stage (n(%)) | ||||
| 0 | 13 (39) | |||
| 1 | 20 (63) | 18 (55) | ||
| 2 | 11 (34) | 2 (6) | ||
| 3 | 1 (3) | |||
| n (%) | n (%) | n (%) | ||
| Pre-menopausal | 19 (59%) | 16 (49%) | 20 (53%) | |
| Post-menopausal | 13 (41%) | 17 (51%) | 18 (47%) | .674 |
| Anti-diabetic medication | 0 (0%) | 2 (6%) | 2 (5%) | |
| Cardiovascular medication | 6 (19%) | 8 (24%) | 9 (24%) | |
| Psychotropic medication | 5 (15%) | 4 (12%) | 2 (5%) | |
| EORTC QLQ-C30 | ||||
| Physical functioningb | 91.7 (11.4)* | 88.1 (11.7)* | 97.0 (7.1) | .001 |
| Role functioningb | 66.1 (35.3)* | 67.2 (29.0)* | 95.2 (19.3) | <.001 |
| Social functioningb | 80.2 (23.0)* | 79.3 (24.3)* | 97.8 (9.6) | <.001 |
| Cognitive functioningb | 81.8 (24.1) | 80.3 (24.8) | 90.4 (14.8) | .104 |
| Global quality of lifec | 75.8 (17.6)* | 73.0 (15.6)* | 87.1 (12.7) | <.001 |
| Paind | 25.5 (27.4)* | 27.8 (27.5)* | 7.0 (13.2) | <.001 |
| Fatigued | 24.7 (23.4) | 34.3 (25.7)* | 15.2 (19.4) | .003 |
| HSCL-25 | 13.5 (13.7) | 11.6 (10.5) | 7.7 (9.4) | .09 |
| PSS | 24.3 (6.6)* | 21.0 (7.5) | 19.2 (5.4) | .006 |
| POMS | ||||
| Fatigue subscalee | 2.5 (3.9) | 3.0 (4.8) | 1.3 (1.5) | .135 |
| Vigor subscalef | 12.0 (4.0)* | 12.3 (3.4)* | 14.6 (2.3) | .001 |
| Total scorese | 17.4 (15.7) | 15.4 (11.8) | 9.6 (5.2) | .014 |
| MOS-cog | 81.4 (16.4) | 74.0 (15.4) | 83.4 (11.9) | .022 |
Values indicate mean ± SD unless indicated otherwise. Pre-ChT+, patients with BC before chemotherapy; pre-ChT−, patients with BC not scheduled to undergo chemotherapy; NCs, no-cancer controls; NART, Dutch version of the National Adult Reading Test: alow = primary school, middle = secondary school, high = university and graduate school; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer health-related Quality-of-life Questionnaire: scores range from 0 to 100, higher score indicates bbetter functioning, cbetter quality of life, or dmore symptoms; HSCL-25, Hopkins Symptom Checklist-25: scores range from 0 to 100, higher score indicates higher levels of anxiety and depression; PSS, Perceived Stress Scale: scores range from 10 to 50, higher scores indicate higher levels of perceived stress; POMS, Profile of Mood States, ehigher scores indicate more problems, flower scores indicate more problems. MOS-cog, Cognitive Functioning Scale of the Medical Outcomes Study, lower scores indicate more problems.
Indicates a significant difference with NC at p < .01
3.2. Patient-reported outcomes
One-way ANOVA showed that the three groups differed on physical, F(2,100) = 7.0, p = .001, role, F(2,100) = 12.3, p < .001, and social functioning, F(2,100) = 10.1, p < .001, global quality of life, F(2,100) = 8.6, p < .001, and pain, F(2,100) = 8.2, p < .001 (as measured with the EORTC QLQ-C30). Post hoc analyses demonstrated significantly lower physical, role, and social functioning, lower global QOL, and more pain in pre-ChT+ as well as pre-ChT− compared to NC. A significant difference in fatigue scores (EORTC QLQ-C30) was found between the groups, F(2,100) = 6.2, p = .003, post hoc testing showed more fatigue in pre-ChT− compared to NC, p = .001. A similar pattern was seen in fatigue scores on the day of the assessment, measured with the POMS, but this did not reach significance. Pre-ChT+ patients reported significantly more stress than the control group, F(2,100) = 5.5, p = .006. A trend was seen for patients reporting more anxiety and depression (measured with the HSCL) than controls. Both patient groups were significantly less active than the control group, as measured with the vigor subscale of the POMS, F(2,100) = 7.2, p = .001. The patient groups indicated more mood disturbances on the POMS total scores, which did not reach significance. None of the other PROs showed significant differences between groups.
3.3. Neuropsychological assessment
Log2 transformed MHD was significantly different between the groups, F(2,100) = 4.0, p = .021, indicating worse cognitive performance in pre-ChT+ and pre-ChT− compared to controls (see Table 2). However, when fatigue, perceived stress, or anxiety and depression were included in the model, this difference was no longer significant. Domain scores and the proportion of cognitively impaired subjects were not significantly different between any of the groups (Table 2).
Table 2.
Overall neuropsychological test performance, standardized cognitive domain scores and raw test scores.
| Pre-ChT+ | Pre-ChT− | NC | p | |
|---|---|---|---|---|
| MHD | 3.92 (1.43) | 3.94 (1.51) | 3.09 (1.40) | .021 |
| % impaired | 3 (9.4%) | 2 (6.1%) | 0 (0%) | .432 |
| Executive function | −.30 (.95) | −.12 (.84) | 0 (.60) | .485 |
| COWAT | 40.00 (10.92) | 44.70 (11.73) | 43.37 (10.29) | |
| BADS zoo test | 2.44 (1.29) | 2.64 (1.17) | 2.45 (1.16) | |
| TMT Ba | 69.13 (23.85) | 70.39 (30.71) | 60.18 (15.59) | |
| Attention | −.29 (.76) | −.10 (.75) | 0 (.64) | .184 |
| Flanker congruent trialsa | 569 (48) | 567 (52) | 558 (42) | |
| Flanker stimulus incongruenta | 578 (45) | 575 (49) | 567 (44) | |
| Flanker response incongruenta | 595 (46) | 594 (53) | 586 (46) | |
| VRT dominant handa | 304 (38) | 295 (32) | 292 (39) | |
| VRT non-dominant handa | 333 (62) | 310 (45) | 309 (43) | |
| Digit span | 13.19 (3.11) | 14.00 (3.81) | 13.74 (3.24) | |
| Visual memory | −.28 (.68) | −.33 (.82) | 0 (.89) | .411 |
| WMS-R immediate recall | 34.13 (2.32) | 34.00 (3.62) | 35.61 (3.70) | |
| WMS-R delayed recall | 31.38 (4.70) | 30.97 (4.34) | 32.13 (4.98) | |
| Verbal memory | .16 (.85) | .02 (.91) | 0 (.86) | .420 |
| HVLT immediate recall | 28.31 (4.88) | 28.15 (4.41) | 27.45 (3.67) | |
| HVLT delayed recall | 10.06 (1.81) | 10.00 (1.89) | 10.00 (1.77) | |
| HVLT delayed recognition | 11.81 (0.47) | 11.55 (0.75) | 11.66 (0.78) | |
| Processing speed | −.34 (1.00) | −.24 (.91) | 0 (.86) | .535 |
| TMT Aa | 33.84 (11.16) | 31.64 (9.41) | 31.32 (9.18) | |
| Digit symbol substitution | 71.75 (13.62) | 71.01 (13.76) | 76.95 (13.08) | |
| Motor speed | −.01 (1.03) | −.51 (.74) | 0 (.93) | .077 |
| Tapping dominant hand | 63.5 (8.2) | 59.7 (6.6) | 63.4 (7.3) | |
| Tapping non-dominant hand | 57.6 (7.3) | 54.2 (5.2) | 57.9 (7.3) |
Values indicate mean ± SD unless indicated otherwise. All analyses were adjusted for age and IQ. Pre-ChT+, patients with BC before chemotherapy; pre-ChT−, patients with BC not scheduled to undergo chemotherapy; NCs, no-cancer controls; MHD, Mahalanobis Distance, higher score indicates worse overall cognitive performance; domain scores are expressed as z-scores, neuropsychological test scores are raw scores.
Higher scores indicate worse performance.
3.4. Task-related fMRI
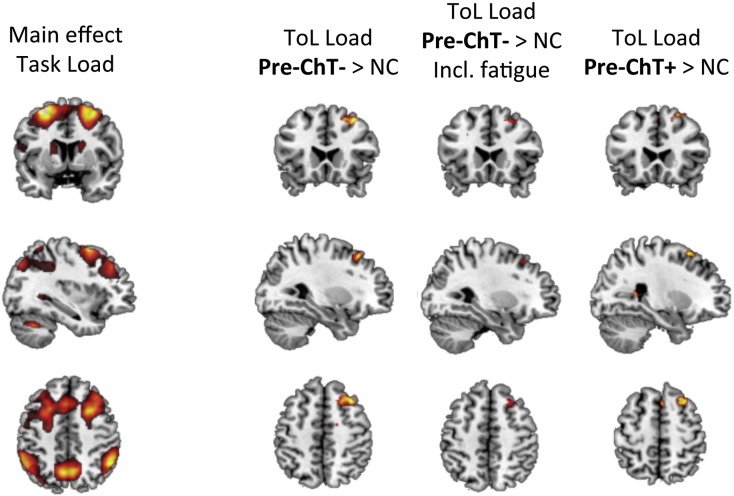
Performance and mean reaction time on the ToL were not significantly different between the three groups (see Supplementary Table 3). For both active versus baseline contrast and task load, all groups showed robust activation of the dorsolateral prefrontal cortex (DLPFC), premotor cortex, precuneus, posterior parietal cortex (PPC), striatum, and cerebellum (see Supplementary Fig. 1 and Supplementary Table 4). Whole-brain as well as ROI analyses showed no significant group differences for the active versus baseline contrast. In the pre-ChT− versus the NC group we observed significant hyperactivation of the dorsomedial prefrontal cortex extending into the DLPFC with increasing task difficulty (see Fig. 2 and Table 3). The pre-ChT+ group demonstrated subthreshold hyperactivation at the same location when compared to NC (see Fig. 2). These differences were no longer significant when fatigue scores were included in the model, while other PROs did not elicit the same effect. ROI analysis did not show any differences between the groups.
Fig. 2.
Tower of London — task load contrast. Main task effect and group comparisons with and without fatigue as a covariate (differences were considered statistically significant at cluster-corrected pfwe < .05; shown at p < .001, except for non-significant difference pre-ChT+ > NC, shown at p < .05; brighter colors indicate higher T-values); pre-ChT+, patients with BC before chemotherapy; pre-ChT−, patients with BC not scheduled to undergo chemotherapy; NCs, no-cancer controls.
Table 3.
fMRI coordinates of significantly different cluster and clusters with significant correlations.
| ToL task load | Region | R/L | MNI coordinates |
Cluster (k) | t value | z value | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Pre-ChT− > NC | Dorsomedial PFC | R | 32 | 20 | 48 | 274 | 4.18 | 3.99 |
| R | 22 | 20 | 52 | 274 | 3.98 | 3.81 | ||
| R | 22 | 28 | 50 | 274 | 3.69 | 3.55 | ||
| Positive correlation fatigue | Dorsomedial PFC | R | 36 | 16 | 52 | 41 | 3.76 | 3.62 |
| R | 32 | 26 | 50 | 41 | 3.49 | 3.37 | ||
BOLD activations (MNI coordinates) for the Tower of London (ToL) task load contrast group differences between pre-ChT−, patients with BC not scheduled to undergo chemotherapy and NCs, no-cancer controls. R, right; L, left; PFC, prefrontal cortex. Group differences are reported at cluster-corrected pfwe < 0.05, correlations are reported at p < 0.001.
Reaction time during memory encoding and retrieval was not significantly different between groups, as was retrieval performance (see Supplementary Table 3). The ventral stream (occipital areas and fusiform gyrus) extending into the parahippocampal gyrus and the hippocampus proper showed significant activation across groups during encoding (see Supplementary Fig. 1 and Supplementary Table 4). During retrieval, the ventral and dorsal stream, and parahippocampal gyrus were significantly activated. Whole brain and ROI analyses revealed no significant group differences during encoding and retrieval.
3.5. Structural MRI
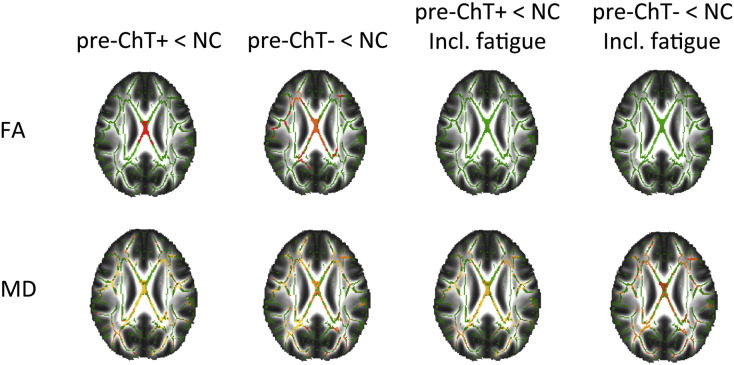
Whole brain analyses of regional GM and white matter volume showed no significant differences. GM volume of ROIs in the DLPFC and superior parietal cortex was not significantly different between groups. Voxel-based analyses showed widespread lower FA and higher MD in both patient groups compared to NCs, indicating lower white matter integrity (see Fig. 3 and Supplementary Table 5). The differences in FA were no longer significant when fatigue, but not other PROs, was added to the model.
Fig. 3.
Group differences in skeletonized FA and MD, with and without fatigue as a covariate (shown at ptfce < .05; green, white matter skeleton; red indicates higher statistical significance, yellow indicates lower statistical significance).
3.6. Other variables
Cortisol concentrations were not significantly different between groups for the previous 2 months, F(2,92) = 2.2, p = .118, and for the previous two to four months, F(2,88) = 1.7, p = .190 (see Supplementary Table 6). 1H-MRS data (see Supplementary Table 7) and Fazekas ratings for white matter lesions, χ2(4) = 5.36, p = .252, (see Supplementary Table 8) did not show significant differences between groups.
3.7. Correlations
Performance on the verbal memory domain was significantly correlated with HSCL depression scores, r = –.269, p = .006. Processing speed was significantly correlated with physical functioning, r = .290, p = .003, emotional functioning, r = .334, p = .001, global QOL, r = .339, p = .001, and fatigue, r = –.292, p = .003, subscales of EORTC QLQ-C30, HSCL anxiety, r = –.293, p = .003, PSS, r = –.334, p = .001, mood, r = –.418, p < .001, and cognitive complaints, r = .260, p = .008. MHD was significantly correlated with emotional functioning, r = –.295, p = .003, social functioning, r = –.293, p = .003, global QOL, r = –.306, p = .002, and fatigue, r = .267, p = .006, subscales of EORTC QLQ-C30, mood, r = .261, p = .008, and cognitive complaints, r = –.277, p = .005. No significant correlations were found between cortisol levels and self-reported outcomes or test performance. A significant correlation between fatigue and ToL task load BOLD activation across all groups was found in the dorsomedial prefrontal cortex (see Fig. 4 and Table 3). None of the other factors was significantly associated with BOLD signal in any of the tasks. Voxel-based analyses did not show significant correlations between FA and MD and PROs or MHD.
Fig. 4.
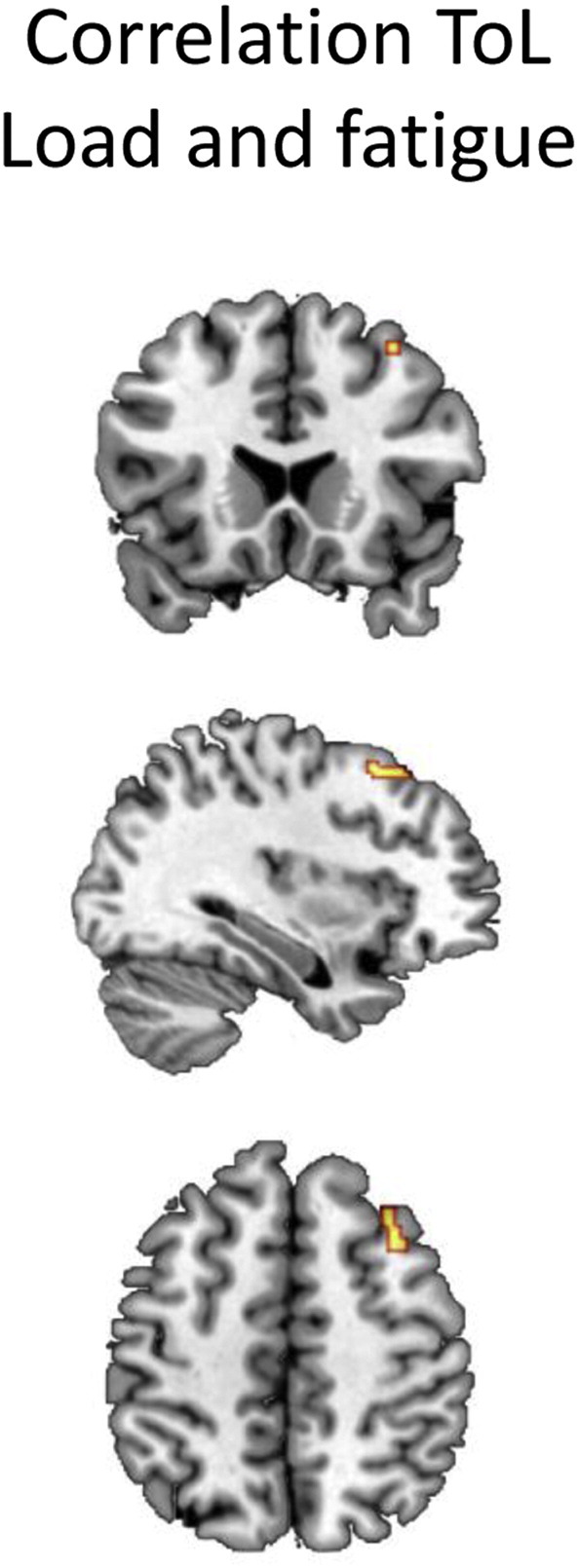
Significant correlation BOLD signal ToL task load contrast and fatigue over all groups (shown at p < .001; brighter colors indicate higher T-values).
4. Discussion
To the best of our knowledge, this is the first study combining different MRI modalities, neuropsychological assessment, and PROs to evaluate pretreatment cognitive function and potential mediating factors in patients with various disease stages. Our findings show worse cognitive performance, prefrontal hyperactivation, and lower white matter integrity in breast cancer patients compared to no-cancer controls, and revealed fatigue as an important factor contributing to these results. No significant differences in regional brain volume or brain metabolites were found. Although sample size of the current study was relatively large, insufficient power might still have played a role considering the arguably subtle effects of various aspects of cancer and treatment on MRI measures.
In agreement with some earlier reports, lower cognitive performance was observed in patients with BC compared to no-cancer controls, but only on a summary measure of cognitive performance and not when group means or percentages of impaired participants were compared. This finding suggests that small deviations across several tests account for the currently observed lower cognitive performance.
Our fMRI findings of prefrontal hyperactivation in patients with BC versus NC during a task of executive function support earlier results and might point to a specific vulnerability of these areas to the effects of cancer and its treatment. Brain hyperactivation has been reported to be due to compensation for white matter damage (Daselaar et al., 2013). Indeed, the patients with BC had widespread lower brain white matter integrity compared to healthy controls. The finding that patients with cancer also differ in their microstructural integrity compared to controls before the start of therapy is new. The only other DTI study observed no differences in white matter integrity between patients with BC and NCs when controlling for depression score, but whether the groups differed when these depression scores were not included was not reported (Deprez et al., 2012).
Interestingly, pre-ChT− patients were most deviant on PROs, brain activation and white matter integrity. As their disease is on average less advanced, these findings make it unlikely that cancer staging was driving the differences between the two patient groups. However, two previous studies have found an association between disease progression and cognitive function (Ahles et al., 2008; Mandelblatt et al., 2014). Combining the results of these studies, the role of cancer staging in cognitive dysfunction remains uncertain.
A second possible explanation for our finding of pretreatment differences might be the side effects of breast surgery or anesthesia. Since both our patient groups had undergone surgery, we could not study this relation directly but looked at time since surgery as a surrogate. Unlike some previous studies (López Zunini et al., 2013; Scherling et al., 2012), we did not find a relation between time since surgery and any of the outcome measures. A general limitation of our study is the lack of a pre-surgery assessment. Breast cancer surgery has previously been found to be associated with lower cognitive performance (Hedayati et al., 2011). These findings indicate that future studies should preferably include a pre-surgery assessment to rule out effects of anesthesia or other surgical factors.
A third factor that could be put forward to explain pretreatment differences, is the higher levels of comorbidity in cancer patients than NC. One report showed that higher rates of comorbidities were associated with cognitive impairment (Mandelblatt et al., 2014). However, patients in that study were on average 15 years older than those in the current sample. Also, the levels of comorbidities were considerably higher than in our sample, as indicated by low levels of medication use in the current sample.
Finally, our findings might also be influenced by differences in reasons for participation between the patient groups. About half of the eligible participants were willing to participate in our study, without differences between the patient groups, and no differences were observed with respect to relevant demographics. Still, patients facing chemotherapy are clearly in a different stage of their overall therapy plan than patients for whom chemotherapy is not required. These differences may influence both symptom perception and expression. Patients who have an intense cancer treatment ahead might still operate in ‘survival mode’. Patients who do not have the prospect of being exposed to chemotherapy might, in contrast, already have moved to another mental state where they allow negative emotions associated with the disease to surface. Also, it could be that patients not receiving chemotherapy feel that the study is less relevant to them. This could lead to a potential bias in the motivation to participate, with more patients already experiencing cognitive problems in the pre-CHT− group, without influencing participation rates per se.
As mentioned before, symptoms of fatigue appear to be related to the observed impairments in patients with BC compared to NCs. Fatigue levels were markedly higher in cancer patients than in controls. Group differences in cognitive function and various MRI measures did not survive our stringent statistical thresholding when the analyses were adjusted for fatigue levels. Furthermore, fatigue levels were modestly but statistically significantly associated with cognitive function and fMRI.
Higher levels of fatigue in patients with cancer compared to the general population are a common finding (Hofman et al., 2007). Pro-inflammatory cytokines have been associated with cancer-related fatigue (Bower et al., 2011). Moreover, pro-inflammatory cytokines are frequently proposed as a possible mechanism underlying cancer-related cognitive dysfunction but clinical studies have not yet shown a clear picture regarding cytokines, cognition and/or fatigue and the way in which these factors may influence one another (Cheung et al., 2013; Vardy et al., 2014). It might be that elevated levels of pro-inflammatory cytokines cause fatigue as well as cognitive problems without a direct relation between the two factors. Another explanation could be that fatigue leads to changes in cerebral blood flow which in turn leads to changes in brain function and structure and consequently has an effect on cognitive function (Ocon, 2013).
In order to obtain a better insight into the wide era of factors that seem to be relevant for pre-treatment cognitive function, assessment of key aspects of health-related quality of life, i.e. fatigue and distress, has to be taken into account in neuroimaging and neuropsychological studies in patients with cancer.
A major strength of this study is the comprehensive coverage of various outcome measures in one report including different MRI modalities, neuropsychological assessment, and patient-reported outcomes. By combining the data and studying different important factors we present a complete assessment of cognitive dysfunction associated with cancer and treatment. Further, the current study encompasses data from a relatively large sample of patients with BC. This large sample size together with consequent correction for multiple comparisons strengthens the results presented here.
To conclude, our findings show worse cognitive performance, prefrontal hyperactivation, and lower white matter integrity in breast cancer patients compared to no-cancer controls. These results were related to fatigue. The role of fatigue in our data suggests cancer-related psychological or biological processes to negatively influence cognitive functioning and associated aspects of brain structure and function. Because even mild cognitive problems can have functional consequences (Marcotte et al., 2010), these findings should be further investigated in specific hypothesis-driven studies. Our results show the importance of the use of PROs to understand cognitive problems BC patients may already experience before treatment. By further studying these problems, it might be possible to identify patients at risk of developing cognitive dysfunction and determine underlying processes that could be used as a target for interventions.
Acknowledgments
We thank Hester Oldenburg, MD, PhD, for her contributions to the study design and set-up of the organization of the patient recruitment. We are indebted to all patients, controls as well as physicians and nurses of the participating hospitals. We thank the research assistants for helping with data collection. This study was funded by the Dutch Cancer Society (KWF 2009-4284).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.02.005.
Appendix A. Supplementary data
Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment - the role of fatigue.
References
- Ahles T.A., Saykin A.J., McDonald B.C., Furstenberg C.T., Cole B.F., Hanscom B.S., Mulrooney T.J., Schwartz G.N., Kaufman P.A. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res. Treat. 2008;110(1):143–152. doi: 10.1007/s10549-007-9686-5. 17674194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.G., Askren M.K., Jung M., Therrien B., Peltier S., Noll D.C., Zhang M., Ossher L., Hayes D.F., Reuter-Lorenz P.A., Cimprich B. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2014;33(3):222–231. doi: 10.1037/a0033425. 23914817 [DOI] [PubMed] [Google Scholar]
- Bower J.E., Ganz P.A., Irwin M.R., Kwan L., Breen E.C., Cole S.W. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y.T., Lim S.R., Ho H.K., Chan A. Cytokines as mediators of chemotherapy-associated cognitive changes: current evidence, limitations and directions for future research. PLOS One. 2013;8(12):e81234. doi: 10.1371/journal.pone.0081234. 24339912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich B., Reuter-Lorenz P., Nelson J., Clark P.M., Therrien B., Normolle D., Berman M.G., Hayes D.F., Noll D.C., Peltier S., Welsh R.C. Prechemotherapy alterations in brain function in women with breast cancer. J. Clin. Exp. Neuropsychol. 2010;32(3):324–331. doi: 10.1080/13803390903032537. 19642048 [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H., Longman R.S., Batty A.M. Some supplementary methods for the analysis of WAIS-IV index scores in neuropsychological assessment. J. Neuropsychol. 2012;6(2):192–211. doi: 10.1111/j.1748-6653.2011.02022.x. 22257377 [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H., Morrice N., Duff K. Some supplementary methods for the analysis of the RBANS. Psychol. Assess. 2012;24:365–374. doi: 10.1037/a0025652. 21942233 [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H., Sutherland D., Borland N. Some supplementary methods for the analysis of the Delis–Kaplan Executive Function System. Psychol. Assess. 2011;23:888–898. doi: 10.1037/a0023712. 21574720 [DOI] [PubMed] [Google Scholar]
- Daselaar S.M., Iyengar V., Davis S.W., Eklund K., Hayes S.M., Cabeza R.E. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht289. 24152545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo L.T. On the meaning and use of kurtosis. Psychol. Methods. 1997;2(3):292–307. [Google Scholar]
- Deprez S., Amant F., Smeets A., Peeters R., Leemans A., Van Hecke W., Verhoeven J.S., Christiaens M.R., Vandenberghe J., Vandenbulcke M., Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J. Clin. Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. 22184379 [DOI] [PubMed] [Google Scholar]
- De Ruiter M.B., Reneman L., Boogerd W., Veltman D.J., Caan M., Douaud G., Lavini C., Linn S.C., Boven E., van Dam F.S.A.M., Schagen S.B. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Human Brain Mapping. 2011;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am. J. Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Hedayati E., Schedin A., Nyman H., Alinaghizadeh H., Albertsson M. The effects of breast cancer diagnosis and surgery on cognitive functions. Acta Oncol. Stockh., Sweden. 2011;50:1027–1036. doi: 10.3109/0284186X.2011.572911. 21554027 [DOI] [PubMed] [Google Scholar]
- Hofman M., Ryan J.L., Figueroa-Moseley C.D., Jean-Pierre P., Morrow G.R. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl. 1):4–10. doi: 10.1634/theoncologist.12-S1-4. 17573451 [DOI] [PubMed] [Google Scholar]
- Jager G., Van Hell H.H., De Win M.M., Kahn R.S., Van Den Brink W., Van Ree J.M., Ramsey N.F. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2007;17(4):289–297. doi: 10.1016/j.euroneuro.2006.10.003. 17137758 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Tietze A., Skoluda N., Dettenborn L. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–37. doi: 10.1016/j.psyneuen.2008.08.024. 18947933 [DOI] [PubMed] [Google Scholar]
- Koppelmans V., Breteler M.M., Boogerd W., Seynaeve C., Gundy C., Schagen S.B. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 2012;30(10):1080–1086. doi: 10.1200/JCO.2011.37.0189. 22370315 [DOI] [PubMed] [Google Scholar]
- Lange M., Giffard B., Noal S., Rigal O., Kurtz J.-E., Heutte N., Lévy C., Allouache D., Rieux C., Le Fel J., Daireaux A., Clarisse B., Veyret C., Barthélémy P., Longato N., Eustache F., Joly F. Baseline cognitive functions among elderly patients with localised breast cancer. Eur. J. Cancer. 2014;50:2181–2189. doi: 10.1016/j.ejca.2014.05.026. 24958735 [DOI] [PubMed] [Google Scholar]
- López Zunini R.A., Scherling C., Wallis N., Collins B., Mackenzie J., Bielajew C., Smith A.M. Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: a prospective fMRI study. Brain Imaging Behav. 2013;7(4):460–477. doi: 10.1007/s11682-012-9213-0. 23242968 [DOI] [PubMed] [Google Scholar]
- Mandelblatt J.S., Stern R.A., Luta G., McGuckin M., Clapp J.D., Hurria A., Jacobsen P.B., Faul L.A., Isaacs C., Denduluri N., Gavett B., Traina T.A., Johnson P., Silliman R.A., Turner R.S., Howard D., Van Meter J.W., Saykin A., Ahles T. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J. Clin. Oncol. 2014;32(18):1909–1918. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte T.D., Scott J.C., Kamat J., Heaton R.K. Neuropsychology and the prediction of everyday functioning. In: Marcotte T.D., Grant I., editors. Neuropsychology of Everyday Functioning. Guilford Press; New York: 2010. pp. 5–38. [Google Scholar]
- Mazaika P., Hoeft F., Glover G., Reiss A. The Organization of Human Brain Mapping, 15th Annual Meeting; 2009 Jun 18–23; 2009; San Francisco, CA. The Organization of Human Brain Mapping; Minneapolis, MN.: 2009. Methods and software for fMRI analysis for clinical subjects. [Google Scholar]
- McDonald B.C., Conroy S.K., Ahles T.A., West J.D., Saykin A.J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J. Clin. Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. 22665542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B.C., Saykin A.J. Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav. 2013;7(4):374–387. doi: 10.1007/s11682-013-9256-x. 23996156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. 11747097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocon A.J. Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front. Physiol. 2013;4:63. doi: 10.3389/fphys.2013.00063. 23576989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomykala K.L., de Ruiter M.B., Deprez S., McDonald B.C., Silverman D.H. Integrating imaging findings in evaluating the post-chemotherapy brain. Brain Imaging Behav. 2013;7(4):436–452. doi: 10.1007/s11682-013-9239-y. 23828813 [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. 8139448 [DOI] [PubMed] [Google Scholar]
- Schagen S.B., Muller M.J., Boogerd W., Mellenbergh G.J., van Dam F.S. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J. Natl. Cancer Inst. 2006;98(23):1742–1745. doi: 10.1093/jnci/djj470. 17148777 [DOI] [PubMed] [Google Scholar]
- Scherling C., Collins B., Mackenzie J., Bielajew C., Smith A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Front. Hum. Neurosci. 2011;5(November):122. doi: 10.3389/fnhum.2011.00122. 22053153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling C., Collins B., Mackenzie J., Bielajew C., Smith A. Prechemotherapy differences in response inhibition in breast cancer patients compared to controls: a functional magnetic resonance imaging study. J. Clin. Exp. Neuropsychol. 2012;37–41(5):543–560. doi: 10.1080/13803395.2012.666227. 22380580 [DOI] [PubMed] [Google Scholar]
- Schmand B., Lindeboom J., Harskamp F., van . De Nederlandse Leestest voor Volwassenen. Swets & Zeitlinger; Lisse: 1992. [Google Scholar]
- Seigers R., Schagen S.B., Van Tellingen O., Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013;7(4):453–459. doi: 10.1007/s11682-013-9250-3. 23949877 [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. 16624579 [DOI] [PubMed] [Google Scholar]
- Van den Heuvel O.A., Groenewegen H.J., Barkhof F., Lazeron R.H., van Dyck R., Veltman D.J. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. NeuroImage. 2003;18(2):367–374. doi: 10.1016/s1053-8119(02)00010-1. 12595190 [DOI] [PubMed] [Google Scholar]
- Vardy J., Dhillon H.M., Pond G.R., Rourke S.B., Xu W., Dodd A., Renton C., Park A., Bekele T., Ringash J., Zhang H., Burkes R., Clarke S.J., Tannock I.F. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann. Oncol. 2014;25(12):2404–2412. doi: 10.1093/annonc/mdu448. 25214544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel J.S., Lenzi R., Theriault R., Buzdar A.U., Cruickshank S., Meyers C.A. “Chemobrain” in breast carcinoma?: a prologue. Cancer. 2004;101(3):466–475. doi: 10.1002/cncr.20393. 15274059 [DOI] [PubMed] [Google Scholar]
- Wefel J.S., Schagen S.B. Chemotherapy-related cognitive dysfunction. Curr. Neurol. Neurosci. Rep. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. 22453825 [DOI] [PubMed] [Google Scholar]
- Wefel J.S., Vardy J., Ahles T., Schagen S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. 21354373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment - the role of fatigue.