Abstract
Objectives:
This study was undertaken to elucidate the adverse effect of lead on female reproductive system following in vivo exposure in rats.
Materials and Methods:
Animals of Group II, III and IV received lead acetate in drinking water (30, 100 and 300 ppm, respectively) for 28 days whereas Group I served as control. Lead levels in digested blood and bone samples were measured using atomic absorption spectrophotometer.
Results:
Marked and a significant decrease in per cent body weight gain was observed in rats of Group IV and III, respectively, compared to that in the control group. Relative uterine weights were found to decrease by 27% in Group III and IV compared to control and low dose lead treated (30 ppm) rats. Lead levels were found to increase in a linear manner in blood along with a marked increase in bone levels in 100 ppm exposure group while there was a decrease in both the blood and bones levels at 300 ppm exposure. Compared to plasma progesterone levels in rats of the control group, a nonsignificant (12.46–21.13%) reduction in plasma progesterone were observed in different lead-treated groups. No apparent gross pathological lesions were observed in any of the vital organs, including uterus. However, histopathological examination of uteri of different groups revealed lead-induced dose-dependent inflammatory changes, which were characterized by thickening of the endometrium, narrowing of uterine lumen, damage to endometrial glands and vacuolar degeneration in endometrial epithelial cells.
Conclusion:
Findings of this study suggest lead-induced pathophysiological alterations in myometrium, which in turn may affect the reproductive efficiency of animals.
KEY WORDS: Blood, bones, histopathology, inflammation, lead, uterus
Introduction
Among the several factors responsible for infertility and consequential productivity losses in dairy animals, malnutrition, ovulatory or hormonal imbalances and infectious agents have been recognized as the major ones. However, information on impact of environmental toxicants on female reproduction and how these affect reproduction is almost obscure. Adverse effects like altered spermatogenesis, increased sperm pathologies and testicular degeneration due to lead have been studied well in males,[1] however, studies on female reproductive toxicology are very less and differ from males due to differences in gametogenesis, access of the germinal cells and also because of the revolving nature of female breeding function.[2,3] Severe cases of lead poisoning have been reported to be associated with sterility, miscarriage, abortion, premature delivery, and infant mortality.[4] Lead crosses the placenta during pregnancy and has been associated with intrauterine deaths, prematurity, and low birth weight.[5] In experimental animals, chronic exposure to lead may cause inhibition of menstruation, ovulation and follicular growth in monkeys,[5] delay in vaginal opening in pubertal rats[6] and decrease in frequency of implanted ova and of pregnancies in mice.[7] In primates, prolonged exposure to lead blocks ovarian and luteal functions by reducing progesterone, luteinizing hormone and follicle-stimulating hormone levels.[8] Lead-induced reduction in number of primordial follicle and increase in number of atretic follicles have been reported in ovaries of mice[9] while in uterus, it damages endometrium, myometrium and perimetrium, along with reduction in uterine gland and decrease in height of columnar cells in mice.[10] In view of the limited laboratory studies on impact of lead on female reproductive system in rats, present study was undertaken in female rats by exposing them to three different lead levels selected based on biomonitoring studies in cows and buffaloes in and around Mathura.[11]
Materials and Methods
Experimental Animals
Healthy adult female Wistar rats were procured from the Laboratory Animal Resource Section, Indian Veterinary Research Institute, Izatnagar, Bareilly and acclimatized in departmental animal house under standard managemental and feeding conditions before starting the experiment. Animals (100–120 g) were divided into four groups; Group I contained six animals while Groups II, III and IV contained eight animals each. Group I was kept as control and rats received triple distilled water only while rats of Group II, III and IV received lead acetate at 30 ppm, 100 ppm and 300 ppm in triple distilled water (w/v), respectively continuously for 28 days. Animals of all the groups had free access to respective drinking water (s). These three dose levels of lead (30, 100 and 300 pm) for the present study were selected based on an earlier study undertaken in our laboratory, where rats were exposed to lead in drinking water at 100 ppm level for 28 days and lead-treated rats were found to have the plasma levels of 2.20 ppm (unpublished observation). Further, cows and buffaloes around Mathura have been observed by us to have the circulating blood lead levels of more than 1.00 ppm (1.37 ± 0.24 ppm in cows and 1.10 ± 0.10 ppm in buffaloes). In view of the above and also to simulate the circulating blood levels of more than 1.0 ppm in experimental rats, we had selected one higher dose and one lower dose of lead for the present study to investigate the toxic effects of lead on rat myometrium and hormonal status.
Blood samples were collected from the retro-orbital plexus on 0, 15th and 29th day in heparinized tubes for the detection of lead levels whereas, at the end of the experiment, all the animals were sacrificed under xylazine-ketamine anesthesia. Uteri were collected for determining its absolute and relative weights and tibia for lead levels. Thereafter, small pieces of uterus were collected in 10% formal saline for histopathological studies. Experimental protocol was approved by the Institutional Animals Ethics Committee as per the provision of CPCSEA of Government of India.
Lead Levels in Blood and Bone
Digestion of blood samples was done by adding heparinized blood samples to concentrated nitric acid (1:1) in a digestion tube and kept overnight at room temperature. Digestion was carried out slowly at low temperature (<90°C) using micro-digestion bench to reduce the volume to 0.5 ml. The volume was adjusted to 5 ml by adding double acid mixture (nitric acid and 70% perchloric acid, 3:1) and digested slowly till white fumes emanated from it. The final volume was adjusted to 10 ml by adding triple distilled water.[12]
For digestion of bone samples, whole tibia was cleaned and dried in hot air oven before weighing and grinding it in a crucible cup, which was kept on a heater for 15–30 min to make it smoke free. The bone ash (1 g), prepared in muffle furnace (550°C for 2 h), was mixed with 1 ml concentrated nitric acid and heated on a hot plate to dry. After cooling, 2 ml concentrated hydrochloric acid was added and kept for 15 min. The resultant mixture was then filtered through Whatman filter paper (No. 1) and the final volume was adjusted to 50 ml by adding triple distilled water.[13]
Lead levels in digested blood and bone samples were measured using atomic absorption spectrophotometer (Model AAS 400; Perkin Elmer, USA).
Plasma Progesterone Levels
Progesterone (P4) levels in plasma samples of different groups of rats were determined using commercially available ELISA kit (Labserv, Fisher Scientific, India).
Histopathological Examination
Uteri of different groups of rats were processed for routine histopathological examination. Approximately, 5–6 μm thick sections were prepared and stained with hemotoxyline and eosin following standard staining technique and examined under light microscope.
Statistical Analysis
Results are expressed as mean ± standard error of the mean and analyzed by one-way ANOVA, followed by Tukey's post-hoc test using SPSS version 19 (SPSS Inc., IBM).
Results
Effect of Lead on Body and Uterine Weights
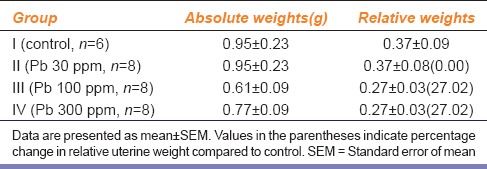
Apparently, no untoward clinical signs were observed in rats of any of the three lead-treatment groups. Per cent change in body weight gain in animals of different experimental groups on days 0, 7, 14, 21 and 28 are presented in Table 1. Absolute and relative weights of uterus as well as per cent change in uterine weights of rats of different groups are summarized in Table 2. Compared with the control group, there was 27% decrease in relative uterine weights in rats of Groups III and IV, but it was not statistically significant.
Table 1.
Effect of different lead treatments on percentage of body weight gain in female rats
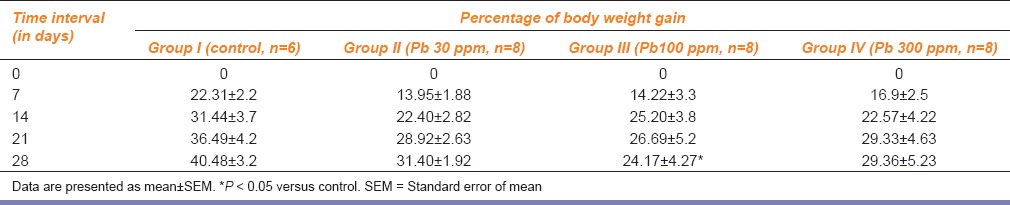
Table 2.
Effect of different lead-treatments on absolute and relative weights of rat uterus
Effect on Blood and Bones Lead Levels
Blood lead levels after 14 and 28 days exposure in rats of different treatment groups (30, 100 and 300 ppm) are illustrated in Figure 1 while lead levels in bone in Figure 2.
Figure 1.
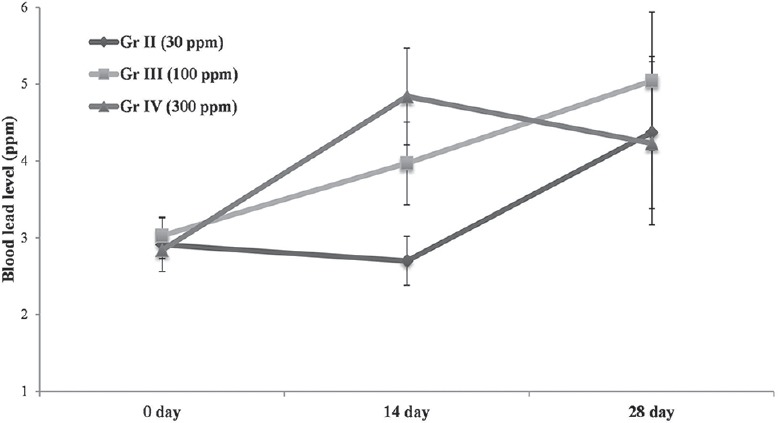
Blood lead levels in rats after 14 and 28 days of exposure at 30, 100, 300 ppm in drinking water. Data are presented as mean ± standard error of the mean (SEM) vertical bars represents SEM
Figure 2.
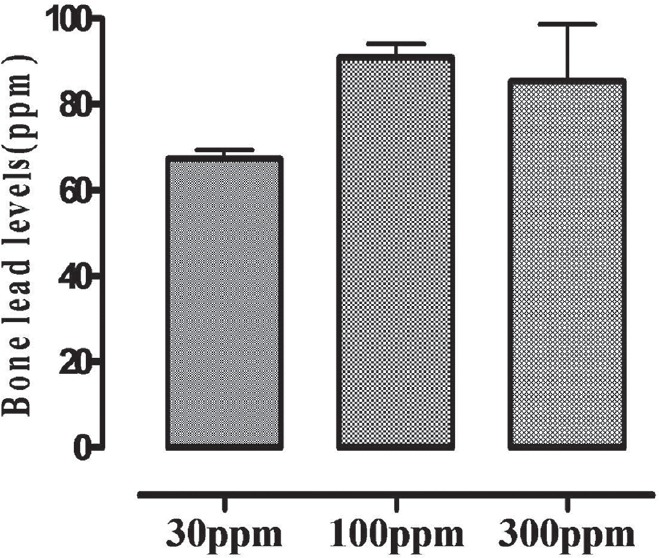
Bone lead levels in rats following 28 days post-exposure at 30, 100, 300 ppm in drinking water. Data are presented as mean ± standard error of the mean (SEM) vertical bars represents SEM
Effect of Lead on Plasma Progesterone Levels
Compared to the control group (10.03 ± 3.93 ng/ml; n = 4), plasma progesterone levels did not differ significantly between different lead-treatment groups, however, these values were markedly lower in 30 and 100 ppm lead-treated groups as the respective values in these groups were found to be 8.78 ± 2.52 ng/ml and 7.91 ± 1.40 ng/ml. However in 300 ppm group, plasma progesterone level was surprisingly almost comparable to that in control group.
Histopathological Studies
Alterations in cellular architecture of uterus of different lead-treatment groups are shown in Figures 3–5. Compared to the uteri of control group, uterus of rats treated with 30 ppm lead acetate (Group II) revealed uniform thickening of endometrium with projections leading to narrowing of lumen, besides chronic inflammation and damage to endometrial glands [Figure 3a and b]. There was extensive thickening of endometrium with projections and marked narrowing of the lumen in rats of Group III (100 ppm). In addition, vacuolar degeneration in endometrial epithelial cells, cystic degeneration of goblet cells, damage to endometrial glands and chronic inflammation were also observed as shown in Figure 4a and b. Uterine inflammatory changes in rats of Group IV (300 ppm) were more severe and characterized by massive thickening of the endometrium with projections and conspicuous narrowing of lumen. In addition, there was sloughing off of the lining endometrial epithelial cells, damage to endometrial glands and marked chronic inflammation as shown in Figure 5.
Figure 3.
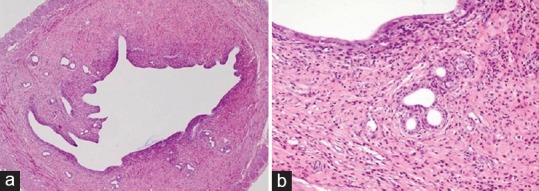
Uterine section of rats showing thickening of endometrium with projections in lumen (a), and chronic endometritis with damage of endometrial glands (b) after exposure to lead at 30 ppm level for 28 days (H and E, ×4 and ×20)
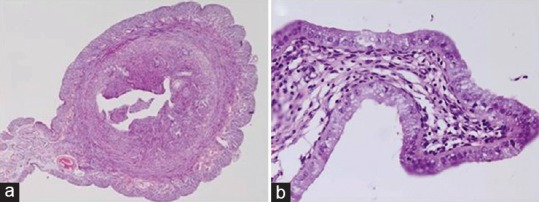
Figure 5.
Uterine section of rats showing marked inflammation leading to narrowing of lumen (a), chronic endometritis with sloughing off of the lining epithelium and damage to glands (b) and severe vacuolar degeneration in the lining endometrial cells (c) after exposure to lead at 300 ppm level for 28 days (H and E, ×4, ×20 and ×40)
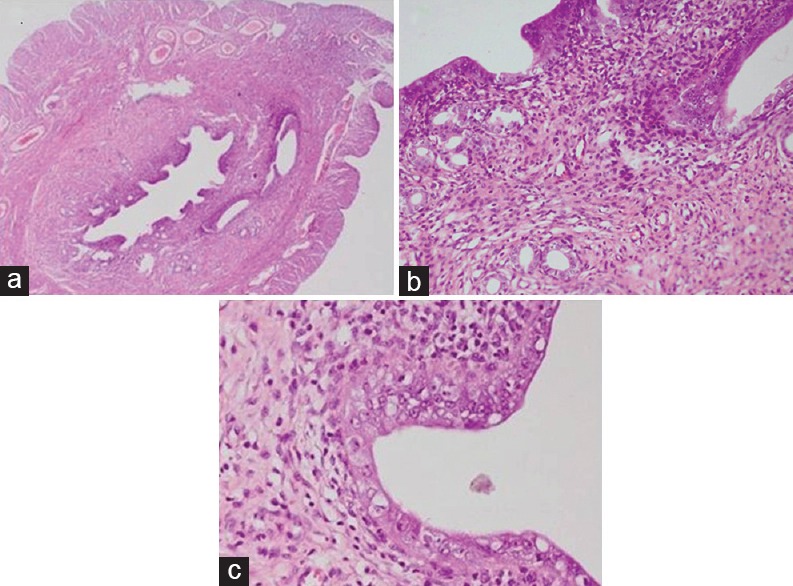
Figure 4.
Uterine section of rats showing chronic endometritis and thickening of endometrium leading to narrowing of the lumen (a) and vacuolar degeneration in endometrial epithelial cells and damage to endometrial glands (b) after exposure to lead at 100 ppm for 28 days (H and E, ×4 and ×40)
Discussion
Oral exposure is one of the important routes of lead entry in humans and animals. Gastrointestinal absorption of lead is around 40% in children and 10% in adults while it is around 10% in nonruminants and <3% in ruminants.[14] After absorption, almost 99% lead binds to erythrocytes and the remaining diffuses into soft tissues and bones, where it equilibrates with blood lead and the lead accumulated in erythrocytes, soft tissues and rapidly growing bones is mostly responsible for its toxic effects.[15]
In the present investigation, compared to control group, 28 days lead exposure produced significant (P < 0.05) reduction in per cent body weight gain only in rats of Group III (100 ppm), while decrease in body weight in other lead-treated Groups (II, IV) was not significant. Reduction in body weights or body weight gain after exposure to lead may be attributed to its influence on feeding behavior via central nervous system or secretion of growth hormone.[16]
Following 28 days exposure to lead, linear increase in blood lead levels was observed in rats of Group III (100 ppm), but at higher exposure level (300 ppm), no further increase in blood levels was observed; rather there was sharp decline in blood lead levels after 14 days onward, thus suggesting maximal lead levels at 100 ppm exposure level. Lead levels in tibia also reflected maximal accumulation at 100 ppm dose level. These observations suggest that possibly beyond 100 ppm exposure levels, body does not bio-accumulate any more lead, and additional lead load may be excreted through urine and bile.[15]
Reproductive impairment like intra-uterine growth restriction, preterm delivery, and spontaneous abortions have been reported in women even with low-to-moderate blood lead levels.[17] Dearth et al.[18] have reported that prepubertal females exposed maternally to low levels of lead exhibited suppressed circulating levels of estradiol-17 β while Franks et al.[8] reported decrease in plasma progesterone concentration. In our study too, compared to control group (10.03 ± 3.93 ng/ml), marked reduction in plasma progesterone levels was observed in rats of Group II (8.78 ± 2.52 ng/ml) and III (7.91 ± 1.40 ng/ml) following 28 days exposure to lead at 30 and 100 ppm, respectively. Decrease in serum progesterone level following lead-treatment has been attributed to increasing in activities of five β-reductase, a progesterone-metabolizing enzyme, in liver and uterine homogenates.[19]
Relative uterine weight was reduced by about 27% in rats of Group III (100 ppm) and Group IV (300 ppm), but decline was not statistically significant. Our observations are somewhat in conformity with the observations of Wiebe et al.[20] who too failed to observe any significant changes in the ovary, uterus and fallopian tubes weights in rats exposed to lead during pregnancy and lactation. But, Dumitrescu et al.[21] observed progressive decline in uterine weight along with fallopian tube following 6 months exposure to lead at 100 and 150 ppb. Differences between our study and those reported by Dumitrescu et al.[21] may be attributed to the difference in dose and duration of lead exposure.
Histopathological studies on uteri of different treatment groups in the present study revealed its dose-dependent deleterious effects on myometrium. Cystic degeneration of goblet cells and sloughing off of endometrial lining epithelial cells was observed in uteri following exposure at 100 and 300 ppm levels. Degenerative and inflammatory changes in uterus including decrease in height of columnar cells and undistinguished areas of blood vessels, lymphatics and connective tissues have been documented by Qureshi and Sharma.[10] In utero or gestational exposure to lead has been reported to cause necrosis of uterine glands and destruction of uterine lining cells.[22] Importance of endometrial glands and their secretions in maintaining estrous cycles, conceptus survival and growth at the peri-implantation stage has been reported by Gray et al.[23] Therefore, lead may produce deleterious effect on in-utero physiological functions by altering uterine glandular secretions like enzymes, growth factors, cytokines, lymphokines, hormones and transport proteins essential for development of conceptus[24] or may even alter dynamics of uterine membrane receptors and ion channels;[25] studies in these aspects are in progress.
Based on the results of present study, it is inferred that accumulation of lead in blood and long bones occurs maximally at 100 ppm level it produces subtle utero-toxic effects which are likely to affect reproduction even without any apparent observable toxic effects.
Footnotes
Source of Support: Financial assistance from Indian Council of Agricultural Research, New Delhi, under Niche Area of Excellence Programme (Grant No. 10(10)/2012-EPD, dated 23rd March, 2012) is thankfully acknowledged.
Conflict of Interest: No.
References
- 1.Assennato G, Paci C, Baser ME, Molinini R, Candela RG, Altamura BM, et al. Sperm count suppression without endocrine dysfunction in lead-exposed men. Arch Environ Health. 1987;42:124–7. doi: 10.1080/00039896.1987.9935808. [DOI] [PubMed] [Google Scholar]
- 2.Pinon-Lataillade G, Thoreux-Manlay A, Coffigny H, Monchaux G, Masse R, Soufir JC. Effect of ingestion and inhalation of lead on the reproductive system and fertility of adult male rats and their progeny. Hum Exp Toxicol. 1993;12:165–72. doi: 10.1177/096032719301200213. [DOI] [PubMed] [Google Scholar]
- 3.Pinon-Lataillade G, Thoreux-Manlay A, Coffigny H, Masse R, Soufir JC. Reproductive toxicity of chronic lead exposure in male and female mice. Hum Exp Toxicol. 1995;14:872–8. doi: 10.1177/096032719501401103. [DOI] [PubMed] [Google Scholar]
- 4.Gerhard I, Waibel S, Daniel V, Runnebaum B. Impact of heavy metals on hormonal and immunological factors in women with repeated miscarriages. Hum Reprod Update. 1998;4:301–9. doi: 10.1093/humupd/4.3.301. [DOI] [PubMed] [Google Scholar]
- 5.Vermande-Van Eck GJ, Meigs JW. Changes in the ovary of the rhesus monkey after chronic lead intoxication. Fertil Steril. 1960;11:223–34. doi: 10.1016/s0015-0282(16)33730-x. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel CA, Grant LD, Sloan CS, Gladen BC. Chronic low-level lead toxicity in the rat. I. Maternal toxicity and perinatal effects. Toxicol Appl Pharmacol. 1980;56:28–41. doi: 10.1016/0041-008x(80)90129-5. [DOI] [PubMed] [Google Scholar]
- 7.Odenbro A, Kihlström JE. Frequency of pregnancy and ova implantation in triethyl lead-treated mice. Toxicol Appl Pharmacol. 1977;39:359–63. doi: 10.1016/0041-008x(77)90130-2. [DOI] [PubMed] [Google Scholar]
- 8.Franks PA, Laughlin NK, Dierschke DJ, Bowman RE, Meller PA. Effects of lead on luteal function in rhesus monkeys. Biol Reprod. 1989;41:1055–62. doi: 10.1095/biolreprod41.6.1055. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Garu U, Panwar K. Developing gonads and lead exposure. World J Environ Biosci. 2012;1:30–7. [Google Scholar]
- 10.Qureshi N, Sharma R. Lead toxicity and infertility in female swiss mice: A review. J Chem Biol Physi Sci. 2012;2:1849–61. [Google Scholar]
- 11.Nakade UP. Mathura: 2013. Lead-induced adrenoceptors modulation in rat and buffaloes myometrium. MVSc Thesis Submitted to U.P. Pandit Deen Dayal Upadhyaya Pashu Chikitsa Vigyan Vishwavidyalaya Evam Go-Anusandhan Sansthan. [Google Scholar]
- 12.Kolmer JA, Spaulding EH, Robinson HW. 5th ed. New York: Aplenton Century Crofts; 1951. Approved Laboratory Techniques. [Google Scholar]
- 13.Francek MA. Soil lead levels in a small town environment: A case study from Mt Pleasant, Michigan. Environ Pollut. 1992;76:251–7. doi: 10.1016/0269-7491(92)90144-y. [DOI] [PubMed] [Google Scholar]
- 14.Washington, D.C: National Academy Press; 1972. National Research Council. Lead in Perspective. [Google Scholar]
- 15.Habal R. Lead Toxicity. 2006. Available from: http://www.emedicine.com/MED/topics1269.htm .
- 16.Huseman CA, Varma MM, Angle CR. Neuroendocrine effects of toxic and low blood lead levels in children. Pediatrics. 1992;90:186–9. [PubMed] [Google Scholar]
- 17.Srivastava S, Mehrotra PK, Srivastava SP, Tandon I, Siddiqui MK. Blood lead and zinc in pregnant women and their offspring in intrauterine growth retardation cases. J Anal Toxicol. 2001;25:461–5. doi: 10.1093/jat/25.6.461. [DOI] [PubMed] [Google Scholar]
- 18.Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR, Dees WL. Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. Reprod Toxicol. 2002;16:343–52. doi: 10.1016/s0890-6238(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 19.Abdou HM, Newairy AA. Hepatic and reproductive toxicity of lead in female rats and attenuation by flaxseed. J Med Res Inst. 2006;27:295–302. [Google Scholar]
- 20.Wiebe JP, Barr KJ, Buckingham KD. Effect of prenatal and neonatal exposure to lead on gonadotropin receptors and steroidogenesis in rat ovaries. J Toxicol Environ Health. 1988;24:461–76. doi: 10.1080/15287398809531177. [DOI] [PubMed] [Google Scholar]
- 21.Dumitrescu E, Alexandra T, Muselin FL. The consequences of chronic lead acetate intake on exposure and morphological integrity biomarkers (lead level and weight of sexual organs) in female rats. Lucr Şt Med Vet Timişoara Luc Stii Medi Veteri. 2008;XLI:619–22. [Google Scholar]
- 22.Eugenia D, Alexandra T, Argherie D, Cristina RT. The consequences of in utero exposure to lead acetate on exposure and integrity biomarkers of reproductive system in female rats at sexual maturity. Lucr Şt Med Vet Timişoara Luc Stii Medi Veteri. 2009;XLII:295–300. [Google Scholar]
- 23.Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction. 2002;124:289–300. [PubMed] [Google Scholar]
- 24.Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82:E4–13. doi: 10.2527/2004.8213_supplE4x. [DOI] [PubMed] [Google Scholar]
- 25.Wiebe JP, Barr KJ. Effect of prenatal and neonatal exposure to lead on the affinity and number of estradiol receptors in the uterus. J Toxicol Environ Health. 1988;24:451–60. doi: 10.1080/15287398809531176. [DOI] [PubMed] [Google Scholar]


