Abstract
Objective:
The aim was to observe the effects of the extract of Ginkgo biloba (EGb761) on the apoptosis of oxygen and glucose-deprived (OGD) human neuroblastoma cells (SH-SY5Y) cells and explore its mechanism.
Materials and Methods:
SH-SY5Y cells were divided into normal control group, OGD group, OGD for 4 h and EGb761-pretreated groups including very low-concentration (20 μg/ml), low-concentration group (25 μg/ml), moderate-concentration group (50 μg/ml) and high-concentration group (100 μg/ml). Twenty four hours after reoxygenation, cell viability was determined with 3-[4, 5-dimehyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide assay, apoptosis rate was detected with annexin V-fluorescein isothiocyanate/propidium iodide double staining flow cytometry and the protein level of apoptosis-inducing factor (AIF) was observed with immunofluorescence technique in each group.
Results:
Cell viability was significantly lower in OGD group than in EGb761-pretreated groups, especially in moderate-concentration group (50 μg/ml) (P < 0.005). Apoptosis rate was significantly lower in EGb761-pretreated groups than in OGD group (P < 0.001). Immunofluorescent staining showed that there was AIF nuclear translocation in both EGb761-pretreated groups and OGD group, but AIF nuclear translocation was less in EGb761-pretreated groups than in OGD group.
Conclusion:
EGb761 can reduce the apoptosis of OGD SH-SY5Y cells probably through inhibiting AIF nuclear translocation. This study provides a theoretical basis for the application of EGb761 in clinical practice.
KEY WORDS: Apoptosis, apoptosis-inducing factor, extract of Ginkgo biloba, oxygen and glucose-deprived model, SH-SY5Y
Introduction
Extract of Ginkgo biloba (EGb761), a kind of a mixture extracted from ginkgo plants, possesses a variety of active ingredients which play an important role in scavenging free radicals, inhibiting excitatory amino acid toxicity and platelet activation, and anti-inflammatory response. EGb761 is widely used in the treatment of cardiovascular and cerebrovascular diseases, because it has protective effects on cerebral ischemia and hypoxia, but its mechanism is not fully clear.[1] Little research has been done about whether EGb761 can inhibit apoptosis through apoptosis-inducing factor (AIF) signal transduction pathway. In this study, we built oxygen and glucose-deprived (OGD) models with SH-SY5Y cells, and then observed the effects of EGb761 on apoptosis of OGD SH-SY5Y cells and explored its possible mechanism.
Materials and Methods
All study methods were approved by Ethics Committee of the First Affiliated Hospital, Liaoning Medical University.
Cell Culture and Grouping
SH-SY5Y cells (provided by Neuropharmacology of Laboratory, China Medical University) were incubated in dulbecco minimum essential medium (DMEM) (provided by Hyclone, Logan, Utah, USA) at 37°C in an atmosphere of 5% CO2. When cells reached 80% confluence, the cells in logarithmic growth phase were used in this experiment. SH-SY5Y cells were divided into normal control group, OGD group and EGb761-pretreated groups including very low-concentration group (20 μg/ml), low-concentration group (25 μg/ml), moderate-concentration group (50 μg/ml) and high-concentration group (100 μg/ml).
Preparation of Oxygen and Glucose-deprived Models and Extract of Ginkgo Biloba Intervention
The SH-SY5Y cells in logarithmic growth phase were incubated in serum and sugar-free artificial cerebrospinal fluid at 37°C in an atmosphere of 95% N2 and 5% CO2 for 4 h, and then incubated in DMEM medium and normoxic atmosphere for 24 h. In EGb761-pretreated groups, of SH-SY5Y cells were inoculated in 18 wells of a 96-well plate (each subgroup with 6 wells) to incubate for 12 h, and then respectively treated with different-concentration EGb761 (20 μg/ml, 25 μg/ml, 50 μg/ml and 100 μg/ml) for 30 min followed by building OGD SH-SY5Y cells as previously described.
Cell Viability Determination
Cell viability was determined with 3-[4, 5-dimehyl -2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay. After the supernatant fluid in SH-SY5Y cells was removed, 200 μl of DMEM and 20 μl of MTT was added to incubate at 37°C for 4 h. After DMEM had been removed, 200 μl of DMSO was added in each well. Ten minutes later, optical density value of each well was determined at 490 nm with Sunrise ELIASA four times. Cell viability was calculated according to the following formula: Cell viability (%) = cells in experimental group/cells in control group × 100%.
Apoptosis Rate Detected with Flow Cytometry
Cells in each group were digested, and then centrifuged at 1000r/min for 5 min. After the supernatant fluid had been removed, cells were collected. Cells were suspended, and then counted. The suspended 5-10 × 104 cells were centrifuged at 1000r/min for 5 min. After the supernatant fluid was removed, cells were suspended with 195 μl of annexin fluorescein isothiocyanate (FITC) mixed liquor, and then 5 μl of Annexin V-EITC was added at room temperature (20-25°C) in the dark for 10 min followed by centrifugation at 1000r/min for 5 min. After the supernatant fluid had been removed, cells were suspended with 190 μl of annexin V-EITC mixed liquor. After 10 μl of propidium iodide was added in ice bath, flow cytometry was performed in triplicate.
Apoptosis Inducing Factor Nuclear Translocation Observed by Immunofluorescent Staining
Cells in each group were digested, washed with phosphate buffered saline two times, fixed, washed, permeabilized, washed and sealed with 5% bovine serum albumin at room temperature for 1–2 h. The primary antibodies were added at 4°C overnight. Cells were washed, and then secondary antibodies were added at 37°C in the dark for 30 min. Cell nucleus was stained with Hoechst followed by sealing with 95% glycerol.
Statistical Analysis
All the data were expressed as (mean ± standard deviation). Statistical analysis was performed with Statistical Package for the Social Sciences 13.0 software (Spss Company, Chicago, US). One-factor analysis of variance was used in the comparison among groups. Statistical significance was established at P < 0.05.
Results
Sh-Sy5y Viability
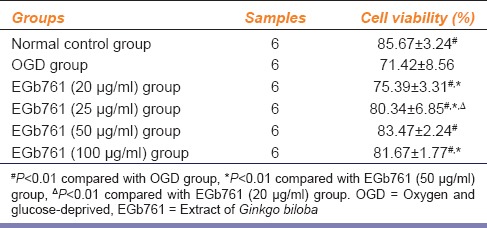
3-[4, 5-dimehyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide assay indicated that SH-SY5Y cell viability was decreased in OGD group. SH-SY5Y cell viability was significantly higher in EGb761-pretreated groups than in OGD group, particularly in moderate-concentration group (50 μg/ml) [Table 1]. SH-SY5Y cell viability was significantly higher in EGb761 (50 μg/ml) group when compared to EGb761 (20 μg/ml) and EGb761 (25 μg/ml) groups (P = 0.006 and P = 0.004), and in EGb761 (25 μg/ml) group than in EGb761 (20 μg/ml) group (P = 0.0028). However, there was no significant difference in SH-SY5Y cell viability between EGb761 (50 μg/ml) group and EGb761 (100 μg/ml) group (P = 0.1550). The results above suggest that 50 μg/ml is the most optimal dose for SH-SY5Y cells.
Table 1.
Effects of EGb761 on cell viability (x̄±s)
Apoptosis
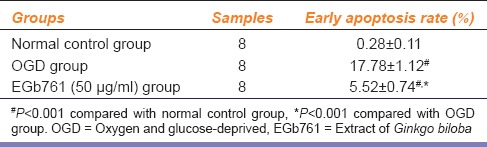
Apoptosis rate was significantly lower in EGb671-pretreated group (50 μg/ml) than in OGD group (P < 0.01) [Table 2].
Table 2.
Effects of EGb761 on apoptosis rate (x̄±s)
Apoptosis-inducing Factor Expression in Each Group
Cells in each group were respectively stained with rabbit anti-human AIF monoclonal antibody and tetramethyl rhodamine isothiocyanate-labeled goat anti-rabbit immunoglobulin G [Figure 1]. In normal control group, the cytoplasm of SH-SY5Y cells emitted red fluorescence, AIF expression was mainly in the cytoplasm. In OGD group, both cytoplasm and cell nucleus of SH-SY5Y cells emitted red fluorescence, AIF expression was mainly in cell nuclei, demonstrating that AIF underwent nuclear translocation and started apoptotic process. In EGb761-pretreated group, partial cell nuclei of SH-SY5Y cells were still lightly stained and AIF expression was in both cell nucleus and cytoplasm; but compared with OGD group, AIF expression in cell nucleus was less, demonstrating that EGb761 inhibited AIF nuclear translocation which inhibited apoptosis.
Figure 1.
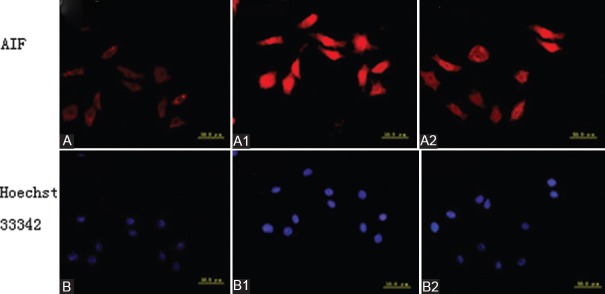
Immunofluorescent staining in each group (apoptosis inducing factor [AIF] nuclear translocation). (A) Cytoplasm emits red fluorescence in normal control group, AIF expression is mainly in cytoplasm and nuclei are olistherozone. (A1) Both cytoplasm and cell nuclei emit red fluorescence, but AIF expression is mainly in cell nuclei in oxygen and glucose-deprived group, and red fluorescence in the cytoplasm is weaker than that in normal control group, demonstrating that AIF nuclear translocation initiates ap-optosis. (A2) Both cytoplasm and cell nuclei emit red fluorescence in extract of Ginkgo biloba-pretreated group, but the red fluorescence in the nuclei is weaker than that in oxygen and glucose-deprived group. (B) Cell nuclei emit dispersed blue fluorescence after Hoechst 33342 staining in normal control group. (B1) Cell nuclei emit blue florescence after Hoechst 33342 staining, and the blue fluorescence is stronger in oxygen and glucose-deprived group than in normal control and Extract of Ginkgo biloba-pretreated groups. (B2) Cell nuclei emit blue fluorescence after Hoechst 33342 staining in extract of Ginkgo biloba-pretreated group, and the blue fluorescence in partial cell nuclei is weaker than that in oxygen and glucose-deprived group (AIF: Apoptosis inducing factor; OGD: Oxygen and glucose-deprived; EGb761: Extract of Ginkgo Biloba)
Discussion
The main pathological process of ischemic cerebrovascular disease is cerebral ischemia-reperfusion injury. There mainly is necrocytosis in the ischemic core and mainly apoptosis in the ischemic penumbra. Relieving neuron apoptosis is the key step for the treatment of ischemic cerebrovascular disease. SH-SY5Y cells, a kind of human neuroblastoma cell, are usually used as a substitution model of nerve cells because they have similar characteristics to nerve cells.[2,3,4] Many factors are involved in ischemic neuronal apoptosis, and its mechanism is complex. Poly adenosine diphosphate-ribose polymerase (PARP)/AIF signal transduction pathway, a pathway independent of caspase protein,[5] is an important apoptotic pathway. AIF-induced apoptosis is not associated with caspase protein. AIF exists in the mitochondrial membrane space to maintain mitochondrial activity and cell survival. In rat ischemic brain injury, AIF leads to karyopyknosis with lots of deoxyribonucleic acid (DNA) fragment.[6] Karyopyknosis is a sign of neuron apoptosis, demonstrating that AIF plays an important role in apoptosis. When apoptotic signals stimulate AIF, AIF is released from the mitochondrion and enters the cell nucleus, causing the chromatin condensation and DNA fragments.[7] Namely that only when apoptotic signals stimulate AIF, AIF enters the cell nucleus to start apoptotic process. Therefore, the study about PARP/AIF apoptotic pathway in cerebral ischemia-reperfusion injury has an important significance for finding effective drugs related to PARP/AIF apoptotic pathway.
Extract of G. Biloba can prevent cerebral ischemia-reperfusion injury through scavenging free radicals, inhibiting inflammatory reaction and apoptosis. Ni et al.[7] and Chen et al.[8] explored the effects of EGb against nerve cell apoptosis and found that hydroxyl radicals could lead to chromatin condensation, but EGb could prevent chromatin condensation, suggesting that EGb could inhibit the hydroxyl radical-induced apoptosis. Mdzinarishvili et al.[9] reported that EGb761 could relieve the brain ischemia-induced edema of nerve cells and reduce the release of glutamate. In this study, viability of OGD SH-SY5Y cells was decrease, but the viability of EGb761-pretreated SH-SY5Y cells was increased. With the increase in the dose of EGb761, the number of survival cells was increased in a certain range. Cell viability was significantly higher in EGb761 (50 μg/ml) group than in EGb761 (25 μg/ml) group (P = 0.006), but cell viability was similar between EGb761 (50 μg/ml) group (83.47 ± 2.24) and EGb761 (100 μg/ml) group (81.67 ± 1.77). Our results suggest that the best effective dose for cell protection was 50 μg/ml, so the dose of 50 μg/ml was used in the next steps.
Our results indicated that SH-SY5Y cell viability was significantly higher in EGb761 (50 μg/ml) group than in EGb761 (20 μg/ml) and EGb761 (25 μg/ml) groups (P = 0.006 and P = 0.0042), and in EGb761 (25 μg/ml) group than in EGb761 (20 μg/ml) group (P = 0.0028). However, there was no significant difference in SH-SY5Y cell viability between EGb761 (50 μg/ml) group and EGb761 (100 μg/ml) group (P = 0.1550). The results above suggest that with the increase in the dose of EGb761 the neuroprotective effects of EGb761 was enhanced, but this dose-effect relationship existed within a certain range, and 50 μg/ml is the most optimal dose of EGb761 for neuroprotective effects. Annexin V-fluorescein isothiocyanate/propidium iodide double staining flow cytometry indicated that early apoptosis rate was significantly lower in EGb761-pretreated group than in OGD group, suggesting that EGb761 had an inhibitory action on ischemia-induced SH-SY5Y apoptosis. Immunofluorescent staining indicated that in normal control group, the cytoplasm of SH-SY5Y cells emitted red fluorescence with pale cell nucleus, suggesting AIF was mainly in the cytoplasm; in OGD group, both cytoplasm and nucleus of SH-SY5Y cells emitted red fluorescence, but the red fluorescence in cell nuclei was stronger in OGD group than in normal control group, suggesting that cells were stimulated with apoptotic signals, AIF entered the cell nucleus to start apoptotic process; in EGb761-pretreated groups, AIF nuclear translocation occurred only in partial cells, suggesting that EGb761 could inhibit AIF nuclear translocation and had anti-apoptotic effects. AIF is located in the mitochondria and hence intracytoplasmic AIF mainly refers to mitochondrial AIF. AIF protein expression was low expression or no expression in normal control group, but was high expressions in OGD group and EGb761-pretreated groups, and was significantly lower in EGb761-pretreated groups than in OGD group, demonstrating that EGb761 exerts anti-apoptotic effects through inhibit AIF nuclear translocation. When the mitochondria is stimulated by apoptotic signals, permeability pores on the mitochondrial member open, and then AIF is released from the mitochondria and enters the cell nucleus with nuclear chromatin agglutination and DNA breakage.[10] We can see that only under the stimulations of apoptotic signals, AIF can be released from the mitochondria and enter the cell nucleus, initiating apoptosis. In short, the apoptosis caused by cerebral ischemia-reperfusion injury is associated with AIF translocation from the mitochondrion to the cell nucleus caused by the excessive activation of PARP and the depletion of intracellular nicotinamide adenine dinucleotide.
The limitations of the study are that the results are not compared with active control.
In summary, EGb761 has anti-apoptotic effects on OGD SH-SY5Y cells through inhibiting AIF nuclear translocation. This study provides a theoretical basis for the application of EGb761 in clinical practice.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Zhang LY, Wang YL. Effects of Ginkgo biloba extract on hippocampal synatic expression in rats. Chin J Pathophysiol. 2008;24:40–3. [Google Scholar]
- 2.Zhu Y, Hoell P, Ahlemeyer B, Sure U, Bertalanffy H, Krieglstein J. Implication of PTEN in production of reactive oxygen species and neuronal death in in vitro models of stroke and Parkinson's disease. Neurochem Int. 2007;50:507–16. doi: 10.1016/j.neuint.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J. Mitochondrial inhibition and oxidative stress: Reciprocating players in neurodegeneration. Antioxid Redox Signal. 2005;7:1117–39. doi: 10.1089/ars.2005.7.1117. [DOI] [PubMed] [Google Scholar]
- 4.Peroni D, Negro A, Bähr M, Dietz GP. Intracellular delivery of neuroglobin using HIV-1 TAT protein transduction domain fails to protect against oxygen and glucose deprivation. Neurosci Lett. 2007;421:110–4. doi: 10.1016/j.neulet.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Yu CJ, Wang CJ, Yang AG. AIF and AIF-dependent apoptosis. J Med Mol Biol. 2003;25:351–4. [Google Scholar]
- 6.Plesnila N, Zhu C, Culmsee C, Gröger M, Moskowitz MA, Blomgren K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:458–66. doi: 10.1097/00004647-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Ni YC, Zhao BL, Hou JW. Protection of Cerebellar neuron by Ginkgo biloba extract against apoptosis induced by hydroxyl radials. Neuron Sci Lett. 1996;214:115–8. doi: 10.1016/0304-3940(96)12897-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Wei T, Gao Z, Zhao B, Hou J, Xu H, et al. Different effects of the constituents of EGb761 on apoptosis in rat cerebellar granule cells induced by hydroxyl radicals. Biochem Mol Biol Int. 1999;47:397–405. doi: 10.1080/15216549900201423. [DOI] [PubMed] [Google Scholar]
- 9.Mdzinarishvili A, Sumbria R, Lang D, Klein J. Ginkgo extract EGb761 confers neuroprotection by reduction of glutamate release in ischemic brain. J Pharm Pharm Sci. 2012;15:94–102. doi: 10.18433/j3ps37. [DOI] [PubMed] [Google Scholar]
- 10.Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, et al. Apoptosis-inducing factor mediates poly (ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103:18314–9. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]


