Abstract
Interspecific territoriality occurs when individuals of different species fight over space, and may arise spontaneously when populations of closely related territorial species first come into contact. But defence of space is costly, and unless the benefits of excluding heterospecifics exceed the costs, natural selection should favour divergence in competitor recognition until the species no longer interact aggressively. Ordinarily males of different species do not compete for mates, but when males cannot distinguish females of sympatric species, females may effectively become a shared resource. We model how reproductive interference caused by undiscriminating males can prevent interspecific divergence, or even cause convergence, in traits used to recognize competitors. We then test the model in a genus of visually orienting insects and show that, as predicted by the model, differences between species pairs in the level of reproductive interference, which is causally related to species differences in female coloration, are strongly predictive of the current level of interspecific aggression. Interspecific reproductive interference is very common and we discuss how it may account for the persistence of interspecific aggression in many taxonomic groups.
Keywords: interspecific territoriality, species recognition, interference competition, character displacement, damselfly, Hetaerina
1. Introduction
Interspecific territoriality [1] is expected to be evolutionarily stable under a narrower range of conditions than intraspecific territoriality, for two principal reasons. First, resource competition is generally weaker between than within species, because of past niche divergence and competitive exclusion [2–4]. Second, attracting and maintaining priority of access to mates is one of the primary benefits of intraspecific territoriality [5], and members of different species generally do not compete for mates [6]. Interspecific territoriality may initially arise as a by-product of intraspecific territoriality when species that still share a common competitor recognition system first come into contact [6–8]. But defence of space is costly, and unless the benefits of excluding individuals of other species exceed the costs, selection should favour divergence in competitor recognition until interspecific aggression is eliminated [3,6–9]. Orians & Willson [6] concluded that interspecific territoriality ought to persist only between species that compete for resources that cannot be partitioned and otherwise should only be seen in cases of very recent sympatry caused by range shifts or where gene flow from allopatry prevents local adaptation in sympatry. The data available on birds 50 years ago appeared to support these predictions, but a taxonomically broader view shows that the theory is incomplete. In insects, fishes, frogs and lizards it is common for males of closely related species to compete over mating territories with no apparent common resources at stake [10–29]. This is often interpreted as a maladaptive by-product of intraspecific territoriality and transient overlap between species in territorial signals [7,16,19,30]. However, an alternative hypothesis is that interspecific territoriality persists in these cases because males of different species actually are in competition for mates [19,31,32].
Indeed, interspecifically territorial species, including birds, often interfere with each other reproductively, i.e. males court, attempt to mate or actually mate with heterospecific females (for examples, see electronic supplementary material, table S1). In hybridizing taxa, the benefits of mating with heterospecifics may outweigh the costs in some contexts [33,34]. In non-hybridizing taxa, reproductive interference is most likely to occur when males cannot easily distinguish between conspecific and heterospecific females. Although females would benefit from being discriminable in a mating context, ecological factors may prevent reproductive character displacement in female traits. For example, selection for crypsis caused by visually orienting predators [35] or prey [36] may constrain divergence in female coloration because mutations that enhance discriminability tend to reduce crypsis [37]. When females cannot easily be distinguished, indiscriminate behaviour on the part of males may be the best tactic for maximizing mating opportunities. Regardless of the reasons, reproductive interference between species is quite common [38].
Species that interfere with each other reproductively effectively compete for mates [39]. Interspecific territoriality may therefore be profitable even when no other resources are defended [19,31,32]. To formally evaluate this hypothesis, we modified an existing individual-based model of agonistic character displacement [40] to simulate the evolutionary effects of secondary contact between two species in which males compete for mating territories. Reproductive interference was incorporated into the model as the fractional reduction (d) in a male's expected mating success caused by sharing a territory with one heterospecific male relative to sharing a territory with one conspecific male. This approach to modelling reproductive interference allowed us to use a single, composite parameter to encapsulate the aggregate effects of multiple factors, such as male mate recognition, microhabitat partitioning, etc., that might influence the intensity of reproductive interference. The evolvable traits in the model are the central location (μ) and width (σ) of the male competitor recognition template and the male trait (z) upon which competitor recognition is based (for further details, including descriptions of population dynamics and the cost of territorial fights, see [40]). In simulations carried out over 104 generations, we systematically varied d and the initial values of μ and z. The results show that moderate levels of reproductive interference are sufficient to allow interspecific territoriality to be maintained or even evolve de novo.
We tested the model in Hetaerina, a damselfly (Zygoptera) genus in which the level of interspecific aggression varies across the species pairs included in our study (electronic supplementary material, table S2). Males compete for small mating territories (1–2 m2) in fast flowing sections of rivers where females oviposit in submerged vegetation. Females usually oviposit outside the territories of their mates and feeding occurs elsewhere [41]. There is no a priori reason to expect interspecific territoriality in Hetaerina, and yet it occurs in most sympatric species pairs [13]. In some cases, interspecific fighting is reduced by divergence in male competitor recognition [13,42] or by species differences in microhabitat use [13], but in most cases, territory holders are equally aggressive to conspecific and heterospecific male intruders (electronic supplementary material, table S3) and interspecific fights often occur just as frequently as intraspecific fights (electronic supplementary material, tables S4 and S5). Evolutionary time lags or gene flow from allopatric populations may explain the failure of particular species pairs to diverge in competitor recognition, but the finding that most sympatric species have not diverged argues for an adaptive explanation. Besides the unexplained variation in interspecific aggression, there are other reasons to think the reproductive interference hypothesis applies to Hetaerina. Males have conspicuous, species-specific coloration, but females are cryptic and variable in coloration and can be difficult to identify to the species level [43]. To examine whether the male damselflies can distinguish between conspecific and heterospecific females, we presented territory holders at eight sympatric sites with live, flying, tethered females. This is a realistic test of male mate recognition because natural mating sequences begin with the male clasping the female (i.e. no pre-clasping courtship) and males usually clasp tethered conspecific females.
The results of this study provide striking support for our model: variation in the level of reproductive interference, caused by variation in the ability of males to distinguish between conspecific and heterospecific females, explains the variability in the level of aggressive interference between species. Hence, we conclude that both divergent and convergent agonistic character displacement processes can occur within a single taxon, depending on the degree to which the interacting species are reproductively isolated.
2. Material and methods
(a). Model
The full details and justifications for the underlying ACD model (without reproductive interference) can be found in [40]. Here, we describe the key features of the model germane to our present study. The model is individual-based [44] and the loci and alleles underlying the evolvable traits are tracked explicitly. We model a sexually reproducing diploid population without overlapping generations, which is appropriate for Hetaerina and many other insects with seasonal reproduction cycles. The agonistic signal (z) and the mean (μ) and width (σ) of the competitor recognition function are each assumed to be quantitative traits whose breeding values are determined by the additive effects of five autosomal, unlinked loci subject to mutation, and allelic values can take on any real number. The width (σ) of the competitor recognition function is expressed as the absolute value of its additive genetic value to ensure that this quantity is non-negative. Mutations occur with a probability 10−4 at each locus. If a mutation occurs, a new allelic value for the locus is drawn from a Gaussian distribution with the mean at the allelic value prior to mutation and a standard deviation given by 10% of the mean initial allelic value. This value thus describes the average magnitude of the mutation-induced variance (e.g. [45]). During the breeding season (90 days), the model proceeds on a daily time step. On each simulated day, mature males either occupy or do not occupy territories. Males without territories attempt to occupy individual territories that may or may not be occupied by other males. If the territory is occupied, three outcomes are possible: mutual recognition as competitors, one-sided recognition as a competitor and mutual non-recognition as competitors. Which of these outcomes is realized is a probabilistic function of the individual values of z, μ and σ of the males encountering each other [40]. Either mutual or one-sided recognition results in a fight, in which males must expend finite energetic reserves, which reduces their future fighting ability. The winner of the fight occupies the territory and the loser is ejected. If mutual non-recognition occurs, the resident and intruding males share the territory. Following the assignment of territories to males on each day, mating occurs. The probability that a given male mates with a given female (and hence his relative reproductive contribution to the next generation) depends on: (i) whether the male occupies a territory or not, (ii) whether the male and the female are conspecifics, and (iii) the number of other males with which the male shares a territory who could potentially interfere with his ability to mate with the female. Thus, the direction and strength of selection on competitor recognition depend on the time-varying relative densities of mates for each species, the frequency distribution in the current generation of the competitor recognition traits (z, μ and σ) in each species, and the variable frequency in territorial encounters.
In contrast to the model in [40], the current model assumes that females cannot control which males attempt to mate with them, and that heterospecific pairings arise from indiscriminate male behaviour. Heterospecific pairs are assumed to break up before sperm transfer, which is realistic for Hetaerina. For a given clutch of eggs, females re-mate until they mate with a conspecific male, at which point the eggs are fertilized by that male's sperm and oviposition occurs. The larval stages of the life cycle, during which density-dependent population regulation is assumed to occur, are modelled implicitly.
We simulated 104 generations following secondary contact, after a 1000-generation allopatric burn-in period. At the start of each simulation, the mean values of μ and z were set to equal each other within species, which means that males initially recognized most conspecific males as competitors. The model is based on a damselfly-like system in which intraspecific territoriality is adaptive [40]. However, because the underlying loci are unlinked, µ and z can diverge from each other within species, resulting in a loss of intraspecific territoriality. The initial magnitude ∂ of divergence between species in μ and z, which determines whether males of the two species initially respond aggressively to each other, was set at 0, 1.5 or 3 standard deviation units. A ∂ value of 1.5 corresponds to probability of approximately 0.33 that encounters between males of the two species will result in heterospecific recognition (one-sided or two-sided), while a ∂ value of 3 corresponds to a heterospecific recognition probability of about 0.01. We varied the level of reproductive interference between species (d) across simulations (d = 0.1, 0.21, 0.27, 0.30, 0.33 or 0.45). A d-value of 0.5 would mean that sharing a territory with one heterospecific male is just as costly, in terms of lost mating opportunities, as sharing a territory with a one conspecific male. We ran 15 replicates for each combination of ∂ and d values.
(b). Study sites
We conducted the fieldwork from March to August in the years 2005–2013 at 11 locations in North America, most with two species of Hetaerina damselflies present at moderate population densities (electronic supplementary material, table S2). We treat one of the locations as two separate sites (PA1 and PA2) because the wing coloration of female H. titia undergoes a dramatic seasonal shift from the spring (PA1) to summer (PA2) months. The seasonal colour shift affects the predictions of our model because males of the sympatric congener (H. occisa) only distinguish between females of the two species after the colour shift (PA2, see electronic supplementary material, table S3). Pooling data from PA1 and PA2 did not change the overall results, however (see electronic supplementary material, figure S1).
(c). Behavioural observations
At each site, we captured most of the adult Hetaerina along a 100–200 m river transect with aerial nets and marked individuals with unique IDs using a previously described method [46]. We conducted behavioural observations (i) to determine which males were defending territories and thus eligible for inclusion in the experiments (see below) and (ii) to record the frequency of naturally occurring conspecific and heterospecific fights. Observers recorded the location of each male to the nearest 0.1 m by reference to numbered flags. We considered males territory holders if they perched near the bank of the river at the same location (within a 1.5 m radius) for 2 or more consecutive days [42]. When fights occurred, we recorded the location, species involved, ID of individuals (if marked) and the level of escalation (1, one-way chase; 2, two-way back-and-forth chase; 3, escalated ‘circle’ fight between two males and 4, escalated fight involving three or more males). Prior to analysis, multiple recorded bouts of fighting between the same two males on the same day were reduced to a single fight. For fights involving unmarked or unidentified individuals, we only recorded one fight within a 5 m radius per day.
To determine whether interspecific fights occur less often than expected by chance, following [13] we generated chance expectations from binomial expansions of the relative frequencies of males of each species and conducted a χ2 goodness-of-fit test on the observed number of fights.
(d). Interspecific aggression
To measure interspecific aggression relative to intraspecific aggression, we followed the protocol of Anderson & Grether [42]: territory holders were presented with live male intruders that were tethered with a transparent thread and flown into the territory with a fishing pole. Each territory holder was presented with one conspecific intruder and one heterospecific intruder, with the order of presentation trials balanced across males. During each trial, a field assistant recorded the behaviour of the territory holder, including the amount of time spent chasing the tethered male and the number of slams (defined as attempts to ram the tethered male, whether successful or not) and grabs (defined as extended physical contact with the tethered male) on a continuously running voice recorder. It was not possible for field assistants to be blind to the treatments, but they had no knowledge of our theoretical model or the prediction being tested. Trials were 2 min in duration with at least a 5-min inter-trial interval. Cases in which we were only able to carry out one of the two trials or in which the territory holder did not chase either tethered intruder for at least 60 s were excluded from the analysis (the latter were interpreted as cases in which the male was not actively defending the site; if possible, these males were retested on a subsequent day).
We tested for differences in the attack rate (slams and grabs divided by the duration of the trial) directed at heterospecific versus conspecific males using paired t-tests when log(x + 0.01)-transformed data met the assumptions of normality and homoscedasticity. Paired Wilcoxon paired signed rank tests were used when the data did not meet parametric assumptions. Sample sizes are given in electronic supplementary material, table S3.
(e). Male mate recognition
We measured male mate recognition by presenting territorial holders with tethered females of both sympatric species at a distance of 0.5 m from the male's perch. The presentation order of conspecific and heterospecific females was balanced. Presentations lasted 5 s each, or until the focal male returned to his perch, whichever came last. If the female was clasped during her first presentation, we ended the trial; otherwise, we presented her to the same male for another 5 s. There is no courtship display in Hetaerina. A mating sequence begins with the male clasping the female, usually in mid-air. Just prior to clasping, the male flies towards the female, curls his abdomen forward and grasps the intersternite region of the female's thorax with his claspers. We considered a male to have responded sexually if he either clasped or attempted to clasp the female—that is, if he pursued her with his abdomen curled forward. In most recorded clasping attempts, the male's claspers made contact with the female's intersternite (96.7%), and in a majority of such cases (63.6%) the male clasped the female at least momentarily. Cases in which the male did not respond sexually to either female or we were unable to complete the set of trials were excluded from the analysis. To test for discrimination between females of different species, we used Fisher's exact tests (for sample sizes, see electronic supplementary material, table S3).
(f). Female wing coloration measurements
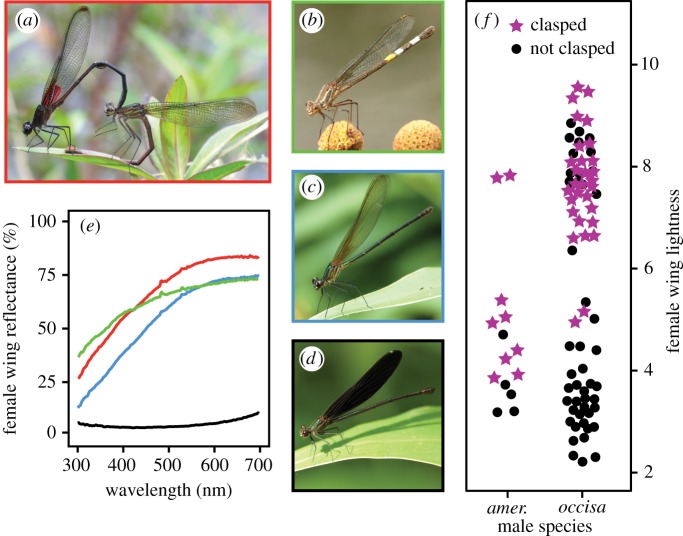
The wings of female Hetaerina vary from nearly clear to nearly black (figure 1a–e). To quantify this variation, we measured wing reflectance spectra using an Ocean Optics spectrometer (USB 2000) equipped with a reflectance probe (Ocean Optics R200–7-UV-VIS) and a pulsed xenon light source (Ocean Optics PX-2), with reference to a Labsphere certified reflectance standard using Ocean Optics' OOIBase32 software. We placed the reflectance standard behind the wings when taking readings, and the light path was oriented 45° relative to the wing surface to eliminate glare. The resulting measurements include both light reflected off the wings and light transmitted through the wings. We took three repeat measurements at three positions (base, middle and tip) on the forewings and hindwings and averaged the repeats. From the average spectra, we calculated ‘lightness' (L) as the sum of per cent reflectance at 2 nm intervals from 300 to 700 nm (scaled by 10−3 for presentation). To account for the proportionally larger mid-wing area, a weighted measure of lightness was obtained with the formula: Ltotal = 0.1Lbase + 0.8Lmiddle + 0.1Ltip, where the coefficients represent the relative area of each region of the wing.
Figure 1.
Female wing coloration and male sexual responses. Photographs females of four Hetaerina species: (a) H. cruentata (mating), (b) H. americana (marked for identification), (c) H. occisa, (d) H. titia. Sample reflectance spectra of female wings (e), with line colours matching the frames of the respective species' photographs (a–d). Wing lightness (f) affects whether H. titia females elicit a sexual response (stars) or not (circles) from H. americana (two-sided Mann–Whitney test, n = 14, p = 0.01) and H. occisa males (n = 77, p < 0.0001). (Online version in colour.)
To examine the effect of female wing coloration on males' responses to females, we measured the coloration of H. titia females that were presented to males in the mate recognition trials. It was not practical to scan the wings of all of the females with a spectrometer, so we instead took measurements from digital wing photographs. Photographs were taken with the wings flattened against a white background using a Canon 10D or 20D digital camera equipped with a Canon 100 mm macro lens and Canon MT-24 macro flash (Canon Inc., Tokyo). In ImageJ (http://imagej.nih.gov/), we used the ‘Color Balance’ plugin in the MBF package to standardize the white balance in each photo relative to the white background of the scale paper included in each photograph. We then used the polygon tool and the ‘Measure RGB’ plugin to analyse the RGB profile of each wing. The average, weighted greyscale calculated in ‘Measure RGB’ provided a photographic measure of wing lightness that correlated well with the spectroradiometric measure of wing lightness (Pearson's product-moment correlation r = 0.78, n = 49, p < 0.001).
(g). Female wing colour manipulation
To determine whether female wing colour per se influenced male mate recognition, we presented territorial males of H. occisa and H. americana at several sites (CT, CV, ES, LM, PA2) with (i) unmanipulated conspecific females and (ii) conspecific females with wings experimentally darkened to resemble dark H. titia females' wings. Females were assigned to treatments at random with respect to their natural wing coloration in an alternating order so as to maintain a balanced design. The same females were also presented to H. titia territory holders at PA2 and CV. The darkening treatment involved colouring the hindwings from the base to the tip with a grey marker (Warm Gray 90%, Prismacolor PM-107) and the forewings from base to the nodus with a grey marker and from the nodus to the tip with a sepia marker (Prismacolor PM-62). We chose these marker colours because their reflectance spectra best approximated the late season wing coloration of female H. titia. We used the same tethering protocol and criteria for male sexual responses and inclusion in analyses as above (for sample sizes, see figure 2).
Figure 2.
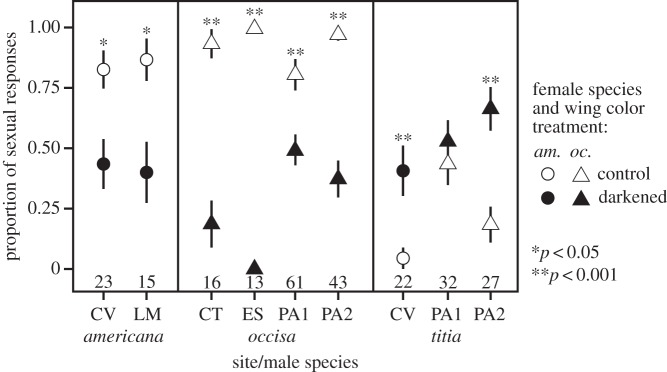
Results of female wing colour manipulation. Female H. americana and H. occisa with experimentally darkened wings elicited fewer sexual responses from conspecific males and more sexual responses from H. titia males than did controls. The plotted values are sample proportions (number of males that responded sexually divided by the total number). Whiskers depict the standard error of the proportion. Some whiskers are covered by the plotted symbols. Sample sizes of males tested are given above the site labels. Significance levels from Fisher's exact tests are shown above the plotted symbols. For study site locations, see electronic supplementary material, table S2.
(h). Statistical analysis
To obtain a relative measure of interspecific aggression, we divided the mean attack rate towards heterospecific tethered males by the mean attack rate towards conspecific tethered males. Likewise, to obtain a relative measure of reproductive interference, we divided the proportion of tethered females that elicited sexual responses in trials with heterospecific males by the proportion of tethered females that elicited sexual responses in trials with conspecific males. We obtained two measures of interspecific aggression and reproductive interference at each study site, one for each species, but only one measure of the species difference in female wing coloration. To test for correlations between these variables, while circumventing potential non-independence caused by the data structure, we used the following randomization approach: one of the two species at each site was dropped at random and a Spearman correlation coefficient (ρ) was calculated using the remaining data points in STATA 12.1 (Statacorp, TX, USA). This procedure was repeated 104 times to yield a distribution of ρ, from which we calculated the mean and standard deviation. We then used phylogenetic simulations to estimate the probability, under Brownian motion (BM) and Ornstein–Uhlenbeck (OU) models of evolution, of obtaining null mean ρ as large as the observed mean ρ (see electronic supplementary material, appendix S1).
3. Results
(a). Model results
With low levels of reproductive interference (d < 0.28), the species diverged in their mean values of μ and z until interspecific aggression was eliminated (figure 3a–c and electronic supplementary material, figure S2). By contrast, in the presence of moderate levels of reproductive interference (d ≥ 0.28), the species converged in their respective values of μ and z until interspecific territoriality was established (figure 3d–f and electronic supplementary material, figure S2). The initial level of divergence (∂) between species had no qualitative effect on the final outcome if d > 0.1 (electronic supplementary material, figure S2). With ∂ = 0 and d ≤ 0.1, intraspecific territoriality was lost in about one-third of the simulation runs (i.e. μ and z diverged within species; electronic supplementary material, figure S3), but ∂ = 0 is biologically unrealistic.
Figure 3.
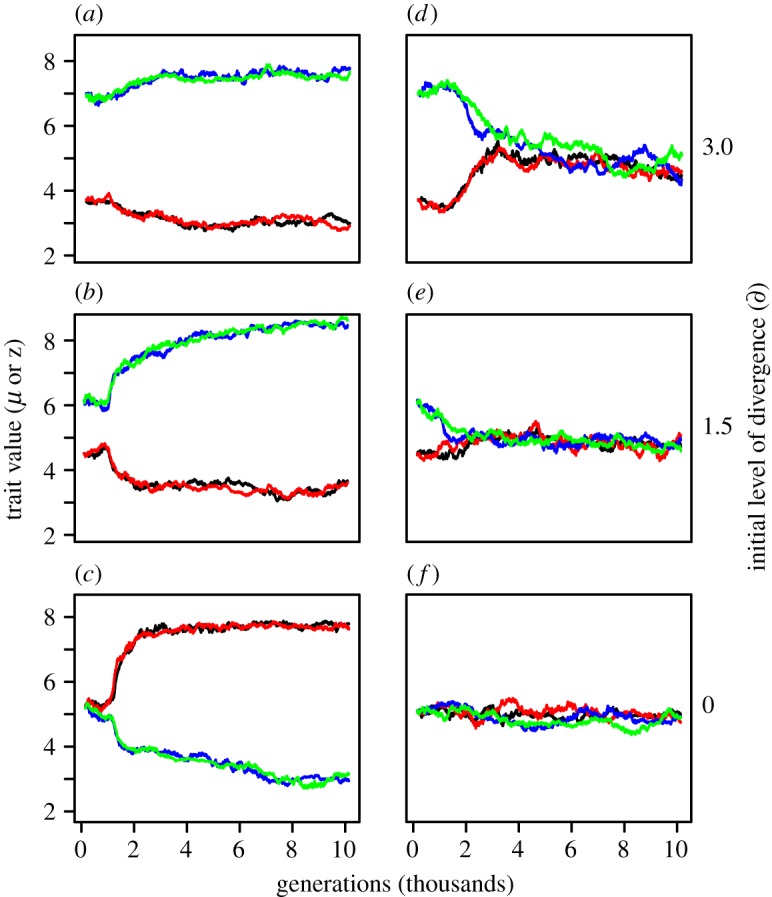
Simulations showing the effects of reproductive interference on the evolution of interspecific aggression. Panels (a–c) illustrate the usual outcome of secondary contact between species with low levels of reproductive interference while (d–f) represent cases with higher levels of reproductive interference. Plotted values: mean of the male trait z (black, species 1; blue, species 2) and mean of the competitor recognition template μ (red, species 1; green, species 2). Generation 0 is the time of secondary contact. In the examples shown here, d = 0.1 (a–c) and d = 0.33 (d–f). (Online version in colour.)
(b). Empirical results
We found that males discriminate between heterospecific and conspecific females in the same two species pairs in which they discriminate between heterospecific and conspecific males (i.e. H. occisa–H. titia, H. americana–H. titia), and not in the other four species pairs tested (electronic supplementary material, table S3). In the species pairs in which males discriminate between conspecific and heterospecific females, females that are more similar to heterospecific females in wing coloration are more likely to be clasped by heterospecific males (figure 1f), and experimental manipulations confirmed that female wing coloration directly affects male sexual responses (figure 2).
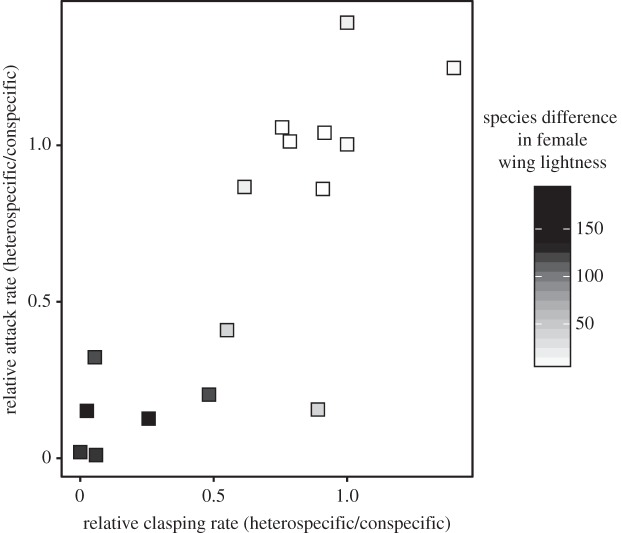
In striking support of our model's predictions, rates of reproductive interference and aggressive interference are strongly, positively correlated across sites (mean ± s.d. Spearman ρ = 0.84 ± 0.11, p < 0.001; figure 4). Both of these rates are negatively correlated with the species differences in female wing lightness (figure 4). The mean Spearman correlation between species differences in female wing lightness and the level of reproductive interference remained highly significant after phylogenetic correction (ρ = −0.77 ± 0.09; BM model of evolution, t = 59.11, d.f. = 999, p < 0.001; OU model of evolution, t = 57.78, d.f. = 999, p < 0.001). Likewise, the mean Spearman correlation between species differences in female wing lightness and the magnitude of interspecific aggression remained highly significant after the phylogenetic correction (ρ = −0.80 ± 0.07; BM model of evolution, t = 55.31, d.f. = 999, p < 0.001; OU model of evolution, t = 53.55, d.f. = 999, p < 0.001).
Figure 4.
Evidence for a link between reproductive interference and interspecific aggression in Hetaerina damselflies. Relative attack rate (a measure of interspecific aggression): the number of attacks elicited by heterospecific male intruders divided by the number of attacks elicited by conspecific male intruders. Relative clasping rate (a measure of reproductive interference): the proportion of tethered females that elicited sexual responses in trials with heterospecific males divided by the proportion of tethered females that elicited sexual responses in trials with conspecific males. Grey scale: species differences in female wing lightness, as measured by reflectance spectrometry. Each point represents a population at a sympatric site. See text for statistical analysis.
4. Discussion
Mutually costly interspecific interactions, such as resource competition and hybridization, can drive divergence between species over evolutionary time [2,47]. It is less intuitive that costly interactions can also prevent divergence or cause evolutionary convergence. Here we formalize the hypothesis that reproductive interference, resulting from indiscriminate male mating behaviour, can render interspecific territoriality adaptive and prevent divergence or cause convergence between species in territorial signals. We then test the model's predictions in the field and find that it explains the pattern of variation in interspecies fighting in Hetaerina damselflies. Recent reviews have highlighted the prevalence of interspecific aggression and reproductive interference [8,14,16,38,48]. Our model formally links these two costly interspecific interactions and provides a mechanism through which aggression between species can be maintained by natural selection.
Overlap between species in female coloration appears to be the root cause of reproductive interference in Hetaerina, and thus it is reasonable to ask why all sympatric species have not diverged substantially in female coloration. A plausible explanation, which has been invoked for other taxa [35,49], is that selection in other contexts, such as visual predation [36,50], overwhelms selection in a mating context and prevents reproductive character displacement in female traits. In the damselflies, divergent selection on female coloration caused by reproductive interference may be quite weak, because the fitness cost of temporary heterospecific pairings is likely to be much lower, for both sexes, than the cost to males of failing to clasp conspecific females. Thus, it pays for males to be relatively non-discriminating, which undermines the potential advantage to females of small increments in discriminability. While some species clearly have diverged sufficiently in female coloration for males to discriminate between the females easily, we have no evidence that this is a product of reproductive character displacement.
Our model predicts a steep sigmoidal relationship between reproductive interference and whether selection favours divergence or convergence between species in competitor recognition (electronic supplementary material, figure S2). While our empirical results are consistent with the existence of such a sigmoidal relationship (figure 4), we cannot yet evaluate whether the switch point occurs at the level of reproductive interference predicted by our model because reproductive interference depends on more than just the relative clasping rate. Other factors, such as microhabitat partitioning and the distance heterospecific pairs travel before the female is released, must also affect the intensity of reproductive interference. Quantifying the influence of such factors, and testing quantitative predictions of the model, is a goal for further research on this system.
The hypothesis that reproductive interference accounts for interspecific aggression and territoriality was first proposed by Payne [31] for parasitic Vidua finches, which, like the damselflies, only defend mating sites. The hypothesis has also been applied to hybridizing species that defend multi-purpose territories, on the basis that excluding heterospecific males is advantageous at the pair formation stage [51] and prevents interspecific extra-pair paternity [51,52]. Yet very few researchers have explicitly linked interspecific aggression to reproductive interference, and ours is the first formal model of the phenomenon. While interspecifically territorial species do not always interfere with each other reproductively, not all species that compete for common resources are interspecifically territorial either [4]. Even when resource defence is the primary function of territoriality, reproductive interference might tip the balance in favour of excluding heterospecifics. Our model can be readily extended to species that defend resources other than mates. Another logical extension of our model would be to evaluate the effects of asymmetries in reproductive interference and/or competitive ability between the interacting species. It is possible for selection to favour trait divergence in one species and convergence in the other, resulting in evolutionary dynamics similar to Batesian mimicry.
Whether character displacement is common or rare remains controversial [47,53,54], but researchers can probably agree that current theory does a poor job of predicting whether species will diverge from each other in sympatry. Indeed, a recent large-scale phylogenetic study of song variation in ovenbirds (Furnariidae) revealed a striking pattern of character convergence between sympatric lineages [55]. Our model shows that evolutionary convergence (or stasis maintained by selection) can result, paradoxically, from species being too similar phenotypically to be fully reproductively isolated. This finding defies conventional thinking on the evolutionary effects of cross-species mating, but it appears to account for the variable patterns of character displacement in Hetaerina damselflies. Our empirical results suggest that selection can favour divergence between some sympatric species and convergence between others within a single genus. Such mixed evolutionary outcomes of within-clade interactions may actually lead to an underestimation of the true effects of species interactions on character evolution in large comparative studies. We anticipate that our combined modelling and empirical results will provide strong impetus for further research on the links between reproductive interference and aggression between species.
Data accessibility
Our data files have been uploaded to Dryad (doi:10.5061/dryad.3rg5m).
Supplementary Material
Acknowledgements
We thank A. González-Karlsson, F. Gould, K. Peiman, T. B. Smith and two anonymous reviewers for comments and discussion. For assistance in the field, we thank T. Alvey, M. Benitez, E. Berlin, A. Chao, S. Giovanetti, P. Green, K. Henderson, S. Hu, L. Karlen, E. Khazan, R. Musker and S. Sanford. For assistance with transcription and image analyses, we thank C. Antaky, S. Ellis, N. Gentry, O. Mckenzie, L. Perng and N. Synstelien. We thank S. Bybee for providing extracted DNA or tissue for several specimens, E. Toffelmier, R. Ellingson, S. Lao and S. Chin for assistance with laboratory work and sample processing, T. B. Smith for use of laboratory facilities, R. Garrison for access to the Hetaerina morphological character data, M. Alfaro and J. Buckner for assistance with the phylogeny reconstruction, and T. Garland for suggesting the phylogenetic simulation approach. We also thank the staff at the Estación Biológica de Los Tuxtlas and Castroville Regional Park and several private landowners for their hospitality.
Author contributions
K.W.O. and G.F.G developed the theoretical model and K.W.O. wrote the code and ran the simulations. J.P.D, G.F.G. and C.N.A. designed and conducted the field experiments and J.P.D. and G.F.G. analysed the empirical data. J.P.D. conducted the laboratory work, constructed the phylogeny and carried out phylogenetic analyses. G.F.G. wrote the main paper and K.W.O, J.P.D. and G.F.G. created the figures. All authors discussed the manuscript drafts at all stages.
Funding statement
J.P.D. received an NSF Graduate Research Fellowship and fellowship support from UCLA's Graduate Division and Department of Ecology & Evolutionary Biology. This research was supported by NSF DEB-1020586 (to G.F.G).
References
- 1.Simmons KEL. 1951. Interspecific territorialism. Ibis 93, 407–413. ( 10.1111/j.1474-919X.1951.tb05443.x) [DOI] [Google Scholar]
- 2.Brown WL, Jr, Wilson EO. 1956. Character displacement. Syst. Zool. 5, 49–64. ( 10.2307/2411924) [DOI] [Google Scholar]
- 3.Lorenz K. 1962. The function of colour in coral reef fishes. Proc. R. Inst. Gt. Britain 39, 282–296. [Google Scholar]
- 4.Dhondt AA. 2012. Interspecific competition in birds. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Payne RB, Groschupf KD. 1984. Sexual selection and interspecific competition: a field experiment on territorial behavior of nonparental finches (Vidua spp.). Auk 101, 140–145. [Google Scholar]
- 6.Orians GH, Willson MF. 1964. Interspecific territories of birds. Ecology 45, 736–745. ( 10.2307/1934921) [DOI] [Google Scholar]
- 7.Murray BG. 1971. The ecological consequences of interspecific territorial behavior in birds. Ecology 52, 414–423. ( 10.2307/1937624) [DOI] [Google Scholar]
- 8.Grether GF, Losin N, Anderson CN, Okamoto K. 2009. The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 84, 617–635. ( 10.1111/j.1469-185X.2009.00089.x) [DOI] [PubMed] [Google Scholar]
- 9.Brown WD, Alcock J. 1990. Hilltopping by the red admiral butterfly: mate searching alongside congeners. J. Res. Lepid. 29, 1–10. ( 10.1016/0163-7827(90)90004-5) [DOI] [Google Scholar]
- 10.Schwartz JJ, Wells KD. 1985. Intra- and interspecific vocal behavior of the Neotropical treefrog Hyla microcephala. Copeia 1985, 27–38. ( 10.2307/1444787) [DOI] [Google Scholar]
- 11.Gerhardt HC, Ptacek MB, Barnett L, Torke KG. 1994. Hybridization in the diploid–tetraploid treefrogs: Hyla chrysoscelis and Hyla versicolor. Copeia 1994, 51–59. ( 10.2307/1446670) [DOI] [Google Scholar]
- 12.Anderson CN, Grether GF. 2010. Character displacement in the fighting colours of Hetaerina damselflies. Proc. R. Soc. B 277, 3669–3675. ( 10.1098/rspb.2010.0935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson CN, Grether GF. 2011. Multiple routes to reduced interspecific territorial fighting in Hetaerina damselflies. Behav. Ecol. 22, 527–534. ( 10.1093/beheco/arr013) [DOI] [Google Scholar]
- 14.Peiman KS, Robinson BW. 2010. Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol. 85, 133–158. ( 10.1086/652374) [DOI] [PubMed] [Google Scholar]
- 15.Dijkstra PD, Groothuis TGG. 2011. Male–male competition as a force in evolutionary diversification: evidence in haplochromine cichlid fish. Int. J. Evol. Biol. 2011, 1–9. ( 10.4061/2011/689254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ord TJ, King L, Young AR. 2011. Contrasting theory with the empirical data of species recognition. Evolution 65, 2572–2591. ( 10.1111/j.1558-5646.2011.01319.x) [DOI] [PubMed] [Google Scholar]
- 17.Lailvaux SP, Huyghe K, Van Damme R. 2012. Why can't we all just get along? Interspecific aggression in resident and non-resident Podarcis melisellensis lizards. J. Zool. 288, 207–213. ( 10.1111/j.1469-7998.2012.00943.x) [DOI] [Google Scholar]
- 18.Iyengar VK, Castle T, Mullen SP. 2014. Sympatric sexual signal divergence among North American Calopteryx damselflies is correlated with increased intra- and interspecific male–male aggression. Behav. Ecol. Sociobiol. 68, 275–282. ( 10.1007/s00265-013-1642-2) [DOI] [Google Scholar]
- 19.Singer F. 1989. Interspecific aggression in Leucorrhinia dragonflies—a frequency-dependent discrimination threshold hypothesis. Behav. Ecol. Sociobiol. 25, 421–427. ( 10.1007/BF00300188) [DOI] [Google Scholar]
- 20.Jones MJ, Lace LA, Harrison EC, Stevens-Wood B. 1998. Territorial behaviour in the speckled wood butterflies Pararge xiphia and P. aegeria of Madeira: a mechanism for interspecific competition. Ecography 21, 297–305. ( 10.1111/j.1600-0587.1998.tb00567.x) [DOI] [Google Scholar]
- 21.Shimoyama R. 1999. Interspecific interactions between two Japanese pond frogs, Rana porosa brevipoda and Rana nigromaculata. Japanese J. Herpetol. 18, 7–15. [Google Scholar]
- 22.Tynkkynen K, Rantala MJ, Suhonen J. 2004. Interspecific aggression and character displacement in the damselfly Calopteryx splendens. J. Evol. Biol. 17, 759–767. ( 10.1111/j.1420-9101.2004.00733.x) [DOI] [PubMed] [Google Scholar]
- 23.McLain DK, Pratt AE. 1999. The cost of sexual coercion and heterospecific sexual harassment on the fecundity of a host-specific, seed-eating insect (Neacoryphus bicrucis). Behav. Ecol. Sociobiol. 46, 164–170. ( 10.1007/s002650050606) [DOI] [Google Scholar]
- 24.Singer F. 1990. Reproductive costs arising from incomplete habitat segregation among three species of Leucorrhinia dragonflies. Behaviour 115, 188–202. ( 10.1163/156853990X00572) [DOI] [Google Scholar]
- 25.Nomakuchi S, Higashi K. 1996. Competitive habitat utilization in the damselfly, Mnais nawai (Zygoptera: Calopterygidae) coexisting with a related species, Mnais pruinosa. Res. Popul. Ecol. 38, 41–50. ( 10.1007/BF02514969) [DOI] [Google Scholar]
- 26.Outomuro D. 2009. Patrones morfológicos latitudinales en poblaciones ibéricas de Calopteryx Leach, 1815 (Odonata, Calopterygidae): posibles causas ambientales y evolutivas. Boln. Asoc. esp. Ent. 33, 299–319. [Google Scholar]
- 27.Tynkkynen K, Grapputo A, Kotiaho JS, Rantala MJ, Väänänen S, Suhonen J. 2008. Hybridization in Calopteryx damselflies: the role of males. Anim. Behav. 75, 1431–1439. ( 10.1016/j.anbehav.2007.09.017) [DOI] [Google Scholar]
- 28.Tynkkynen K, Kotiaho JS, Luojumaki M, Suhonen J. 2006. Interspecific territoriality in Calopteryx damselflies: the role of secondary sexual characters. Anim. Behav. 71, 299–306. ( 10.1016/j.anbehav.2005.03.042) [DOI] [Google Scholar]
- 29.Svensson EI, Karlsson K, Friberg M, Eroukhmanoff F. 2007. Gender differences in species recognition and the evolution of asymmetric sexual isolation. Curr. Biol. 17, 1943–1947. ( 10.1016/j.cub.2007.09.038) [DOI] [PubMed] [Google Scholar]
- 30.Schultz JK, Switzer PV. 2001. Pursuit of heterospecific targets by territorial amberwing dragonflies (Perithemis tenera Say): a case of mistaken identity. J. Insect Behav. 14, 607–620. ( 10.1023/A:1012223217250) [DOI] [Google Scholar]
- 31.Payne RB. 1980. Behavior and songs of hybrid parasitic finches. Auk 97, 118–134. [Google Scholar]
- 32.Nishikawa KC. 1987. Interspecific aggressive behaviour in salamanders: species-specific interference or misidentification? Anim. Behav. 35, 263–270. ( 10.1016/S0003-3472(87)80232-4) [DOI] [Google Scholar]
- 33.Pfennig KS. 2007. Facultative mate choice drives adaptive hybridization. Science 318, 127–134. ( 10.1126/science.1146035) [DOI] [PubMed] [Google Scholar]
- 34.Willis PM. 2013. Why do animals hybridize? Acta Ethol. 16, 127–134. ( 10.1007/s10211-013-0144-6) [DOI] [Google Scholar]
- 35.Stamps JA, Gonn SMI. 1983. Sex-biased pattern variation in the prey of birds. Ann. Rev. Ecol. Syst. 14, 231–253. ( 10.1146/annurev.es.14.110183.001311) [DOI] [Google Scholar]
- 36.Grether GF, Grey RM. 1996. Novel cost of a sexually selected trait in the rubyspot damselfly Hetaerina americana: conspicuousness to prey. Behav. Ecol. 7, 465–473. ( 10.1093/beheco/7.4.465) [DOI] [Google Scholar]
- 37.Endler JA. 1991. Interactions between predators and prey. In Behavioural ecology, an evolutionary approach (eds Krebs JR, Davies NB.), pp. 169–201. Oxford: Blackwell. [Google Scholar]
- 38.Gröning J, Hochkirch A. 2008. Reproductive interference between animal species. Q. Rev. Biol. 83, 257–282. ( 10.1086/590510) [DOI] [PubMed] [Google Scholar]
- 39.Reitz SR, Trumble JT. 2002. Competitive displacement among insects and arachnids. Annu. Rev. Entomol. 47, 435–465. ( 10.1146/annurev.ento.47.091201.145227) [DOI] [PubMed] [Google Scholar]
- 40.Okamoto KW, Grether GF. 2013. The evolution of species recognition in competitive and mating contexts: the relative efficacy of alternative mechanisms of character displacement. Ecol. Lett. 16, 670–678. ( 10.1111/ele.12100) [DOI] [PubMed] [Google Scholar]
- 41.Grether GF. 1996. Intrasexual competition alone favors a sexually dimorphic ornament in the rubyspot damselfly Hetaerina americana. Evolution 50, 1949–1957. ( 10.2307/2410753) [DOI] [PubMed] [Google Scholar]
- 42.Anderson CN, Grether GF. 2010. Interspecific aggression and character displacement of competitor recognition in Hetaerina damselflies. Proc. R. Soc. B 277, 549–555. ( 10.1098/rspb.2009.1371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrison RW. 1990. A synopsis of the genus Hetaerina with descriptions of four new species (Odonata: Calopterygidae). Trans. Am. Entomol. Soc. 116, 175–259. [Google Scholar]
- 44.DeAngelis DL, Mooij WM. 2005. Individual-based modeling of ecological and evolutionary processes. Annu. Rev. Ecol. Evol. Syst. 36, 147–168. ( 10.1146/annurev.ecolsys.36.102003.152644) [DOI] [Google Scholar]
- 45.Bürger R. 2000. The mathematical theory of selection, recombination, and mutation. Chichester, UK: Wiley. [Google Scholar]
- 46.Anderson CN, Cordoba-Aguilar A, Drury JP, Grether GF. 2011. An assessment of marking techniques for odonates in the family Calopterygidae. Entomol. Exp. Appl. 141, 258–261. ( 10.1111/j.1570-7458.2011.01185.x) [DOI] [Google Scholar]
- 47.Pfennig DW, Pfennig KS. 2012. Evolution‘s Wedge. Competition and the origins of diversity. Berkeley, CA: University of California Press. [Google Scholar]
- 48.Grether GF, Anderson CN, Drury JP, Kirschel ANG, Losin N, Okamoto K, Peiman KS. 2013. The evolutionary consequences of interspecific aggression. Ann. NY. Acad. Sci. 1289, 48–68. ( 10.1111/nyas.12082) [DOI] [PubMed] [Google Scholar]
- 49.Seddon N, et al. 2013. Sexual selection accelerates signal evolution during speciation in birds. Proc. R. Soc. B 280, 20131065 ( 10.1098/rspb.2013.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grether GF. 1997. Survival cost of an intrasexually selected ornament in a damselfly. Proc. R. Soc. Lond. B 264, 207–210. ( 10.1098/rspb.1997.0029) [DOI] [Google Scholar]
- 51.Sedlacek O, Cikanova B, Fuchs R. 2006. Heterospecific rival recognition in the black redstart (Phoenicurus ochruros). Ornis Fenn. 83, 153–161. [Google Scholar]
- 52.Baker MC. 1991. Response of male indigo and lazuli buntings and their hybrids to song playback in allopatric and sympatric populations. Behaviour 119, 225–242. ( 10.1163/156853991X00454) [DOI] [Google Scholar]
- 53.Stuart YE, Losos JB. 2013. Ecological character displacement: glass half full or half empty? Trends Ecol. Evol. 28, 402–408. ( 10.1016/j.tree.2013.02.014) [DOI] [PubMed] [Google Scholar]
- 54.Gerhardt HC. 2013. Geographic variation in acoustic communication: reproductive character displacement and speciation. Evol. Ecol. Res. 15, 605–632. [Google Scholar]
- 55.Tobias JA, Cornwallis CK, Derryberry EP, Claramunt S, Brumfield RT, Seddon N. 2013. Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. Nature 506, 359–363. ( 10.1038/nature12874) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data files have been uploaded to Dryad (doi:10.5061/dryad.3rg5m).