Abstract
Objective:
Statins have anti-inflammatory effects that are not directly related to their cholesterol lowering activity. This study was carried out to evaluate the effect of simvastatin or rosuvastatin on the extent of colonic mucosal damage and on the inflammatory response in trinitrobenzene sulfonic acid (TNBS)-induced ulcerative colitis.
Materials and Methods:
Ulcerative colitis was induced by single intrarectal injection of 120 mg/kg TNBS. Test groups were treated with simvastatin (10 mg/kg, p.o.) or rosuvastatin (10 mg/kg, p.o.). Colonic mucosal inflammation was evaluated clinically, biochemically, and histologically.
Result:
Disease activity index score in TNBS-treated rats, as determined by weight loss, stool consistency, fecal occult blood, were significantly lowers in simvastatin or rosuvastatin-treated rats than TNBS-treated animals. Simvastatin or rosuvastatin counteracted the reduction in colon length, decreased colon weight, neutrophil accumulation, and tumor necrosis factor-alpha level in TNBS-induced colitis. Simvastatin and rosuvastatin also inhibited the increase in oxidative stress levels after TNBS administration.
Conclusions:
These results suggest that simvastatin and rosuvastatin significantly ameliorate experimental colitis in rats, and these effects could be explained by their anti-inflammatory and antioxidant activity.
KEY WORDS: Rosuvastatin, simvastatin, trinitrobenzene sulfonic acid, tumor necrosis factor-α, ulcerative colitis
Introduction
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), are immune-mediated chronic relapsing intestinal disorders, characterized by rectal bleeding and diarrhea resulting in disruption of the epithelial barrier and the formation of epithelial ulceration.[1] However, little is known about etiology of these diseases, it is believed to involve an abnormal host response to endogenous or environmental antigens or microbes, causing initial tissue injury followed by amplification of this response.[2,3] There is an evidence for intense local immune response associated with transmural infiltration of macrophages, lymphocytes, neutrophils, and mast cells, ultimately giving rise to mucosal disruption and ulceration.[4,5] Activation of these infiltrating cells results in the release of different pro-inflammatory mediators, plays a crucial role in tissue destruction and propagation of the inflammatory response.[6] Among these pro-inflammatory mediators, inflammatory cytokines play a crucial role in the development of UC. Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 β (IL-1 β), an IL-6 significantly increased in the colonic mucosa in UC. In particular, TNF-α is regarded as the most prominent “first-line” cytokines participating in the inflammatory process in UC.[7] Furthermore, prolong and chronic UC may progress to colorectal cancer.
Although extensive development has been made in the treatment of disease, no definitive therapies until now are available for this disorder, and limiting drug-induced toxicity is a continuous challenge. As a result, the current treatment options for the patient with either CD or UC are limited.[8,9] For this reason, there is a need to develop new strategies that in combination with safety, could restore the altered immune response that emerges in the inflamed intestine in these conditions.
Statins are known to be 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and are widely used to reduce cholesterol in patients with hyperlipidemia leading to cardiovascular morbidity.[10] Recent clinical studies have suggested that statins may also have anti-inflammatory and endothelial cell protective actions, independent of their antihyperlipidemic effects.[11] Statins also reduced highly sensitive C-reactive protein levels and vascular proinflammatory cytokines such as TNF-α, IL-1, and IL-6 in hyperlipidemia patients. Recently, one of the studies has shown that rosuvastatin reduced disease activity and colonic inflammation in dextran sulfate sodium (DSS)-induced colitis in mice.[12] Therefore, it was thought worthwhile to study the protective effect of simvastatin or rosuvastatin against colitis induced by trinitrobenzene sulfonic acid (TNBS) in rats.
Materials and Methods
Drugs and Chemicals
Rosuvastatin and simvastatin were obtained as a gift sample from Zydus Cadila (Ahmedabad, India). 2, 4, 6-TNBS was purchased from Sigma Co., (St Louis, USA). All other chemicals used were of analytical grade.
Experimental Animals
Female Wistar rats, 200–250 g, were placed in polypropylene cages in controlled room (temperature 24–25°C, humidity 35–60%, 12 h light/dark cycle) and were fed a CHAKKAN diet (Nav Maharashtra Oil Mills Pvt. Ltd., Pune). Rats were deprived of food for 12 h prior to the induction of colitis but were allowed free access to tap water throughout experiments. Experiments followed a protocol observed by Institutional Animal Ethics Committee of University and all experiments were in accordance with the approval of the Committee for the Purpose of Control and Supervision of Experiments on Animals.
Induction of Colitis
Colitis was induced according to the procedure described by Arita et al.[13] Briefly, rats were slightly anesthetized with ether, and then a flexible silicone plastic tube with an external diameter of 2 mm was inserted rectally into the colon. The tip was 8 cm proximal to the anus verge. TNBS (120 mg/kg) dissolved in ethanol (50%, v/v) was instilled into the colon via the cannula to induced acute colitis. To distribute the agents within the entire colon rats were held in a head-down position for 2–3 min after the instillation of TNBS. Control rats received 50% ethanol (0.5 ml) rectally.
Experimental Protocol
All the animals were divided into four groups (n = 6/group). Group 1 served as normal control and received no treatment and second group served as a colitis control and received only vehicle (0.5% sodium carboxymethylcellulose). Groups 3 and 4 were treated, respectively, with simvastatin (10 mg/kg, p.o.) or rosuvastatin (10 mg/kg, p.o.) daily from 5 days before to 7 days after rectal administration of TNBS. All treatments were given orally for 12 days. Administration of TNBS developed the colitis characterized by significant change in body weight score, stool consistency score, and rectal bleeding score. All animals were decapitated 7 days after TNBS administration. Colon was removed aseptically and placed on an ice-cold plate, longitudinally opened and cleaned from their luminal contents with cold saline. Afterward, the colonic segment was weighed, and its length measured. Then colon was fixed in 10% formaldehyde for histopathological examination.
Assessment of Disease Activity Index Score of Colitis
Colitis was assessed daily using disease activity index (DAI) based on a scoring system by Meerveld and Tyler.[14] This score reflects the body weight loss, stool consistency, and rectal bleeding. Occult blood in feces was assessed using Fecal Occult Blood Test Kit.
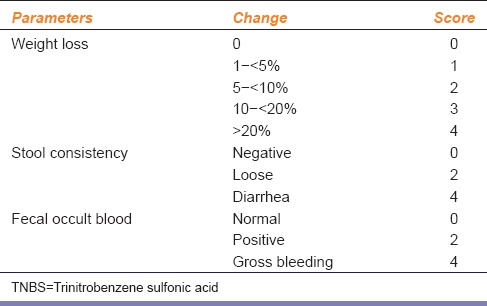
DAI = (combined score of weight loss, stool consistency, and bleeding)/3. Normal stool was well formed pellets; loose was pasty stools that do not stick to the anus; diarrhea was liquid stools that stick to the anus [Table 1].
Table 1.
Effect of TNBS on weight loss, stool consistency, and fecal occult blood (markers of disease activity index)
Measurement of Malondialdehyde, Superoxide Dismutase, and Reduced Glutathione
The colon tissues were removed and kept in cold conditions. The tissues were cross-chopped with a surgical scalpel into fine slices in chilled 0.25 M sucrose and homogenized in chilled 10 mM Tris-HCl buffer (10% w/v, pH 7.4). The homogenates were centrifuged at 10,000 ×g at 0°C for 30 min using Remi cooling centrifuge. The clear supernatant was used for the assay of malondialdehyde (MDA)[15], activity of endogenous antioxidant enzymes such as superoxide dismutase (SOD)[16] and reduced glutathione (GSH).[17]
Estimation of Myeloperoxidation Assay
Myeloperoxidation (MPO) activity in colonic tissue was determined as described.[18] The colon tissue was homogenized in 0.5% hexadecyltrimethylammonium bromide containing 50 mM potassium phosphate buffer (pH 6) using a polytron tissue homogenizer. After three freeze-thawing 3 times, the samples were centrifuged at 20,000 ×g for 15 min at 4°C, and the resulting supernatant was assayed spectrophotometrically for MPO activity. In brief, 0.1 ml of sample was mixed with 2.9 ml of 50 mM potassium phosphate buffer (pH 6) containing 0.167 mg/ml O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was then measured for 5 min using spectrophotometer. Myeloperoxidase activity data are presented as U/g tissue.
Determination of Tumor Necrosis Factor-Alpha by Enzyme-Linked Immunosorbent Assay
The TNF-α level in homogenized colon tissues was determined by quantitative enzyme-linked immunosorbent assay kits according to the manufacturer's instructions (Rat TNF-α kit, Endogen, USA).
Histopathology
The colon tissue was rapidly dissected out and washed with saline immediately and fixed in 10% buffered formaldehyde. Paraffin-embedded specimens were cut into 5 μm-thick sections and stained with hematoxylin and eosin. The sections were examined under the light microscope (Olympus Bx 10, Japan) for histopathological changes, and photomicrographs (Olympus DP12 camera, Japan) were taken. The pathologist performing histopathological evaluation was blinded to the treatment as assignment of different study groups.
Statistical Analysis
All the data are expressed as mean ± standard error of the mean. Statistical significance between more than two groups was tested using one-way ANOVA followed by the Bonferroni multiple comparisons test or unpaired two-tailed Student's t-test as appropriate using a computer-based fitting program (Prism, GraphPad 5).
Results
Effects of Simvastatin and Rosuvastatin on Disease Activity Index, Colon Length, and Colon Weight
We did not observe any mortality among the experimental groups. However, single dose administration of TNBS in colitis control group caused acute colitis and diarrhea followed by rectal bleeding and severe weight loss from day 6 to day 12. DAI score, an indicator of the severity of intestinal inflammation, was a significant increase in the colitis control group. In the simvastatin or rosuvastatin-treated groups, we did not observe bloody stools, stools were better formed, and weight loss was lessened. DAI score was significantly decreased in the rats treated with simvastatin or rosuvastatin than TNBS alone treated rats [Figure 1].
Figure 1.
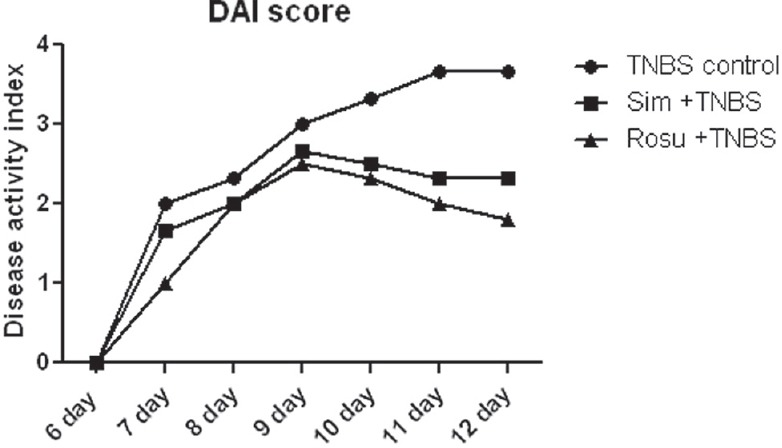
Effect of simvastatin or rosuvastatin on disease activity index score of trinitrobenzene sulfonic acid -induced colitis in rats
Intrarectal administration of TNBS produced severe inflammation in rat colon, with hemorrhagic spots and extensive disruption [Figure 2]. There was a significant (P < 0.001) decrease of colon length 7 days after TNBS administration as compared to control the group. In contrast, decreased in colon length after TNBS administration was significantly reversed by simvastatin or rosuvastatin [Figure 3a]. The colon weight was significantly (P < 0.001) increased after TNBS administration as compared to control group, while in treatment with simvastatin or rosuvastatin significantly (P < 0.05, P < 0.01) decreased in colon weight, respectively, as compared to TNBS alone treated rats [Figure 3b].
Figure 2.
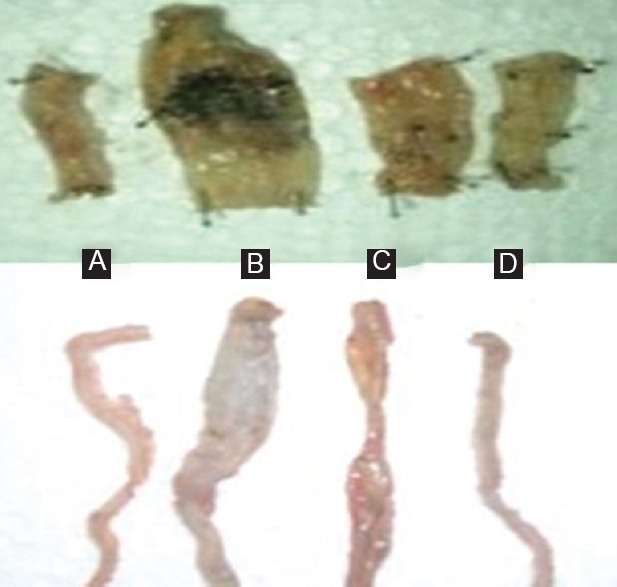
Effect of simvastatin or rosuvastatin on colon in colitic rats induced with trinitrobenzene sulfonic acid (TNBS). (A) Control rat colon; (B) TNBS control rat colon; (C) colitic rat treated with simvastatin (10 mg/kg, p.o.); (D) colitic rat treated with rosuvastatin (10 mg/kg, p.o.)
Figure 3.
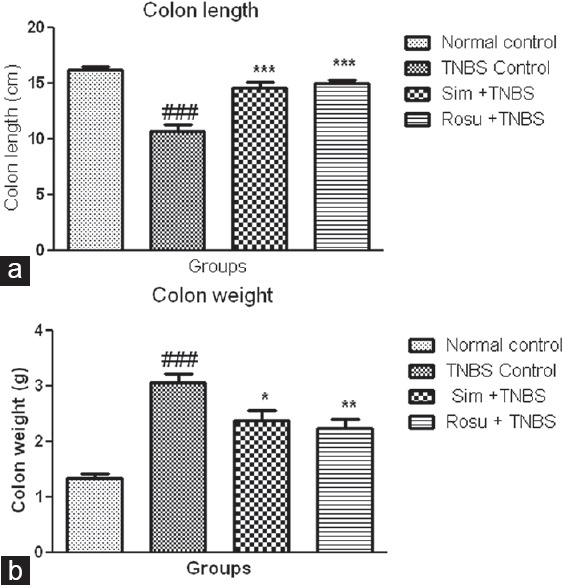
Effect of simvastatin or rosuvastatin on colon length and weight. Values are expressed as mean±SEM, n=6. ###P<0.001 as compared to control group, *P<0.05, **P<0.01, ***P<0.001 as compared to TNBS control group
Effects of Simvastatin or Rosuvastatin on Malondialdehyde, Superoxide Dismutase, and Glutathione in Colonic Tissue
The levels of MDA and GSH along with the activity of antioxidant enzyme SOD in normal and experimental rats are listed in Table 2. TNBS-treated rats showed a significant (P < 0.001) increase in the levels of MDA, end product of lipid peroxidation and marker of oxidative stress in colonic tissue as compared to control rats. TNBS-treated rats also showed a significant reduction (P < 0.001) in the levels of GSH, an endogenous antioxidant as compared to control rats. Activity of antioxidant enzyme SOD was significantly (P < 0.001) decreased in colon tissue of TNBS-treated rats as compared to control rats.
Table 2.
Effect of simvastatin or rosuvastatin on MDA, SOD, GSH, MPO, and TNF-α in colonic tissue
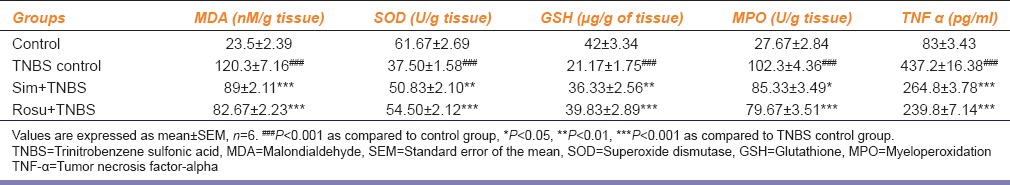
The treatment with simvastatin or rosuvastatin showed a significant reduction in oxidative stress level. The treatment with simvastatin showed a significant increase in SOD (P < 0.01), GSH (P < 0.01) activities and a significant decrease in MDA (P < 0.001) levels as compared to TNBS-treated group. The treatment with rosuvastatin showed a significant increase in SOD (P < 0.001), GSH (P < 0.001) activities and a significant decrease in MDA (P < 0.001) level as compared to TNBS-treated group [Table 2].
Effects of Simvastatin or Rosuvastatin on Tumor Necrosis Factor-Alpha and Myeloperoxidase Activity in Colonic Tissue
Trinitrobenzene sulfonic acid administration showed a significant (P < 0.001) increase the inflammatory marker such as colonic TNF-α level and MPO activity as compared to normal control rats. The treatment with simvastatin or rosuvastatin in TNBS-treated rats showed a significant decrease in colonic TNF-α (P < 0.01) and MPO activity (P < 0.05, P < 0.001), respectively, as compared to TNBS-treated rats [Table 2].
Effects of Simvastatin or Rosuvastatin on Histopathology of Colonic Tissue
Histopathological sections of the colon from the control group did not have any change. However, administration of TNBS showed gross ulcers and proliferous granulomas, necrosis of the epithelium (→) at mucosal surface and inflammatory cell infiltration. Animals treated with simvastatin or rosuvastatin there was a significant reduction of necrosis and inflammation of the epithelial cell [Figure 4].
Figure 4.
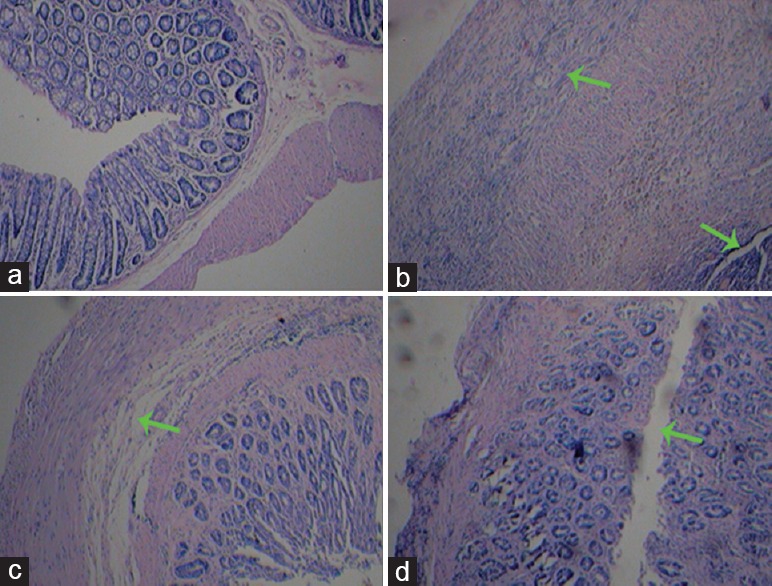
Histological appearance of colon mucosa: (a) Normal control; (b) trinitrobenzene sulfonic acid control; (c) colitic rat treated with simvastatin (10 mg/kg, p.o.); (d) colitic rat treated with rosuvastatin (10 mg/kg, p.o.)
Discussion
In the present study, treatment with simvastatin and rosuvastatin attenuates TNBS-induced colitis in rats. UC was assessed by measurement of DAI, colon length, colon weight, and histology. DAI score, an indicator of the severity of intestinal inflammation, was a significantly increased in the TNBS alone treated rats, while in simvastatin or rosuvastatin-treated groups, DAI score was significantly lower than TNBS control group. Both the statins significantly increased colon length and decreased the colon weight. However, simvastatin or rosuvastatin-treatment significantly inhibited colonic injury.
In present study, simvastatin or rosuvastatin-treatment showed a significant reduction in oxidative stress levels by virtue of increasing SOD, GSH, and reducing MDA. Therefore, antioxidant effect of both statins might be playing a significant role in the amelioration of TNBS-induced colitis.
The present study has shown that MPO activity, an index of tissue associated neutrophil accumulation, significantly increased in the colonic mucosa after TNBS administration. Treatment with simvastatin or rosuvastatin attenuated the increase MPO activity. These results indicate that the inhibition of neutrophil accumulation by both statins may be responsible for protecting TNBS-induced colonic mucosal injury. It was reported that pretreatment with simvastatin inhibited the increase in tissue MPO content in the experimental model in lungs ischemia-reperfusion injury.[19]
Recently, TNF-α has been shown to play a role in the pathogenesis of IBD. It was previously reported that the contribution of T-helper 1 responses in the generation of TNF-α in the DSS-induced colitis in mice.[20] It was also demonstrated that suppression of TNF-α gene expression by pioglitazone, accompanied by considerable inhibition of intestinal inflammation in DSS-induced colitis.[21] Therefore, we evaluated the effects of simvastatin or rosuvastatin on colonic inflammation after induction of colitis. In the present study, we confirmed that the reduction of mucosal TNF-α might be responsible for anti-inflammatory effects of both statins. In atherosclerotic mice, it was shown in the previous study that rosuvastatin inhibited the TNF-α expression in blood vessel wall.[22]
We conclude that antioxidants and anti-inflammatory properties of statins might be responsible in protection against TNBS-induced colitis. It was also concluded that rosuvastatin appears to be more effective in colitis than simvastatin. This result suggests that both statins may be an alternative therapeutic strategy for UC.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Da Silva MS, Sánchez-Fidalgo S, Talero E, Cárdeno A, da Silva MA, Villegas W, et al. Anti-inflammatory intestinal activity of Abarema cochliacarpos (Gomes) Barneby and Grimes in TNBS colitis model. J Ethnopharmacol. 2010;128:467–75. doi: 10.1016/j.jep.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen OH, Rask-Madsen J. Mediators of inflammation in chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1996;216:149–59. doi: 10.3109/00365529609094569. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 5.Katz JA, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15:291–7. doi: 10.1097/00001574-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kolios G, Petoumenos C, Nakos A. Mediators of inflammation: Production and implication in inflammatory bowel disease. Hepatogastroenterology. 1998;45:1601–9. [PubMed] [Google Scholar]
- 7.Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde KH, et al. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27:1743–50. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 8.Robinson M. Medical therapy of inflammatory bowel disease for the 21st century. Eur J Surg Suppl. 1998:90–8. doi: 10.1080/11024159850191517. [DOI] [PubMed] [Google Scholar]
- 9.Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996;334:841–8. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 10.Amarenco P, Labreuche J, Lavallée P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: Systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–9. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 11.Oda H, Keane WF. Recent advances in statins and the kidney. Kidney Int Suppl. 1999;71:S2–5. doi: 10.1046/j.1523-1755.1999.07101.x. [DOI] [PubMed] [Google Scholar]
- 12.Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, et al. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:997–1004. [PubMed] [Google Scholar]
- 13.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–6. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meerveld BG, Tyler KR. CPI-1189 protects against DSS-induced colitis in mice. Am J Pharmacol Toxicol. 2006;1:54–9. [Google Scholar]
- 15.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogeno alkanes or peroxidative reactions in rat liver fractions in vitro. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 17.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 18.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 19.Naidu BV, Woolley SM, Farivar AS, Thomas R, Fraga C, Mulligan MS. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg. 2003;126:482–9. doi: 10.1016/s0022-5223(03)00699-8. [DOI] [PubMed] [Google Scholar]
- 20.Kojouharoff G, Hans W, Obermeier F. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi T, Naito Y, Tomatsuri N, Handa O, Ichikawa H, Yoshida N, et al. Pioglitazone, a PPAR-gamma ligand, provides protection from dextran sulfate sodium-induced colitis in mice in association with inhibition of the NF-kappaB-cytokine cascade. Redox Rep. 2002;7:283–9. doi: 10.1179/135100002125000802. [DOI] [PubMed] [Google Scholar]
- 22.Kleemann R, Princen HM, Emeis JJ, Jukema JW, Fontijn RD, Horrevoets AJ, et al. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice: Evidence for antiinflammatory effects of rosuvastatin. Circulation. 2003;108:1368–74. doi: 10.1161/01.CIR.0000086460.55494.AF. [DOI] [PubMed] [Google Scholar]


