Abstract
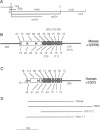
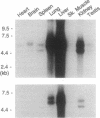
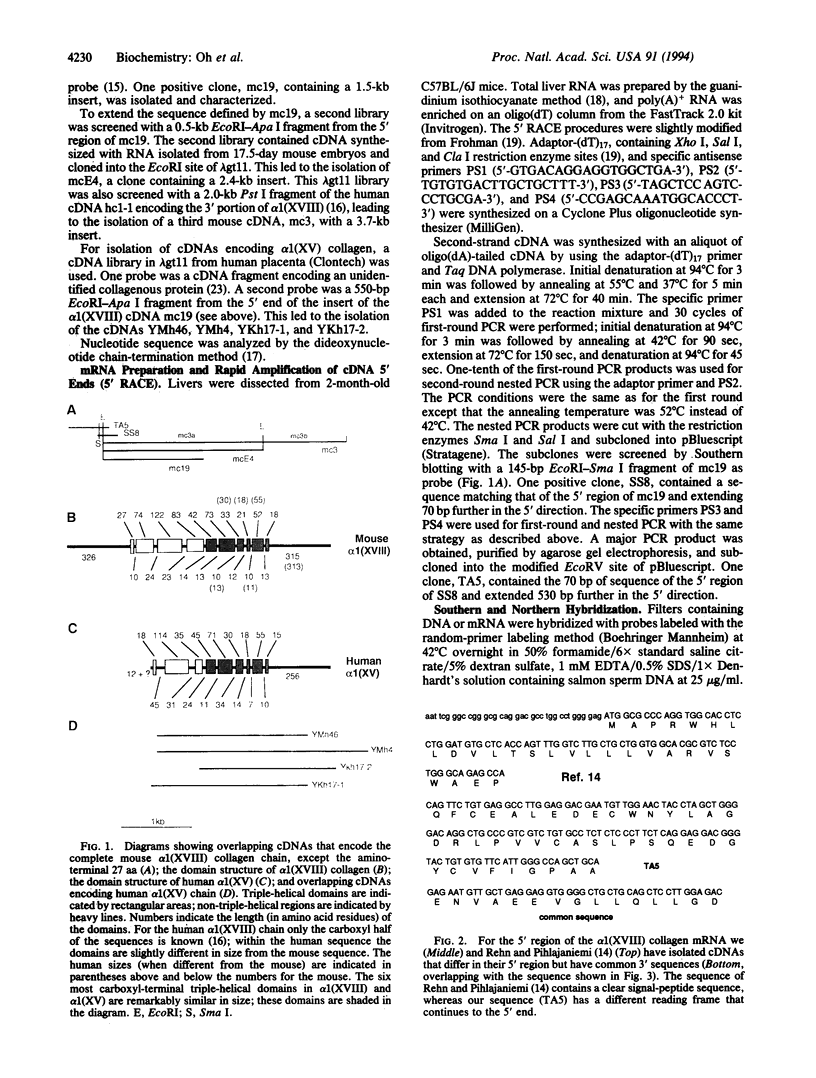
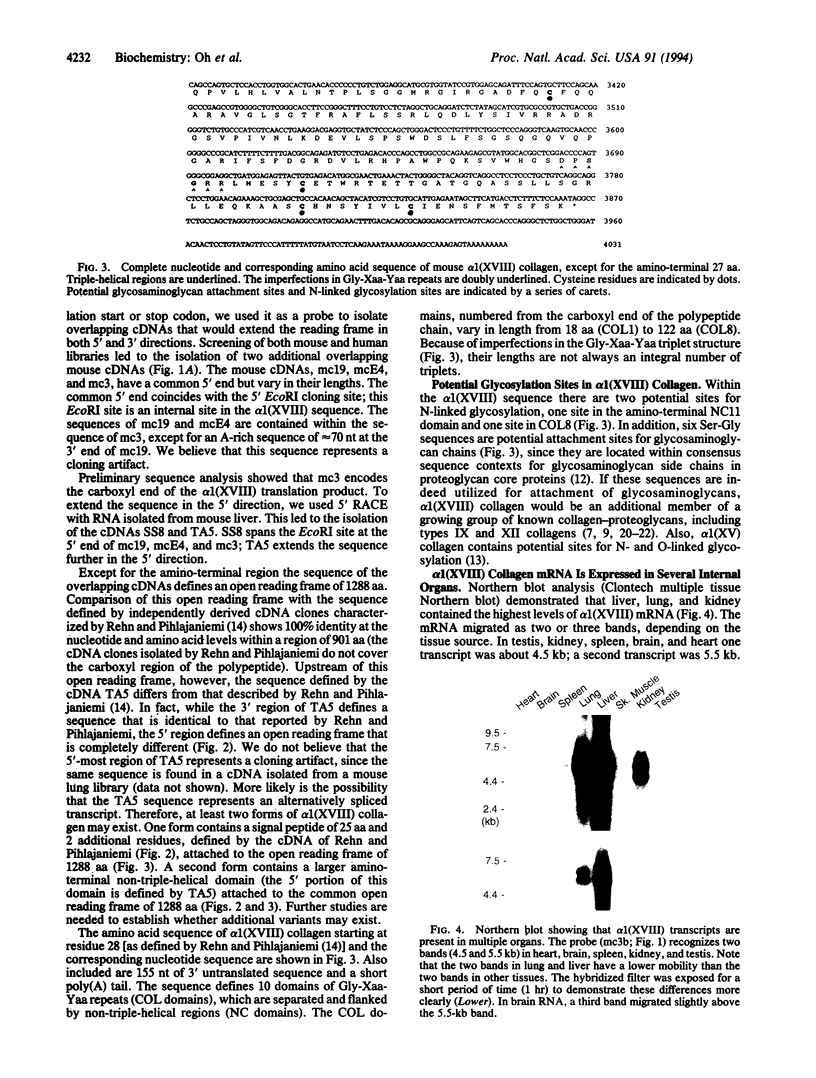
We have isolated overlapping mouse cDNAs encoding a collagenous polypeptide that we have designated alpha 1(XVIII) collagen. Nucleotide sequence analysis shows that alpha 1(XVIII) collagen contains 10 triple-helical domains separated and flanked by non-triple-helical regions. Within the non-triple-helical regions, there are several Ser-Gly-containing sequences that conform to consensus sequences for glycosaminoglycan attachment sites in proteoglycan core proteins. Northern blots show that alpha 1(XVIII) transcripts are present in multiple organs, with the highest levels in liver, lung, and kidney. We have also isolated overlapping cDNAs encoding human alpha 1(XV) collagen, and their sequence extends a published partial alpha 1(XV) sequence to the 3' end. Comparison of the alpha 1(XV) and alpha 1(XVIII) sequences reveals a striking similarity in the lengths of the six most carboxyl-terminal triple-helical domains. In addition, within the carboxyl non-triple-helical domain NC1 of the two chains, a region of 177 amino acid residues shows about 60% identity at the amino acid level. We suggest, therefore, that alpha 1(XV) and alpha 1(XVIII) collagens are structurally related. Their structure is different from that of other known collagen types. We conclude that they belong to a subfamily of extracellular matrix proteins and we suggest the designation multiplexins (for protein with multiple triple-helix domains and interruptions) for members of this subfamily.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bächinger H. P., Morris N. P., Lunstrum G. P., Keene D. R., Rosenbaum L. M., Compton L. A., Burgeson R. E. The relationship of the biophysical and biochemical characteristics of type VII collagen to the function of anchoring fibrils. J Biol Chem. 1990 Jun 15;265(17):10095–10101. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dublet B., Oh S., Sugrue S. P., Gordon M. K., Gerecke D. R., Olsen B. R., van der Rest M. The structure of avian type XII collagen. Alpha 1 (XII) chains contain 190-kDa non-triple helical amino-terminal domains and form homotrimeric molecules. J Biol Chem. 1989 Aug 5;264(22):13150–13156. [PubMed] [Google Scholar]
- Irwin M. H., Mayne R. Use of monoclonal antibodies to locate the chondroitin sulfate chain(s) in type IX collagen. J Biol Chem. 1986 Dec 15;261(35):16281–16283. [PubMed] [Google Scholar]
- Jacenko O., Olsen B. R., LuValle P. Organization and regulation of collagen genes. Crit Rev Eukaryot Gene Expr. 1991;1(4):327–353. [PubMed] [Google Scholar]
- Koch M., Bernasconi C., Chiquet M. A major oligomeric fibroblast proteoglycan identified as a novel large form of type-XII collagen. Eur J Biochem. 1992 Aug 1;207(3):847–856. doi: 10.1111/j.1432-1033.1992.tb17116.x. [DOI] [PubMed] [Google Scholar]
- Li K., Tamai K., Tan E. M., Uitto J. Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5'-end of the gene and the 3'-untranslated region of the mRNA. J Biol Chem. 1993 Apr 25;268(12):8825–8834. [PubMed] [Google Scholar]
- Muragaki Y., Abe N., Ninomiya Y., Olsen B. R., Ooshima A. The human alpha 1(XV) collagen chain contains a large amino-terminal non-triple helical domain with a tandem repeat structure and homology to alpha 1(XVIII) collagen. J Biol Chem. 1994 Feb 11;269(6):4042–4046. [PubMed] [Google Scholar]
- Myers J. C., Kivirikko S., Gordon M. K., Dion A. S., Pihlajaniemi T. Identification of a previously unknown human collagen chain, alpha 1(XV), characterized by extensive interruptions in the triple-helical region. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10144–10148. doi: 10.1073/pnas.89.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. P., Taylor R. W., Gerecke D. R., Rochelle J. M., Seldin M. F., Olsen B. R. The mouse alpha 1(XII) and human alpha 1(XII)-like collagen genes are localized on mouse chromosome 9 and human chromosome 6. Genomics. 1992 Oct;14(2):225–231. doi: 10.1016/s0888-7543(05)80210-1. [DOI] [PubMed] [Google Scholar]
- Oh S. P., Warman M. L., Seldin M. F., Cheng S. D., Knoll J. H., Timmons S., Olsen B. R. Cloning of cDNA and genomic DNA encoding human type XVIII collagen and localization of the alpha 1(XVIII) collagen gene to mouse chromosome 10 and human chromosome 21. Genomics. 1994 Feb;19(3):494–499. doi: 10.1006/geno.1994.1098. [DOI] [PubMed] [Google Scholar]
- Pan T. C., Zhang R. Z., Mattei M. G., Timpl R., Chu M. L. Cloning and chromosomal location of human alpha 1(XVI) collagen. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6565–6569. doi: 10.1073/pnas.89.14.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn M., Pihlajaniemi T. Alpha 1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4234–4238. doi: 10.1073/pnas.91.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. M., Olsen B. R. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem Sci. 1991 May;16(5):191–194. doi: 10.1016/0968-0004(91)90074-6. [DOI] [PubMed] [Google Scholar]
- Vuorio E., de Crombrugghe B. The family of collagen genes. Annu Rev Biochem. 1990;59:837–872. doi: 10.1146/annurev.bi.59.070190.004201. [DOI] [PubMed] [Google Scholar]
- Watt S. L., Lunstrum G. P., McDonough A. M., Keene D. R., Burgeson R. E., Morris N. P. Characterization of collagen types XII and XIV from fetal bovine cartilage. J Biol Chem. 1992 Oct 5;267(28):20093–20099. [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]