Abstract
Objectives:
The objective was to investigate the protective effects of rhein on renal histology change and the effects of rhein on renal tissue toll-like receptor (TLR) 4, TLR9, transforming growth factor-β1 (TGF-β1) expression in immunoglobulin A nephropathy (IgAN) rats.
Materials and Methods:
Bovine serum albumin-lipopolysaccharide-carbon tetrachloride 4 method was used to establish IgAN model. Thirty-two male sprague dawley rats were randomly divided into the control group, IgAN model group, rhein-prevented group, and rhein-treated group. 24-h urinary protein (UP), creatinine, urea, alanine aminotransferase (ALT), total protein (TP) contents in the serum of rats were detected with automatic biochemical analyzer and renal pathological changes were observed by the hematoxylin and eosin and periodic acid-Schiff staining. The glomerular deposition of IgA was measured by immunofluorescence staining. Real-time polymerase chain reaction and immunohistochemistry were used to detect renal tissue contents of TLR4, TLR9, TGF-β1 messenger ribonucleic acid and protein expression.
Results:
The biochemical parameters results of IgAN model rats showed that the 24-h UP excretion and ALT concentration were much higher, and TP concentration was much lower than those of the control group (P < 0.05). Granule-like or mass-like IgA depositions in the mesangial area, glomerular hypercellularity, hyperplasia of mesangial matrix, and tubulointerstitial fibrosis were found in IgAN group. Rhein-prevented and rhein-treated both improved the biochemical parameters and relieved renal pathological injury. The expressions of renal tissue TLR4, TGF-β1, but not TLR9 were significantly elevated in IgAN model rats (P < 0.05). Rhein-prevented and rhein-treated both inhibited TLR4 and TGF-β1 expressions.
Conclusion:
Rhein significantly improved the serum and urine biochemical parameters, and attenuated the glomerular pathological changes and tubulointerstitial fibrosis in IgAN rats. The mechanism may involve inhibition of renal TLR4 and TGF-β1 secretion.
KEY WORDS: Immunoglobulin A nephropathy, rhein, toll-like receptor, transforming growth factor-β1
Introduction
Immunoglobulin A nephropathy (IgAN) is probably the most frequently occurring primary glomerulonephritis worldwide, accounting for 39.6–44.6% of patients with the primary glomerulonephritis in China.[1] Moreover, IgAN is one of the most important causes of end-stage renal disease (ESRD). Especially patients with IgAN of Pacific Asian origin have a higher risk of progression to ESRD than others.[2] In China, 36% of adult patients with IgAN progress to ESRD within 20 years.[3] It is widely recognized that renal histology changes determine IgAN progress to ESRD, such as mesangial and capillary endothelium cells proliferation, especially the glomerulosclerosis, tubulointerstitial fibrosis.[4] The pathogenesis of IgAN is complicated and remains to be fully elucidated, and no specific treatment exists. Delaying renal histology progress and inhibiting chronic fibrotic changes become an important strategy for treatment of IgAN. However, there is no accepted therapy for this problem till now. Rhein (4,5-dihydroxyanthraquinone-2-carboxylic acid), an anthraquinone compound, isolated from rhubarb, has been broadly used in the treatment of diabetic nephropathy and other chronic renal diseases.[5,6,7] In previous study, it is demonstrated that rhein prevents the development of glomerulosclerosis and halts the progression of IgAN via inhibition of fibronectin (FN) and α-smooth muscle actin (α-SMA) expression.[8] It is well-known that toll-like receptors (TLRs) have been reported to be involved in the pathogenesis of IgAN.[9,10] TLR4 promotes mesangial cell injury and renal fibrosis by induction of pro-inflammatory cytokines, profibrotic molecule, and transforming growth factor-β1 (TGF-β1) signaling in Chronic Kidney Disease.[11,12,13] Thus, blocking of TLR4-mediated inflammatory and profibrotic signals is a promising strategy for treatment of IgAN. In the present study we explored the protective effects of rhein on renal histology changes and the effects of rhein on renal tissue TLR4, TLR9, TGF-β1 expression in IgAN rats.
Materials and Methods
Materials
Rhein (the purity of more than 95%) was extracted and identified by Chengdu Mansite Pharmaceutical Co. Ltd, China; fluorescein isothiocyanate (FITC)-conjugated mouse against rat IgA antibody was purchased from Antigenix, USA; Antibodies rabbit against rat TLR4, TLR9, and TGF-β1 were purchased from Abcam, UK; bovine serum albumin (BSA) and lipopolysaccharide (LPS) were purchased from Solarbio, China; carbon tetrachloride (CCl4) and castor oil were purchased from Shanghai Reagents, China; Horseradish peroxidase-conjugated goat against rabbit secondary antibodies were purchased from Beijing Zhongshan, China.
Animal Model
Thirty-two male Sprague-Dawley rats (specific pathogen free) weighing 180–220 g were purchased from the Experimental Animal Center of Nanchang University. Rats were given free access to food and water. Animals were treated humanely by use of protocols approved by the Institutional Animal Use and Care Committee of Nanchang University. Rats were randomly divided into a control group, IgAN group, Rhein-prevented group and Rhein-treated group (n = 8 each). The IgAN experimental animal model was established by administration of BSA, LPS and CCl4 as described previously.[14] In brief: BSA was intragastrically administered at 400 mg/kg once on alternate days for 6 consecutive weeks, 0.05 mg of LPS was given at the 6th week and the 8th week by intravenous injection and 0.1 mL CCl4 dissolved in 0.5 mL castor oil was given once weekly for 9 weeks by subcutaneous injection. The Rhein-treated rats were given rhein (100 mg/kg/day) from the 7th week up to 10th week. The Rhein-prevented group received rhein (100 mg/kg/day) from the 1st week to the 6th week. The control and IgAN groups were given the same volume of normal saline. All the rats were sacrificed with method of barbiturate overdose intravenously at 10th week.
Serum and Urine Sampling
The 24-h urine was collected using metabolic cages before sacrificing for measuring the volume of urine and 24-h urinary protein (UP) excretion. 3 ml serum samples from each rat were collected by abdominal aortic method at 10th week for measuring the concentration of alanine aminotransferase (ALT), urea, creatinine, and total protein (TP). The above parameters were measured using standard laboratory methods with the automatic biochemical analyzer (Hitachi 7500, Japan).
Histology Examination
Three μm thickness kidney sections from paraformaldehyde (4%)-fixed, paraffin-embedded tissues were used to perform hematoxylin and eosin (H and E), and periodic acid-Schiff (PAS) staining for general histology observation.
Direct immunofluorescence staining was performed as described previously.[8] In brief: Frozen kidney sections fixed with cold acetone for 10 min at 4°C were treated with 10% normal sheep serum in phosphate buffer sodium (PBS) to block nonspecific reaction and then incubated with the FITC-conjugated mouse against rat IgA antibodies (1:100) overnight at 4°C. Stained kidney sections were viewed under fluorescence microscope, and the description of results is represented in Table 1.
Table 1.
IgA immunofluorescence staining of the kidney tissue

Immunohistochemical Studies
This assay was performed as described previously.[8] Briefly, paraffin-embedded kidney sections were processed in sodium citrate buffer (0.01 mol/L, potential of hydrogen 6) for 15 min at maximum power in the microwave. The sections were incubated with 3% H2 O2 deionized water at room temperature for 10 min and treated with 10% normal goat serum at room temperature for 20 min to block endogenous peroxidase and nonspecific reaction. Then, the sections were incubated with rabbit antirat TLR4 (1:100), TLR9 (1:100), TGF-β1 (1:50) overnight at 4°C. After washing three times with PBS, sections were incubated with horseradish peroxidase-conjugated goat against rabbit secondary antibodies and incubated with diaminobenzidine and hydrogen peroxide until brown bindings were visualized. The stained sections were viewed with an optical microscope equipped with a digital camera and were analyzed by a morphological analysis system for semi-quantitatively determining the expression of brown bindings. The integrated optical density represented the quantity of positive material.
Ribonucleic Acid Isolation and Real-time Polymerase Chain Reaction
Total ribonucleic acid (RNA) of the renal cortex was extracted using Total RNA Kit (Omega, USA), according to the manufacturer's instructions. Then, 5 μl of total RNA was reverse-transcribed to complementary deoxyribonucleic acid (cDNA) using a Reverse Transcription kit (Trans, China), according to the manufacturer's instructions. Real-time polymerase chain reaction (RT-PCR) using SYBR® Premix Ex Taq™ (Takara, JP) was performed to measure the messenger RNA (mRNA) levels of TLR4, TLR9, TGF-β1, and β-actin for each sample in triplicate on an Applied Bio systems 7500 RT-PCR system. The reaction conditions were a single cycle of 95°C for 30 s followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. The relative mRNA expression of target genes was evaluated using the comparative quantification method (2−ΔΔCt), including ΔCt = Ct of the target gene minus Ct of the housekeeping gene, ΔΔCt = ΔCt (experimental sample) −ΔCt (control sample). β-actin was used for normalization of all target gene expression levels. The primer sequences used in the present study were as follows: TLR4 forward: CCGCTCTGGCATCATCTTCAT, reverse: AAGGCTTTTCCATCCAACAGG. TLR9 forward: CATGCAGGGGATAGGCCACAAC, reverse: GCCCACACCGTTGCCGCTGAA. TGF-β1 forward: CCGGCTGCTGACCCCCACTGA, reverse: GGGGGTGGCCATGAGGAGCAG. β-actin forward: CGCGCTCCCTCATGCCATCCT, reverse: GGGGCATCGGAACCGCTCAT.
Statistical Analysis
All data were expressed as mean and standard deviation. Analysis was performed using Statistical Package for the Social Sciences version 17.0 software. A t-test was used to compare all parameters between control group and experimental groups, and one-way analysis of variance followed by Student-Newman-Keuls test was used to compare three or more groups. A two-tailed P < 0.05 was considered to be statistically significant.
Results
Serum and Urine Biochemical Parameters
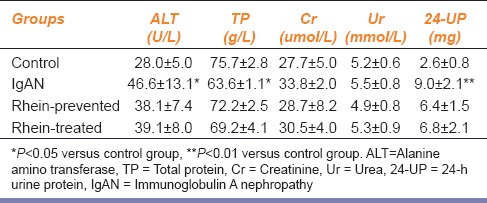
The experimental IgAN model was established using the immunity combination method of intragastric administration of BSA and injection of LPS and CCl4. The biochemical parameters of IgAN model rats showed that the 24-h UP excretion and ALT concentration in serum samples were much higher than those of the control group (P < 0.05) and TP concentration in serum samples was much lower than that of the control group (P < 0.05). While compared with the control group, the 24-h UP excretion and biochemical parameters of the rhein-prevented group rats and rhein-treated group rats were not statistically significant (P > 0.05) [Table 2]. These results indicate that rhein successfully improved the serum and urine biochemical parameters in IgAN model rats.
Table 2.
Serum and urine biochemical parameters
Histology Observation
Hematoxylin and eosin and PAS staining of kidney sections were performed to show the general histology observation. Figure 1a and b show representative micrographs of the H and E and PAS staining of renal tissue sections. Normal renal histology structure of control rats was found without any tubular or glomerular injury. The renal pathological changes of IgAN rats were very obvious and characterized by expansion of the renal corpuscle, glomerular hypercellularity, moderate to severe hyperplasia of mesangial matrix, and proliferation of the mesangial cells. Swelling and necrosis of some renal tubular epithelial cells existed. The above-mentioned renal pathological changes were markedly ameliorated in rhein-prevented and rhein-treated rats.
Figure 1.
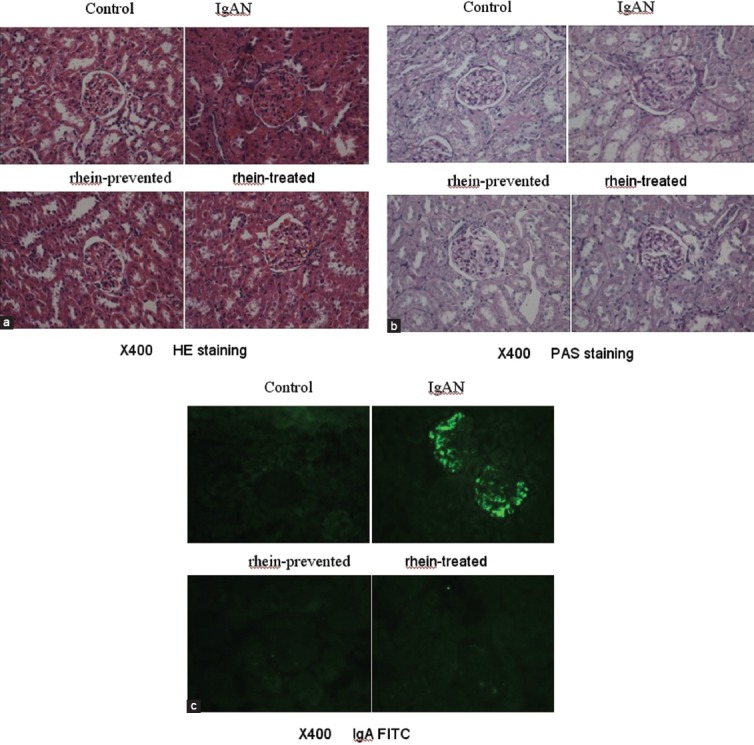
(a) Hematoxylin and eosin staining of kidney sections (×400) (b) Periodic acid-Schiff staining of kidney sections (×400) (c) Immunoglobulin A fluorescein isothiocyanate (×400)
Direct immunefluorescence staining was used to examine the IgA deposits in the glomeruli. Figure 1c indicates that there was very little green fluorescence found in the glomeruli of all control rats. However, there were clear granule-like or bright mass-like green fluorescence found in the glomeruli of all IgAN rats, the fluorescence intensity grade of which ranged from “++” to “+++.” There was obvious weaker green fluorescence found in the glomeruli of rhein-prevented and rhein-treated rats than IgAN rats, the fluorescence intensity grade of which ranged from “+” to “++.”
Rhein Down-regulated Expression of Toll-like Receptor 4 and Transforming Growth Factor-β1 Protein of Renal Cortex
Immunohistochemical assay with paraffin-embedded kidney sections was used to detect TLR4, TLR9, and TGF-β1 protein expression in the renal cortex. Immunohistochemical stain showed that TGF-β1 protein was obviously detected in the cytoplasm of most tubular epithelial cells and tubulointerstitial of all biopsies, and slightly detected in the glomeruli of some biopsies from IgAN rats. However, the control group sections stain showed that TGF-β1 protein was slightly expressed within the cytoplasm of a few renal tubular cells with scattered and sparse patterns and not detected in the glomeruli. The quantitative value of integrated optical density of IgAN rats was much higher than that of the control group (P < 0.05). Compared with the IgAN rats, there was an obvious decrease in TGF-β1 protein expression within the cytoplasm of some renal tubular cells of rhein-prevented and rhein-treated rats. The quantitative values of integrated optical density of TGF-β1 protein were not statistically significant between the rhein-prevented or rhein-treated rats and control rats (P > 0.05) [Figure 2a, Table 3]. TLR4 protein was found in the cytoplasma of most renal tubular cells and glomeruli. The quantitative values of integrated optical density demonstrated that renal tubular and glomerular TLR4 protein expression of IgAN rats was significantly up-regulated in comparison to control rats. With rhein-prevented and rhein-treated, TLR4 protein expression became weaker than IgAN rats, and there was no significant difference compared with controls [Figure 2b, Table 3]. TLR9 protein weakly existed in the cytoplasma of some renal tubular cells and glomeruli from all the four group rats. The quantitative value of integrated optical density between the four groups was not statistically significant [Figure 2c, Table 3].
Figure 2.
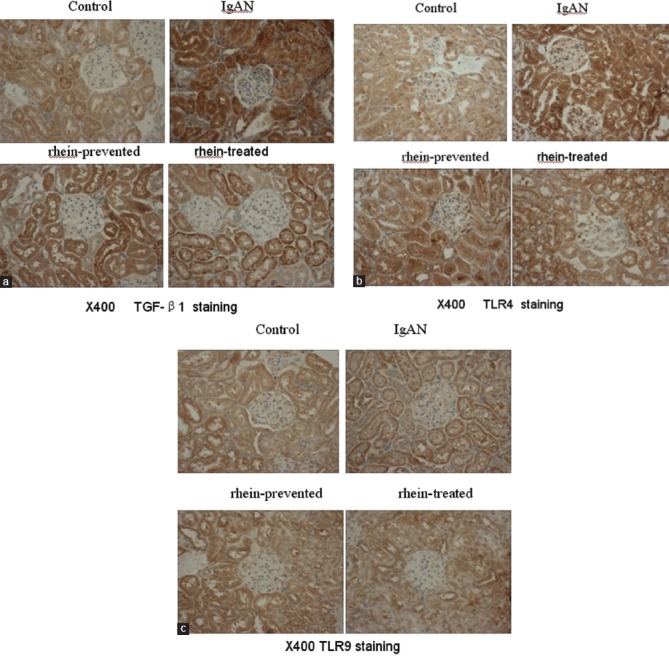
(a) Transforming growth factor-β1 immunohistochemical staining of kidney sections (b) Toll-like receptor 4 immunohistochemical staining of kidney sections (c) Toll-like receptor 9 immunohistochemical staining of kidney sections
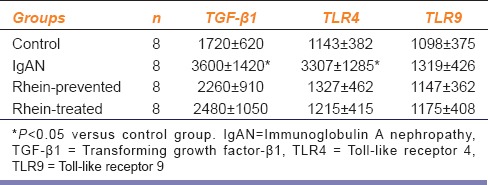
Table 3.
The integrated optical density of renal TGF-β1, TLR4 and TLR9 protein expression
Rhein Down-regulated Expression of Toll-like Receptor 4 and Transforming Growth Factor-β1 Messenger Ribonucleic Acid of Renal Cortex
To detect TLR4, TLR9, and TGF-β1 mRNA expression, RT-PCR was performed using total RNA extracted from the renal cortex. Compared with the control group, the relative mRNA expression of TLR4 and TGF-β1 genes from IgAN model rats reached 3.1 and 2.6 folds, respectively. There were significantly statistically differences (P < 0.05). In contrast, the relative mRNA expression of TLR4 and TGF-β1 genes from rhein-prevented and rhein-treated rats were similar to those of control rats. There was no obvious difference in TLR9 mRNA expression among four groups [Figure 3].
Figure 3.
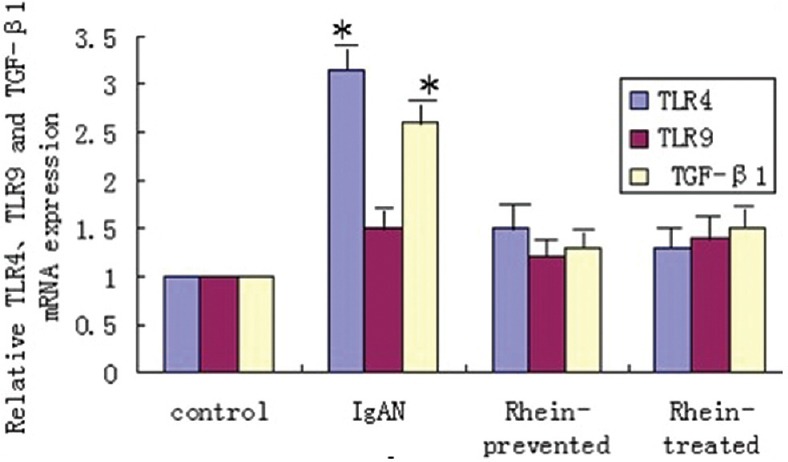
Rhein down-regulated of toll-like receptor 4 and transforming growth factor-β1 messenger ribonucleic acid expression from immunoglobulin A nephropathy model
Discussion
Immunoglobulin A nephropathy, first described in 1968 by Berger and Hinglais, is the most common primary chronic glomerulonephritis and one of the most important causes of ESRD worldwide.[15] IgAN is characterized by a diffuse deposition of IgA in the glomerular mesangial area and can only be diagnosed by immunohistological examination of a kidney biopsy at present. Histopathologically, IgAN is characterized by the expansion of the glomerular mesangial matrix and mesangial cell proliferation. The risk factors of IgAN progress to ESRD are demonstrated to be mainly associated with proteinuria, hypertension, impairment of renal function and glomerulosclerosis, and tubulointerstitial fibrosis.[16] The IgAN experimental animal model in the present study was innovated on bases of oral immunization and secondary hepatocellular injury elicited by CCl4 that led to hepatic cirrhosis, and it was widely used.[17,18,19,20] IgAN model rats showed the higher 24-UP excretion, lower TP concentration in serum, strong IgA deposits in glomeruli and obvious pathological changes. Taken together, these results indicated successful establishment of our IgAN model.
The pathogenesis of IgAN is unclear. Delaying IgAN progress to ESRD is an important strategy for treatment. Rhein has been broadly used in the treatment of chronic fibrotic diseases in animal and clinical experiments. Rhein ameliorated the degree of liver fibrosis induced by CCl4 and experimental pancreatic fibrosis through down-regulation of α-SMA and TGF-β1.[21,22] Rhein also significantly improved renal function and markedly ameliorated renal tubulointerstitial fibrosis in chronic allograft nephropathy rats and unilateral ureteral obstruction mice by suppressing α-SMA, FN and TGF-β1expression.[7] In the present study, we found that rhein improved the serum and urine biochemical parameters and alleviated IgA deposits in glomeruli and ameliorated renal histology pathological changes of IgAN rats. The results suggest that rhein may be a potential therapeutic agent for the IgAN pathologies. However, the therapeutic mechanism is obscure. In previous study, we demonstrated that rhein inhibited renal FN and α-SMA expression.[8]
It is well-established that α-SMA-positive myofibroblasts are the principal effector cells in fibrotic kidney and TGF-β1 plays a determinant role in the accumulation of extracellular matrix and renal fibrosis. TGF-β1-dominated cytokine networks activated by stimulus transform renal resident cells, including fibroblasts, renal tubular epithelial, and endothelial cells, into myofibroblasts, which exacerbates renal fibrosis. Up-regulation of TGF-β1 expression has been demonstrated to be involved in podocyte injury and the development of glomerulosclerosis in most of the IgAN patients and animal models.[23,24] TGF-β1 expression in the kidneys was increased in accordance with increased World Health Organization pathologic class of IgAN.[25] The removal of elevated TGF-β1 from the serum and kidneys is considered to be a fundamental approach in the treatment of IgAN. In the present study, we found that TGF-β1 protein was strongly detected in the cytoplasm of most tubular epithelial cells and tubulointerstitial of IgAN rats. Rhein inhibited renal TGF-β1 expression.
Toll-like receptor is a key component of the mammalian innate immune system. TLR4 recognizes LPS of Gram-negative bacteria. TLR9 has been identified as a receptor for bacterial and viral cytosine-phosphate-guanosine (CpG)-DNA. Binding with its ligands, TLRs initiate an intracellular signaling cascade, release of cytokines, enhance the expression of cell surface costimulatory molecules, and initiate an adaptive immune response.[26] There has been circumstantial but supportive evidence for the involvement of TLRs in the pathogenesis of IgAN. Up-regulation of TLR4 expression on circulating mononuclear cells was significantly correlated with proteinuria or phases of clinical activity in patients with IgAN.[9] TLR4 is involved in mesangial cell injury by induction of pro-inflammatory cytokines in IgAN.[12] Nasal challenge with ligand of TLR9 aggravated renal injury with elevation of albuminuria, serum IgA level and mesangial IgA deposition in an IgAN-prone mouse model.[27] In humans, TLRs are expressed on a wide range of cell types, including renal epithelial cells. TLR4 is expressed primarily in the proximal and distal tubules, whereas the expression in glomeruli and endothelial cells is minor.[28] Recently, renal TLR4-mediated inflammatory and profibrotic signaling in chronic kidney disease have been demonstrated. TLR4 expression correlated significantly with expression of the profibrotic molecule TGF-β1 or altered the susceptibility of renal cells toward TGF-β1 signaling.[29,30] These data suggest that renal TLR4 signaling may be a therapeutic target for the prevention of IgAN progress to ESRD. In the present study, we found that TLR4 and TLR9 protein was found in the cytoplasma of most renal tubular cells and weakly found in glomeruli. TLR4, but not TLR9 expression of IgAN rats was significantly up-regulated in comparison to control rats. Rhein-prevented and rhein-treated both inhibited renal TLR4 expression in IgAN rats.
In summary, this study demonstrated the protective effects of rhein on IgAN. Rhein ameliorated renal histological damage, alleviated IgA deposits in glomeruli, and improved biochemical parameters in sera. Its therapeutic mechanism is, at least in part, related to inhibiting TLR4-mediated profibrotic signals and expression of the profibrotic molecule TGF-β1.
Footnotes
Source of Support: National Natural Science Foundation of China (No. 30900574,81160050), Scientific Research Project of Jiangxi Provincial Education Department (GJJ14102) and Jiangxi Technology Support Program (20111BBG70015-3,20133BBG70055).
Conflict of Interest: No.
References
- 1.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–3. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 2.Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, et al. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013;84:1017–24. doi: 10.1038/ki.2013.210. [DOI] [PubMed] [Google Scholar]
- 3.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27:1479–85. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 4.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 5.Zheng JM, Zhu JM, Li LS, Liu ZH. Rhein reverses the diabetic phenotype of mesangial cells over-expressing the glucose transporter (GLUT1) by inhibiting the hexosamine pathway. Br J Pharmacol. 2008;153:1456–64. doi: 10.1038/bjp.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia ZH, Liu ZH, Zheng JM, Zeng CH, Li LS. Combined therapy of rhein and benazepril on the treatment of diabetic nephropathy in db/db mice. Exp Clin Endocrinol Diabetes. 2007;115:571–6. doi: 10.1055/s-2007-981469. [DOI] [PubMed] [Google Scholar]
- 7.He D, Lee L, Yang J, Wang X. Preventive effects and mechanisms of rhein on renal interstitial fibrosis in obstructive nephropathy. Biol Pharm Bull. 2011;34:1219–26. doi: 10.1248/bpb.34.1219. [DOI] [PubMed] [Google Scholar]
- 8.Sheng-Nan P, Hui-Hong Z, Ai-Xiang F, Xiao-Wen C, Qing-Xian Z. Protection of rhein on IgA nephropathy mediated by inhibition of fibronectin expression in rats. Indian J Pharmacol. 2013;45:174–9. doi: 10.4103/0253-7613.108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppo R, Camilla R, Amore A, Peruzzi L, Daprà V, Loiacono E, et al. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol. 2010;159:73–81. doi: 10.1111/j.1365-2249.2009.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato D, Suzuki Y, Kano T, Suzuki H, Matsuoka J, Yokoi H, et al. Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant. 2012;27:1090–7. doi: 10.1093/ndt/gfr403. [DOI] [PubMed] [Google Scholar]
- 11.Lepenies J, Eardley KS, Kienitz T, Hewison M, Ihl T, Stewart PM, et al. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-ß1 in patients with chronic kidney disease. Nephron Clin Pract. 2011;119:c97–104. doi: 10.1159/000324765. [DOI] [PubMed] [Google Scholar]
- 12.Lim BJ, Lee D, Hong SW, Jeong HJ. Toll-like receptor 4 signaling is involved in IgA-stimulated mesangial cell activation. Yonsei Med J. 2011;52:610–5. doi: 10.3349/ymj.2011.52.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21:1299–308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y, Lou TQ, Cheng CL. Improvement of experimental IgA nephropathy model. J Sun Yat Sen Univ (Med Sci) 2006;27:184–7. [Google Scholar]
- 15.Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–97. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Chen N. Treatment of progressive IgA nephropathy: An update. Contrib Nephrol. 2013;181:75–83. doi: 10.1159/000348460. [DOI] [PubMed] [Google Scholar]
- 17.Emancipator SN, Gallo GR, Lamm ME. Experimental IgA nephropathy induced by oral immunization. J Exp Med. 1983;157:572–82. doi: 10.1084/jem.157.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iida H, Izumino K, Matsumoto M, Takata M, Mizumura Y, Sugimoto T. Glomerular deposition of IgA in experimental hepatic cirrhosis. Acta Pathol Jpn. 1985;35:561–7. doi: 10.1111/j.1440-1827.1985.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Chen LL, Jiang XY, Mo Y, Ling YH, Sun LZ. Temporal and spatial expression of podocyte-associated molecules are accompanied by proteinuria in IgA nephropathy rat model. Physiol Res. 2013;62:35–45. doi: 10.33549/physiolres.932380. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Peng Y, Long XD, Liu Z, Wen X, Jia M, et al. Tonsillar CD4+CD25+regulatory T cells from IgA nephropathy patients have decreased immunosuppressive activity in experimental IgA nephropathy rats. Am J Nephrol. 2013;37:472–80. doi: 10.1159/000350533. [DOI] [PubMed] [Google Scholar]
- 21.Guo MZ, Li XS, Xu HR, Mei ZC, Shen W, Ye XF. Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats. Acta Pharmacol Sin. 2002;23:739–44. [PubMed] [Google Scholar]
- 22.Tsang SW, Zhang H, Lin C, Xiao H, Wong M, Shang H, et al. Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis. PLoS One. 2013;8:e82201. doi: 10.1371/journal.pone.0082201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalliakmani P, Nakopoulou L, Tsakas S, Gerolymos M, Papasotiriou M, Goumenos DS. Urinary interleukin-6 (IL-6) and transforming growth factor (TGF-ß) levels in corticosteroidtreated patients with IgA nephropathy. Clin Nephrol. 2011;76:144–50. doi: 10.5414/cn106983. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, Zhang Q, Shi S, Liu L, Lv J, Zhang H. Synergistic effect of mesangial cell-induced CXCL1 and TGF-ß1 in promoting podocyte loss in IgA nephropathy. PLoS One. 2013;30(8):e73425. doi: 10.1371/journal.pone.0073425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng H, Zhang L, E X, Ye F, Li H, Han C, et al. Application of Oxford classification, and overexpression of transforming growth factor-β1 and immunoglobulins in immunoglobulin A nephropathy: Correlation with World Health Organization classification of immunoglobulin A nephropathy in a Chinese patient cohort. Transl Res. 2014;163:8–18. doi: 10.1016/j.trsl.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–65. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384–95. doi: 10.1681/ASN.2007121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zager RA, Johnson AC, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: Acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 2007;292:F304–12. doi: 10.1152/ajprenal.00237.2006. [DOI] [PubMed] [Google Scholar]
- 29.Campbell MT, Hile KL, Zhang H, Asanuma H, Vanderbrink BA, Rink RR, et al. Toll-like receptor 4: A novel signaling pathway during renal fibrogenesis. J Surg Res. 2011;168:e61–9. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skuginna V, Lech M, Allam R, Ryu M, Clauss S, Susanti HE, et al. Toll-like receptor signaling and SIGIRR in renal fibrosis upon unilateral ureteral obstruction. PLoS One. 2011;6:e19204. doi: 10.1371/journal.pone.0019204. [DOI] [PMC free article] [PubMed] [Google Scholar]


