Abstract
Background
Disrupted cortical connectivity is thought to underlie the complex cognitive and behavior profile observed in individuals with autism spectrum disorder (ASD). Previous neuroimaging research has identified patterns of both functional hypo- and hyper-connectivity in individuals with ASD. A recent theory attempting to reconcile conflicting results in the literature proposes that hyper-connectivity of brain networks may be more characteristic of young children with ASD, while hypo-connectivity may be more prevalent in adolescents and adults with the disorder when compared to typical development (TD) (Uddin etal., 2013). Previous work has examined only young children, mixed groups of children and adolescents, or adult cohorts in separate studies, leaving open the question of developmental influences on functional brain connectivity in ASD.
Methods
The current study tests this developmental hypothesis by examining within- and between-network resting state functional connectivity in a large sample of 26 children, 28 adolescents, and 18 adults with ASD and age- and IQ-matchedTD individuals for the first time using an entirely data-driven approach. Independent component analyses (ICA) and dual regression was applied to data from three age cohorts to examine the effects of participant age on patterns of within-networkwhole-brain functional connectivity in individuals with ASD compared with TD individuals. Between-network connectivity differences were examined for each age cohort by comparing correlations between ICA components across groups.
Results
We find that in the youngest cohort (age 11 and under), children with ASD exhibit hyper-connectivity within large-scale brain networks as well as decreased between-network connectivity compared with age-matchedTD children. In contrast, adolescents with ASD (age 11–18) do not differ from TD adolescents in within-network connectivity, yet show decreased between-network connectivity compared with TD adolescents. Adults with ASD show no within- or between-network differences in functional network connectivity compared with neurotypical age-matched individuals.
Conclusions
Characterizing within- and between-network functional connectivity in age-stratified cohorts of individuals with ASD and TD individuals demonstrates that functional connectivity atypicalities in the disorder are not uniform across the lifespan. These results demonstrate how explicitly characterizing participant age and adopting a developmental perspective can lead to a more nuanced understanding of atypicalities of functional brain connectivity in autism.
Keywords: Autism spectrum disorder, Independent component analysis, Resting state fMRI, Functional connectivity, Salience network
1. Introduction
Early neuroimaging research comparing functional brain connectivity in individuals with autism spectrum disorder (ASD) and typically developing (TD) individuals led to the hypo-connectivity hypothesis proposing that fronto-posterior connectivity deficits are partly responsible for cognitive deficits in ASD(Belmonte etal., 2004; Just etal., 2004). Previous task-based functional magnetic resonance imaging (fMRI) studies primarily using region-of-interest (ROI) analyses found support for the hypo-connectivity theory (Just etal., 2012; Minshew and Williams, 2007). These experiments found hypo-connectivity within the temporal–parietal junction in a theory of mind task (Kana etal., 2009), the limbic system in a face perception task (Kleinhans etal., 2008), between the frontal and parietal regions in a working memory task (Koshino etal., 2005), and between the frontal, parietal and occipital regions in a cognitive control task (Solomon etal., 2009). However, later task-based fMRI studies found hyper-connectivity in connections involving the posterior superior temporal sulcus in visual search tasks (Shih etal., 2011), the medial temporal lobe in face perception tasks (Welchew etal., 2005), within the left hemisphere in a source recognition task (Noonan etal., 2009), between the inferior frontal gyrus, and between the inferior parietal lobule and the superior temporal sulcus in semantic judgments and letter decision tasks (Shih etal., 2010). These results suggested that ASD could also be characterized by hyper-connectivity, providing evidence against a pure hypo-connectivity account (Kana etal., 2011).
Recently, resting-state fMRI (rsfMRI), has emerged as a powerful tool for examining intrinsic functional brain connectivity in clinical pediatric populations. Resting-state fMRI offers two advantages over task-based fMRI. First, it allows easier data collection from special populations such as young children with ASD, who have difficulties with long task-based fMRI experiments (Yerys etal., 2009). Second, it identifies underlying intrinsic functional networks that are not confounded by differences in task performance or strategy differences commonly found between individuals with ASD and neurotypical controls. Resting-state fMRI typically involves instructing participants to rest for 5–8 min while blood oxygen level dependent (BOLD) signals are acquired with MRI. By focusing on temporal correlations of the BOLD signal between functionally coupled brain regions, it is possible to identify intrinsically connected functional networks that are not confounded by cognitive tasks (Biswal etal., 1995; Bressler and Menon, 2010).
Resting state fMRI studies in neurotypical individuals have identified several major intrinsically connected networks related to visual, motor, auditory, memory and executive processes (Damoiseaux etal., 2006). Research examining individuals with ASD has recently focused on hypo- and hyper-connectivity differences observed in two major large-scale brain networks. The default mode network (DMN) consists of key nodes in the posterior cingulate cortex (PCC), the medial temporal lobes (MTL) and the medial prefrontal cortex (MPFC) and is active in self-related tasks such as autobiographical memories or social tasks such as theory of mind (Fox and Raichle, 2007; Spreng etal., 2009). The salience network (SN) involves the anterior insula (AI) and the anterior cingulate cortex (ACC) and is thought to regulate switching of endogenous and exogenous attention to relevant stimuli that helps in guiding behavior (Uddin, 2015). Studies using rsfMRI have found both hypo- and hyper-connectivity of these and other functional networks when comparing individuals with ASD with neurotypical (NT) individuals. Hypo-connectivity in ASD compared with TD individuals has been identified in connections between the insula and amygdala (Ebisch etal., 2011) and most consistently between connections of nodes within the DMN(Assaf etal., 2010; Ebisch etal., 2011; Kennedy and Courchesne, 2008a; Monk etal., 2009; Weng etal., 2010). Hyper-connectivity in ASD compared with TD individuals has been identified in motor and visual networks, as well as the DMN and SN(Uddin etal., 2013; Washington etal., 2014), and between striatal areas and the insula (Di Martino etal., 2011). Finally, one study has even demonstrated extremely small to no differences in functional connectivity in ASD compared with neurotypical adults (Tyszka etal., 2014), producing additional conflicting evidence.
Although methodological differences surely contribute to the mixed findings in the literature (Muller etal., 2011), a more recent idea attempting to reconcile these discrepant findings proposes that hyper-connectivity may be more characteristic of young children with ASD, while hypo-connectivity may begin to emerge in adolescence and persist into adulthood. In a review of the rsfMRI functional connectivity literature in ASD it is suggested that studies demonstrating hyper-connectivity have typically examined children less than 12 years of age, while studies demonstrating hypo-connectivity have typically examined adolescents and adults over the age of 12 (Uddin etal., 2013). The authors proposed that a developmental account hypothesizing early childhood hyper-connectivity and later adolescent and adult hypo-connectivity in ASD compared with TD could partially account for the mixed functional connectivity findings in the literature.
Previous functional connectivity studies have focused on a single age group (e.g.,childhood, adolescence, or adulthood), mixed age groups (e.g.,combining childhood and adolescence, or adolescence and adulthood), or used a linear regression correlational approach across a single group of subjects containing various age ranges (Assaf etal., 2010; Kennedy and Courchesne, 2008b; Monk etal., 2009). Several studies exploring whole-brain connectivity have included subjects with a wide range of ages, allowing for the possibility that a certain age group was driving the functional connectivity findings in the results (Assaf etal., 2010; Gotts etal., 2012). Therefore, an important gap in the literature concerns the principled examination of functional connectivity alterations in ASD across different developmental stages. In the current work we stratify individuals into age cohorts to directly test if some of the mixed findings throughout the literature can be accounted for by explicitly sorting groups of ASD and TD participants according to their ages.
An additional aspect of network connectivity that has received less attention in ASD research is how correlations between networks compare to that observed in the neurotypical population. Previous research has shown that the DMN, referred to as a ‘task-negative network’ (TNN), typically exhibits negative correlations with task-positive networks (TPN) such as the dorsal attention network (DAN) (Fox etal., 2005). The TNN nomenclature refers to the fact that nodes of the DMN typically show reductions in activity when a participant is focused on a task demanding exogenous attention while TPN refers to the fact that nodes of the DAN typically show increases in activity during such a task. Thus these networks are often referred to as “anti-correlated” because when a TPN is active the TNN is not, and vice versa. The relationship between these networks relates to behavioral performance in the neurotypical population (Kelly etal., 2008), but is not well understood in ASD. Characterization of relationships between these two networks may have important implications for understanding brain dynamics in ASD.
A challenge to synthesizing the functional connectivity literature in autism is that several studies have used ROI-based analyses that are difficult to compare with each other, as they are often linked to hypotheses about specific functional circuits (Abrams etal., 2013; Kennedy and Courchesne, 2008b; Lynch etal., 2013). The current study sought to compare whole-brain functional connectivity in ASD and TD individuals using an entirely data-driven approach. We explored the nature and extent of functional differences both within- and between-networks when comparing ASD and TD individuals across three age groups— children (under 11), adolescents (11–18), and adults (over 18). In order to assess within-network group differences in functional connectivity, we used independent component analysis (ICA) (Beckmann etal., 2005) across three different age groups to examine if the developmental trajectory of hyper- to hypo-connectivity in children to adults respectively, as predicted by Uddin and colleagues (Uddin etal., 2013) would be present. To assess between-network group differences in functional connectivity, we applied a network analysis to examine how correlations between networks potentially differ across the three age groups. This approach of exploring within- and between-network functional connections was adapted to elucidate how large-scale brain networks in ASD compare to those observed in age-matchedTD individuals across development.
2. Methods
2.1. Participants
We used data from the Autism Brain Imaging Data Exchange (ABIDE), a publicly available data set (http://fcon_1000.projects.nitrc.org/indi/abide/) (Di Martino etal., 2014). Only data collected at the New York University Langone Medical Center were utilized to avoid cross-study methodological acquisition differences. To explore the effects of participant age on functional connectivity, we divided the data into three age groups of ASD and TD participants: young children under 11 years of age (n = 52), adolescents from 11–18 years of age (n = 56), and adults over 18 years of age (n = 36; Table1). Individuals with ASD had a clinical DSM-IV diagnosis of Autistic Disorder, Asperger's syndrome, Pervasive Developmental Disorder Not-Otherwise Specified (PDD-NOS) while TD participants were required to have no Axis-I disorders based on the KSADS-PL questionnaire.
Table 1.
Participant demographics.
| ASD | TD | p value | |
|---|---|---|---|
| Children (<11) | |||
| Mean age | 9.51 (1.12) | 9.10 (1.32) | .22 |
| Age range | 7.15–10.96 | 6.47–10.86 | |
| Gender | 24M/2F | 19M/7F | |
| Full IQ | 107.77 (16.16) (76–142) | 113.04 (13.67) (80–136) | .10 |
| ADI social scorea | 19.4 (5.42) (7–27) | ||
| ADI verbal scorea | 16.16 (3.92) (8–22) | ||
| ADI RRBa | 5.92 (2.27) (3–10) | ||
| ADOS communication | 3.31 (1.85) (0–7) | ||
| ADOS social | 7.58 (2.67) (4–14) | ||
| Adolescent (11–18) | |||
| Mean age | 13.71 (1.79) | 14.01 (1.74) | .53 |
| Age range | 11.01–17.88 | 11.32–16.93 | |
| Gender | 23M/5F | 23M/5F | |
| Full IQ | 103.57 (15.45) (78–132) | 105.18 (9.90) (80–121) | .65 |
| ADI social scoreb | 20.46 (5.53) (13–28) | ||
| ADI verbal scorea | 15.78 (4.06) (8–23) | ||
| ADI RRBa | 6.07 (2.66) (0–12) | ||
| ADOS communication | 3.64 (1.52) (1–6) | ||
| ADOS social | 8.64 (2.98) (2–14) | ||
| Adults (>18) | |||
| Mean age | 24.13 (3.92) | 25.41 (5.87) | .45 |
| Age range | 18.58–39.1 | 18.59–31.78 | |
| Gender | 14M/4F | 14M/4F | |
| Full IQ | 108.06 (13.86) (80–137) | 116.11 (14.20) (81–139) | .09 |
| ADI social scorec | 18 (6.14) (9–27) | ||
| ADI verbal score | 6.46 (5.95) (8–25) | ||
| ADI RRB score | 4.62 (2.36) (2–9) | ||
| ADOS communication | 3.72 (1.36) (2–6) | ||
| ADOS social | 7.44 (3.14) (2–12) | ||
Score missing for 1 participant.
Score missing for 2 participants.
Score missing for 5 participants.
Within each of the three groups, there were no significant within-group differences between age and full-scaleIQ between participants with ASD and TD participants (ps > .09). A 2 × 3 mixed-modelANOVA on IQ showed that there was a marginally significant main effect of age (p = .06) but no main effect of group (p = .1) and no age × group interaction (p = .8). Follow up t-tests showed that the children (M = 110.40) had higher IQ scores than adolescents (M = 104.38; p = .03), and adults (M = 112.08) had higher IQ scores than adolescents (p = .01), with no differences between children and adults (p = .6). As is typical in young children with ASD, these children had greater RMS relative and absolute motion than TD individuals (Supplementary Table1; relative ASD = .10, TD = .06, p = .001; absolute ASD = .39, TD = .23, p = .008). There were no motion differences between the adolescent and adult groups (ps > .15) (Supplementary Table1). There were no differences between individuals with ASD across the three age groups for all ASD diagnostic questionnaires (ADI and ADOS; one way ANOVAs, p > .2).
For the overall group ICA used to create templates for subsequent analyses, we randomly selected 18 participants from each age group (18 × 6 = 108) to derive the independent components (ICs). This was done in order to avoid non-network noise differences across the three age groups by creating a common template of ICs for use in the dual regression analysis. There were no differences in full-scaleIQ for any group of 18 participants (2 × 3 ANOVA: p = .59), nor were there any differences for all ASD diagnostic questionnaires (ADI and ADOS) across the three age cohorts (one way ANOVAs, p > .38).
2.2. Data acquisition
Resting state fMRI data were collected on a 3 T Siemens Allegra scanner using an echo-planner imaging (EPI) sequence (TR = 2000 ms; TE = 15 ms; flip angle = 90°; FOV = 240 mm; voxel size = 3 × 3 × 4 mm; number of slice = 33, 4 mm slice thickness). Each resting-state scan lasted for 6 min, consisting of 180 volumes collected while participants were asked to relax with their eyes open and fixate on a projection screen displaying a white cross hair on a black background.
Anatomical images were acquired using a magnetization prepared gradient echo sequence (TR = 2530 ms; TE = 3.25 ms; inversion time = 8.07 min; flip angle = 7°; 128 slices; 1 volume; FOV = 256 mm) (Di Martino etal., 2014).
2.3. Image preprocessing
Functional MRI data were preprocessed using FSL 5.06 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). The first three volumes of each data set were deleted. Preprocessing steps included motion correction, interleaved slice-timing correction, spatial smoothing (full width at half maximum = 5 mm), and high pass temporal filtering using a local fit of a straight line (Gaussian-weighted least-squares straight line fitting with sigma = 100 s). Images were then normalized to the Montreal Neurological Institute (MNI) 152 stereotactic space (2 mm) using the default settings in FSL's FEAT toolbox by applying a linear transformation with 12 degrees of freedom. Global signal regression was not applied (Saad etal., 2012).
2.4. Within-network connectivity: dual regression ICA
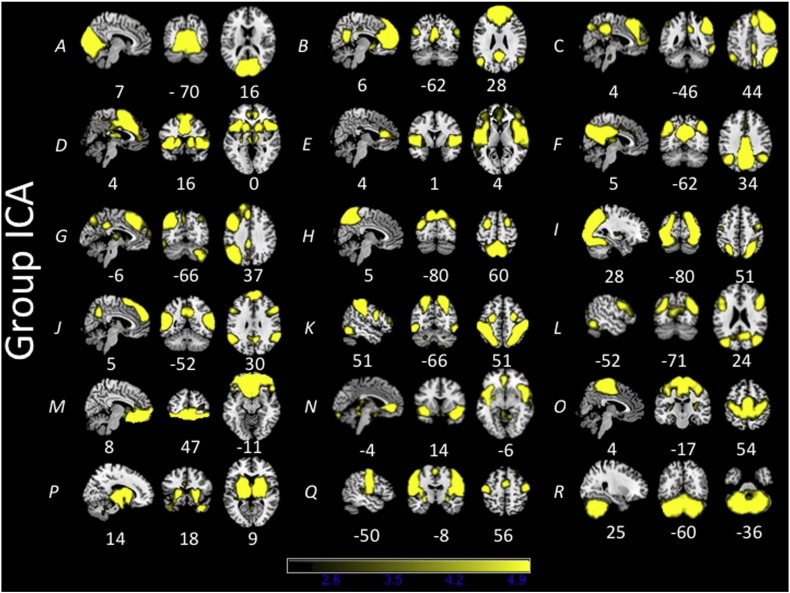
In order to examine within-network differences in functional connectivity, the dual regression approach in FSL (v 5.06) was applied to preprocessed images (Beckmann and Smith, 2004). Currently, dual regression is the preferred data-driven approach for exploring between-population differences in large-scale functional connectivity patterns (Filippini etal. 2009). The overall group preprocessed data consisting of 108 subjects were concatenated and subjected to an ICA using MELODIC(http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC) in FSL. Next, 25 ICs were created representing large-scalegroup-level functional networks. Visual inspection of these group-level ICs was used to identify those best representing previously identified functional networks (Damoiseaux etal., 2006). The remaining components were considered noise or artifacts such as movement, white matter, or ventricles and were not subjected to further analysis.
To account for head motion differences between the child ASD and TD groups, and keep analyses consistent between age groups, the time courses of all 144 subjects were subjected to a covariate regression of the Friston 24 motion parameters (6 typical motion parameters for each volume, the preceding volume, and each of the 12 derivatives) using the DPARSF-A toolbox (http://rfmri.org/DPARSF). This 24-parameter model has been shown to better reduce the influence of motion effects than other models (Satterthwaite etal., 2012; Yan etal., 2013). The covariate regression was not applied to the initial ICA because the initial ICA creates motion components that were then discarded from further analysis.
The ICs of interest were then compared to motion-regressedparticipant-specific time courses and spatial maps with a dual regression algorithm (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/DualRegression) producing group difference maps for each component within each age group. Permutation testing using the randomize feature in FSL was conducted using the default settings (5000 permutations) to create difference maps between groups for each component of interest. The resulting group difference maps were thresholded using threshold-free cluster enhancement with an alpha level of .05 (corrected). Correction for multiple component testing was not applied in this case, as in previous similar studies (Uddin etal., 2013).
2.5. Between-network connectivity: FSL Nets
To examine between-network differences in functional connectivity for each age cohort, the FSL Nets analysis package was implemented in Matlab (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). This analysis takes the participants' time courses from the dual regression analysis and subjects them to between-network comparisons that determine how independent components from the overall ICA are correlated with each other (Smith et al., 2013). Between-subject testing is then conducted across correlation values acquired for pairs of independent components.
3. Results
3.1. Group ICA
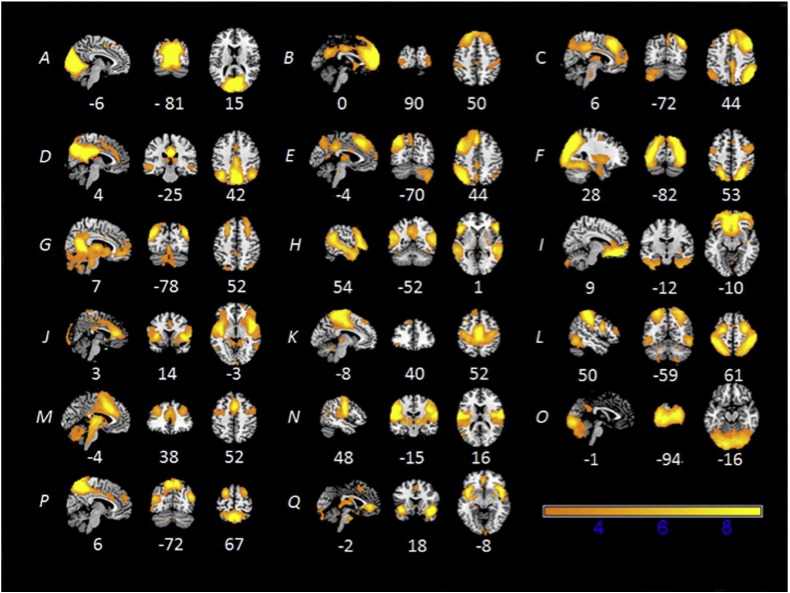
The group ICA from the subset of 108 subjects produced 25 ICs. Of these 25 ICs, 7 were determined to be noise-related artifacts representing cerebral spinal fluid, ventricles, and head motion. These ICs were discarded from further analysis, leaving 18 ICs of interest used in each dual regression analysis (Fig.1). Group ICAs were also conducted for each age cohort separately for comparison purposes. Each age cohort exhibited similar components to the overall ICA(Supplementary Figs.1–3).
Fig. 1.
Functional networks observed in an overall group of 108 subjects (18 from each age group) using ICA.
3.2. Overall between-network comparisons
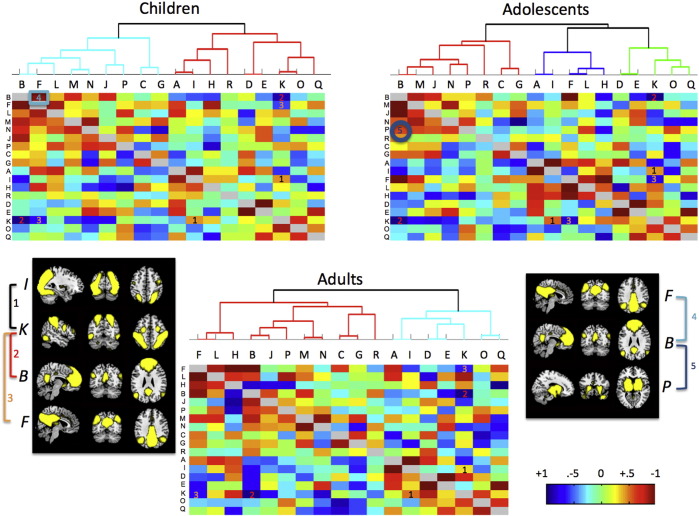
The overall FSL Nets analysis was conducted on each age group (Fig.2). Boxes below the diagonal line represent full correlation comparisons, while boxes above the diagonal line represent partial correlation values. Full correlation comparisons allow for the influence of other network values on pairs of interest, while partial correlations are a more direct measure of the relationship between pairs of networks. Positive correlations for each age group can be seen between the DAN and higher-level visual areas (labeled 1) while negative correlations between the DAN and two DMN components (labeled 2 and 3) are also present in both the full and partial correlation matrices.
Fig. 2.
FSL Nets between network correlations for each age group. Full correlations are shown below the diagonal line with partial correlations shown above the diagonal line. Letters on each axis indicate specific components from Fig.1. Number 1 within each correlation matrix represents a positive correlation between the dorsal attention network (K) and higher order visual areas (I). Numbers 2 and 3 represent negative correlations between default mode networks (B and F) and the dorsal attention network (K). Number 4 represents a significant difference in partial correlations between default mode networks (B and F) for children (TD > ASD). Number 5 represents a significant difference in full correlations between default mode (B) and subcortical/insula networks (P) for adolescents (TD > ASD). Groupings on top of each matrix represents hierarchical clustering of component timeseries.
3.3. Within-network connectivity: children with ASD vs. TD children
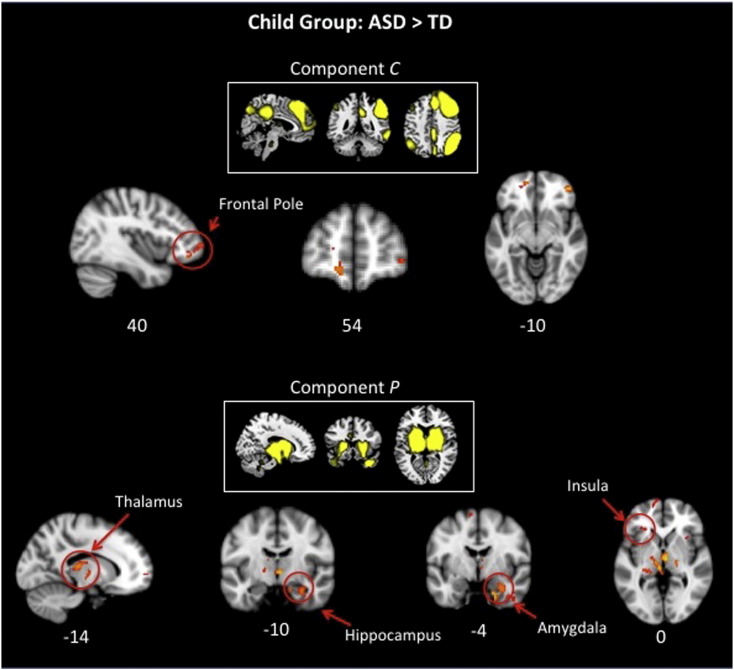
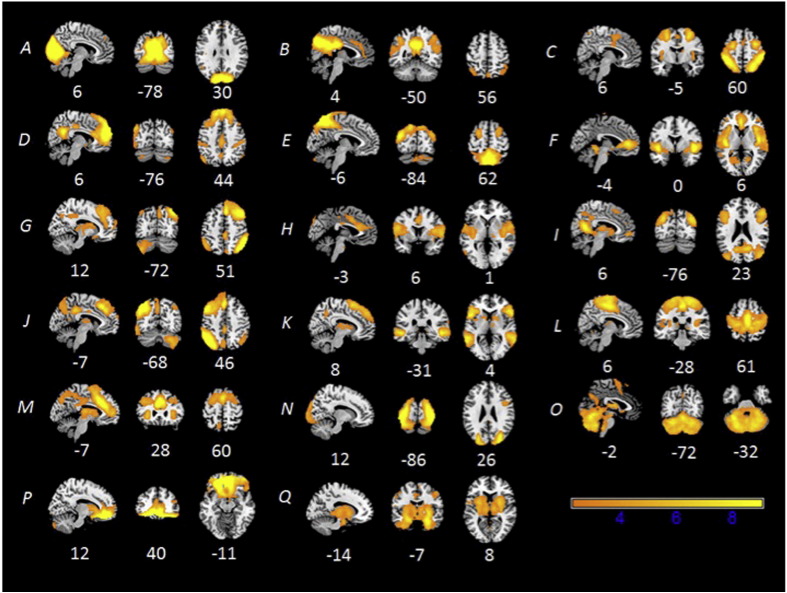
For the within-network comparison, two components showed significant hyper-connectivity in ASD compared with TD (ASD > TD, Fig.3). Component C representing a network that included nodes of both the DMN and central-executive network showed hyper-connectivity in the right frontal pole. Component P representing the insula and subcortical areas showed hyper-connectivity in bilateral areas that included the insula, thalamus, hippocampus, and amygdala. No TD > ASD functional connectivity differences were observed in any of the networks examined.
Fig. 3.
Functional networks showed greater connectivity for children with ASD compared with TD children in 2 out of 18 networks examined: default mode (top, C), and insula/subcortical (bottom, P).
3.4. Between-network connectivity: children with ASD vs. TD children
The between-network comparison showed only one significant difference for partial correlation values between components F and B representing the DMN(Fig.2, labeled 4; FWE corrected: p = .017). The difference was such that children with ASD showed a significantly smaller correlation between these two networks compared with TD children. No other differences emerged for full or partial correlation comparisons (FWE corrected: p > .07.).
3.5. Within-network connectivity: adolescents with ASD vs. TD adolescents
There were no significant within-network differences between adolescents with ASD and TD adolescents, in either direction (ASD > TD or TD > ASD).
3.6. Between-network connectivity: adolescents with ASD vs. TD adolescents
The between-network comparison showed one significant difference for full correlation values between components B and P representing the DMN and a subcortical/insula network. The difference was such that individuals with ASD had a significantly smaller correlation between the two components (Fig.2, labeled 5; FWE corrected: p = .004). No other differences emerged for between-network comparisons (FWE corrected: p > .08).
3.7. Adults with ASD vs. NT adults
No significant group differences in within- or between-network connectivity were observed for any of the networks examined, in either direction (ASD > TD or TD > ASD).
4. Discussion
The results of the current study demonstrate age-specific patterns of whole-brain functional connectivity atypicalities in ASD. Hyper-connectivity within large-scale brain networks in ASD was observed in young children under the age of 11, with no within-network differences in functional connectivity in adolescents and adults with ASD compared with neurotypical individuals. Abetween-network analysis showed that children with ASD had a smaller correlation between two DMN networks, while adolescents with ASD had a smaller correlation between the DMN and a subcortical/insula network compared with TD individuals. Adults with ASD showed no differences in either within- or between-network functional connectivity compared to neurotypical controls. Overall, the results demonstrate that children with ASD exhibit atypical within- and between-network functional connectivity, adolescents with ASD show atypical between-network functional connectivity, and adults with the disorder do not differ from their age-matched peers on either of these measures.
4.1. Within-network functional connectivity
The results from this data-driven developmental approach to investigating whole-brain functional connectivity between ASD and TD individuals are an important first step in helping to resolve discrepancies in the ASD rsfMRI literature finding hyper- and hypo-connectivity of intrinsically functional networks across different studies. Importantly, these results are partially in accord with the developmental trajectory hypothesis proposed by Uddin etal. (2013) predicting hyper-connectivity in young children with ASD and hypo-connectivity in adults with ASD. Although hypo-connectivity was not found in the adult group, the general developmental trajectory shows widespread hyper-connectivity in children with ASD that reduces with age. The results also confirm the findings of previous rsfMRI studies showing hyper-connectivity in young children with ASD(Di Martino etal., 2011; Lynch etal., 2013; Supekar etal., 2013; Uddin et al., 2013; Washington et al., 2014) along with no differences in whole-brain functional connectivity in adults with ASD (Tyszka et al., 2014).
The current study found hyper-connectivity within the DMN in children with ASD. This replicates findings of Uddin etal. (2013), and other studies showing hyper-connectivity of DMN nodes in children with ASD(Lynch etal., 2013; Washington etal., 2014). Although the exact function of the DMN is still not clear, activity in key nodes of the DMN such as the MPFC and PCC is found to decrease during tasks with a high cognitive demand (Raichle etal., 2001) and increase during rest and also during tasks of a social nature involving self-reflection(Buckner etal., 2008; Harrison etal., 2008; Northoff etal., 2006). Previously, most studies finding aberrant DMN function in ASD have found hypo-connectivity across nodes of the DMN(Kennedy and Courchesne, 2008a; Monk etal., 2009; von dem Hagen etal., 2013; Weng etal., 2010). However, these studies were conducted in adolescents and adults. Thus, a developmental account predicting within-networkhyper-connectivity in children with ASD that gradually recedes over time would be a plausible explanation as to why some previous studies have found hyper- opposed to hypo-connectivity across nodes of the DMN.
The mechanisms underlying the observed widespread functional hyper-connectivity in children with ASD are still unclear. Previous research has shown that ASD is characterized by increased head circumference in childhood (Lainhart etal., 1997) while in-vivo(Courchesne etal., 2003) and post-mortem studies (Courchesne etal., 2011) have shown increased neuronal growth in children with ASD from 2–5 years and 2–16 years respectively. Additionally, another post-mortem study has shown both increased spine density and decreased synaptic pruning across childhood and adolescence (Tang etal., 2014). Although no direct evidence exists that links neuronal density to functional connectivity within neural networks in the context of rsfMRI studies, it is plausible that changes in neural density are related to changes in functional connectivity. Thus, increased neural density found early in life in children with ASD in these post-mortem studies could be related to increased functional connectivity found in rsfMRI studies.
Additionally, mouse models have also demonstrated that decreases in synaptic pruning (Gogolla etal., 2014; Tang etal., 2014) and increases in synaptic turnover (Isshiki etal., 2014) during critical developmental periods are related to atypical behavior similar to those found in human individuals with ASD. These studies suggest that critical periods of synaptic development may influence cortical function in ASD, making the study of developmental trajectories imperative for research examining the disorder in humans. Within the context of the current study, changes in synaptic pruning could be related to changes in functional connectivity found across the lifespan such that increased synaptic pruning could be responsible for normalizing the increased neuronal growth found in children with ASD. Thus, increased synaptic pruning of extra neuronal growth could help to normalize the amount of neurons present in individuals with ASD during development that would result in a more typical pattern of functional connectivity of individuals with ASD as they get older. Unfortunately, a large amount of ASD research is conducted on adolescents and adults, probably due to the difficulty in acquiring artifact-free fMRI data from younger children, especially young children with psychiatric disorders such as ASD(Yerys etal., 2009). The few studies that have examined rsfMRI functional connectivity in young children with ASD have generally found hyper-connectivity between brain areas (Di Martino etal., 2011; Lynch etal., 2013; Uddin etal., 2013; Washington etal., 2014) demonstrating the importance of exploring child populations in ASD research.
One previously proposed explanation for the findings of changing functional connectivity across development in ASD is that the pubertal period during adolescence is responsible for changes in underlying brain organization, and that pubertal hormones may differentially affect developmental trajectories in the disorder (Uddin etal., 2013). Hormonal changes during puberty have been linked with changes in both gray and white matter (Herting etal., 2012). However, there have been no cross-sectional or longitudinal studies examining changes in functional connectivity that accompany the pubertal transition in humans, in either typical or atypical development.
Previous studies showing hypo-connectivity in adults with ASD using rsfMRI data have mostly used seed ROI-based functional connectivity analysis while also including mixed groups of participants younger than 18 (Cherkassky etal., 2006; Ebisch etal., 2011; Kennedy and Courchesne, 2008a; Monk etal., 2009) whereas the current study examined resting state data using a whole brain ICA approach with subjects 18 years and older in the adult group. The current study and the previous study finding a lack of hypo-connectivity in adults (Tyszka et al., 2014) used only participants older than 18 years in a data-drivenICA analysis. However, two other studies using ICA with participants over 18 have found hypo-connectivity in ASD(Mueller etal., 2013; von dem Hagen etal., 2013). Thus, it is still unclear why some studies have found hypo-connectivity in adults with ASD, compared with the results of the current study and the previous study (Tyszka et al., 2014) which found no differences in functional connectivity between adults with ASD and neurotypical adults. In addition to the proposed developmental account which finds empirical support from the current study, others have suggested that hypo-connectivity is generally found in task-driven studies using seed-based analysis approaches compared to studies not finding hypo-connectivity that have used whole brain analysis approaches combined with task-regression analysis to remove task-related activity (Muller etal., 2011). These and other methodological factors may contribute to some of the inconsistencies in the literature.
Three earlier studies examined functional connectivity in ASD using linear regression correlations between functional connectivity and age. Wiggins etal. (2011) examined the DMN in groups of ASD and TD adolescents (10.25–18.97) and found that connectivity between posterior DMN nodes such as the inferior parietal lobule had decreased connectivity with the superior frontal gyrus as age increased in ASD, but connectivity increased with age in the TD group. Padmanabhan etal. (2013) examined cortico-striatal connectivity in groups of ASD and TD individuals (8–36 years) and found that both groups showed general decreases in cortico-striatal connections as age increased. Additionally, the ASD group had increased connectivity of the striatum with the cerebellum, fusiform gyrus, and inferior/superior temporal gyri with age while these connections decreased in TD individuals. However, when controlling for age, there was ASD striatal hyper-connectivity with the parietal cortex but hypo-connectivity with the pre-frontal cortex compared with TD individuals. Bos etal. (2014) found that adolescents with ASD (8.3–15.1) had little to no within-network connectivity differences compared to TD adolescents (6.4–15.8) using a group ICA, but found that the ASD group had connectivity between the right insula and other nodes of the DMN that increased with age, while decreasing connectivity with age was observed in the TD group.
Although previous studies have also found hyper-connectivity in the DMN and insula (Bos etal., 2014), these results were found using a linear correlation approach as opposed to the whole brain ICA used in the current study. Although Bos etal. (2014) found no group differences between ASD and TD in an overall group ICA, they used age ranges that combined children with adolescents (6.4–15.8) while the current study separated children under 11 and adolescents from 11–18 and found hyper-connectivity in children, and no differences in adolescents. Thus, future research is needed to further explore the effects of stratifying samples by age.
4.2. Between-network functional connectivity
The overall between-network results in the current study showed that both ASD and TD groups for all ages had similar anti-correlations between task-positive and task-negative networks represented by the DAN and DMN respectively. Additionally, both ASD and TD groups for all ages had positive correlations between the DAN and higher-levelvisual areas. This shows that individuals with ASD show typical anti-correlations between the DAN and DMN and typical positive correlations between the DAN and higher-level visual areas. Both of these results replicate previous work exploring between-network connectivity of resting state data in the neurotypical population (Smith etal., 2013).
Previously, Kennedy and Courchesne (2008b) utilized a hypothesis-driven seed ROI-based approach to demonstrate that adults with ASD show typical anti-correlations between the DMN and DAN. The current study extends this work by using a completely data-driven method to demonstrate typical anti-correlations between the DMN and DAN, in addition to typical positive correlations between the DAN and higher-level visual areas. In addition, the novel contribution of the current study demonstrates these results apply to children and adolescents with ASD in addition to adults.
Between-network differences were observed in the current study between DMN components for the child cohort and between a DMN and subcortical/insula component for the adolescent cohort. The difference for children (ASD < TD) was found in the partial-correlationcomparison, while the difference for adolescents was found in the full-correlation comparison. The partial-correlation difference for children suggests that DMN networks had less direct functional relationships with each other, demonstrating atypical cooperation between DMN components in children with ASD. The full-correlation difference in adolescents would suggest that a weaker functional relationship between the DMN and subcortical/insula component for children with ASD is moderated by another network.
Starck etal. (2013) used an ICA approach to examine the DMN and its subnetworks in adolescents with ASD compared with TD adolescents. Their study found no differences in within-networkDMN functional connectivity compared with TD adolescents, consistent with the current findings. They did find significantly reduced correlations between anterior and posterior DMN subnetworks in ASD.
Additionally, the current results are in accord with previous ideas suggesting that increased within-network connectivity in children with ASD could be responsible for reduced between-network connectivity as tighter coupling within networks could lead to reduced coupling between networks (Uddin etal., 2013). In the current study, this may explain the reduced coupling between DMN components for children with ASD. The lack of within-networkhyper-connectivity in adolescents suggests that reductions in between-network coupling can still occur in the absence of within-network hyper-connectivity. In the case of adolescents, the between-network differences were found between the DMN and a subcortical/insula network. Both of these networks are related to cognitive flexibility and may contribute to some of the behavioral patterns found in ASD, as discussed in the following section. However, it is not possible to determine from the current study if there is a direct relationship that relates within- and between-network coupling.
4.3. Insula and the default mode network
Although the current study did not find any functional connectivity group differences within the context of the salience network, there were within- and between-network differences involving a subcortical/insula component. Thus, although the current findings implicate the insula as a source of atypical functional connectivity in ASD, this does not occur within the context of the SN. The exact reason for this divergence is unclear. One possible reason is that insula activation within the SN in the context of the current study was generally related to homogeneous activity across the entire insula— a finding typical in ICA based studies of the SN (Seeley etal., 2007; Uddin etal., 2013; Uddin etal., 2011). However, other work suggests that insula subregions such as the dorsal anterior, ventral anterior, and posterior insula regions vary substantially in their functional roles and patterns of connectivity (Deen etal., 2011; Uddin etal., 2014). The ventral anterior insula has been shown to have direct structural connections with subcortical areas in primate studies (Mesulam and Mufson, 1982) as well as sharing task-based subcortical activations in fMRI studies of humans (Uddin etal., 2014; Uddin, 2015). Additionally, the ventral anterior insula has been shown to have connections to subcortical limbic areas in task-based activation studies (Uddin etal., 2014). Thus, it may be possible that different insula subregions may be driving the functional connections of the insula with the ACC in the context of the SN and subcortical areas with the insula in the context of the subcortical/insula component found in the current study. Thus, different insula subregions may be driving the atypical functional connections found in the current study compared to previous studies finding atypical functional connections of the insula within the context of the SN(Uddin etal., 2013).
The findings of the current study and previous studies strongly implicate atypical within- and between-network functional connectivity of the DMN and insula as possible brain markers of ASD. The major nodes of the DMN include the posterior parietal and lateral prefrontal cortices while the nodes of the SN include the anterior cingulate and anterior insular cortices; areas that are important in flexibly switching between internal cognitive and external information (Cole etal., 2013; Dosenbach etal., 2007; Uddin etal., 2014; Uddin etal., 2011). Accordingly, Uddin etal. (2014) have demonstrated that atypical effective connectivity of these brain areas is correlated with restrictive and repetitive patterns of behavior in children with ASD such that greater atypical connectivity is related with more severe behavior deficits. This suggests that the atypical functional connectivity of these networks that are important in cognitive flexibility contribute to the restrictive and repetitive behaviors that often characterize the disorder. The current study shows both within- and between-network differences involving the DMN and insula that further implicate the atypical functional connections of the two as a possible biomarker of brain function in ASD.
5. Conclusions
In sum, the current findings support adopting a developmental perspective to help reconcile the heterogeneous findings of functional hypo- and hyper-connectivity observed in the rsfMRI literature in ASD. These results demonstrating group differences specific to certain age cohorts highlight the utility of carefully considering developmental stage in studies of functional brain connectivity in ASD. We find that while children show atypical within and between-network functional relationships, adolescents exhibit fewer such differences and adults are indistinguishable from age-matched neurotypical peers on such measures. The fact that both within- and between-network differences diminish across the lifespan could offer an explanation for some of the improved function often found in adults with ASD compared to children with ASD. These results also highlight the importance of considering within- and between-network whole brain functional connections in conjunction with a developmental approach in order to better characterize brain connectivity in ASD.
Although the current study is an important first step in taking a developmental approach to investigating differences in functional connectivity between individuals with ASD and neurotypical individuals, future studies should explore the influence of various age groupings to more precisely determine where differences in hyper- and hypo-connectivity begin to emerge between specific brain areas. As an increasing awareness of the impact of development on brain function in ASD has begun to emerge (Picci and Scherf, 2014; Uddin etal., 2013) more studies that explore the impact of age on brain-based biomarkers in ASD are needed in order to provide a better picture of the developmental maturation of functional connectivity patterns that emerge across the lifespan in individuals with ASD.
Conflicts of interest
None.
Acknowledgments
This work was supported by a National Institute of Mental Health Career Development award (K01MH092288), a NARSAD Young Investigator Award, and a Slifka/Ritvo Innovation in Autism Research Award from the International Society for Autism Research to LQU. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the NIH.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.02.024.
Contributor Information
Jason S. Nomi, Email: jxn131@miami.edu.
Lucina Q. Uddin, Email: l.uddin@miami.edu.
Appendix A. Supplementary data
Supplementary Fig. 1.
Functional networks observed in a group of children with ASD(n = 26) and TD children (n = 26) using ICA.
Supplementary Fig. 2.
Functional networks observed in a group of adolescents with ASD(n = 28) and TD adolescents (n = 28) using ICA.
Supplementary Fig. 3.
Functional networks observed in a group of adults with ASD(n = 18) and neurotypical adults (n = 18).
Motion parameters for individuals with autism spectrum disorder (ASD) and typically developing (TD) individuals.
References
- Abrams D.A., Lynch C.J., Cheng K.M., Phillips J., Supekar K., Ryali S., Uddin L.Q., Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc. Natl. Acad. Sci. U S A. 2013;110(29):12060–12065. doi: 10.1073/pnas.1302982110. 23776244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M., Jagannathan K., Calhoun V.D., Miller L., Stevens M.C., Sahl R., O’Boyle J.G., Schultz R.T., Pearlson G.D. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05.067. 20621638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. 16087444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. I. E.E.E. Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. 14964560 [DOI] [PubMed] [Google Scholar]
- Belmonte M.K., Allen G., Beckel-Mitchener A., Boulanger L.M., Carper R.A., Webb S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. 15496656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. 8524021 [DOI] [PubMed] [Google Scholar]
- Bos D.J., van Raalten T.R., Oranje B., Smits A.R., Kobussen N.A., Belle J.v, Rombouts S.A., Durston S. Developmental differences in higher-orderresting-state networks in autism spectrum disorder. Neuroimage Clin. 2014;4:820–827. doi: 10.1016/j.nicl.2014.05.007. 24936432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. 20493761 [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. 18400922 [DOI] [PubMed] [Google Scholar]
- Cherkassky V.L., Kana R.K., Keller T.A., Just M.A. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. 17047454 [DOI] [PubMed] [Google Scholar]
- Cole M.W., Reynolds J.R., Power J.D., Repovs G., Anticevic A., Braver T.S. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 2013;16(9):1348–1355. doi: 10.1038/nn.3470. 23892552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Carper R., Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. 12865374 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Mouton P.R., Calhoun M.E., Semendeferi K., Ahrens-Barbeau C., Hallet M.J., Barnes C.C., Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. 22068992 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. 16945915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B., Pitskel N.B., Pelphrey K.A. Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex. 2011;21(7):1498–1506. doi: 10.1093/cercor/bhq186. 21097516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Kelly C., Grzadzinski R., Zuo X.N., Mennes M., Mairena M.A., Lord C., Castellanos F.X., Milham M.P. Aberrant striatal functional connectivity in children with autism. Biol. Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. 21195388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Yan C.G., Li Q., Denio E., Castellanos F.X., Alaerts K., Anderson J.S., Assaf M., Bookheimer S.Y., Dapretto M., Deen B., Delmonte S., Dinstein I., Ertl-Wagner B., Fair D.A., Gallagher L., Kennedy D.P., Keown C.L., Keysers C., Lainhart J.E. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry. 2014;19(6):659–667. doi: 10.1038/mp.2013.78. 23774715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. 17576922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch S.J., Gallese V., Willems R.M., Mantini D., Groen W.B., Romani G.L., Buitelaar J.K., Bekkering H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum. Brain Mapp. 2011;32(7):1013–1028. doi: 10.1002/hbm.21085. 20645311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. 19357304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. 15976020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N., Takesian A.E., Feng G., Fagiolini M., Hensch T.K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83(4):894–905. doi: 10.1016/j.neuron.2014.06.033. 25088363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts S.J., Simmons W.K., Milbury L.A., Wallace G.L., Cox R.W., Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135(9):2711–2725. doi: 10.1093/brain/aws160. 22791801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., López-Solà M., Hernández-Ribas R., Deus J., Ortiz H., Soriano-Mas C., Yücel M., Pantelis C., Cardoner N. Consistency and functional specialization in the default mode brain network. Proc. Natl. Acad. Sci. U. S. A. 2008;105(28):9781–9786. doi: 10.1073/pnas.0711791105. 18621692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Maxwell E.C., Irvine C., Nagel B.J. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. 22002939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki M., Tanaka S., Kuriu T., Tabuchi K., Takumi T., Okabe S. Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nat. Commun. 2014;5:4742. doi: 10.1038/ncomms5742. 25144834 [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence ofunderconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. 15215213 [DOI] [PubMed] [Google Scholar]
- Just M.A., Keller T.A., Malave V.L., Kana R.K., Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. 22353426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Soc. Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. 18633829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Libero L.E., Moore M.S. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys. Life Rev. 2011;8(4):410–437. doi: 10.1016/j.plrev.2011.10.001. 22018722 [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. 17919929 [DOI] [PubMed] [Google Scholar]
- Kennedy D.P., Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc. Cogn. Affect. Neurosci. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. 19015108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. 18083565 [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–1012. doi: 10.1093/brain/awm334. 18234695 [DOI] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. 15652316 [DOI] [PubMed] [Google Scholar]
- Lainhart J.E., Piven J., Wzorek M., Landa R., Santangelo S.L., Coon H., Folstein S.E. Macrocephaly in children and adults with autism. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. 9031582 [DOI] [PubMed] [Google Scholar]
- Lynch C.J., Uddin L.Q., Supekar K., Khouzam A., Phillips J., Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol. Psychiatry. 2013;74(3):212–219. doi: 10.1016/j.biopsych.2012.12.013. 23375976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M., Mufson E.J. Insula of the old world monkey. III: efferent cortical output and comments on function. J. Comp. Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. 7174907 [DOI] [PubMed] [Google Scholar]
- Minshew N.J., Williams D.L. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch. Neurol. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. 17620483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Peltier S.J., Wiggins J.L., Weng S.J., Carrasco M., Risi S., Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. 19409498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Keeser D., Samson A.C., Kirsch V., Blautzik J., Grothe M., Erat O., Hegenloh M., Coates U., Reiser M.F., Hennig-Fast K., Meindl T. Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLOS One. 2013;8(6):e67329. doi: 10.1371/journal.pone.0067329. 23825652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R.A., Shih P., Keehn B., Deyoe J.R., Leyden K.M., Shukla D.K. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. 21378114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan S.K., Haist F., Müller R.A. Aberrant functional connectivity in autism: evidence from low-frequencyBOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. 19401185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain— a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. 16466680 [DOI] [PubMed] [Google Scholar]
- Padmanabhan A., Lynn A., Foran W., Luna B., O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front. Hum. Neurosci. 2013;7:814. doi: 10.3389/fnhum.2013.00814. 24348363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci G., Scherf K.S. A two-hit model of autism: adolescence as the second hit. Clinical Psychological Science. 2014 doi: 10.1177/2167702614540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. 11209064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., Chen G., Jo H.J., Martin A., Cox R.W. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. 22432927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. 22233733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. 17329432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P., Keehn B., Oram J.K., Leyden K.M., Keown C.L., Müller R.A. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol. Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. 21601832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P., Shen M., Ottl B., Keehn B., Gaffrey M.S., Müller R.A. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48(10):2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. 20558187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Beckmann C.F., Andersson J., Auerbach E.J., Bijsterbosch J., Douaud G., Duff E., Feinberg D.A., Griffanti L., Harms M.P., Kelly M., Laumann T., Miller K.L., Moeller S., Petersen S., Power J., Salimi-Khorshidi G., Snyder A.Z., Vu A.T., Woolrich M.W. Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013;80(0):144–168. doi: 10.1016/j.neuroimage.2013.05.039. http://dx.doi.org/10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M., Ozonoff S.J., Ursu S., Ravizza S., Cummings N., Ly S., Carter C.S. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. 19410583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. 18510452 [DOI] [PubMed] [Google Scholar]
- Starck T., Nikkinen J., Rahko J., Remes J., Hurtig T., Haapsamo H., Jussila K., Kuusikko-Gauffin S., Mattila M.L., Jansson-Verkasalo E., Pauls D.L., Ebeling H., Moilanen I., Tervonen O., Kiviniemi V.J. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. Front. Hum. Neurosci. 2013;7:802. doi: 10.3389/fnhum.2013.00802. 24319422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Khouzam A., Phillips J., Gaillard W.D., Kenworthy L.E., Yerys B.E., Vaidya C.J., Menon V. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. 24210821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Gudsnuk K., Kuo S.H., Cotrina M.L., Rosoklija G., Sosunov A., Sonders M.S., Kanter E., Castagna C., Yamamoto A., Yue Z., Arancio O., Peterson B.S., Champagne F., Dwork A.J., Goldman J., Sulzer D. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83(5):1131–1143. doi: 10.1016/j.neuron.2014.07.040. 25155956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka J.M., Kennedy D.P., Paul L.K., Adolphs R. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cereb. Cortex. 2014;24:1894–1905. doi: 10.1093/cercor/bht040. 23425893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. 25406711 [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kinnison J., Pessoa L., Anderson M.L. Beyond the tripartite cognition–emotion–interoception model of the human insular cortex. J. Cogn. Neurosci. 2014;26(1):16–27. doi: 10.1162/jocn_a_00462. 23937691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J., Cheng K.M., Odriozola P., Barth M.E., Phillips J., Feinstein C., Abrams D.A., Menon V. Brain state differentiation and behavioral inflexibility in autism. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu161. 25073720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J., Khouzam A., Phillips J., Feinstein C., Ryali S., Menon V. Salience network-based classification and prediction of symptom severity in children with autism. J.A.M.A. Psychiatry. 2013;70(8):869–879. doi: 10.1001/jamapsychiatry.2013.104. 23803651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. 23966925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. 22171056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von dem Hagen E.A., Stoyanova R.S., Baron-Cohen S., Calder A.J. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc. Cogn. Affect. Neurosci. 2013;8(6):694–701. doi: 10.1093/scan/nss053. 22563003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington S.D., Gordon E.M., Brar J., Warburton S., Sawyer A.T., Wolfe A., Mease-Ference E.R., Girton L., Hailu A., Mbwana J., Gaillard W.D., Kalbfleisch M.L., VanMeter J.W. Dysmaturation of the default mode network in autism. Hum. Brain Mapp. 2014;35(4):1284–1296. doi: 10.1002/hbm.22252. 23334984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchew D.E., Ashwin C., Berkouk K., Salvador R., Suckling J., Baron-Cohen S., Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol. Psychiatry. 2005;57(9):991–998. doi: 10.1016/j.biopsych.2005.01.028. 15860339 [DOI] [PubMed] [Google Scholar]
- Weng S.J., Wiggins J.L., Peltier S.J., Carrasco M., Risi S., Lord C., Monk C.S. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. 20004180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L., Peltier S.J., Ashinoff S., Weng S.J., Carrasco M., Welsh R.C., Lord C., Monk C.S. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. 21047495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. 23499792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys B.E., Jankowski K.F., Shook D., Rosenberger L.R., Barnes K.A., Berl M.M., Ritzl E.K., Vanmeter J., Vaidya C.J., Gaillard W.D. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum. Brain Mapp. 2009;30(10):3426–3435. doi: 10.1002/hbm.20767. 19384887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Motion parameters for individuals with autism spectrum disorder (ASD) and typically developing (TD) individuals.