Abstract
Varicella zoster virus (VZV) causes varicella upon first exposure and may reactivate later in life into herpes zoster (HZ), with a risk that is thought to be reduced by re-exposures to VZV. Given the decades-long time scales of reactivation and its dependence on the accumulation of re-exposure episodes, adopting a long-term perspective may be useful to correctly interpret current epidemiological trends of VZV. In this study, we investigate the possible impact of demographic changes on varicella and HZ in Spain, using an age-structured mathematical model informed with historical demographic data and calibrated against age-specific profiles of varicella seroprevalence and HZ incidence data. The model qualitatively reproduces the remarkable growth of HZ incidence observed in Spain between 1997 and 2004, before the introduction of varicella vaccination programmes. We demonstrate that this growth may be partially ascribed to the reduction of varicella circulation that followed the overall decline of the birth rate in the twentieth century. Model predictions further suggest that, even under the most optimistic projections, HZ incidence will continue its rise until at least 2040. Considering the effect of demographic changes can help interpreting variations in epidemiological trends of HZ, contributing to a more accurate evaluation of vaccination programmes against VZV.
Keywords: varicella, herpes zoster, demographic transition, mathematical modelling
1. Background
Varicella zoster virus (VZV) is responsible for two clinically different diseases. The first exposure to VZV usually occurs in childhood and causes varicella disease. After recovery, the virus remains dormant in sensory ganglia and in about 30% of cases it will reactivate later in life, causing herpes zoster (HZ) [1], an inflammatory skin disease associated with more serious morbidity [2]. In 1965, Hope-Simpson hypothesized that secondary exposures to VZV (also called ‘exogenous boosting events') boost the host's VZV-specific antibodies, thereby reducing the risk of developing HZ [3]. Later, it was found that the main component of immunity against HZ was cell-mediated [4,5], but Hope-Simpson's exogenous boosting hypothesis has been corroborated by immunological studies showing an increase of VZV-specific T cells in people exposed to contacts with the virus [6–9], as well as by epidemiological [10,11] and modelling studies [11–14].
Mathematical models of VZV transmission dynamics, all based on the exogenous boosting hypothesis, unanimously predict that mass immunization against varicella, which has been already introduced in some countries [15,16], including some regions of Spain [17], may result in a temporary increase of HZ incidence [12,13,18,19] as a consequence of the expected reduction of VZV re-exposures after vaccination. Nonetheless, empirical evidence coming from surveillance programmes of HZ provides ambiguous results, with some studies showing an increase of HZ following mass immunization and others not [20,21]. However, an increase in HZ incidence has been detected in several areas long before the introduction of the varicella vaccine [22–25] and, more recently, in areas without relevant VZV vaccination history [21,26]: for example, in the Community of Madrid (where until 2005 the vaccine was introduced only for individuals at high risk of complications), the annual HZ incidence has risen from about 2.5 to about 3.6 cases per 1000 individuals per year between 1997 and 2004, and the trend was statistically significant [26]. This suggests the presence of other factors that might confound trend interpretations. One such factor may be represented by demographic changes. During the twentieth century, industrialized countries completed their fertility transitions [27], with a massive decline in birth rates and substantial population ageing. In particular, since 1980, several central and southern European countries have experienced sustained below-replacement fertility [28].
This work aims at exploring the role of demographic changes in VZV transmission and reactivation dynamics by making use of a mathematical model. The developed model, informed with country-specific epidemiological and demographic data, is used to investigate the past dynamics of varicella and HZ in Spain, and to predict their future burden under different demographic and vaccination scenarios.
2. Methods
(a). Data
Demographic changes for the period 1900–2009 were modelled using data on yearly birth rates [29], age-specific mortality rates [30] and age-specific migration flows [31–33] over time. Historical age structures of the Spanish population [29] were used to validate the demographic model. Available projections on mortality, birth and migration rates [34] relative to the period 2010–2050 were used for model predictions about future dynamics. For what concerns the transmission model, as no survey data on social mixing patterns [35,36] are available for Spain, we parametrized age-specific contact patterns with estimates based on census-type data provided by a previously published study [37]. Calibration was done using age-specific VZV seroprevalence data in 1996 for Spain, made available by the European Seroepidemiological Network 2 [38], and age-specific data on HZ incidence from 2005 to 2006 [39]. Model estimates were also qualitatively compared against an independent dataset that included age-specific HZ incidence data in the years 1997–2004 [26].
(b). The model
We use a stochastic individual-based age-structured epidemiological model for the natural history of varicella and HZ, explicitly accounting for demographic changes. Epidemiological transitions are modelled as in [14]: individuals are born susceptible to VZV and develop varicella upon contacts with VZV-infectious individuals. Contacts are assumed to be structured by age according to a mixing matrix previously estimated for Spain [37] and rescaled at each time step by the current age distribution of the population, following the approach proposed in [40]. After recovery, individuals gain lifelong immunity against varicella and become susceptible to HZ. The risk of HZ increases with age and time elapsed since the last exposure episode. We assume that the risk of HZ declines progressively upon repeated contacts with VZV-infectious individuals, in order to take into account the accumulation of protection against reactivation that Hope-Simpson postulated to follow from successive re-exposures to VZV [2,3]. Only a fraction of contacts that would result in varicella infection in susceptible individuals lead to an effective immune boosting in VZV-experienced individuals. After recovery from HZ, individuals are assumed to become lifelong immune to new episodes of VZV reactivation [11–14]. Indeed, secondary HZ episodes, although possible, are relatively rare in immunocompetent individuals [2].
The vital dynamics of the population is explicitly modelled in order to reproduce the observed changes in the demographic structure of the Spanish population over the period 1900–2009, following the approach of a previously published model for Italy [41]. As in [41], the model is initialized in 1900 at both demographic and epidemiologic equilibrium, whereas for the following years, demographic dynamics are driven by variations of birth, age-specific mortality and migration rates. Postulating that the level of the birth rate is a main determinant of transmission in childhood infections, we believe the hypothesis of epidemiological equilibrium in 1900 is motivated by the fact that most of the fertility decline in Spain occurred after that date [42]. Full details about the developed model are reported in the electronic supplementary material, together with a validation of the demographic model against observed longitudinal data on the age structure of the Spanish population.
(c). Model calibration and validation
Model calibration was carried out by a Bayesian approach using uniform prior distributions on the six model parameters combined with the likelihood of varicella and zoster data. The latter is defined as the product between the binomial likelihood of the observed age-specific VZV serological profile in 1996 [38] and the Poisson likelihood of the observed age-specific HZ incidence in 2005–2006 [39]. Computation of posterior distributions was carried out by means of a Monte Carlo Markov chain approach with random-walk Metropolis–Hastings sampling with normal jump distributions. Convergence of MCMC (22 000 iterations) was assessed by considering several different starting points and by visual inspection, after a burn-in period of 2000 iterations. Details on model calibration and the estimated posterior distribution of parameters are available in the electronic supplementary material.
(d). Demographic projections
The model is used to retrospectively analyse the impact of demographic changes on varicella and HZ (period 1900–2009) and to predict their future dynamics (period 2010–2050). The latter were simulated by using both published demographic projections [34] and theoretical scenarios, based for simplicity on temporal changes during the prediction period (2010–2050) of the birth rate only. Theoretical scenarios have been formulated by assuming that all other demographic indicators are kept equal to their 2009 values, based on the assumption that mortality and migration play a minor role on dynamics of varicella infection. In fact, the circulation of infectious diseases depends on the fraction of susceptibles within the population, which, in the case of childhood infections, is shaped by the birth rate. As varicella occurs predominantly in children, either an increase or a decrease of the birth rate has the potential of leading respectively to higher or lower VZV circulation, which can affect the dynamics of HZ through the effect of boosting.
Figure 1 shows the reported birth rate in Spain over the period 1900–2009, along with projected scenarios for the period 2010–2050. In particular, we considered a baseline projection scenario that assumes a constant birth rate; one that assumes a linear decrease of the birth rate up to a minimum of 0 in 2050 (denoted by ‘L’ for ‘lowest’); and one assuming a linear increase with a doubling of the 2009 birth rate over the same period (denoted by ‘H’ for ‘highest’). Although these scenarios are demographically naive, they represent useful illustrative limit cases for HZ. A more realistic scenario, based on published population projections made available from the United Nations [34] for Spain, has also been considered; this scenario includes changes in mortality rates and migration fluxes, and projects a decrease of the birth rate between 2010 and 2030, followed by a partial recovery between 2030 and 2050 (figure 1).
Figure 1.
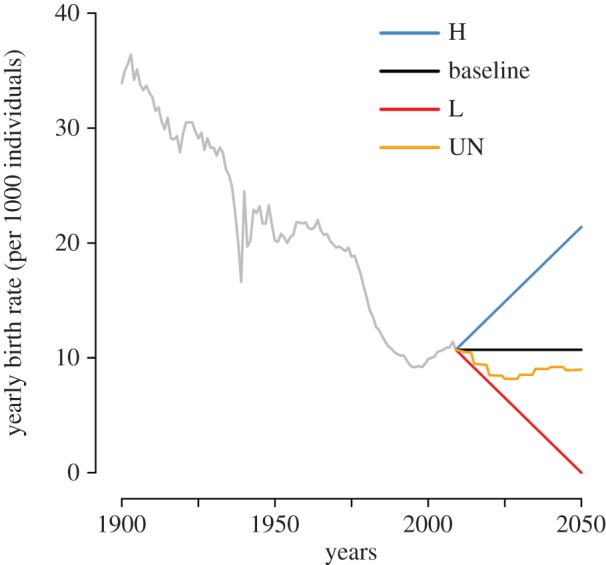
Birth rate over time in Spain. Yearly birth rate as observed in Spain during the period 1900–2009 (grey line), and as assumed by different prediction scenarios considered (2010–2050). Illustrative scenarios: H, baseline and L. Scenario based on published population projections of the UN [34].
(e). Vaccination
The model is applied to evaluate the potential long-term effects of a varicella vaccination programme starting in 2010 on future HZ incidence under the baseline scenario for demographic projections. Vaccine is administered to children at the age of 15 months. We assume different combined levels of vaccine efficacy and coverage, namely 80%, 90% and 100%. Successfully immunized individuals are assumed to be fully protected against VZV infection (i.e. we do not explicitly consider breakthrough varicella infections) [43]. Moreover, vaccinated individuals can develop HZ from vaccine strain, although at a lower rate compared with natural VZV, as observed in [44].
3. Results
Figure 2 shows the ability of the model to fit the VZV serological profile observed in Spain in 1996. Observed and estimated 95% CI intersect for all younger age classes (age 20 years or below). The model also reproduces the observed age-specific HZ incidence in 2005–2006. The observed yearly incidence lies in the 95% CI of the estimated incidence for most age groups. Moreover, the model captures the decreasing trend of incidence in the elderly, which may be ascribed to exogenous boosting effects [13,14].
Figure 2.
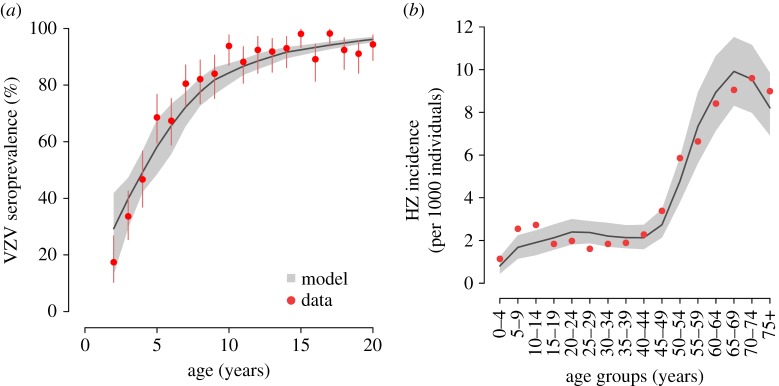
VZV seroprevalence and HZ incidence. (a) Age-specific VZV seroprevalence as observed in data from Spain in 1996 [38] and as estimated by the model. Vertical lines represent 95% CI of the data computed by exact binomial test. (b) HZ incidence by age group as observed in 2005–2006 [39] and as estimated by the model. In both panels, grey areas show the 95% CI of model estimates.
(a). Historical period (1900–2009)
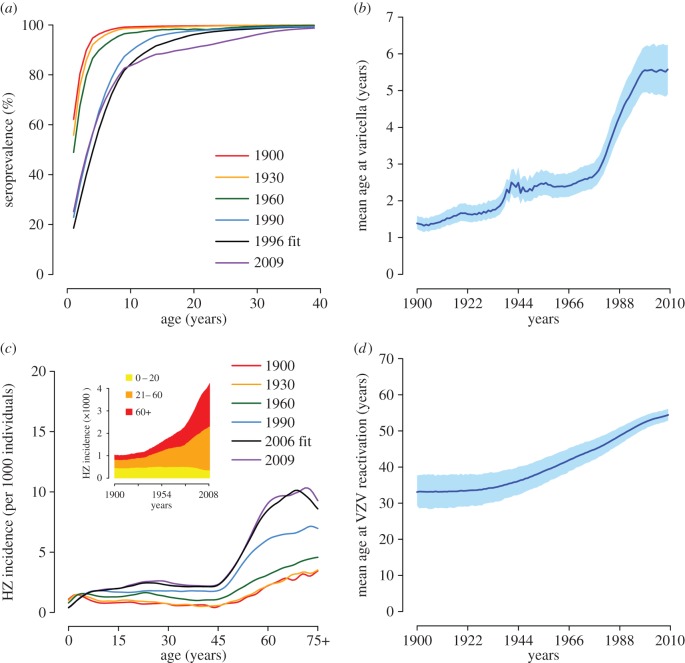
Figure 3 shows model results for the period 1900–2009. According to the model, the overall decreasing trend of the birth rate observed since 1900 has led to a reduction of the fraction of VZV seropositive individuals at all ages (figure 3a). For instance, the estimated fraction of children who have acquired varicella by 10 years of age dropped from 99.2% (95% CI 98.8–99.6) in 1900 to a minimum of 81.5% (95% CI 76.5–85.8) in 2004. Consequently, the mean age at varicella infection is estimated to increase from about 1.4 (95% CI 1.2–1.6) to a maximum of 5.6 (95% CI 4.9–6.2) years (figure 3b). Most of this increase (from about 2.5 to 5.6 years) occurs during the prolonged epoch of low fertility started in the second half of the 1970s [29]. The modelled effect of changes in the birth rate on varicella is due to a depletion of new susceptible individuals that sustain the circulation of the infection, and occurs with a delay of a few years. Major fertility fluctuations observed in the past century are reflected in the estimated mean age at infection. For example, the increase in the mean age at varicella infection that is estimated around 1940 (figure 3b) is a consequence of the temporary brisk drop of the birth rate during the Spanish civil war in 1936–1939 (figure 1); similarly, the stabilization in the mean age estimated in the decade 2000–2009 is due to the recovery of the birth rate started in the late 1990s. As regards HZ, the model estimates a gradual increase of the incidence rates since 1900 (figure 3c, inset). This trend, at least for recent years, has been documented for Spain by a surveillance study [26] reporting a remarkable growth in the total yearly HZ incidence over the period 1997–2004. In particular, HZ incidence is approximately stable for younger individuals, with virtually all of the HZ growth concentrated at ages above 65 [26]. In comparison, our model estimates an increase in the total incidence of about 12% (up to 18% by assuming different contact patterns by age; see electronic supplementary material) over the same period, from 3.52 (95% CI 3.18–3.87) to 3.93 (95% CI 3.58–4.31) cases per 1000 individuals, also concentrated in older adults. These changes in HZ incidence are reflected in a progressive increase of the mean age at VZV reactivation from about 33.6 (95% CI 29.3–37.7) years in 1900 to 54.4 (95% CI 52.9–56.1) years in 2009 (figure 3d). The estimated HZ growth is mainly ascribable to the declining birth rate via the complex dynamics of exogenous boosting. Indeed, the individual risk of HZ is higher when the number of experienced episodes of VZV re-exposures is low. The sustained decrease of the birth rate substantially reduced VZV circulation and frequency of re-exposures in the population, therefore contributing to increase the overall HZ risk at the population level. In other words, each cohort has been replaced over time by a new cohort with a lower level of protection, which has caused the increase of HZ incidence throughout the past century at virtually all ages.
Figure 3.
Estimated impact of demographic changes on VZV epidemiology (1900–2009). (a) Estimated age-specific VZV seroprevalence at different years. (b) Estimated mean age (and 95% CI, shaded areas) at varicella over time. (c) Estimated age-specific HZ incidence at different years. The inset shows the total HZ incidence over time and disaggregated by age group. (d) Estimated mean age (and 95% CI, shaded areas) at HZ over time.
It is worth noting that, owing to these indirect and cumulative effects, the result of a changing birth rate becomes visible on HZ incidence after a significant delay of a few decades. For instance, the model estimates that despite the increasing birth rate in 2000–2009, HZ incidence keeps growing, although at a slower rate (figure 3c, inset). The delay in the effect of birth rate changes on HZ incidence is intrinsic in the long time scales of VZV reactivation, which critically depends on the accumulation of individual immunological protection through VZV re-exposures over several decades.
(b). Prediction period (2010–2050)
Model predictions on the future epidemiology of varicella are shown in figure 4, under the three illustrative demographic scenarios presented in the Methods section. Results for scenarios based on realistic population projections [34] are qualitatively similar to the scenario where a constant birth rate is assumed and they are subjected to the same general considerations characterizing the analysis of the illustrative scenarios. They are therefore reported for brevity in the electronic supplementary material.
Figure 4.
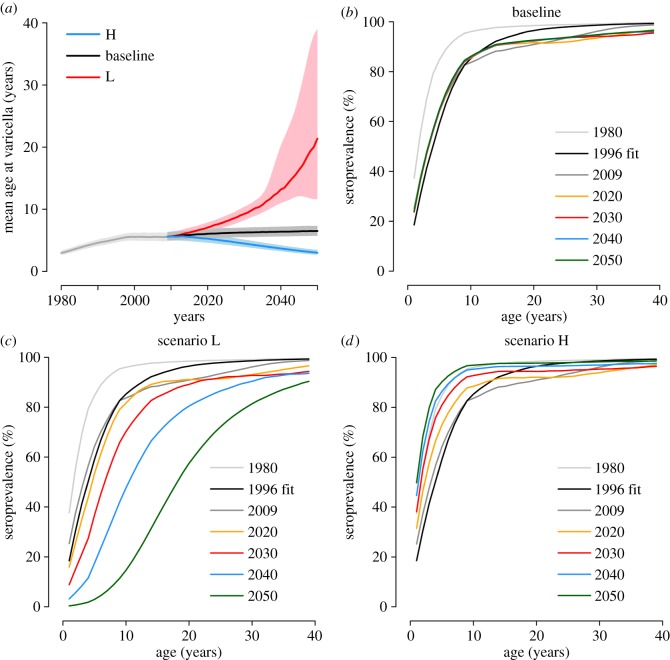
Predicted impact of demographic changes on the future epidemiology of varicella (2010–2050). (a) Predicted mean age (and 95% CI, shaded areas) at varicella over time. Different colours correspond to different projection scenarios. (b–d) Predicted age-specific VZV seroprevalence at different years for (b) the baseline scenario, (c) the ‘lowest birth rate’ scenario L and (d) the ‘highest birth rate’ scenario H.
In the baseline scenario (where a constant birth rate is assumed), the predicted mean age at VZV infection (figure 4a) and the serological profile (figure 4b) remain substantially stable after 2010. Scenario L, which assumes a decreasing birth rate, results in a remarkable rise of the mean age at VZV infection (figure 4a) and in a decrease in the seropositive fraction at all ages (figure 4c). On the opposite, scenario H, characterized by a growing birth rate, results in a decrease of the mean age at VZV infection (figure 4a) and a growing fraction of seropositive individuals at all ages (figure 4d).
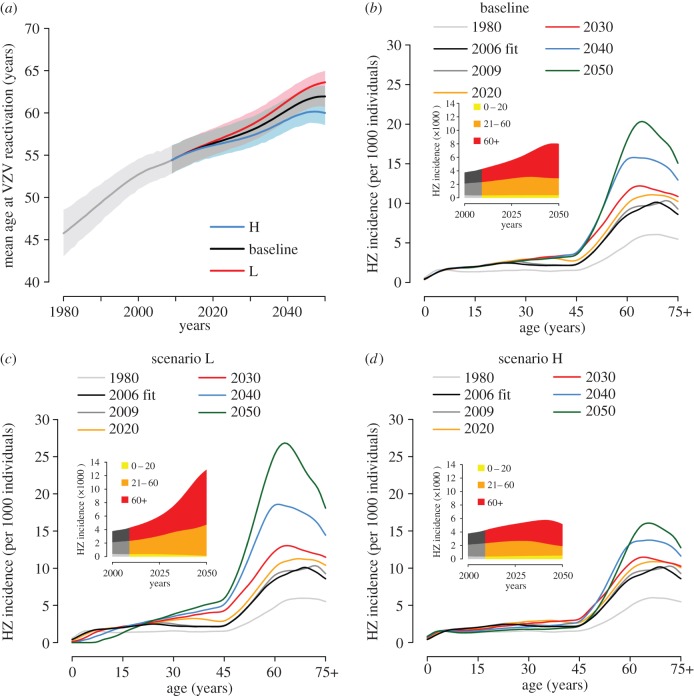
The predicted impact of these scenarios on future HZ epidemiology is shown in figure 5. In the baseline scenario, the model predicts an increase of the mean age at VZV reactivation, from 54.4 years (95% CI 52.9–56.1) in 2009 to 62.0 years (95% CI 60.7–63.2) in 2050 (figure 5a). Correspondingly, the age-specific HZ incidence (figure 5b) also increases, especially in adults and in the elderly, with peak values rising from about 10 to about 20 cases per 1000 individuals. This trend of HZ growth is consistent with the observed historical trend [26] and occurs despite the relative stability of the underlying varicella dynamics (figure 4a) as a result of the delayed effect of the decreasing birth rate in the second half of the past century on the risk of HZ development.
Figure 5.
Predicted impact of demographic changes on the future epidemiology of HZ (2010–2050). (a) Predicted mean age (and 95% CI, shaded areas) at HZ over time. Different colours correspond to different projection scenarios. (b–d) Predicted age-specific HZ incidence at different years for (b) the baseline scenario, (c) the ‘lowest birth rate’ scenario L and (d) the ‘highest birth rate’ scenario H. The insets show the total HZ incidence over time disaggregated by age group.
In scenario L, the increase in HZ incidence is inflated by the further decrease of VZV circulation predicted during the period 2010–2050 (figure 5c). In scenario H, the increase of VZV circulation started in the first decade of the twenty-first century mitigates the growth of HZ incidence and eventually counterbalances it after a few decades (figure 5d, inset).
(c). Predictions under varicella vaccination
Figure 6 shows the age-specific and total HZ incidence over time in the case of a varicella vaccination programme starting in 2010 and assuming a combined level of vaccine effectiveness and coverage of 90%, under the baseline demographic scenario. The model predicts a further dramatic increase of HZ incidence in all non-vaccinated cohorts, as a consequence of the increased risk owing to the rapid decline of varicella circulation (see the electronic supplementary material). In particular, the total HZ incidence is predicted to increase by 185% between 2010 and 2050, from 4.24 (95% CI 3.86–4.67) to 12.09 (95% CI 11.02–13.30) cases per 1000 individuals, compared with an 89% increase in the baseline scenario without vaccination (figure 5b, inset). Similarly, the peak age-specific incidence soars to 37.5 cases per 1000 individuals in 2050 compared with a maximum of about 20 cases per 1000 individuals in the same year in the corresponding vaccination-free scenario. HZ incidence caused by the reactivation of the vaccine strain is negligible, contributing less than 1.4% of all HZ cases that occurred throughout the prediction period. Results based on a different combined level of vaccine effectiveness and coverage are shown in the electronic supplementary material.
Figure 6.
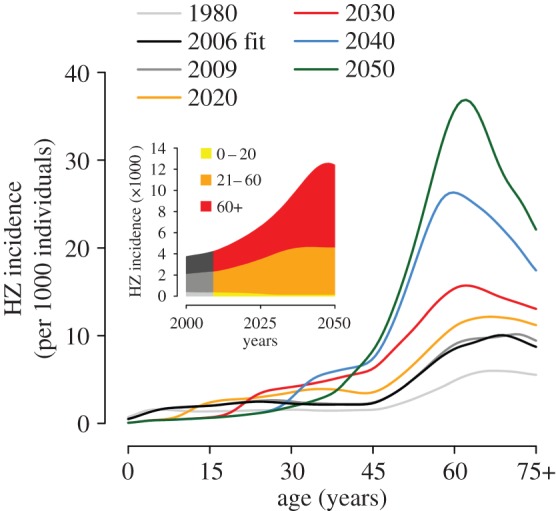
Joint predicted impact of demographic changes and vaccination on the future epidemiology of HZ (2010–2050). Predicted age-specific HZ incidence at different years under the baseline demographic scenario and a vaccination programme starting in 2010 with 90% coverage and 100% lifelong efficacy. The inset shows the total HZ incidence over time disaggregated by age group.
4. Discussion
In the past century, a range of demographic changes has occurred in most industrialized countries [27], yielding a massive decrease of fertility and a progressive ageing of the population [29]. In this work, we investigate the impact of demographic dynamics on the epidemiology of varicella and HZ in Spain, by using an age-structured individual-based stochastic model of VZV transmission dynamics. The model is calibrated against the age-specific profiles of VZV seroprevalence and HZ incidence, and is able to qualitatively reproduce the age-specific increase in HZ observed between 1997 and 2004 [26]. In the model, a decrease in the birth rate results in a decrease of VZV circulation, owing to the depletion of susceptible individuals that fuel transmission. Under Hope-Simpson's exogenous boosting hypothesis, the reduced VZV circulation results in an increase of the reactivation risk. In particular, the model suggests that HZ incidence has been increasing in Spain since the beginning of the past century, in association with a strong reduction of varicella circulation, mainly caused by the progressive decline in fertility. While varicella-related epidemiological changes follow demographic changes with a delay of a few years, consequences on HZ incidence require several decades to reveal. As a consequence, under all demographic projections considered for the future, the model predicts a further increase of HZ incidence until at least 2040. This trend is expected to be eventually reverted only in the case of a massive, sustained growth of the birth rate over the next decades.
Model results are a consequence of the assumption that varicella and HZ dynamics are linked through the exogenous boosting hypothesis. The existence of a relationship between VZV re-exposures and reduced HZ risk is largely supported by data [6–8,10,11], even though some opposing evidence [45] shows that the question is far from being completely elucidated and requires further study. Published mathematical models of VZV [11–14] unanimously integrate the exogenous boosting hypothesis, as it represents a simple and plausible explanation of the observed age-specific HZ incidence patterns in several European countries [11–14,19,26,39,46]. In this study, we chose to adopt a formulation that accounts for the cumulative increase of protection with repeated exposures, termed ‘progressive immunity’ in a previously proposed study [14]. The progressive immunity model has been shown to accurately fit HZ data in several European countries, including Spain [14]. However, we found similar qualitative results when using a different formulation of the exogenous boosting hypothesis (following [13,18,19]), where immunity does not cumulate with successive re-exposure episodes (see the electronic supplementary material).
A limitation of this model concerns the representation of contact patterns for the transmission dynamics of VZV. Actual mixing patterns may change significantly over time, following changes in socio-demographic characteristics [37,47] (e.g. household size and composition, characteristics of the educational system, school attendance levels). However, changes in contact patterns by age that occurred during the past century are difficult to reconstruct quantitatively, because neither historical data on mixing patterns themselves nor detailed historical epidemiological data useful to identify these patterns are available. In the absence of sufficient information on historical mixing patterns, and because the construction of time-dependent contact matrices is beyond the scope of this paper, we adopted recent estimates of age-specific mixing patterns [37], rescaled by the fraction of population by age group at each time step [40]. However, the main results of this work are robust with respect to this assumption. To support this claim, we performed a further analysis where mixing patterns are assumed to be homogeneous by age. Model estimates obtained under this alternative contact pattern scheme support the same qualitative conclusions with limited quantitative variations (see the electronic supplementary material).
The choice of Spain for this study was dictated by the availability of longitudinal HZ incidence data [26], which show a strong trend of growth before the introduction of varicella vaccination. Similar trends have been reported in the pre-vaccine era in other geographical settings (e.g. USA [24], UK [25], Canada [22,23,25]), but factors underlying the growth of HZ have not been elucidated yet. Our study shows that demographic changes have contributed, at least partially, to this trend in Spain. Because many industrialized countries underwent comparable demographic changes during the past century [27,28], we suggest that our conclusions are likely to be generalizable to other geographical settings.
In addition, retrospective evaluations of the effects of varicella immunization on HZ epidemiology have been confounded by pre-existing trends in HZ epidemiology [20,48]. Model simulations reproduce this situation by showing that, in the years immediately following the introduction of vaccination, the variation in HZ trends owing to vaccination may be subtle and hard to distinguish from that occurring in the pre-vaccine era. Therefore, we suggest that the approach proposed here may help to disentangle the effect of natural epidemiological processes from that of vaccination, eventually allowing a more accurate assessment of past and future interventions.
Supplementary Material
Funding statement
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007 2013)/ERC Grant agreement no. 283955 to P.P.
Author's contributions
P.P., G.G., M.A. and S.M. conceived of the study. V.M. performed the experiments. V.M., P.P. and G.G. analysed results. V.M., P.P., G.G., M.A., P.M. and S.M. wrote the manuscript, and read and approved the final manuscript.
Competing interests
We have no competing interests.
References
- 1.Donahue JG, Choo PW, Manson JE, Platt R. 1995. The incidence of herpes zoster. Arch. Intern. Med. 155, 1605–1609. ( 10.1001/archinte.1995.00430150071008) [DOI] [PubMed] [Google Scholar]
- 2.Oxman MN. 2009. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J. Am. Osteopath. Assoc. 109, S13–S17. [PubMed] [Google Scholar]
- 3.Hope-Simpson RE. 1965. The nature of herpes zoster: a long-term study and a new hypothesis. Proc. R. Soc. Med. 58, 9–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Oxman MN. 1995. Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurology 45, S41–S46. ( 10.1212/WNL.45.12_Suppl_8.S41) [DOI] [PubMed] [Google Scholar]
- 5.Burke BL, et al. 1982. Immune responses to varicella-zoster in the aged. Arch. Intern. Med. 142, 291–293. ( 10.1001/archinte.1982.00340150091017) [DOI] [PubMed] [Google Scholar]
- 6.Terada K, Kawano S, Yoshihiro K, Morita T. 1993. Proliferative response to varicella-zoster virus is inverse related to development of high levels of varicella-zoster virus specific IgG antibodies. Scand. J. Infect. Dis. 25, 775–778. ( 10.3109/00365549309008578) [DOI] [PubMed] [Google Scholar]
- 7.Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. 2004. Development of virus-specific CD4+ T cells on reexposure to varicella-zoster virus. J. Infect. Dis. 190, 72–82. ( 10.1086/421277) [DOI] [PubMed] [Google Scholar]
- 8.Ogunjimi B, Smits E, Hens N, Hens A, Lenders K, Ieven M, Van Tendeloo V, Van Damme P, Beutels P. 2011. Exploring the impact of exposure to primary varicella in children on varicella-zoster virus immunity of parents. Viral Immunol. 24, 151–157. ( 10.1089/vim.2010.0031) [DOI] [PubMed] [Google Scholar]
- 9.Ogunjimi B, et al. 2014. Influence of frequent infectious exposures on general and varicella-zoster virus-specific immune responses in pediatricians. Clin. Vaccine Immunol. 21, 417–426. ( 10.1128/CVI.00818-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas S, Wheeler JG, Hall A. 2002. Contacts with varicella or with children and protection against herpes zoster in adults: a case–control study. Lancet 360, 678–682. ( 10.1016/S0140-6736(02)09837-9) [DOI] [PubMed] [Google Scholar]
- 11.Brisson M, Gay N, Edmunds WJ, Andrews NJ. 2002. Exposure to varicella boosts immunity to herpes zoster: implications for mass vaccination against chickenpox. Vaccine 20, 2500–2507. ( 10.1016/S0264-410X(02)00180-9) [DOI] [PubMed] [Google Scholar]
- 12.Karhunen M, Leino T, Salo H, Davidkin I, Kilpi T, Auranen K. 2010. Modelling the impact of varicella vaccination on varicella and zoster. Epidemiol. Infect. 138, 469–481. ( 10.1017/S0950268809990768) [DOI] [PubMed] [Google Scholar]
- 13.Poletti P, et al. 2013. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PLoS ONE 8, e60732 ( 10.1371/journal.pone.0060732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzzetta G, Poletti P, dal Fava E, Ajelli M, Scalia-Tomba G, Merler S, Manfredi P. 2013. Hope-Simpson's progressive immunity hypothesis as a possible explanation for herpes zoster incidence data. Am. J. Epidemiol. 177, 1134–1142. ( 10.1093/aje/kws370) [DOI] [PubMed] [Google Scholar]
- 15.Rasch G, Hellenbrand W. 2004. Germany adds varicella vaccine to the national vaccination programme. Euro Surveill. 8, 2511. [Google Scholar]
- 16.Seward JF, et al. 2002. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. J. Am. Med. Assoc. 287, 606–611. ( 10.1001/jama.287.5.606) [DOI] [PubMed] [Google Scholar]
- 17.Stefanoff P, et al. 2011. Varicella and herpes zoster surveillance and vaccination recommendations 2010–2011. Rome, Italy: VENICE II Consortium. [Google Scholar]
- 18.Brisson M, Melkonyan G, Drolet M, De Serres G, Thibeault R, De Wals P. 2010. Modeling the impact of one-and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine 28, 3385–3397. ( 10.1016/j.vaccine.2010.02.079) [DOI] [PubMed] [Google Scholar]
- 19.Van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. 2011. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 29, 2411–2420. ( 10.1016/j.vaccine.2011.01.037) [DOI] [PubMed] [Google Scholar]
- 20.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. 2005. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J. Infect. Dis. 191, 2002–2007. ( 10.1086/430325) [DOI] [PubMed] [Google Scholar]
- 21.Reynolds MA, Chavez SS, Harpaz R, Lopez AS, Seward JF. 2008. The impact of varicella vaccination program on herpes zoster epidemiology in the United States: a review. J. Infect. Dis. 197, S224–S227. ( 10.1086/522162) [DOI] [PubMed] [Google Scholar]
- 22.Law BJ, Chateau D, Walld R, Roos L. 2004. Temporal trends in the annual population-based incidence of herpes zoster by age and gender: Manitoba, 1979–1998. Can. J. Infect. Dis. Med. Microbiol. 15, 357–358. [Google Scholar]
- 23.Russell ML, Schopflocher DP, Svenson L, Virani SN. 2007. Secular trends in the epidemiology of shingles in Alberta. Epidemiol. Infect. 135, 908–913. ( 10.1017/S0950268807007893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragozzino MW, Melton LJ, III, Kurland LT, Chu CP, Perry HO. 1982. Population-based study of herpes zoster and its sequelae. Medicine 61, 310–316. ( 10.1097/00005792-198209000-00003) [DOI] [PubMed] [Google Scholar]
- 25.Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos L, de Serres G. 2001. Epidemiology of varicella-zoster virus in Canada and the United Kingdom. Epidemiol. Infect. 127, 305–314. ( 10.1017/S0950268801005921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Farinós N, Ordobás M, García-Fernández C, García-Comas L, Cañellas S, Rodero I, Gutiérrez-Rodríguez Á, García-Gutiérrez J, Ramírez R. 2007. Varicella and herpes zoster in Madrid, based on the sentinel general practitioner network: 1997–2004. BMC Infect. Dis. 7, 59 ( 10.1186/1471-2334-7-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livi-Bacci M. 2012. A concise history of world population. Cambridge, MA: Wiley-Blackwell. [Google Scholar]
- 28.Kohler HP, Billari FC, Ortega JA. 2002. The emergence of lowest-low fertility in Europe during the 1990s. Popul. Dev. Rev. 28, 641–681. ( 10.1111/j.1728-4457.2002.00641.x) [DOI] [Google Scholar]
- 29.Instituto Nacional de Estadística. 2014. INEbase. See http://ine.es/inebmenu/indice.htm.
- 30.University of California, Berkeley (USA) and Max Planck Institute for Demographic Research (Germany). 2014 Human mortality database. See http://www.mortality.org.
- 31.Statistical Office of the European Commission (Eurostat). 2011. Migrants in Europe. A statistical portrait of the first and second generation. Technical report See http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-31-10-539/EN/KS-31-10-539-EN.PDF.
- 32.World Bank 2014. Net migration. See http://data.worldbank.org/indicator/SM.POP.NETM.
- 33.Bover O, Velilla P. 1999. Migration in Spain: historical background and current trends. Bonn, Germany: Institute for the Study of Labor. [Google Scholar]
- 34.United Nations Department of Economic and Social Affairs. 2012. UN world population prospects: the 2012 revision See http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm Accessed: 29 December 2014.
- 35.Mossong J, et al. 2008. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74 ( 10.1371/journal.pmed.0050074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melegaro A, Jit M, Gay N, Zagheni E, Edmunds WJ. 2011. What types of contacts are important for the spread of infections? Using contact survey data to explore European mixing patterns. Epidemics 3, 143–151. ( 10.1016/j.epidem.2011.04.001) [DOI] [PubMed] [Google Scholar]
- 37.Fumanelli L, Ajelli M, Manfredi P, Vespignani A, Merler S. 2012. Inferring the structure of social contacts from demographic data in the analysis of infectious diseases spread. PLOS Comput. Biol. 8, e1002673 ( 10.1371/journal.pcbi.1002673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nardone A, et al. 2007. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine 25, 7866–7872. ( 10.1016/j.vaccine.2007.07.036) [DOI] [PubMed] [Google Scholar]
- 39.García Cenoz M, et al. 2008. Incidencia de la varicela y el herpes zóster antes de la introducción de la vacunación sistemática infantil en Navarra, 2005–2006. Anales Sis San Navarra 31, 71–80. ( 10.4321/S1137-66272008000100006) [DOI] [PubMed] [Google Scholar]
- 40.Manfredi P, Williams JR. 2004. Realistic population dynamics in epidemiological models: the impact of population decline on the dynamics of childhood infectious diseases: measles in Italy as an example. Math. Biosci. 192, 153–175. ( 10.1016/j.mbs.2004.11.006) [DOI] [PubMed] [Google Scholar]
- 41.Merler S, Ajelli M. 2014. Deciphering the relative weights of demographic transition and vaccination in the decrease of measles incidence in Italy. Proc. R. Soc. B 281, 20132676 ( 10.1098/rspb.2013.2676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livi-Bacci M. 1999. The population of Europe: a history. Oxford, UK: Blackwell. [Google Scholar]
- 43.Brisson M, Edmunds WJ, Gay NJ, Law B, De Serres G. 2000. Analysis of varicella vaccine breakthrough rates: implications for the effectiveness of immunization programmes. Vaccine 18, 2775–2778. ( 10.1016/S0264-410X(00)00100-6) [DOI] [PubMed] [Google Scholar]
- 44.Civen R, Chaves S, Jumaan A, Wu H, Mascola L, Gargiullo P, Seward JF. 2009. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr. Infect. Dis. J. 28, 954–959. ( 10.1097/INF.0b013e3181a90b16) [DOI] [PubMed] [Google Scholar]
- 45.Gaillat J, Gajdos V, Launay O, Malvy D. 2011. Does monastic life predispose to the risk of Saint Anthony's fire (herpes zoster)? Clin. Infect. Dis. 53, 405–410. ( 10.1093/cid/cir436) [DOI] [PubMed] [Google Scholar]
- 46.Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, Serradell L, di Marzo R, Volpi A. 2010. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect. Dis. 10, 230–240. ( 10.1186/1471-2334-10-230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iozzi F, Trusiano F, Chinazzi M, Billari FC, Zagheni E, Merler S, Ajelli M, Del Fava E, Manfredi P. 2010. Little Italy: an agent-based approach to the estimation of contact patterns-fitting predicted matrices to serological data. PLoS Comput. Biol. 6, e1001021 ( 10.1371/journal.pcbi.1001021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. 2005. The incidence of varicella and herpes zoster in Massachusetts as measured by the behavioral risk factor surveillance system (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 5, 68–76. ( 10.1186/1471-2458-5-68) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.