Abstract
Amazonia contains one of the world's richest biotas, but origins of this diversity remain obscure. Onset of the Amazon River drainage at approximately 10.5 Ma represented a major shift in Neotropical ecosystems, and proto-Amazonian biotas just prior to this pivotal episode are integral to understanding origins of Amazonian biodiversity, yet vertebrate fossil evidence is extraordinarily rare. Two new species-rich bonebeds from late Middle Miocene proto-Amazonian deposits of northeastern Peru document the same hyperdiverse assemblage of seven co-occurring crocodylian species. Besides the large-bodied Purussaurus and Mourasuchus, all other crocodylians are new taxa, including a stem caiman—Gnatusuchus pebasensis—bearing a massive shovel-shaped mandible, procumbent anterior and globular posterior teeth, and a mammal-like diastema. This unusual species is an extreme exemplar of a radiation of small caimans with crushing dentitions recording peculiar feeding strategies correlated with a peak in proto-Amazonian molluscan diversity and abundance. These faunas evolved within dysoxic marshes and swamps of the long-lived Pebas Mega-Wetland System and declined with inception of the transcontinental Amazon drainage, favouring diversification of longirostrine crocodylians and more modern generalist-feeding caimans. The rise and demise of distinctive, highly productive aquatic ecosystems substantially influenced evolution of Amazonian biodiversity hotspots of crocodylians and other organisms throughout the Neogene.
Keywords: Miocene, caimanine crocodylians, proto-Amazonia, Pebas System, molluscs, durophagy
1. Introduction
In Western Amazonia, the beginning of the Neogene (at approx. 23 Ma) was marked by a peak in Andean uplift that favoured onset and development of the Pebas Mega-Wetland System [1]. Ten million years later, just prior to establishment of the transcontinental Amazon River drainage, this inland ecosystem attained huge size (more than 1 million km2) and extreme complexity with multiple environments, such as lakes, embayments, swamps and rivers that drained towards the Caribbean [2,3]. The exceptional depositional and fossil record of the Pebas/Solimões Formations around the Peruvian–Colombian–Brazilian junction permits detailed reconstructions of these Miocene palaeoenvironments and their distinctive biotas [2,4–8]. Aquatic invertebrates (ostracods and molluscs) are extremely abundant and diverse within those deposits [2,4,9–11], denoting an extensive radiation of endemic lacustrine taxa by about 13 Ma [2]. The biostratigraphic framework for the Pebas/Solimões Formation is based on molluscs and pollen [4,5]. Although fishes [12] had been reported prior to our exploration of this region, other fossil vertebrates were unknown other than a teiid lizard later discovered [13]. Since 2002, systematic survey of Peruvian localities of the Iquitos area in northwestern Amazonia has yielded well preserved remains of mammals, turtles, fishes and crocodylians, the latter in great abundance. Two nearby, correlative and contemporaneous lignitic bonebeds in outcrops of approximately 20 and approximately 200 m2 each document at least seven co-occurring crocodylian species, contrasting with the three species that rarely occur sympatrically within the Amazon biodiversity hotspot today. Here, we report discovery of this new highly diverse and endemic crocodylian community, dominated by small blunt-snouted taxa with crushing dentitions that inhabited the Pebas Mega-Wetland System at its climax, just after the Middle Miocene Climate Optimum (MMCO). We describe three new, sympatric, blunt-snouted caimans to assess distinctive trophic dynamics of proto-Amazonian wetlands and identify a key interval of ecological turnover at the dawn of the Amazon River drainage.
2. Results
(a). Systematic palaeontology
Crocodyliformes Hay, 1930; Alligatoroidea Gray, 1844; Globidonta Brochu, 1999; Caimaninae Brochu, 1999.
Gnatusuchus pebasensis, gen. et sp. nov.
Etymology: Gnatusuchus from Quechua ‘Ñatu’ for small nose, and Greek ‘souchos’, crocodile; pebasensis from Pebas, after the old Amazonian village of Pebas, Peru, for which the source geological formation was named.
Holotype: Vertebrate Palaeontology Collection of the Natural History Museum of San Marcos University (MUSM) 990, nearly complete skull (figures 1a,b and 2a; electronic supplementary material, figure S2 and table S1).
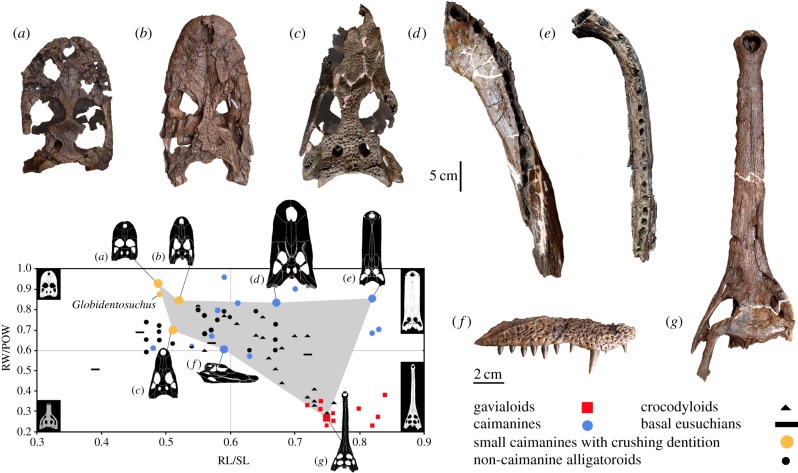
Figure 1.
Pebasian crushing-dentition caimans. Gnatusuchus pebasensis gen. et sp. nov. (a–d). Skull (holotype, MUSM 990) in dorsal (a) and ventral (b) view. (c) Right mandible (MUSM 1979) in dorsal view. (d) Comparison among coloured bone photographs of mandible of G. pebasensis (right) and Caiman crocodilus (left) in dorsal views and cross sections at the level of the adductor fossa. Values of 70° and 90° indicate the corresponding lateral vertical angle of the mandibular ramus with the horizontal plane. Kuttanacaiman iquitosensis gen. et sp. nov. (e,f,i). Skull and mandibles (holotype, MUSM 1490) in dorsal (e), ventral (f) and left lateral (i) view. Caiman wannlangstoni sp. nov. (g,h, j). Skull (holotype, MUSM 2377) in dorsal (g), ventral (h) and right lateral (j) view. an, angular; ar, articular; bo, basioccipital; co, coronoid; cr, canthi rostralii; d, dentary; d1–7, d11–14, dentary tooth positions; ec, ectopterygoid; emf, external mandibular fenestra; eo, exoccipital; f, frontal; FAD, mandibular adductor fossa; j, jugal; j.mx, maxilla surface for jugal; l, lacrimal; m1–9, m12, maxillary tooth positions; mx, maxilla; n, nasal; na, narial opening; p, parietal; pa, palatine; pf, prefrontal; pm, premaxilla; pm1–5, premaxillary tooth positions; po, postorbital; pt, pterygoid; q, quadrate; qj, quadratojugal; qj.q, quadrate surface for quadratojugal; QK, knob of quadrate crest A; s, splenial; sa, surangular; so, supraoccipital; sq, squamosal. Scale bars, 5 cm. Slightly smaller scale bar for (c) and (d).
Figure 2.
Pebasian crocodylian diversity and snout morphotypes. Positions of the six caimanines (a–f) and the sole gavialoid (g) from Iquitos are indicated in a plot of relative snout width and length within the Eusuchia (electronic supplementary material, figure S3 and text S1). (a) Gnatusuchus pebasensis gen. et sp. nov., (MUSM 990), skull. (b) Kuttanacaiman iquitosensis gen. et sp. nov. (MUSM 1490), skull. (c) Caiman wannlangstoni sp. nov. (MUSM 2377), skull. (d) Purussaurus neivensis (MUSM 1392), right dentary. (e) Mourasuchus atopus (MUSM 2379), right dentary. (f) Pebas Paleosuchus sp. (MUSM 1985), right maxilla in lateral view. (g) Pebas gavialoid (MUSM 1981), skull. Bivariate plot modified from [14]. Quadrants correspond to the four potential combinations of the bidimensional snout-shaped morphospace. RW/POW, rostral width–postorbital width index; RL/SL, rostral length–skull length index.
Locality and horizon: Locality IQ114 (electronic supplementary material, figure S1 and text S1), Iquitos area, Peru; Pebas Formation, late Middle Miocene, ca 13 Ma; Mollusc Zone 8 (MZ8) [4].
Referred specimens: MUSM 1979, right mandible (figure 1c,d), Locality IQ114 (electronic supplementary material, table S2); MUSM 662, partial left mandible (figure 3a–c), Locality IQ116 (electronic supplementary material, table S2); MUSM 2040, partial left mandible (figure 3d), Locality IQ114 (see electronic supplementary material, text S1).
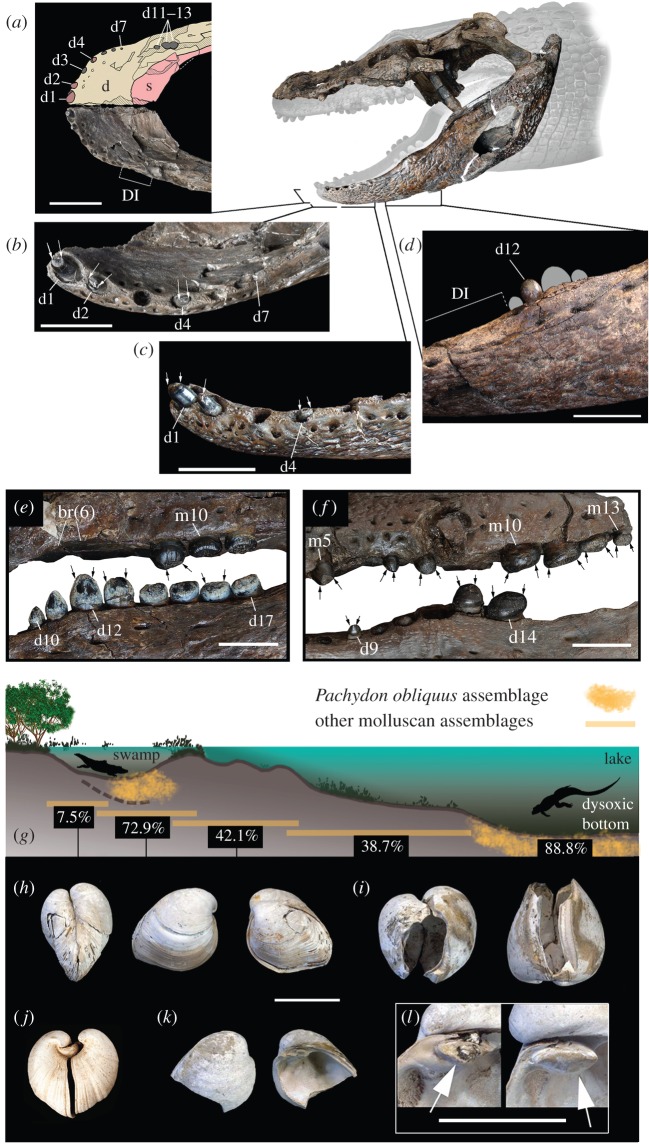
Figure 3.
Crushing-dentition caimanines and co-occurring pachydontine molluscs. (a–d) Gnatusuchus pebasensis. MUSM 662, anterior mandibular anatomy in dorsal (a), anterodorsal (b) and lateral view (c). (d) MUSM 2040, posterior mandibular dentition in lateral view. (e) Kuttanacaiman iquitosensis, MUSM 1942, posterior dentition. (f) Caiman wannlangstoni, MUSM 1983, posterior dentition. (g) Inferred distribution of molluscan assemblages within the typical depositional environments of the Pebas System [2]. Percentage values correspond to the estimated average abundance of the Pachydon group within each assemblage. Thick-shelled Pachydon obliquus (h,i,k,l) and Pachydon cuneatus (j) with convex outline making them well capable to withstand external pressure. (h,i) Crushing type predation scars in specimens that survived and then resumed growth. (k) Sharp edges typical of this type of predation. (l) Detail of cardinal tooth with (left) and without (right) crushing damage. See electronic supplementary material, and [2] and [4] for molluscan data and localities. Arrows in (b,c,e,f) indicate severe crown tooth wear. d, dentary; d1–4, d7, d9–14, d17, dentary tooth positions; DI, diastema; br(6), bone resorption in alveolar position 6; m5, m10, m13, maxillary tooth positions; s, splenial. Scale bars: 5 cm for (a), 2 cm for (b–f) and 1 cm for (h–l).
Diagnosis: Gnatusuchus is a blunt-snouted caimanine alligatoroid diagnosed by the following combination of characters (autopomorphies within Crocodyliformes are demarcated with an asterisk): skull short and broad, parallel-sided with a reduced rostrum and a wide rounded snout; upturned orbital rims absent; rostral canthi or ‘spectacle’ absent; thick laterosphenoid; attachment scars on ventral surface of quadrate forming a prominent knob; anterior teeth peg-shaped with blunt crowns and posterior teeth globular, both with no carinae*; dentary with an extensive diastema that separates seven anterior alveoli from four close-packed posterior ‘cheek teeth’ alveoli*; anterior dentary teeth strongly procumbent; posterior mandibular teeth completely surrounded by the dentary; shovel-like mandible with a long symphysis reaching the level of the eleventh dentary tooth alveolus (of related taxa); large participation of splenial in symphysis; posterior half of the mandibular ramus tilted lateroventrally*.
General description: The snout of G. pebasensis is substantially wider than long, with a length–breadth index [15] of 1.55—a slightly higher value than the 1.45 index of the bizarre pug-nosed Malagasy Cretaceous crocodyliform Simosuchus clarki [16]. As in Acynodon iberoccitanus [17], the skull is so short that the orbits are situated midway between the anterior tip and posterior margin of the skull. The index of rostral-skull length is 0.49. Skull bone sculpturing is generally moderate, but a little stronger in the jugal bones and skull table. The external naris is apple-shaped. Orbits are large and nearly circular, but with long axis oriented mediolaterally. The suborbital fenestrae are short, lacking a posterior notch and appearing to be widely separate from each other. The anterior and lateral rims of the choana lie flush with the surrounding pterygoid bones; the posterior rim is deeply incised. Mandibular articulation of the quadrates is proportionally larger than usually observed in caimans. Tooth loci count probably consisted of a total of five and nine in each premaxilla and maxilla, respectively. The mandible is short and wide. Symphyseal region is dorsoventrally compressed, with dentary and splenial forming a continuous concave dorsal surface. The surangular reaches the tip of the short, massive retroarticular process. Gnatusuchus shares with other caimanines small supratemporal fenestrae with overhanging rims (character 152–1), trapezoid supraoccipital on skull table (character 160-4) and slender process of exoccipital ventrally to basioccipital tubera (character 176-2). Total body length estimate is 148.9–167.7 cm (electronic supplementary material, table S7 and text S1).
Specialized dentition: Compared with other blunt-snouted or ‘generalized’ caimans, which possess around 18–20 tooth alveoli, the mandibular dentition of G. pebasensis is extremely reduced in number. Mandibular teeth include 11 tooth alveoli grouped into seven anterior teeth and four posterior ‘cheek’ teeth, with these groups separated by a long diastema. Tooth positions, dentary shape and vestigial dental alveoli indicate that the diastema results from the evolutionary loss of at least three tooth alveolus loci (the eighth, ninth and tenth loci of related taxa). Significant evolutionary loss of several alveoli posterior to the 14th tooth locus also occurred within the rear mandibular dentition, as the ancestral caimanine condition may have included four to six more tooth loci posterior to the 14th locus [18,19]. Additional alveolar closure within tooth loci posterior to the fourth is observed among most individuals, but it might be a consequence of secondary bone filling (i.e. bone resorption) after tooth loss that probably occurred during in vivo feeding activity on hard food (e.g. durophagy).
Teeth are distinctly modified in shape from the generalized crocodylian pattern. Anterior teeth are thin and peg-like in shape, bearing blunt crowns with no carinae ridges or striae. Preserved teeth show apical wear. Anterior teeth are notably procumbent. In MUSM 2040, one posterior tooth is preserved in the eleventh dentary position (figure 3d). This tooth is globular in shape with a distinct neck at the base of the crown. Similar morphology is expected for adjoining teeth from edentulous loci.
Kuttanacaiman iquitosensis, gen. et sp. nov.
Etymology: Kuttana from Quechua for ‘grinding or crushing machine’, and caiman, referring to tropical American alligatoroids; iquitosensis from the Iquitos native ethnic group inhabiting the Maynas province close to the Peruvian Amazonian city of Iquitos near the specimen locality.
Holotype: MUSM 1490, nearly complete skull and mandibles in anatomical connection (figures 1e,f,i and 2b; electronic supplementary material, table S3).
Locality and horizon: Locality IQ26 (electronic supplementary material, figure S1 and text S1), Iquitos area, Peru; Pebas Formation, late Middle Miocene, ca 13 Ma; MZ8 [4].
Referred specimens: MUSM 1942, associated left mandible, maxilla and skull table (figures 3e and S3a; electronic supplementary material, table S4), Locality IQ114 (see electronic supplementary material, text S1).
Diagnosis: Kuttanacaiman is a small caimanine diagnosed by the following combination of characters: robust, blunt and short snout; interorbital bridge flat and slender; posterior maxillary and dentary teeth closely packed, globular, low and laterally compressed. Symphysis reaching level of the sixth dentary alveolus, splenial excluded from mandibular symphysis, anterior dorsal process of splenials turned medially; abrupt elevation of surangular dorsal margin posterior to dentary series; first and fourth dentary teeth piercing premaxilla; angular–surangular suture contacting external mandibular fenestra at posterior angle; maxillary bearing a broad shelf extending into suborbital fenestra; palatine lateral margins extending into suborbital fenestra anteriorly and posteriorly; parietal excluded from posterior margin of skull table.
General description: The snout is blunt and rounded. Lateral rostral margins are slightly convex, without significant transverse expansion posteriorly. The index of rostral-skull length is 0.52 (figure 2). The narial opening projects dorsally and appears longer than wide. The nasal bones form the posterior rim of narial opening. Nasals and lacrimals are not in contact. The orbits are large, long and subtriangular in shape, with anterior angle displaced laterally as in Globidentosuchus brachyrostris and Eocaiman cavernensis. The skull table is parallel-sided, proportionally small and flat. The supratemporal fenestrae are circular and small owing to overlap of squamosal, parietal and postorbital bones. The incisive foramen is teardrop-shaped. Suborbital fenestrae are relatively small and roughly triangular. The choana is transversely wide. Skull bones are heavily sculpted with subcircular pits, particularly on rostrum and skull table. The skull bears five and 13 alveoli in premaxilla and maxilla, respectively, and 18 alveoli in dentary. Mandibular symphysis is relatively flat as in G. brachyrostris and E. cavernensis. Kuttanacaiman differs from E. cavernensis in having palatine processes projecting anteriorly into suborbital fenestrae (character 119-1), ectopterygoid–pterygoid flexure present throughout ontogeny (character 126-1), enlarged 12th dentary alveolus (character 51-1) and posterior teeth laterally compressed (character 79-1) and globular (character 198-2). It differs from G. pebasensis and G. brachyrostris in lacking splenial symphysis (character 54-2). Total body length estimate is 171.2–189.1 cm (see electronic supplementary material, table S7).
Caiman wannlangstoni, sp. nov.
Etymology: wannlangstoni after Wann Langston Jr, for his invaluable contributions to the knowledge of South American fossil crocodylians.
Holotype: MUSM 2377, partial skull (figures 1g,h,j, 2c; electronic supplementary material, figure S3b and table S5).
Locality and horizon: Locality IQ26 (electronic supplementary material, figure S1 and text S1), Iquitos area, Peru; Pebas Formation, late Middle Miocene, ca 13 Ma; MZ8 [4].
Referred specimens: MUSM 1983, associated cranial and mandibular elements (figure 3f), locality IQ114 (see electronic supplementary material). Also AMU-CURS-49, a right premaxilla and maxilla, from the Urumaco Formation [20].
Diagnosis: Small- to medium-sized Caiman species diagnosed by the following combination of characters: high and blunt snout with lateral margins strongly sinuous and diverging posteriorly; canthi rostralii very prominent; maxilla bearing broad shelf extending into suborbital fenestra, prefrontals contacting medially; edges of orbits upturned; narial opening oriented anterodorsally; crown teeth smooth to ribbed within crown upper half; dentary and maxillary posterior teeth large, globular, tightly packed and rounded in section; pterygoid surface pushed inward anterolateral to choana; dentary symphysis extended to level of sixth alveolus.
General description: The skull is roughly triangular in dorsal view. It bears a markedly high and robust rostrum. The nasal bones project into a fairly large narial opening. The orbits are oval and large. The jugal barely reaches the anterior margin of the orbits, thus its medial contact with lacrimal is reduced. The supratemporal fenestrae are constricted, as is typical in caimanines. Posterior margin of skull table is semicircular and overhangs the occipital plate. Configuration of the skull table bones resembles that of Caiman latirostris and Melanosuchus niger. Caiman wannlangstoni differs from K. iquitosensis in having upturned orbital edges (character 137-1), canthi rostralii (character 96-1), alveoli circular in cross section (character 79-0), pterygoid surface lateral and anterior to internal choana pushed inward (character 123-1) and surangular–angular suture intersecting external mandibular fenestra along its ventral margin (character 60-1). Here, we refer AMU-CURS-49, a right premaxilla and maxilla, from the Urumaco Formation [20] to C. wannlangstoni based on the presence of strong sinuous rostral margins and robust globular posterior teeth. UCMP 39978, a partial skull from La Venta (Colombia), was originally referred to Caiman lutescens [15] and more recently to C. latirostris [21]. It shares several features with C. wannlangstoni and might belong to this new species as well. However, La Venta Caiman lacks the distinctive high rostrum and anterodorsally narial opening observed in C. wannlangstoni. Although preserved teeth are blunt, there is no conclusive evidence that La Venta Caiman had enlarged and globular posterior teeth. Consequently, we propose to treat it as a distinct entity of uncertain taxonomic affinities until more anatomical data are available. Caiman brevirostris from Acre (Brazil) and probably Urumaco (Venezuela) [22] can be distinguished from C. wannlangstoni in having a proportionally shorter and parallel-sided rostrum as well as long dorsal premaxillary processes (character 90-1), prefrontals separated by frontal bone (character 129-1), and pterygoid surface lateral and anterior to internal choana flush with choanal margin (character 123-1). The estimated total body length of C. wannlangstoni is 210.5–226.7 cm (see electronic supplementary material, table S7).
(b). Other crocodylians of the Pebas System
Besides the blunt-snouted caimanines with crushing dentition and stout jaws, we also recovered the first unambiguous fossil of the extant smooth-fronted caiman Paleosuchus, which possesses a relatively more generalist snout shape (figure 2f). It bears a lightly built maxilla with vertically oriented walls, and distinctive sharply pointed teeth. As in other species of Paleosuchus, it has four premaxillary teeth (character 87-1) and a notched palatine anterior process (character 113-1). Although still poorly known, this fossil taxon (Paleosuchus sp.) differs from its extant relatives in having fewer maxillary teeth and a proportionally longer anterior process of the ectopterygoid running medially to posterior alveoli (electronic supplementary material, figure S4). Large caimanines are represented by Purussaurus neivensis and Mourasuchus atopus, the only two Pebasian taxa previously known from Miocene localities in the region [15]. Purussaurus possessed a hulking skull and a mandible with large robust anterior teeth and smaller blunt posterior teeth (figure 2d). Among a set of Purussaurus teeth from IQ125 (electronic supplementary material, figure S1), we recognized teeth with ‘bulb-shaped crowns’ that are indistinguishable from those of coeval Balanerodus longimus [15], suggesting that material of this latter species [23,24] pertains instead to specific regions of Purussaurus jaws, undermining recognition of it as a distinct taxon. The ‘duck-faced’ taxon Mourasuchus occupies one extreme of crocodylian snout morphotypes, with an exceptionally long and wide rostrum (figure 2e). Its feeding habits are controversial, although it was considered to eat small organisms (e.g. fishes) by some kind of filtering strategy [15]. A new, unnamed gavialoid documents the only crocodylian with a longirostral morphotype in this community (figure 2g), a fact that contrasts with the high diversity of longirostrine crocodylians characteristic of Late Miocene Neotropical assemblages [20,25].
3. Discussion
The crocodylian assemblage of the Iquitos bonebeds is extraordinary in representing both the highest taxonomic diversity and the widest range of snout morphotypes ever recorded in any crocodyliform community, recent or extinct. Other previously proposed peaks in sympatric diversity (e.g. in Late Miocene faunas of Venezuela and Brazil) are based on material from correlated strata of various localities and multiple horizons within basins rather than from a single site [18,25]. The hyperdiverse Iquitos assemblage (six caimanines and one gavialoid; figure 2) includes five new taxa that form the endemic Pebasian crocodylian fauna of the long-lived proto-Amazonian lakes that occupied most of western Amazonia during the Middle Miocene. Taxonomic distinctions from coeval assemblages within the same Neotropical realm, such as La Venta, Colombia [15] and Fitzcarrald, Peru [24], probably represent more fluvial-dominated palaeoenvironments. The extraordinary heterogeneity of snout shapes at Iquitos covers most of the morphospace range known for the entire Crocodylia clade (figure 2), reflecting the combined influences of long-term evolution, resource abundance and variety, and niche partitioning in a complex ecosystem.
Small caimanines with posterior globular teeth were conspicuous components of the Pebasian crocodylian assemblage (figure 3). These posterior teeth resemble those of the extant teiid lizard Dracaena, which has a strictly malacophagous diet [26]. In addition to the globular dentition, these taxa share several other distinctive traits (i.e. massive jaws, long symphysis, blunt snouts) of particular ecological relevance in the context of the peculiar Pebas palaeoenvironment as they together strongly suggest durophagy [27,28]. We propose that this array of crushing-toothed caimans predominantly fed on endemic molluscs that were copious in this time interval (MZ8; figure 4). Within the diverse fauna of approximately 85 co-occurring endemic species [4,9], corbulid pachydontine bivalves were especially abundant [2]. These bivalves display high morphological disparity and distinct anti-predatory adaptations, including thick shells, profuse ornamentation, overlapping valves, globose shape and a rostrum projecting siphons [10]. Successful and unsuccessful (i.e. healed) crushing predation scars are common and the proportion of shell fragments with sharp edges typical of this kind of damage reach up to 93% in valves of some molluscan samples (figure 3h–l; see electronic supplementary material). This extremely intense predation had been attributed to fishes and decapod crustaceans [10], but the Pebasian ichthyofauna does not differ significantly from its modern Amazonian counterpart [12], which lacks large-shell crushing fishes and is poor in molluscan species [33]. Instead, an array of crushing-dentition Pebasian caimans co-evolved with and exploited this trophically distinct, long-lasting, proto-Amazonian episode of increasing molluscan diversity and abundance, resulting in the high mollusc predation intensity observed. Consistent with these predator–prey interactions, the crocodylian ‘crusher’ morphotype exhibits anterior and posterior teeth with severe wear (figure 3e,f). The Iquitos bonebeds are also rich in isolated globular caiman teeth that were worn flat, suggesting active crushing or grinding during normal feeding activity. Small globidontan crocodylians from the Cretaceous and Palaeogene of the Great Plains of United States were interpreted as shell crushers owing to similar feeding-related traits and heavy surface wear pattern [27,28]. At least during the Palaeocene epoch, the huge freshwater systems of the Great Plains hosted three genera of corbulid bivalves that also occurred in the Pebasian Mega-Wetland System (Pachydon, Ostomya and Anticorbula), possibly indicating a much longer corbulid–globidontan interaction [34]. Similar interactions are hypothesized for molluscs and durophagous freshwater stingrays, co-occurring in early Palaeogene deposits of both the Great Plains (Palaeocene) and Western Amazonia (Middle Eocene-onward) [35].
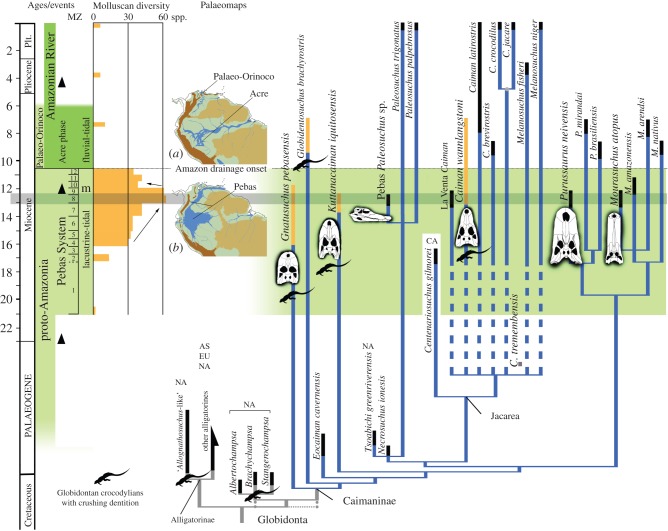
Figure 4.
Phylogenetic position of the Pebasian caimanines within the Alligatoroidea. Time-calibrated, strict consensus cladogram of 70 most parsimonious trees (see electronic supplementary material, figure S6 and test S1). Gnatusuchus pebasensis is the most basal caimanine and Globidentosuchus brachyrostris is the next outgroup to all remaining caimanines; these two taxa reveal unknown character states ancestrally present within the caimans (i.e. long splenial symphysis, posterior globular teeth) and their inclusion influenced the topology of relationships within the caimanine clade. Character support provides a novel sister-grouped relationship between the South American caimans and the North American Cretaceous globidontan alligatoroids (i.e. Brachychampsa, Albertochampsa and Stangerochampsa), whereas prior analyses showed either the monophyly of Alligatoridae (Caimaninae + Alligatorinae) exclusive of Cretaceous globidontans [29,30] or, more recently, a polytomy within the globidontan alligatoroids (Caimaninae + [alligatorines and Cretaceous globidontans]) [18,31]. Here, this polytomy (dotted lines) is obtained when the alligatorine Allognathosuchus wartheni is excluded from the analysis. Results also suggest an early diversification of major groups within the Caimaninae dating back to the end of the Cretaceous or Palaeocene interval. (a) The Acre Phase (ca 9 Ma) after intense Andean uplift and onset of the transcontinental Amazon River System. (b) The Pebas Mega-Wetland System in northwestern South America during MZ8 (ca 13 Ma). Stratigraphic distribution of taxa (yellow bars for crushing-dentition caimanines, black lines for other taxa) relative to major Neogene stages and events in Amazonia. Palaeogeographical reconstructions, Andean uplift peaks (black triangles) and marine incursions (m) are from Hoorn et al. [1]. Molluscan Zones and diversity for the Pebas System (MZ1–12) are from Wesselingh et al. [4]. When suitable, internal nodes were time-calibrated with molecular data from Oaks [32]. Darker grey marks MZ8. Alligatoroids are from South America, Asia (AS), Central America (CA), Europe (EU) or North America (NA).
Even though correlations between morphotype and ecology cannot be stated with certainty in extinct taxa, the singular G. pebasensis anatomy not only further supports durophagy but also reveals other distinctive aspects of its feeding strategy. Unique among crocodyliforms, Gnatusuchus possesses a dentary bearing a large edentulous gap between the seven procumbent anterior and four globular posterior teeth. This mammal-like diastema of about 30 mm results from the evolutionary loss of most of the alveoli lying between the dual (anterior and posterior) regions of maximum alveolar diameter of most crocodylians [36]. Mandibular rami are firmly sutured, yielding the longest symphysis observed within globidontan alligatoroids, and a stable shovel-like structure for the lower jaws (figure 3a–c). Posteriorly, the mandibular ramus is high and robust. In this region, the entire ramus is tilted lateroventrally and houses a wider and more capacious adductor fossa (figure 1d). In Gnatusuchus, strong adductor muscles and robust mandibular joints might have facilitated any feeding activity involving powerful dislocating jaw forces. This distinctive dental and craniomandibular anatomy is consistent with a durophagous diet, as well as with head burrowing activity in search of prey. Infaunal pachydontine bivalves (length approx. 7–25 mm) were diverse and abundant in unconsolidated bottoms of dysoxic lakes of the Pebas System (figure 3h–l) [2,10]. Gnatusuchus probably fed on them by ‘shoveling’ with the jaw and the procumbent anterior teeth, then crushing shells with the globular, tightly packed posterior teeth. During durophagy, traumatic tooth avulsion and severe damage involving tooth replacement might provide explanations for cases of bone resorption of posterior tooth loci in Gnatusuchus mandibles. Alveolar remodelling is also observed in one specimen of K. iquitosensis (figure 3e). Although this condition is common among crushing-dentition Pebasian caimans, it is unusual among extinct or extant reptiles [37]. Oxygen-stressed environments might be adverse for many potential predators feeding on mud bottoms (e.g. benthic fishes or crustaceans) but not for air-breathing caimans.
This new fauna highlights co-occurrence at approximately 13 Ma of every phylogenetic lineage currently recognized within the Caimaninae, emphasizing the role these proto-Amazonian mega-wetlands played in fostering the persistence of basal lineages simultaneously with the initial diversification of their modern relatives (figure 4; electronic supplemental material, figure S6). Phylogenetic analysis of a morphological dataset (see electronic supplemental material) positions Gnatusuchus as the most basal caiman, suggesting that a blunt-snouted rostrum with crushing dentition could have been the ancestral condition for the entire clade, while the more generalized morphology of the caiman crown-group is derived (figure 4). A long mandibular splenial symphysis also might be associated with early stages of caimanine evolution [18]. An evolutionary pattern in which generalist taxa, such as the extant species of Caiman, originated from blunt-snouted ‘crushers’ was similarly proposed for a different crocodylian clade: the alligatorines [38]. This distinctive caimanine morphotype, closer to that of Cretaceous alligatoroids, was unknown prior to the discovery of the Miocene taxa Gnatusuchus and Globidentosuchus, probably owing to the scarce Palaeogene fossil record in tropical South America. Similarly, Palaeocene or even Cretaceous origins and diversification of some caimanine groups consequently are expressed as long ghost lineages within the time-calibrated phylogenetic tree (figure 4), predicting currently unrecovered high morphotypic and taxonomic diversity continuously along caimanine evolutionary history until the Late Miocene. Regarding the evolution of globular dentitions, results of this analysis also suggest that a reversal occurred later within jacarean caimanines. Posterior globular teeth of the new crushing-dentition taxon C. wannlangstoni would have evolved from a generalized dental pattern as an opportunistic adaptation to the increasing abundance and diversity of molluscs throughout the Middle Miocene (figure 4).
The Pebas fossil record further underlines the occurrence of a key ecological turnover in western Amazonia around the Middle–Late Miocene transition, providing new insights on establishment of modern ecosystems. The Iquitos bonebeds immediately underlie strata documenting episodes of marine incursions and the first decline in endemic mollusc diversity [3,4]. This stage (MZ9, ca 12 Ma) represents the initial demise of dysoxic lacustrine Pebas environments [4] and coincides with events of intense Andean uplift that dissected proto-Amazonia into the modern Magdalena, Orinoco and Amazonian river basins. Major reorganization of drainage patterns at approximately 10.5 Ma included initiation of the transcontinental Amazon River drainage (figure 4) [1,39,40]. Lignite-poor outcrops just above MZ9 [41] in the Nueva Unión area south of Iquitos yield the youngest record of Gnatusuchus, but do not contain other crushing-dentition caimanines. Giant caimans like Purussaurus and Mourasuchus are common at Nueva Unión, as is also characteristic in the Late Miocene Solimões Formation of Acre that represents the fluvio-tidal Acre Phase, when a transcontinental river system first became established [3]. Contrary to the Pebas System, small- to medium-sized caimanines in Acre are represented by two Caiman species, including only one short-snouted species (i.e. C. brevirostris) with blunt posterior teeth considered ecologically similar to the extant C. latirostris [22,25]. Relatively depauperate fluvial mollusc assemblages dominate the Acre Phase [42], resembling modern Amazonian faunas. In northern South America, the Urumaco Formation (coeval with the Late Miocene Solimões Formation) documents life in the palaeo-Orinoco basin, including at least three ‘crushers’ among both basal (i.e. G. brachyrostris) and advanced caimanines, such as the Pebasian C. wannlangstoni [18,20]. Freshwater molluscs have not been described there yet, but shells are abundant throughout the Urumaco Formation [43]. As a whole, Acre and Urumaco crocodylian faunas are highly similar (i.e. several longirostrine crocodylians, giant taxa among gavialoids and caimanines) [25], although evidence suggests that an equivalent array of the Pebas crushing-dentition caimanines persisted during the Late Miocene within the palaeo-Orinoco [18], whereas they decayed in the Amazonian Acre Phase, suggesting faunal provincialism and persistence of Pebas-like ecosystems throughout the Late Miocene only in the northernmost Neotropics.
Morphological diversification of ‘crusher’ crocodylians during the Pebas System, including the singular anatomy of the shoveling caiman G. pebasensis, appears to have been largely driven by adaptation to the abundance of molluscan food sources in dysoxic lake bottom habitats. Crocodylian peak diversity was reached near the end of the MMCO, prior to fragmentation of these proto-Amazonian wetlands. Central and northern Andean uplift at approximately 12 Ma (Middle–Late Miocene transition) not only contributed to retreat of the Pebasian system to northernmost South America but also fostered the origin of the transcontinental flow of the modern Amazon River [1,44]. This transition ultimately led to the development of early Amazonian-type trophic dynamics that favoured fluvial faunas, including the initial replacement of more archaic, dietarily specialized crocodylians by the more generalist-feeding caimans that dominate modern Amazonian ecosystems.
Supplementary Material
Acknowledgements
We thank A. Balcarcel, A. Goswami, B. Shockey, R. Varas, A. Wyss and everybody who helped us during fieldwork; A. Balcarcel and W. Aguirre for the fossil preparation; M. Ellison and A. Benites for specimen photographs; C. de Muizon (MNHN) and R. Hulbert (UF) for access to comparative collections; J. Clarke, A. Valdés-Velasquez, G. Billet, S. Jouve and J. Martin for valuable discussions; K. Montalbán-Rivera for modelling the life reconstruction of Gnatusuchus pebasensis and O. Delgado for painting it. We are much indebted to G. Vermeij and an anonymous reviewer for their constructive critical reviews. Iquitos specimens are permanently deposited at the Museo de Historia Natural, UNMSM (MUSM), Lima, Peru. This is ISEM publication 2015-006 SUD.
Author contributions
J.J.F., P.-O.A. and R.S.-G. designed the research; R.S.-G., J.J.F., P.-O.A., P.B. and J.V.T.-L. contributed to the survey and collecting the crocodylian fossil material. F.P.W. provided data on Pebasian molluscan fossil record and ecology. P.B. provided geological data. R.S.-G. wrote the manuscript and performed anatomical descriptions and the systematic research, with additional writing contributions from all authors. All authors contributed to the discussions and interpretation of the results.
Funding statement
The study was financially supported by the National Aeronautics and Space Administration, Field Museum (Chicago), Frick Fund (Vertebrate Paleontology, AMNH), ‘Environnements et CLImats du Passé: hiStoire et Evolution’ (ECLIPSE) Program of France, Centre National de la Recherche Scientifique and Institut de Recherche pour le Développement. R.S.-G. benefits from a doctoral grant of the Escuela Doctoral Franco-Peruana en Ciencias de la Vida, and from financial support of the Frick Fund (AMNH) for visiting collections and of CampusFrance.
Competing interests
The authors declare no competing interests.
References
- 1.Hoorn C, et al. 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931. ( 10.1126/science.1194585) [DOI] [PubMed] [Google Scholar]
- 2.Wesselingh FP, Räsänen ME, Irion G, Vonhof HB, Kaandorp R, Renema W, Romero Pittman L, Gingras M. 2002. Lake Pebas: a palaeoecological reconstruction of a Miocene, long-lived lake complex in western Amazonia. Cainoz. Res. 1, 35–81. [Google Scholar]
- 3.Hoorn C, Wesselingh FP, Hovikoski J, Guerrero J. 2010. The development of the Amazonian mega-wetland (Miocene; Brazil, Colombia, Peru, Bolivia). In Amazonia, landscape and species evolution (eds Hoorn C, Wesselingh FP.), pp. 123–142. Oxford, UK: Wiley. [Google Scholar]
- 4.Wesselingh FP, Hoorn MC, Guerrero J, Räsänen ME, Romero Pittmann L, Salo JA. 2006. The stratigraphy and regional structure of Miocene deposits in western Amazonia (Peru, Colombia and Brazil), with implications for late Neogene landscape evolution. Scr. Geol. 133, 291–322. [Google Scholar]
- 5.Hoorn MC. 1993. Marine incursions and the influences of Andean tectonics on the Miocene depositional history of northwestern Amazonia: results of a palynostratigraphic study. Palaeogeogr. Palaeoclim. Palaeoecol. 105, 267–309. ( 10.1016/0031-0182(93)90087-Y) [DOI] [Google Scholar]
- 6.Pons D, De Franceschi D. 2007. Neogene woods from western Peruvian Amazon and paleoenvironmental interpretation. Bull. Geosci. 82, 343–354. ( 10.3140/bull.geosci.2007.04.343) [DOI] [Google Scholar]
- 7.Muñoz-Torres FA, Whatley RC, van Harten D. 2006. Miocene ostracod (Crustacea) biostratigraphy of the upper Amazon Basin and evolution of the genus Cyprideis. J. S. Am. Earth Sci. 21, 75–86. ( 10.1016/j.james.2005.08.005) [DOI] [Google Scholar]
- 8.Antoine PO, et al. 2006. Amber from western Amazonia reveals Neotropical diversity during the middle Miocene. Proc. Natl Acad. Sci. USA 103, 13 595–13 600. ( 10.1073/pnas.0605801103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesselingh FP, Salo JA. 2006. A Miocene perspective on the evolution of the Amazonian biota. Scr. Geol. 133, 439–458. [Google Scholar]
- 10.Wesselingh FP. 2006. Evolutionary ecology of the Pachydontinae (Bivalvia, Corbulidae) in the Pebas lake/wetland system (Miocene, western Amazonia). Scr. Geol. 133, 395–417. [Google Scholar]
- 11.Nuttall CP. 1999. A review of the Tertiary non-marine molluscan faunas of the Pebasian and other inland basins of north-western South America. Bull. Br. Mus. Nat. Hist. Geol. 45, 165–371. [Google Scholar]
- 12.Monsch KA. 1998. Miocene fish faunas from northwestern Amazonia basin (Colombia, Peru, Brazil) with evidence of marine incursions. Palaeogeogr. Palaeoclim. Palaeoecol. 143, 31–50. ( 10.1016/S0031-0182(98)00064-9) [DOI] [Google Scholar]
- 13.Pujos F, Albino AM, Baby P, Guyot JL. 2009. Presence of the extinct lizard Paradracaena (Teiidae) in the Middle Miocene of the Peruvian Amazon. J. Vertebr. Paleontol. 29, 594–598. ( 10.1671/039.029.0227) [DOI] [Google Scholar]
- 14.Busbey AB. 1995. The structural consequences of skull flattening in crocodilians. In Functional morphology in vertebrate paleontology (ed. Thomason JJ.), pp. 173–192. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Langston W., Jr 1965. Fossil crocodilians from Colombia and the Cenozoic history of the Crocodilia in South America. Univ. Calif. Publ. Geol. Sci. 52, 1–157. [Google Scholar]
- 16.Buckley GA, Brochu CA, Krause DW, Pol D. 2000. A pug-nosed crocodyliform from the Late Cretaceous of Madagascar. Nature 405, 941–944. (doi:10:1038/35016061) [DOI] [PubMed] [Google Scholar]
- 17.Martin JE. 2007. New material of the Late Cretaceous globidontan Acynodon iberoccitanus (Crocodylia) from southern France. J. Vertebr. Paleontol. 27, 362–372. ( 10.1671/0272-4634(2007)27[362:NMOTLC]2.0.CO;2) [DOI] [Google Scholar]
- 18.Scheyer TM, Aguilera OA, Delfino M, Fortier DC, Carlini AA, Sánchez R, Carrillo-Briceño JD, Quiroz L, Sánchez-Villagra MR. 2013. Crocodylian diversity peak and extinction in the late Cenozoic of the northern Neotropics. Nat. Commun. 4, 1907 ( 10.1038/ncomms2940) [DOI] [PubMed] [Google Scholar]
- 19.Simpson GG. 1933. A new crocodilian from the Notostylops beds of Patagonia. Am. Mus. Novit. 623, 1–9. [Google Scholar]
- 20.Sánchez-Villagra MR, Aguilera OA. 2006. Neogene vertebrates from Urumaco, Falcón State, Venezuela: diversity and significance. J. Syst. Palaeontol. 4, 213–220. ( 10.1017/s1477201906001829) [DOI] [Google Scholar]
- 21.Bona P, Riff D, Brandoni de Gasparini Z. 2013. Late Miocene crocodylians from northeast Argentina: new approaches about the austral components of the Neogene South American crocodylian fauna. Earth Environ. Sci. Trans. R. Soc. 103, 1–20. ( 10.1017/S175569101300042X) [DOI] [Google Scholar]
- 22.Fortier DC, Souza-Filho JP, Guilherme E, Maciente AAR, Schultz CL. 2014. A new specimen of Caiman brevirostris (Crocodylia, Alligatoridae) from the Late Miocene of Brazil. J. Vertebr. Paleontol. 34, 820–834. ( 10.1080/02724634.2014.838173) [DOI] [Google Scholar]
- 23.Langston W, Gasparini Z. 1997. Crocodilians, Gryposuchus, and the South American gavials. In Vertebrate paleontology in the neotropics—the Miocene fauna of La Venta, Colombia (eds Kay RF, Madden RH, Cifelli RL, Flynn JJ.), pp. 113–115. Washington, DC: Smithsonian Institution. [Google Scholar]
- 24.Salas-Gismondi R, et al. 2007. Middle Miocene crocodiles from the Fitzcarrald Arch, Amazonian Peru. Cuad. Museo Geom. Inst. Geol. Min. Esp. 8, 355–360. [Google Scholar]
- 25.Cozzuol MA. 2007. The Acre vertebrate fauna: age, diversity, and geography. J. S. Am. Earth Sci. 21, 185–203. ( 10.1016/jsames.2006.03.005) [DOI] [Google Scholar]
- 26.Dalrymple GH. 1979. On the jaw mechanism of the snail-crushing lizards, Dracaena Daudin 1802 (Reptilia, Lacertilia, Teiidae). J. Herpetol. 13, 303–311. ( 10.2307/1563324) [DOI] [Google Scholar]
- 27.Carpenter K, Lindsey D. 1980. The dentary of Brachychampsa montana Gilmore (Alligatorinae, Crocodylidae), a Late Cretaceous turtle-eating alligator. J. Paleontol. 54, 1213–1217. [Google Scholar]
- 28.Abel O. 1928. Allognathosuchus, ein an die cheloniphage Nahrungsweise angepaßter Krokodiltypus de nordamerikanischen Eozäns. Paläontol. Zeitschr. 9, 367–374. ( 10.1007/BF03041562) [DOI] [Google Scholar]
- 29.Brochu CA. 1999. Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. Soc. Vertebr. Paleontol. Mem. 6, 9–100. ( 10.1080/02724634.1999.10011201) [DOI] [Google Scholar]
- 30.Brochu CA. 2010. A new alligatorid from the lower Eocene Green River formation of Wyoming and the origin of caimans. J. Vertebr. Paleontol. 30, 1109–1126. ( 10.1080/02724634.2010.483569) [DOI] [Google Scholar]
- 31.Brochu CA. 2011. Phylogenetic relationships of Necrosuchus ionensis Simpson, 1937 and the early history of caimanines. Zool. J. Linn. Soc. 163, 228–256. ( 10.1111/j.1096-3642.2011.00716.x) [DOI] [Google Scholar]
- 32.Oaks JR. 2011. A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution 65, 3285–3297. ( 10.1111/j.1558-5646.2011.01373.x) [DOI] [PubMed] [Google Scholar]
- 33.Fittkau EJ. 1981. Armut in der Vielfalt—Amazonien als Lebensraum fur Weichtiere. Mitt. Zool. Ges. Braunau 3, 197–200. [Google Scholar]
- 34.Anderson LC, Hartman JH, Wesselingh FP. 2006. Close evolutionary affinities between freshwater corbulid bivalves from the Neogene of western Amazonia and Paleogene of western Amazonia and Paleogene of northern Great Plains, USA. J. S. Am. Earth Sci. 21, 28–48. ( 10.1016/j.jsames.2005.07.008) [DOI] [Google Scholar]
- 35.Adnet S, Salas-Gismondi R, Antoine PO. 2014. River stingrays (Chondrichthyes: Potamotrygonidae) from the middle Eocene of Peruvian Amazonia and an overview of potamotrygonid dental morphology. Naturwissenschaften 101, 33–45. ( 10.1007/s00114-013-1127-1) [DOI] [PubMed] [Google Scholar]
- 36.Iordansky NN. 1964. The jaw muscles of the crocodiles and some relating structures of the crocodilian skull. Anat. Anz. 115, 256–280. [PubMed] [Google Scholar]
- 37.Xing LD, Bell PR, Rothschild BM, Ran H, Zhang JP, Dong ZM, Zhang W, Currie PJ. 2013. Tooth loss and alveolar remodeling in Sinosaurus triassicus (Dinosauria, Theropoda) from the Lower Jurassic strata of the Lufeng Basin, China. Chin. Sci. Bull. 58, 1931–1935. ( 10.1007/s11434-013-5765-7) [DOI] [Google Scholar]
- 38.Brochu CA. 2004. Alligatorine phylogeny and the status of Allognathosuchus Mook, 1921. J. Vertebr. Paleontol. 14, 857–873. ( 10.1671/0272-4634(2004)024[0857:APATSO]2.0.CO;2) [DOI] [Google Scholar]
- 39.Figueiredo J, Hoorn C, van der Ven P, Soares E. 2010. Late Miocene onset of the Amazon River and the Amazon deep-sea fan: evidence from the Foz do Amazonas Basin: Reply. Geol. Forum 38, e213 ( 10.1130/G31057Y.1) [DOI] [Google Scholar]
- 40.Shephard GE, Müller RD, Liu L, Gurnis M. 2010. Miocene drainage reversal of the Amazon River driven by plate–mantle interaction. Nat. Geosci. 3, 870–875. ( 10.1038/NGEO1017) [DOI] [Google Scholar]
- 41.Rebata LA, Räsänen ME, Gingras MK, Vieira V, Jr, Berberi M, Irion G. 2006. Sedimentology and ichnology of tide-influenced Late Miocene successions in western Amazonia: the gradational transition between the Pebas and Nauta formations. J. S. Am. Earth Sci. 21, 116–129. ( 10.10116/jsames.2005.07.011) [DOI] [Google Scholar]
- 42.Wesselingh FP, Ranzi A, Räsänen ME. 2006. Miocene freshwater Mollusca from western Brazilian Amazonia. Scr. Geol. 133, 419–437. [Google Scholar]
- 43.Quiroz LI, Jaramillo CA. 2010. Stratigraphy and sedimentary environment of Miocene shallow to marginal marine deposits in the Urumaco trough, Falcon Basin, Western Venezuela. In Urumaco & Venezuelan paleontology (eds Sánchez-Villagra M, Aguilera OA, Carlini AA.), pp. 153–172. Bloomington, IN: Indiana University Press. [Google Scholar]
- 44.Mora A, Baby P, Roddaz M, Parra M, Brusset S, Hermoza W, Espurt N. 2010. Tectonic history of the Andes and sub-Andean zones: implications for the development of the Amazon drainage basin. In Amazonia, landscape, and species evolution (eds Hoorn C, Wesselingh FP.), pp. 38–60. Oxford, UK: Wiley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.