Abstract
Objective:
The aim of this study was to investigate the molecular mechanism of bleomycin A5 in inducing the apoptosis of human umbilical vein endothelial cells (ECV304).
Materials and Methods:
ECV304 cells were cultured and passaged, and then were divided into control group and three treatment groups. The later three groups were treated with 15, 75, and 150 μg/ml bleomycin A5 for 24 hours, respectively. The expressions of caspase-3, p53, and bcl-2 in ECV304 cells were detected by flow cytometry, and the activity of telomerase was determined using telomere repeat amplification protocol (TRAP)-silver staining method.
Results:
After treatment with different concentrations of bleomycin A5, the expression of caspase-3 in ECV304 cells was increased. It was significantly decreased with the increase of bleomycin A5 concentration, but the difference between 75 μg/ml and 150 μg/ml groups was not significant. Bleomycin A5 could significantly increase the expression of p53, with concentration dependence. It had no obvious effect on bcl-2 expression. There was high expression of telomerase in control group. After treatment with different concentration of bleomycin A5, the telomerase activity was significantly decreased.
Conclusion:
Bleomycin A5 can increase caspase-3 and p53 levels and inhibit telomerase activity to induce ECV304 apoptosis.
KEY WORDS: Apoptosis, bleomycin A5, caspase-3, hemangioma, p53, vascular endothelial cell
Introduction
Head and neck hemangioma is a refractory disease, which can cause appearance malformation, abnormal function, or psychological barriers. The surgical resection of this disease may lead to massive blood loss, incomplete resection, disfiguring, or recurrence. At present, the intratumoral injection of hardener has been widely applied to treatment of hemangioma, which has obtained exact efficacy.[1,2,3] Many studies have indicated that bleomycin A5 has strong anti-tumor effect, with low toxicity and damage to the lung,[4] and is an ideal drug to hemangioma,[5,6,7] of which the possible mechanism is related to its inhibition of proliferation of vascular endothelial cells and induction of cell apoptosis.[8,9,10] Therefore, further investigating the molecular mechanism of bleomycin A5 on hemangioma has significant importance for providing valuable clues to find new target in treatment of hemangioma. Human umbilical vein endothelial cell (ECV304) line is a model of in vitro vasculogenic mimicry.[11,12] In this study, the caspase-3, p53, and bcl-2 expressions and telomerase activity in ECV304 cells were observed, and the molecular mechanism of bleomycin A5 in inducing the apoptosis of ECV304 cells was investigated. The objective is to further apply bleomycin A5 to treatment of hemangioma.
Materials and Methods
Cell Culture and Treatment
ECV304 cells were provided by Institute of Biochemistry, Institutes for Biological Sciences of Chinese Academy of Sciences, China, which were prepared as follows: The umbilical cord veins were infused with digestive enzyme for digestion, and the vascular endothelial cells were obtained, followed by culture in M199 containing 20% fetal bovine serum. Then the cells were identified as ECV304 cells by factor VIII immunofluorescence staining and scanning electron microscopy. After adding cryopreservation solution containing 10% dimethyl sulfoxide (DMSO), the cells were placed in liquid nitrogen and stored for use. ECV304 cells were cultured and passaged in Corning cell culture flasks (Corning Company, Ltd., China) under the same conditions. These cells were divided into control group and three treatment groups, three specimens in each group. After the cells covered the bottom of flask, the later three groups were treated with 15, 75, and 150 μg/ml bleomycin A5 for 24 hours, respectively, without any treatment of the control group. The shed cells were collected for test. This study was conducted in accordance with the declaration of Helsinki and with the approval from the Ethics Committee of Eye and ear, nose, throat (ENT) of 118 Military Hospital of China. Written informed consent was obtained from all participants.
Determination of Caspase-3, p53, and bcl-2 Expression
The caspase-3, p53, and bcl-2 expressions were determined using BD FACSCalibur flow cytometer (Jieweifu Company, China). The shed ECV304 cells (5 × 106) were added with 200 μL Cytofix/Cytoperm solution (Shanghai Leihao Information Technology Co., Ltd., China), followed by standing (4°C, 30 min) for fixation and perforation. After centrifugation at 1500 × g for 15 min, the supernatant was removed, and then 1 ml Perm/Wash solution (Shanghai Hao Ran Biological Technology Co., Ltd., China) was added, followed by centrifugation at 1500 ×g for 5 min. After removing the supernatant, the caspase-3, p53, and bcl-2 antibodies were added to the experimental tube, respectively (the isotype antibody was added to the control tube), followed by incubation (4°C, dark) for 30 min. Five hundred microliter Perm/Wash solution was added, followed by washing for two times. Three hundred microliter washing liquid was added to suspend the cells. The Cell quest software was used to analyze the percentage of caspase-3-and bcl-2-positive cells and p53-positive cell fluorescence intensity. Each test was repeated for two times.
Determination of Telomerase Activity
After treatment with bleomycin A5, the cells were observed under inverted microscope (Leica Microsystems CMS GmbH, Mannheim, Germany). The shed cells were could be observed in the culture medium. These shed cells were collected and added with S-100 extraction solution (20 μl of 1 × CHAPS lysis buffer, 2 μl of 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 μl of 0.1 M β-mercaptoethanol solution). The mixture was treated by ice bath for 30 min, followed by pipetting for 3–5 times. After centrifugation at 4°C and 12,000 r/min for 30 min, the supernatant was collected. The protein concentration was determined by Bradford method, using BSA as standard protein. The activity of telomerase was determined using telomere repeat amplification protocol (TRAP)-silver staining method. The staining results were observed and photographed.
Statistical Analysis
Statistical analysis was performed using statistical package of social sciences (SPSS) 10.0 statistical software. A paired t-test was used to analyze the differences among different groups, and P < 0.05 was considered as statistically significant.
Results
Effects of Bleomycin A5 on Caspase-3, p53 and bcl-2 Expressions
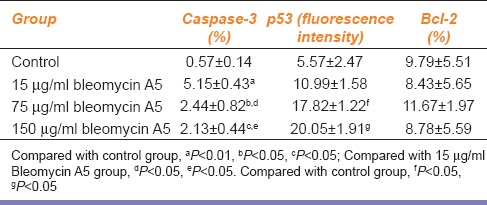
As shown in Table 1, after treatment with different concentration of bleomycin A5, the expression of caspase-3 in ECV304 cell was increased. It was significantly decreased with the increase of bleomycin concentration, but the difference between 75 μg/ml and 150 μg/ml groups was not significant. Bleomycin A5 could significantly increase the expression of p53, with concentration dependence. It had no obvious effect on bcl-2 expression in ECV304 cells.
Table 1.
Effects of bleomycin A5 on caspase-3, p53 and Bcl-2 expressions
Effects of Bleomycin A5 on Telomerase Activity
Figure 1 shows that, in control group, the transverse streaks indicated the telomerase stripes, which had obvious color. This suggests that there was high expression of telomerase in control group. After treatment with bleomycin A5 (15 μg/ml), the color of telomerase stripes was significantly lighted, indicating decreased telomerase activity. In the 75 and 150 μg/ml bleomycin A5 groups, there was almost no telomerase strip, which indicated that the telomerase activity was significantly inhibited. Bleomycin A5 could significantly inhibit the telomerase activity in ECV304 cells, with concentration dependence.
Figure 1.
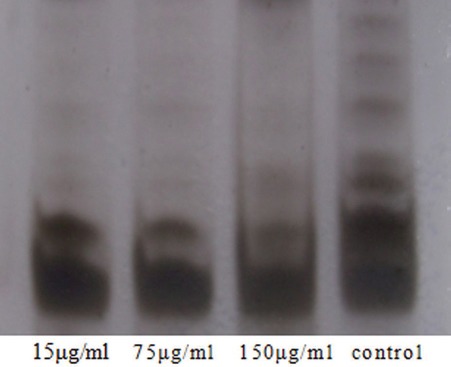
Effects of bleomycin A5 on telomerase activity
Discussion
Cysteine-aspartic specific protease, also called caspase, plays an essential role in apoptosis and is related to cell morphological feature.[13] Some studies demonstrated that there is a correlation between ECV304 cell apoptosis induced by many factors and activated caspase.[14,15] We compared the expression of caspase-3 before and after bleomycin A5 treatment and found that expression of caspase-3 was increased in all groups treated with bleomycin A5, but there was no dose-dependent effect. This result indicated that caspase-3 might be involved in the apoptosis process of vascular endothelial cell induced by bleomycin A5, which seems be independent of drug concentration. This cause needs to be further investigated. p53 gene, also called wild type p53, which encodes p53 protein, is made up of 393 amino acids. The p53 protein is crucial in multicellular organisms, and its function is to regulate cell cycle, deoxyribonucleic acid (DNA) repair and apoptosis induction.[16]
While bleomycin A5-induced cell apoptosis through p53 pathway is never reported, we conclude that bleomycin A5 is the subtype of bleomycin which significantly induced cell apoptosis in the wild-type mouse cerebellar internal granular layer (IGL) and Purkinje cell layer (PL) through p53 pathway. However, in p53-deficient mice, these responses were not observed.[17] This suggested that bleomycin-induced cell apoptosis depending on p53 but some studies reported that bleomycin was independent on p53 to induce cell apoptosis in head and neck squamous cell carcinoma.[18]
In this study, we observed that the p53 level was up-regulated after treatment of bleomycin A5, especially treatment of high concentration of bleomycin A5 in ECV304. p53 level was a significant difference between control group and treatment group, and bleomycin A5 concentration was dose-dependent. This result suggested that bleomycin A5-induced ECV304 cell line apoptosis through p53 pathway. However, according to previous studies,[19,20] we speculated that bleomycin A5-induced cell apoptosis in different cell types might have been independent or dependent on p53 pathway. Therefore, we need further studies to prove it.
Anti-apoptotic protein, bcl-2, contains BH3 domain necessary for dimerization with Bax to inhibit pro-apoptosis protein activity. Furthermore, bcl-2 also inhibits the release of cytochrome C to protect cell against apoptosis process, and bcl-2 blocks oxygen-free radicals to cause cell death through antioxidation pathway.[21] In the process of cell apoptosis induced by bleomycin A5, we detected bcl-2 expression. Result showed that there was no significant different between control group and treatment groups. This indicated that bleomycin A5 didn’t increase Blc-2 level, which was in line with previous study.[22] However, Chang et al.,[23] found that bcl-2 was down-regulated after treated with licochalcones that induced ECV304 cell line apoptosis. Therefore, whether there results were caused by different cell lines or by different chemotherapy drugs need to be studied further.
Kim et al.,[24] established an amazing method that called TRAP to detect telomerase activity in 1994, which was an important milestone. In our study, we measured telomerase activity through TRAP-silver stain method in ECV304 cells. Results showed that the telomerase expression was observed in control group, but not seen in treatment groups which were treated with different concentrations bleomycin A5. In control group, band was deep, which suggested telomerase activity was high expression. In 15 μg/ml bleomycin A5 group, color of band was weak, and there was no band in 75 μg/ml bleomycin A5 group and 150 μg/ml bleomycin A5 group. This indicated that the telomerase activity of ECV304 was inhibited by bleomycin A5.
In conclusion, bleomycin A5 can increase caspase-3 and p53 levels and inhibit telomerase activity to induce ECV304 apoptosis. In addition, the expression of caspase-3 is closely related with the cell apoptosis induced by bleomycin A5. These results give us more ideas to make further studies. For example, recombinant caspase-3 gene can suppress the growth of breast tumor in vivo. It is necessary to use this method to choose an effective and direct way to treat hemangioma, and avoid using chemotherapeutic drugs which probably cause severe adverse effects.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Sachin K, Rashmi S, Manish S, Siddhartha W, Uday L. Haemangiomas and venous malformations of the head and neck: A retrospective analysis of endovascular management in 358 patients. Indian J Plast Surg. 2013;46:109–16. doi: 10.4103/0970-0358.113727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Y, Wang R, Yang SF, Zhao YF, Zhao JH. Sclerotherapy for the mucoceles of the anterior lingual salivary glands with pingyangmycin. Oral Dis. 2014;20:473–6. doi: 10.1111/odi.12155. [DOI] [PubMed] [Google Scholar]
- 3.Liu XJ, Qin ZP, Tai MZ. Angiographic classification and sclerotic therapy of maxillofacial cavernous haemangiomas: A report of 204 cases. J Int Med Res. 2009;37:1285–92. doi: 10.1177/147323000903700503. [DOI] [PubMed] [Google Scholar]
- 4.Zhu QQ, Sun GY. The effect of intrapleural pingyangmycin administration on activity of fibrinolytic system and transforming growth factor-beta1 in malignant pleural effusion. Zhonghua Jie He He Hu Xi Za Zhi. 2009;32:674–8. [PubMed] [Google Scholar]
- 5.Luo Q, Zhao F. How to use bleomycin A5 for infantile maxillofacial haemangiomas: Clinical evaluation of 82 consecutive cases. J Craniomaxillofac Surg. 2011;39:482–6. doi: 10.1016/j.jcms.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Hou J, Wang M, Tang H, Wang Y, Huang H. Pingyangmycin sclerotherapy for infantile hemangiomas in oral and maxillofacial regions: An evaluation of 66 consecutive patients. Int J Oral Maxillofac Surg. 2011;40:1246–51. doi: 10.1016/j.ijom.2011.07.906. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Liu J, Pan W, Wang X, Gao Q, Hou S. Pingyangmycin loaded bovine serum albumin microspheres for chemoembolization therapy-- in vitro and in vivo studies. Int J Pharm. 2008;351:219–26. doi: 10.1016/j.ijpharm.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Xiao XE, Guo ZT. Effect of bleomycin A5 on the hemangioma-derived endothelial cell line XTPS-1. Zhonghua Kou Qiang Yi Xue Za Zhi. 2012;47:324–8. doi: 10.3760/cma.j.issn.1002-0098.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Li DF, Guo ZT, Xiao XE. Therapeutic mechanism of bleomycin A5 on infancy hemangioma: An experimental study. Zhonghua Kou Qiang Yi Xue Za Zhi. 2013;48:18–22. [PubMed] [Google Scholar]
- 10.Huang Y, Li P, Xia S, Zhuo Y, Wu L. Proapoptotic effect and the mechanism of action of pingyangmycin on cavernous hemangiomas. Exp Ther Med. 2014;7:473–7. doi: 10.3892/etm.2013.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RA, Wang Z, Dookie S, Griffin M. The role of TG2 in ECV304-related vasculogenic mimicry. Amino Acids. 2013;44:89–101. doi: 10.1007/s00726-011-1214-6. [DOI] [PubMed] [Google Scholar]
- 12.Heng XP, Chen KJ, Hong ZF, He WD, Chu KD, Chen WL, et al. Anticolchicine cytotoxicity enhanced by Dan Gua-Fang, a Chinese herb prescription in ECV304 in mediums. Chin J Integr Med. 2011;17:126–33. doi: 10.1007/s11655-011-0646-X. [DOI] [PubMed] [Google Scholar]
- 13.Lockshin RA, Williams CM. Programmed cell death—II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J Insect Physil. 1964;10:643–9. [Google Scholar]
- 14.Shim D, Kang HY, Jeon BW, Kang SS, Chang SI, Kim HY. Protein kinase B inhibits apoptosis induced by actinomycin D in ECV304 cells through phosphorylation of caspase 8. Arch Biochem Biophys. 2004;425:214–20. doi: 10.1016/j.abb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Li ZF, Wang ZD, Ji YY, Zhang S, Huang C, Li J, et al. Induction of apoptosis and cell cycle arrest in human HCC MHCC97H cells with Chrysanthemum indicum extract. World J Gastroenterol. 2009;15:4538–46. doi: 10.3748/wjg.15.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Xu L, Yin L, Xu Y, Han X, Qi Y, et al. iTRAQ-based proteomic analysis of dioscin on human HCT-116 colon cancer cells. Proteomics. 2014;14:51–73. doi: 10.1002/pmic.201300101. [DOI] [PubMed] [Google Scholar]
- 17.Inamura N, Araki T, Enokido Y, Nishio C, Aizawa S, Hatanaka H. Role of p53 in DNA strand break-induced apoptosis in organotypic slice culture from the mouse cerebellum. J Neurosci Res. 2000;60:450–7. doi: 10.1002/(SICI)1097-4547(20000515)60:4<450::AID-JNR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Kelekar A, Thompson CB. Bcl-2-family proteins: The role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–30. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 19.Levine AJ, Perry ME, Chang A, Silver A, Dittmer D, Wu M, et al. The 1993 Walter Hubert Lecture: The role of the p53 tumor-suppressor gene in tumorigenesis. Br J Cancer. 1994;69:409–16. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Tee C, Zeng F, Sherry JP, Dixon B, Bols NC, et al. Characterization of p53 expression in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol. 2011;154:326–32. doi: 10.1016/j.cbpc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Mabeta P. Decreased secretion of vascular endothelial growth factor is associated with increased apoptosis in vascular tumor derived endothelial cells. J Physiol Pharmacol. 2013;64:473–7. [PubMed] [Google Scholar]
- 22.García-Sáez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19:1733–40. doi: 10.1038/cdd.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang HJ, Yoon G, Park JS, Kim MH, Baek MK, Kim NH, et al. Induction of apoptosis by the licochalcone E in endothelial cells via modulation of NF-kappaB and Bcl-2 Family. Biol Pharm Bull. 2007;30:2290–3. doi: 10.1248/bpb.30.2290. [DOI] [PubMed] [Google Scholar]
- 24.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]


