Abstract
We report that two species of mouse-tailed bats (Rhinopoma microphyllum and R. cystops) hibernate for five months during winter in geothermally heated caves with stable high temperature (20°C). While hibernating, these bats do not feed or drink, even on warm nights when other bat species are active. We used thermo-sensitive transmitters to measure the bats’ skin temperature in the natural hibernacula and open flow respirometry to measure torpid metabolic rate at different ambient temperatures (Ta, 16–35°C) and evaporative water loss (EWL) in the laboratory. Bats average skin temperature at the natural hibernacula was 21.7 ± 0.8°C, and no arousals were recorded. Both species reached the lowest metabolic rates around natural hibernacula temperatures (20°C, average of 0.14 ± 0.01 and 0.16 ± 0.04 ml O2 g−1 h−1 for R. microphyllum and R. cystops, respectively) and aroused from torpor when Ta fell below 16°C. During torpor the bats performed long apnoeas (14 ± 1.6 and 16 ± 1.5 min, respectively) and had a very low EWL. We hypothesize that the particular diet of these bats is an adaptation to hibernation at high temperatures and that caves featuring high temperature and humidity during winter enable these species to survive this season on the northern edge of their world distribution.
Keywords: Rhinopoma, geothermal heat, hibernation, torpor, arousals, evaporative water loss
1. Introduction
Torpor and hibernation are common physiological adaptations used by mammals to conserve energy. These behaviours have been studied mostly in the context of adaptation to negative energy balance and extended periods of water and food shortage, and have been described in species from desert and tropical habitats. In recent years, different ecological interactions were found to affect the use of torpor and hibernation [1–5]. The Rhinopomatidae (mouse-tailed bats) are a monotypic family of subtropical insectivorous bats inhabiting semi-arid and warm regions in Asia and Africa. Two species, the greater mouse-tailed bat (Rhinopoma microphyllum) and the lesser mouse-tailed bat (Rhinopoma cystops) are medium-sized insectivorous bats with body masses of approximately 25 g and 12 g, respectively, and are both well adapted to arid environments [6,7]. Rhinopoma microphyllum and R. cystops inhabit the dry and warm regions of Israel, which is the northern edge of their world distribution [8]. These species have limited tolerance to low ambient temperatures and it has been suggested that they are unable to perform deep torpor or hibernation [6].
We have previously found that during summer, R. microphyllum exhibit complete sexual segregation, inhibiting warm and dry caves (28–32°C). We also found that lactating females remain almost normothermic during the day and perform long foraging bouts during the night, while males and non-lactating females use daily torpor during the day and perform short foraging bouts during the night [9,10]. During late summer (August) both species accumulate large amounts of body fat, sometimes reaching 50% of their body mass, a phenomenon typical for hibernators. This increase in body fat is achieved by switching diet preference during summer (mid-July) from coleopterans and heteropterans to mainly fat-rich winged carpenter ants [11]. This fat-rich food contains a high fraction of saturated and mono-unsaturated fatty acids (SFA and MUFA, respectively), but almost no polyunsaturated fatty acids (PUFA) [12] which are considered important for hibernation success at low temperatures [13–17]. As a result, mouse-tailed bat pre-winter body fat is lacking in PUFAs and is extremely saturated [11,12], which is a-typical for hibernators.
During late October, both mouse-tailed bat species move to caves in which they remain throughout the winter [12]. Based on our observations of no foraging activity during the winter months, and the accumulation of large amounts of saturated body fat before winter [11,12], we hypothesized that mouse-tailed bats hibernate during winter at high ambient temperatures. To test these hypotheses, we monitored skin and hibernaculum temperature of free-ranging mouse-tailed bats during winter, and examined the effect of ambient temperature on energy expenditure and water loss under controlled conditions in these two species.
2. Material and methods
(a). Winter hibernacula
A winter colony of both R. microphyllum and R. cystops was discovered during a field survey on 2003 on a cliff by the Sea of Galilee (32°46′ N 35°32′ E, 100 m below sea level). The cave is a wet karstic cave in Eocene limestone formed by a pheratic and hypogenic dissolving process in a confined aquifer. The dissolving has created a maze network of mainly horizontal branched tunnels with some halls and typical domes in which the bats hang. The temperature in this cave is relatively high and constant due to geothermic activity (speleological data from the Israeli Cave Research Unit, The Hebrew University, Jerusalem). From 2003 to 2013, we visited this cave three to eight times a year, and counted the bats directly by observation or photography. We also acoustically identified and counted bats emerging from this cave during winter nights, using Pettersson D500 (Pettersson Elektronik, Sweden) and ANABAT bat detectors (Titley Scientific, Australia). During 2003 and 2004, we placed paper sheets on the cave floor beneath the roosting bats for faeces collection.
(b). Cave and skin temperature
We use thermal sensitive transmitters to record skin temperature (Tsk) in hibernating mouse-tailed bats. Skin temperature of small mammals (including bats) was validated and is considered to be a good predictor for body temperature, and is used in numerous studies to measure the use of torpor and hibernation in free-ranging small mammals including bats [18–20].
Nine adult R. cystops (five females and four males) were captured during daytime (15 January 2012) in their natural hibernacula using a hand net, and body mass was measured using an electronic scales (Micron, China, ±0.01 g). To attach the temperature transmitters for skin temperature detection, we removed a patch of hair between the bats’ shoulder blades using a depilatory cream (Veet®, Reckitt Benckiser, UK). The area was then washed gently with a wet cotton ball and dried. Then, a calibrated temperature sensitive radio transmitter (BD-2T 0.35 g, Holohil, Canada) was attached between the bat’s shoulder blades using medical adhesive (Perma-Type Company, USA). After attaching the transmitter, the bats were immediately returned to the hibernacula.
To cover a large area of the cave, we positioned two RX-900 receivers (Televilt, Sweden) inside the cave, one in the outer section and the other deeper inside the cave maze. We used four dipole antennas (two per receiver) connected to the receivers with 20 m long cables. Voltage to each receiver was supplied by a 12 V car battery (replaced every 5–8 days).
Ambient temperature was recorded every 90 min using four I-Button temperature data loggers (0.5°C resolution, Maxim Integrated, USA) in four different rooms next to each antenna. Relative humidity was measured on December 2004 and again on June 2013, using a mechanical hygrometer (Fischer, Germany).
Of the nine R. cystops fitted with temperature sensitive radio transmitters on 15 January 2012, we were able to monitor skin temperatures for only eight. The ninth bat disappeared the day it was tagged and probably moved to another cave on that cliff. On 19 January, one of the receivers stopped working (chewed up by Hystrix indica inhabiting this cave), and we lost the signal of three more bats. Five remained within the range of the other receiver throughout the 25 days of transmitter battery life.
(c). Torpid metabolic rate
For metabolic measurements 14 adult bats (R. microphyllum, five males and four females; R. cystops, two males and three females) were captured on 12 February 2012 in their hibernacula as described previously. The bats were brought to the laboratory, marked individually with bat rings (Porzana, UK, for R. microphyllum—4.2 mm, for R. cystops—2.6 mm) and placed in a custom-made wooden cage in a temperature-controlled room at 20°C, which resembles the ambient temperature of the bats' natural hibernacula. An animal was considered in torpor when its metabolic rate decreased under 0.7 ml O2 g−1 h−1 [21] and/or was performing intermittent breathing patterns. TMR of the bats was measured using an open-flow respirometry system. Bats (all fasted at least 2 days in the laboratory and probably much longer in their hibernacula) were weighed using electronic scales (Micron, China, ±0.01 g) and introduced individually into a metabolic chamber (Perspex chambers; 250 ml for R. microphyllum and 210 ml for R. cystops). O2 consumption and CO2 production were measured by gas analyser (FoxBox, Sable Systems Int., USA). Dry air passed through the metabolic chamber at a constant flow of 100 ml min−1. Data were collected at 5-s intervals using a PC and ADAMview software (Advantech, USA). After placing a bat in the chamber, the chamber was immersed in a temperature-controlled water bath at 20°C, reflecting natural hibernacula temperature.
To detect the influence of ambient temperature on TMR the metabolic rate of each bat was measured at various temperatures on 2 consecutive days, each starting with 120 min of acclimation to 20°C. On day 1, TMR was recorded at 19°C, 18°C, 17°C and 16°C for 120 min each. We did not reduce Ta below 16°C as at this temperature the bats started to get stressed and as this temperature was reported as lethal to this species [6]. On the second day, TMR was recorded at 20°C, 25°C, 30°C and 35°C (33°C is the highest temperature we recorded in the summer roosts [9]) for 120 min each. The experiment was stopped when a bat aroused from torpor, i.e. no apnoea cycles were detectable and CO2 levels in the chamber rose above 0.7%.
For data analysis alone the last 30 min of each temperature were used. VO2 ml O2 g−1 h−1 was calculated according to Withers [22]. For comparisons of TMR, we used the average value of VO2 for the last 30 min of measurement at each temperature.
During November and February 2013, 10 R. cystops were measured for TMR as described above at 20°C. At the end of each measurement Tsk was taken from a hairless area of the dorsal side, by an infra-red thermometer GM300 (Benetech, China). We avoided invasive procedures as these are protected species in Israel.
At the end of the 2-day experiment, we prepared the bats for release back to nature: bats were hand-fed daily with a syringe for two weeks with 1 ml of squeezed darkling beetle larvae (Zophobas morio) enriched with high nutrient food for pets (Recovery, Royal Canin). The bats were fed in order to increase their weight which, we assumed, will increase their chance for survival in nature. The bats were returned to the hibernacula in late March. Two of the bats used in the experiments were recaptured in good shape 1 year later. We assume that no harm was caused to the bats from the captivity period.
(d). Evaporative water loss
For EWL measurements an additional 10 adult bats (R. microphyllum, three males and two females; R. cystops three males and two females) were captured in February 2013 in their winter hibernacula as described before. The bats were placed in individual Perspex metabolic chambers (250 ml for both species) with constant air flow of 100 ml min−1 of a CO2 free, dry air from a cylinder. Air supply to the animals was controlled by an eight-channel gas controller and monitor (Flow bar-8, Sable Systems Int.). The air flowed to an eight-channel gas multiplexer (V5, Sable Systems Int.) that selected channels sequentially for sampling. Air samples were passed through a water vapour analyser (RH-300, Sable Systems Int.). To make sure the bats were hibernating during the measurements, we also measured O2 and CO2 levels in the air sample: the air coming out from the RH-300 was dried with a drierite (W. A. Hammond, USA) column and passed through an O2/CO2 analyser (FoxBox, Sable Systems Int.). Data regarding water vapour, O2 and CO2 were collected every 5 s using Expedata software (Sable Systems Int.). EWL was calculated using Lighton's 10.11 equation [23]. The bats were kept unfed during the 2 days of measurements in the laboratory and then returned to their hibernacula.
3. Results
In the winters of 2003–2013, we counted 200–500 individuals of R. cystops and 50–500 of R. microphyllum in the observed winter roost. Rhinopoma microphyllum was often found in clusters while R. cystops always maintained a distance of forearm and tail from each other. During all our visits, the bats' eyes were opened and they were able to swiftly fly (within seconds) in response to disturbance. Bats were making audible calls, which were detectable before entering the cave during both day and night. In contrast to the summer roosts, in the hibernacula no fresh faeces were observed on the cave floor or on the collection paper sheets positioned under the clusters of bats at any point of their stay there. However, much fresh urine was absorbed onto these sheets.
Six other species of insectivorous bats were identified visually and acoustically in the same cave during winter: Rhinolophus ferumequinum, R. blassi, R. hipposideros, Miniopterus schreibersii, Taphozous perforatus and Myotis capaccini. Unlike all of these latter species, which were observed emerging from the cave to feed on winter nights and whose faeces were found under their roosting spots, R. cystops and R. microphyllum remained inside the cave even on relatively warm nights (greater than or equal to 20°C), when many aerial insects were active.
During the study we found another five caves with winter colonies of Rhinopoma in Israel, four of them along the Syrian–African rift valley and all of which are warm and humid. This area is geologically active and contains many hot springs and underground warm-water cisterns.
(a). Cave and skin temperature
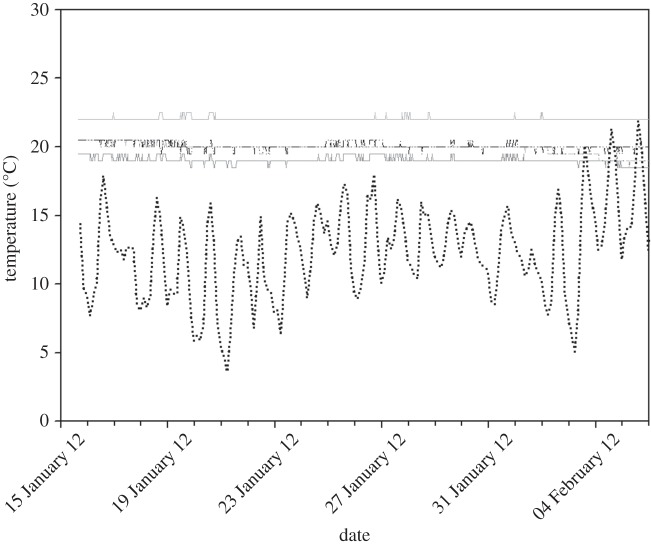
While ambient temperatures outside the cave fluctuated considerably during winter from 4 to 22°C (figure 1), the temperature inside the cave remained relatively constant at about 19°C in the outer parts and at about 22°C in the inner parts of the cave (figure 1). Relative humidity in the cave was approximately 100%.
Figure 1.
Ambient temperature outside the cave (dashed black line) and temperature at four different rooms in the hibernacula cave (grey lines) during the study. The two middle rooms are almost identical in their temperature. The temperature in this cave is higher than that outside the cave owing to geothermic activity.
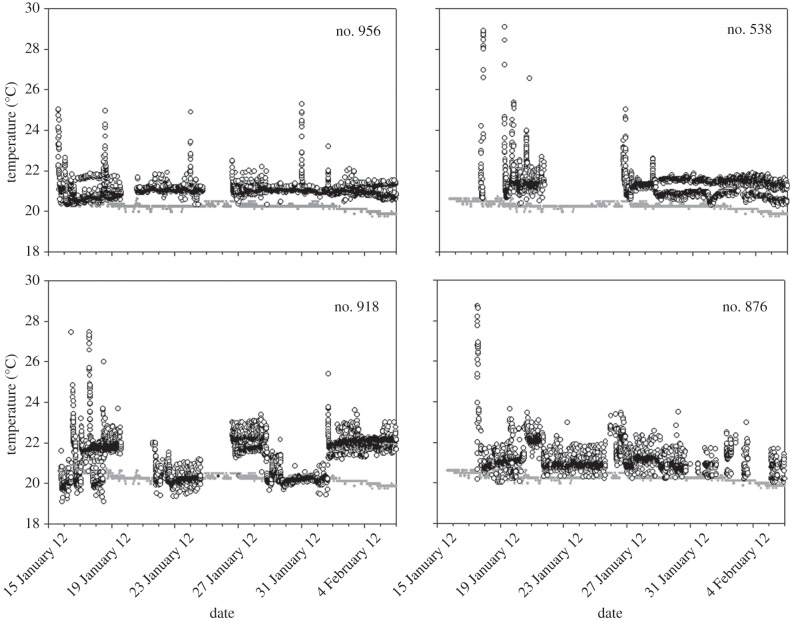
Tsk of all bats recorded was 1–3°C above average cave temperature (table 1). All bats exhibited sporadically short events of higher Tsk. These events were more common during the first days after transmitter attachment and always lasted less than 40 min (figure 2).
Table 1.
Forearm length, body mass, monitoring period and skin temperature of nine hibernating Rhinopoma cystops during winter 2012.
| no. | sex | start | end | mass (g) | forearm (mm) | average Tsk (°C) | min Tsk (°C) | max Tsk (°C)* |
|---|---|---|---|---|---|---|---|---|
| 339 | F | 15 Jan | 19 Jan | 11.20 | 55.3 | 22.02 | 20.54 | 24.96 |
| 379 | F | 15 Jan | 19 Jan | 11.60 | 55.8 | 21.2 | 20.35 | 23.5 |
| 499 | F | 15 Jan | 19 Jan | 11.90 | 57.9 | 22.57 | 19.5 | 23.73 |
| 538 | M | 15 Jan | 6 Feb | 10.90 | 57.7 | 21.21 | 19.51 | 29.09 |
| 738 | M | 15 Jan | 31 Jan | 14.90 | 59 | 23.18 | 19.78 | 25.48 |
| 877 | F | 15 Jan | 6 Feb | 10.40 | 55.2 | 21.22 | 19.49 | 23.6 |
| 918 | F | 15 Jan | 6 Feb | 11.70 | 56.9 | 21.11 | 19.09 | 27.4 |
| 938 | M | 15 Jan | 15 Jan | 15.20 | 60.8 | — | — | — |
| 957 | M | 15 Jan | 6 Feb | 13.50 | 59.5 | 21.03 | 19.41 | 25.2 |
Figure 2.
Skin temperature (Tsk) of four Rhinopoma cystops during January–February 2013 (black circles). Grey line, cave temperature (average of four sensors).
(b). Torpid metabolic rate
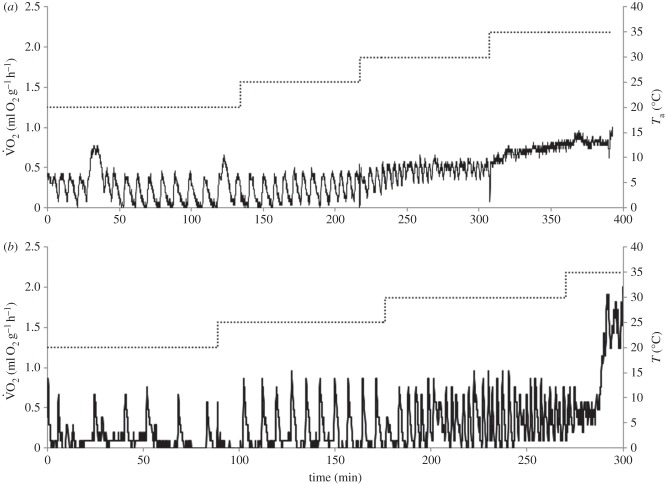
After the mouse-tailed bats were introduced into the metabolic chamber at 20°C they reduced their metabolic rate within 2 to 25 min and entered torpor. Tsk of torpid bats at 20°C was 22.3 ± 1.7. The torpid bats performed typical cycles of apnoea followed by a short ventilation period. These cycles were temperature-dependent. Both species showed the longest cycles around natural hibernacula temperature of 20°C with an average of 14 ± 1.6 min for R. cystops and 16 ± 1.5 min for R. microphyllum (figure 3), and with a maximum cycle length of 28 min.
Figure 3.
An example of oxygen consumption VO2 ml O2 g−1 h−1 of Rhinopoma microphyllum (a) and Rhinopoma cystops (b) in the laboratory at different ambient temperatures (20–35°C at 5°C intervals, 120 min at each temperature). Dashed line, ambient temperature, solid line, oxygen consumption. Peaks represent ventilations between apnoeas.
The two species differed significantly in their torpid metabolic rates (TMRs) at different temperatures (two-way repeated measure ANOVA F = 7.9, p < 0.001). R. cystops had significantly lower TMRs at the lowest measured temperature of 16°C (figure 4, post hoc with Holm–Sidak method: difference of means = 1.59, t = 7.1, p < 0.001), and R. microphyllum had significantly lower TMRs at the highest measured temperature of 35°C (figure 4, post hoc Holm–Sidak method: difference of means = 0.345, t = 2, p = 0.04).
Figure 4.
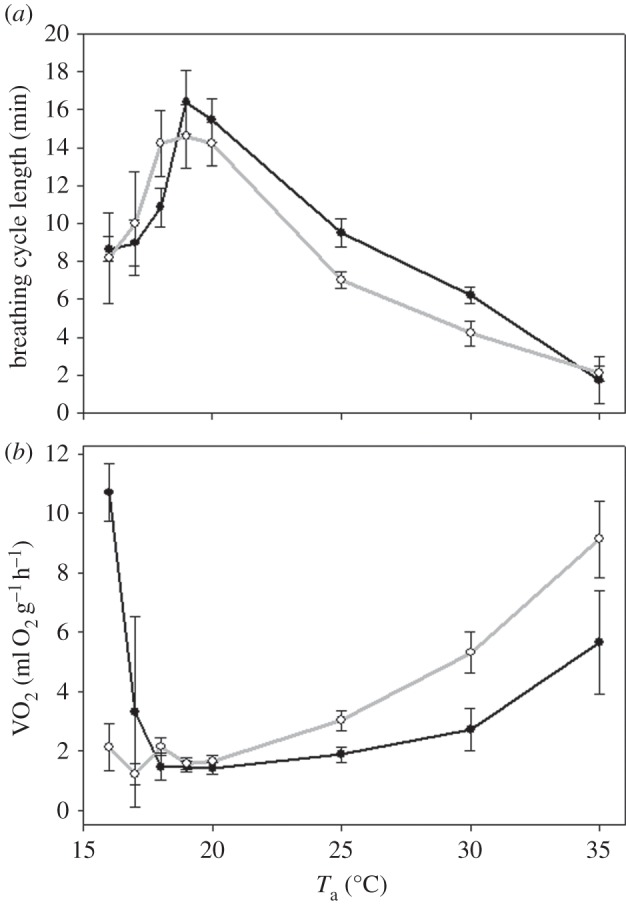
Average (±s.e.) of (a) breathing cycle length (min) and (b) metabolic rates (oxygen consumption VO2 ml O2 g−1 h−1 of R. microphyllum (close symbols, black line) and Rhinopoma cystops (open symbols, grey line) at different temperatures: 20–35°C at 5°C intervals, 120 min at each temperature. For calculations, the last 30 min at each temperature were used. Asterisks (*) indicates significant difference from the other species, (**) indicates significant difference from 20°C in the same species, p < 0.05. Two-way repeated measure ANOVA with post hoc Holm–Sidak method.
Average TMR at 20°C was 0.14 ± 0.01 and 0.16 ± 0.04 ml O2 g−1 h−1 for R. microphyllum and R. cystops, respectively (figure 4b). In R. microphyllum, TMR increased significantly at 16°C, while in R. cystops it increased significantly at 35°C. Six out of eight R. microphyllum started arousing from torpor when the ambient temperature was lower than 17°C, and only two remained torpid. However, TMR of these two individuals was significantly higher than TMR of R. cystops at the same temperature (post hoc with Holm–Sidak method: difference of means = 1.59 t = 7.1, p < 0.001). When ambient temperature was raised above 20°C, while TMR of both species increased, at these temperatures that of R. cystops was significantly higher than that of R. microphyllum, and reached normothermic values at 35°C of about 0.91 ± 0.2 ml/O2 h (figure 4).
We found significantly lower TMR in R. microphyllum at 20°C in the 2013 group (see below, EWL experiment) compared with the 2012 group at the same time of the year (0.14 ± 0.01 versus 0.087 ± 0.01 ml O2 g−1 h−1, t-test t = −5.19, p < 0.001). This may be related to the fact that the first group was kept in the laboratory for a few days before measurements in low humidity and constant noise from the climate chamber compressor, which might stress the bats. No significant difference between the years was found in R. cystops (t-test, p = 0.8). Q10 values for the torpid mouse-tailed bats were higher than 2 in both species (table 2).
Table 2.
Q10 values (±s.d.) of Rhinopoma microphyllum and Rhinopoma cystops.
| temperature range | R. microphyllum | R. cystops |
|---|---|---|
| Q10 20–30°C | 2.5 ± 0.5 | 3.5 ± 1.3 |
| Q10 25–35°C | 4.0 ± 0.8 | 3.1 ± 1.2 |
(c). Evaporative water loss
Total EWL at hibernacula temperature (20°C) was 0.29 ± 0.05 mg H2O(h g)−1 for R. microphyllum and 0.38 ± 0.1 mg H2O(h g)−1 for R. cystops. There was no significant difference in total EWL between the species (t-test t = −1.5, p = 0.16). We suggest that the main channel for EWL in mouse-tailed bats is cutaneous, as most of the time the bats were in apnoea, with their mouth and nostrils shut.
4. Discussion
Our results show that both R. cystops and R. microphyllum hibernate during winter. During hibernation, the bats showed intermitted breathing, with bouts of ventilation and apnoea, as reported in several other hibernating rodents, bats and marsupial species [24]. During the ventilation and apnoea, uptake and clearing of O2 and CO2 was rapid and greatly reduced respectively, and we assume that as reported in other species, blood oxygen and CO2 tension change accordingly [24]. The fact that apnoea cycles length changed with temperatures suggests that breathing bouts are correlated with metabolic demands, and most probably with blood gas levels.
Even though these two species have the option to hibernate in cold caves nearby, they prefer to hibernate in a geothermally heated cave with warm and a stable ambient temperature of 19–23°C. This behaviour suggests that this Ta is of advantage for the mouse-tailed bats during their winter hibernation, which is indeed supported by our findings that lowest TMR, and hence highest energy conservation, and longest cycles of apnoea are achieved around 20°C. It is possible that the existence of these geothermally heated caves enables mouse-tailed bats to extend their distribution northwards along the Syrian–African rift valley. In contrast to the outmoded assumption that hibernation is associated with low temperatures, we present an example of a bat that is not only able to hibernate at high temperatures, but prefers to hibernate at high ambient temperature and is able to maintain low TMRs at temperatures of, and above, 30°C, while arousing at temperatures below 16°C.
Although it is well established that mammals and birds can reduce their metabolic rates by the use of torpor and hibernation, the mechanism underlying this reduction is still controversial. It was argued that reduction of energy metabolism during hibernation cannot be explained by Q10 effect alone (a measure of the rate of change of a biochemical system as a consequence of changing the temperature by 10°C), since Q10 value of biochemical systems is typically 2, while the Q10 values measured for daily heterotherms and hibernating mammals in temperatures range of 20–30°C are usually higher then 2 (average of 4.11 and 2.24 in hibernators and daily heterotherms, respectively [25]), which implies that animals use metabolic inhibition to maximize energy savings [23]. The Q10 values in the mouse-tailed bats were between 2.5 and 4 in the temperature range measured (table 2) which is close to the ones measured in real hibernators, and suggests that the bats used metabolic inhibition.
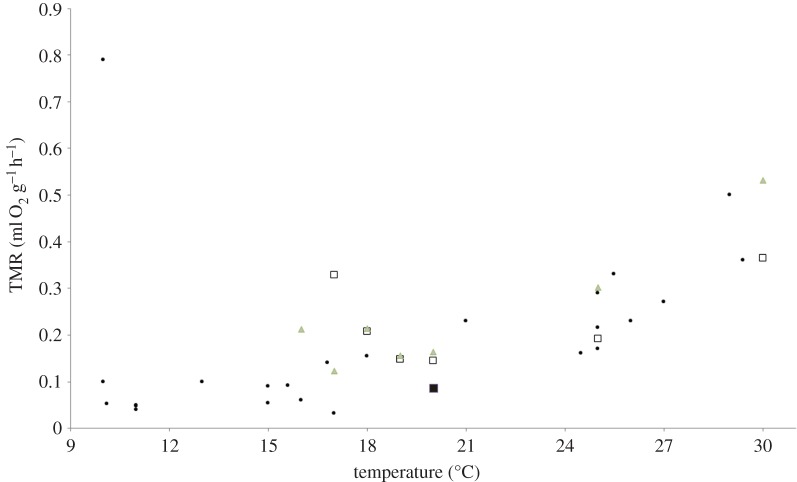
Even though both R. cystops and R. microphyllum hibernate at high temperatures compared with most other hibernating mammals, their TMR at their natural hibernacula temperature is comparable with the TMR of other small (less than 150 g) hibernators at the same temperature range (figure 5). We found R. cystops to be more tolerant to low temperatures than R. microphyllum, which may explain its wider distribution in Israel: during summer it is found at higher altitudes and in larger areas of the Mediterranean region [26]. Kulzer [6] reported that mouse-tailed bats die at ambient temperature of 15°C, and that when their Tb is below 19°C, their re-warming rate is significantly low in comparison with other bat families. Based on our previous work on summer torpor and activity Tsk [9] and on the present observations on hibernation Tsk in mouse-tailed bats, we suggest that mouse-tailed bats defend torpor body temperature of about 20°C, and start thermoregulating if Ta drops below 19°C. This finding is unusual for hibernators as for most hibernators, the minimum Tb is limited by the freezing point of body fluids (approx. 0°C), which prevents a further drop of Tb [27]. For the mouse-tailed bats, using geothermally heated caves and keeping body temperature at this relatively high level will have adaptive significance, since it will significantly reduce the energetic cost of arousals, and therefore, the total cost of hibernation.
Figure 5.
Body temperature and metabolic rate in hibernating mammals in the temperature range of 10–30°C (black filled circles, data from [21]). Open squares, ambient temperature and TMR measured in Rhinopoma microphyllum on 2012, black square, on 2013. Grey triangles, ambient temperatures and TMR measured in Rhinopoma cystops on 2012. (Online version in colour.)
We suggest that mouse-tailed bats started arousing and increased TMR at 16°C owing to their body fatty acid composition. We previously found that towards the end of summer, during the pre-hibernation period, mouse-tailed bats feed mainly on queens of carpenter ants [11]. These queen ants are an extremely poor source of PUFA (0.5%) and rich in SFA (approx. 44%) and MUFA (approx. 55.5%) [12]. We also found that this extremely saturated lipid profile is reflected in the mouse-tailed bats’ pre-hibernation tissue, which is, to the best of our knowledge, the most saturated ever recorded in a mammal [12] and differs from the typical PUFA-rich food preference and body composition of other hibernating mammals [17]. High levels of dietary PUFAs in pre-hibernating mammals are known to positively affect torpor and hibernation, for example, by increasing depth and duration of torpor bouts [14,15,28], but the function of these fatty acids during hibernation is still unknown [29,30]. Since mouse-tailed bats fat is saturated, unlike other hibernators, it should restrict their ability to hibernate at low temperatures.
During hibernation, hibernating mammals are motionless and their eyes are closed, while mouse-tailed bats hibernate with their eyes open, instantly react to stimuli, and swiftly become active after being disturbed, even when their body temperature is still far below normothermy (approx. 37°C). This can be explained by the relatively high Tb of mouse-tailed bats during hibernation. We observed mouse-tailed bats actively flying when their body temperature was still low (approx. 23°C) and argue that they can perform many basic functions at relatively low Tb (see also [9]). Quantitative data on locomotor performance at low Tb in torpid marsupials suggest that they can run at Tb far below 20°C [31]. The ability to react fast to threat may be especially important for a small mammal hibernating at a cave entrance in a subtropical winter. Unlike winters in temperate regions, in a subtropical climate other mammals, reptiles and birds of prey are present and active during the winter and might be a threat to the hibernating bats. Therefore, vocal communication between individuals like we heard when entering the hibernacula and a fast response may be crucial for survival and adds another adaptive significance of hibernation at high temperatures.
Why do these bats hibernate during the Israeli temperate winter? This is especially interesting as other bat species inhabiting the same cave remain active and forage. A possible explanation is that for a subtropical bat at the northern edge of its distribution, the Mediterranean winter is too cold to be active and to survive so they hibernate in geothermally heated warm protected caves. However, another subtropical bat, T. perforatus, inhabits the same cave during winter but remains active. This may suggest that other factors influence the use of torpor in these species. One possibility is that in contrast to the active bat species, the mouse-tailed bats in northern Israel are diet-specialists. During early summer they consume mainly coleopterans and heteropterans, which are scarce during winter, and from July they feed almost solely on winged ants, which are absent during the winter months [11]. Other factors, like predation risk, were suggested as possible contributors to the use of torpor and hibernation [1], but these remain to be studied.
We did not record any periodic arousals during hibernation in any of the mouse-tailed bats in their natural hibernacula. We expected to find more than few such arousals during the 25 days of Tsk recording. In other temperate zone bats hibernating at ambient temperature around 15°C and above, arousals appear almost every day [32–35]. One exception is the large (60 g) subtropical insectivore bat Hipposideros terasensis, hibernating at relatively warm hibernacula around 20°C. In this bat, torpor bouts lasted from 1.6 to 19 days, and arousals and foraging bouts are observed throughout winter (70% of torpor bouts were shorter than 10 days) [36]. We monitored mouse-tailed bats Tsk for 25 days and did not record a single arousal in any of the eight bats recorded. Nevertheless, we did record sporadic increases of 3–8°C in body temperature up to 25–29°C for a very short duration (20–60 min, figure 2 and table 1). Based on these findings, we suggest that mouse-tailed bats do not perform arousals, or that they perform unusual, extremely long torpor bouts for hibernation in high ambient temperatures. Except for the energy saving, reducing arousal frequency can reduce possible oxidative stress or other damage to the brain and other tissues due to hypoxia during arousals (reviewed by [37,38]).
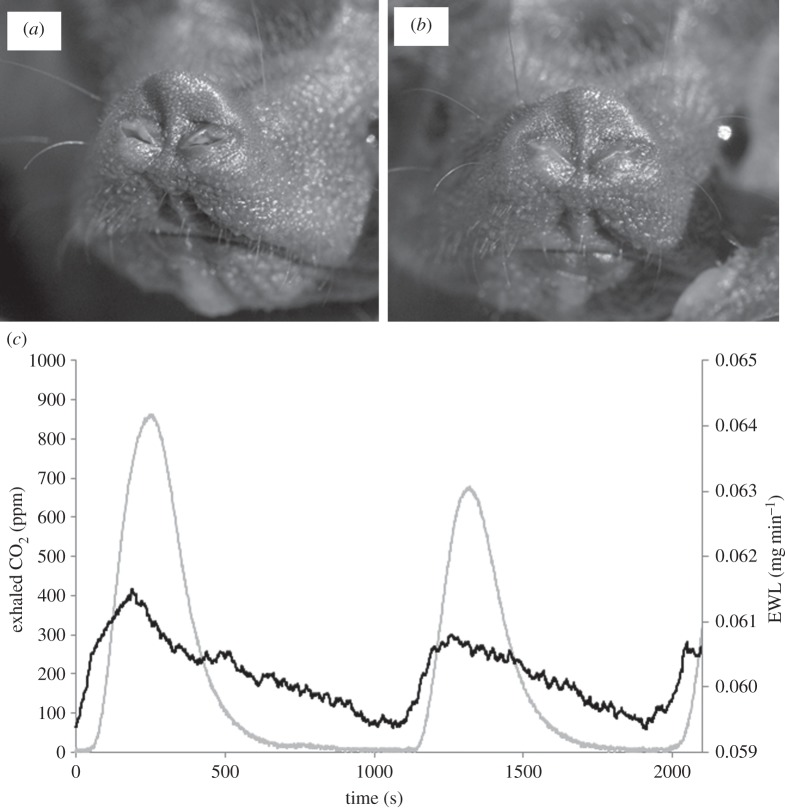
In contrast to other hibernating bats [32,36,39] mouse-tailed bats remain in their hibernacula all winter long and avoid foraging or drinking. During our study the mouse-tailed bats were never observed drinking water, neither during warm winter nights above 20°C nor during the summer, suggesting that they are very efficient at conserving water. It was previously suggested that hibernating mammals lose water through EWL until a specific threshold at which they must arouse to drink [40–42]. According to this hypothesis, if mouse-tailed bats are very efficient in reducing water loss during hibernation, this might significantly contribute to reducing arousals during hibernation or even to avoiding them. Indeed, mouse-tailed bats possess a very low density of capillaries in their skin, which reduces EWL [43]. Accordingly, we found that the total EWL of mouse-tailed bats is exceptionally low, even under the zero relative humidity conditions we used in the laboratory. As cave relative humidity is close to 100%, the values of EWL in wild hibernating mouse-tailed bats should be even lower and close to zero. Even though most of the water loss is through the skin, we suggest that during apnoea in which nostrils are closed, pulmonary water loss should be greatly reduced. It was previously suggested in other bat species that during bat apnoea the glottis is closed and prevents water loss, but there is contradicting evidence to this theory [41,44,45]. Mouse-tailed bats have distinctive valves in their nostrils (figure 6). During apnoea these valves are shut and only flutter (open and shut rhythmically with diaphragm movement) during the ventilation cycles between the apnoeas. This results in greater total EWL than during apnoea (figure 6). We suggest that in mouse-tailed bats the nostril valves function analogically with the spiraculum of the insects’ trachea [46] and contribute to reducing pulmonary water loss. We observed these nostril valves in another two arid zone bat species; Taphozous nudiventris and T. preforatus. In Israel, these two species occupy the same habitats as mouse-tailed bats, and we suggest this might be a common mechanism for reducing water loss in arid zone bats.
Figure 6.
Rhinopoma microphyllum nostrils. (a) Valves are open and (b) valves are shut. (c) Total evaporative water loss (black line) and CO2 exhaled (grey line) during hibernation. There is a clear effect of apnoea and ventilation (as indicated by CO2 exhalation) on pulmonary water loss.
In summary, both species of mouse-tailed bats enter a very efficient hibernation, which they perform in geothermally heated, stable microclimate of warm hibernacula, maintaining their body temperature above 20°C. Unlike other hibernators, we observed no periodic arousals or periods of normothermy during hibernation, and even though it is possible that the recording period was too short to record such arousals, we suggest that their ability to function at low Tb, combined with their efficient water economy reduces the need for periodic arousals in these subtropical hibernating species. Their pre-hibernation diet in Israel, and as a result their body fatty acid composition, may contribute to their hibernation success, but at the same time we hypothesize it forces them to maintain their body temperature above 16°C. Hibernating in the moderate Israeli winter raises questions regarding the selective forces and adaptive significance of using hibernation in this genus, and remains to be studied. The finding that mouse-tailed bats use specific caves with constant high temperature and humidity during winter, enabling these species to survive on the northern edge of their distribution, highlights the importance of protecting such caves for the conservation of these species.
Ethics statement
All procedures were carried out under permit no. 2009/31867 from the Israel Nature Reserves and Park Authority (NPA) and the Ethics Committee license no. L-09–002.
Acknowledgements
We thank Boaz Langford and Amos Frumkin from the Israeli Cave Research Unit, The Hebrew University, Jerusalem, for spelaeological data.
Funding statement
This research was supported by the Israel Science Foundation (grant no. 232/08).
References
- 1.Kronfeld-Schor N, Dayan T. 2013. Thermal ecology, environments, communities, and global change: energy intake and expenditure in endotherms. Annu. Rev. Ecol. Evol. Syst. 44, 461–480. ( 10.1146/annurev-ecolsys-110512-135917) [DOI] [Google Scholar]
- 2.Geiser F. 2013. Hibernation. Curr. Biol. 23, R188–R193. ( 10.1016/j.cub.2013.01.062) [DOI] [PubMed] [Google Scholar]
- 3.Grimpo K, Legler K, Heldmaier G, Exner C. 2013. That's hot: golden spiny mice display torpor even at high ambient temperatures. J. Comp. Physiol. B 183, 567–581. ( 10.1007/s00360-012-0721-4) [DOI] [PubMed] [Google Scholar]
- 4.Dechmann DKN, Ehret S, Gaub A, Kranstauber B, Wikelski M. 2011. Low metabolism in a tropical bat from lowland Panama measured using heart rate telemetry: an unexpected life in the slow lane. J. Exp. Biol. 214, 3605–3612. ( 10.1242/jeb.056010) [DOI] [PubMed] [Google Scholar]
- 5.Amichai E, Levin E, Kronfeld-Schor N, Roll U, Yom-Tov Y. 2013. Natural history, physiology and energetic strategies of Asellia tridens (Chiroptera). Mamm. Biol. Z. Saugetierkunde 78, 94–103. ( 10.1016/j.mambio.2012.06.006) [DOI] [Google Scholar]
- 6.Kulzer E. 1965. Temperaturregulation bei Fledermausen (Chiroptera) aus Verschiedenen Klimazonen. Z. Vergleichende Physiol. 50, 1–34. ( 10.1007/BF00388050) [DOI] [Google Scholar]
- 7.Schliter A, Qumsiyeh M. 1996. Rhinopoma microphyllum. Mamm. Species 542, 1–5. ( 10.2307/3504243) [DOI] [Google Scholar]
- 8.Levin E, Yom-Tov Y, Barnea A, Huchon D. 2008. Genetic diversity and phylogeography of the greater mouse-tailed bat (Rhinopoma microphyllum, Brunnich, 1782) in the Levant. Acta Chiropterol. 10, 207–212. ( 10.3161/150811008X414791) [DOI] [Google Scholar]
- 9.Levin E, Ar A, Yom-Tov Y, Kronfeld-Schor N. 2012. Summer torpor and sexual segregation in the subtropical bat (Rhinopoma microphyllum). In Living in a seasonal word (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 167–174. Berlin, Germany: Springer. [Google Scholar]
- 10.Levin E, Roll U, Dolev A, Yom-Tov Y, Kronfeld-Shcor N. 2013. Bats of a gender flock together: sexual segregation in a subtropical bat. PLoS ONE 8, e54987 ( 10.1371/journal.pone.0054987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin E, Yom-Tov Y, Barnea A. 2009. Frequent summer nuptial flights of ants provide a primary food source for bats. Naturwissenschaften 96, 477–483. ( 10.1007/s00114-008-0496-3) [DOI] [PubMed] [Google Scholar]
- 12.Levin E, Yom-Tov Y, Hefetz A, Kronfeld-Schor N. 2012. Changes in diet, body mass and fatty acid composition during pre-hibernation in a subtropical bat in relation to NPY and AgRP expression. J. Comp. Physiol. B 183, 157–166. ( 10.1007/s00360-012-0689-0) [DOI] [PubMed] [Google Scholar]
- 13.Dark J. 2005. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu. Rev. Nutr. 25, 469–497. ( 10.1146/annurev.nutr.25.050304.092514) [DOI] [PubMed] [Google Scholar]
- 14.Florant GL. 1998. Lipid metabolism in hibernators: the importance of essential fatty acids. Am. Zool. 38, 331–340. [Google Scholar]
- 15.Frank CL. 1992. The influence of dietry fatty acids on hibernation by golden mantled ground squirrles (Spermophilus lateralis). Physiol. Zool. 65, 906–920. [Google Scholar]
- 16.Geiser F, Mcallan BM, Kenagy GJ. 1994. The degree of dietary fatty acid unsaturation affects torpor patterns and lipid composition of a hibernator. J. Comp. Physiol. B 164, 299–305. ( 10.1007/BF00346446) [DOI] [PubMed] [Google Scholar]
- 17.Ruf T, Arnold W. 2008. Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am. J. Physiol. 294, R1044–R1052. ( 10.1152/ajpregu.00688.2007) [DOI] [PubMed] [Google Scholar]
- 18.Barclay RMR, Kalcounis MC, Crampton LH, Stefan C, Vonhof MJ, Wilkinson L, Brigham RM. 1996. Can external radiotransmitters be used to assess body temperature and torpor in bats? J. Mammal. 77, 1102–1106. ( 10.2307/1382791) [DOI] [Google Scholar]
- 19.Audet D, Thomas DW. 1996. Evaluation of the accuracy of body temperature measurement using external radio transmitters. Can. J. Zool. 74, 1778–1781. ( 10.1139/z96-196) [DOI] [Google Scholar]
- 20.Dausmann KH. 2005. Measuring body temperature in the field—evaluation of external vs. implanted transmitters in a small mammal. J. Thermal Biol. 30, 195–202. ( 10.1242/jeb.056010) [DOI] [Google Scholar]
- 21.Geiser F. 2004. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. ( 10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- 22.Withers PC. 2001. Design calibration and calculation for flow-through respirometry systems. Austr. J. Zool. 49, 445–461. ( 10.1071/ZO00057) [DOI] [Google Scholar]
- 23.Lighton JRB. 2008. Mesuring metabolic rates—a manual for scientists. New York, NY: Oxford University Press. [Google Scholar]
- 24.Malan A. 2014. The evolution of mammalian hibernation: lessons from comparative acid–base physiology. Integr. Compar. Biol. 54, 484–496. ( 10.1093/icb/icu002) [DOI] [PubMed] [Google Scholar]
- 25.Geiser F. 1988. Reduction of metabolism during hibernation and daily torpor in mammals and birds: temperature effect or physiological inhibition. J. Comp. Physiol. B 158, 25–37. ( 10.1007/BF00692726) [DOI] [PubMed] [Google Scholar]
- 26.Mendelssohn H, Yom-Tov Y. 1999. Mammals of Israel, p. 448 Jerusalem, Israel: Israel Academy of Sciences & Humanities. [Google Scholar]
- 27.Geiser F, Ruf T. 1995. Hibernation versus daily torpor in mammals and birds—physiological variables and classification of torpor patterns. Physiol. Zool. 68, 935–966. [Google Scholar]
- 28.Frank CL, Dierenfeld ES, Storey KB. 1998. The relationship between lipid peroxidation, hibernation, and food selection in mammals. Am. Zool. 38, 341–349. [Google Scholar]
- 29.Giroud S, et al. 2009. Dietary palmitate and linoleate oxidations, oxidative stress, and DNA damage differ according to season in mouse lemurs exposed to a chronic food deprivation. Am. J. Physiol. 297, R950–R959. ( 10.1152/ajpregu.00214.2009) [DOI] [PubMed] [Google Scholar]
- 30.Munro D, Thomas DW. 2004. The role of polyunsaturated fatty acids in the expression of torpor by mammals: a review. Zoology 107, 29–48. ( 10.1016/j.zool.2003.12.001) [DOI] [PubMed] [Google Scholar]
- 31.Rojas AD, Körtner G, Geiser F. 2012. Cool running: locomotor performance at low body temperature in mammals. Biol. Lett. 8, 868–870. ( 10.1098/rsbl.2012.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunbar MB, Tomasi TE. 2006. Arousal patterns, metabolic rate and energy budget of eastern red bats (Lasiurus borealis) in winter. J. Mammal. 87, 1096 ( 10.1644/05-MAMM-A-254R3.1) [DOI] [Google Scholar]
- 33.Turbill C, Geiser F. 2008. Hibernation by tree-roosting bats. J. Comp. Physiol. B 178, 597–605. ( 10.1007/s00360-007-0249-1) [DOI] [PubMed] [Google Scholar]
- 34.Park KJ, Jones G, Ransome RD. 2000. Torpor, arousal and activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum). Funct. Ecol. 14, 580–588. ( 10.1046/j.1365-2435.2000.t01-1-00460.x) [DOI] [Google Scholar]
- 35.Arlettaz R, Ruchet C, Aeschimann J, Brun E, Genoud M, Vogel P. 2000. Physiological traits affecting the distribution and wintering strategy of the bat Tadarida teniotis. Ecology 81, 1004–1014. ( 10.1890/0012-9658(2000)081[1004:PTATDA]2.0.CO;2) [DOI] [Google Scholar]
- 36.Liu J-N, Karasov W. 2011. Hibernation in warm hibernacula by free-ranging Formosan leaf-nosed bats, Hipposideros terasensis, in subtropical Taiwan. J. Comp. Physiol. B 181, 125–135. ( 10.1007/s00360-010-0509-3) [DOI] [PubMed] [Google Scholar]
- 37.Dave KR, Christian SL, Perez-Pinzon MA, Drew KL. 2012. Neuroprotection: lessons from hibernators. Comp. Biochem. Physiol. B 162, 1–9. ( 10.1016/j.cbpb.2012.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew K, Zuckerman J, Shenk P, Bogren L, Jinka T, Moore J. 2013. Hibernation: a natural model of tolerance to cerebral ischemia/reperfusion. In Innate tolerance in the CNS (eds Gidday JM, Perez-Pinzon MA, Zhang JH.), pp. 37–50. New York, NY: Springer. [Google Scholar]
- 39.Boyles JG, Dunbar MB, Whitaker JO. 2006. Activity following arousal in winter in North American vespertilionid bats. Mamm. Rev. 36, 267–280. ( 10.1111/j.1365-2907.2006.00095.x) [DOI] [Google Scholar]
- 40.Ben-Hamo M, Muñoz-Garcia A, Williams JB, Korine C, Pinshow B. 2013. Waking to drink: rates of evaporative water loss determine arousal frequency in hibernating bats. J. Exp. Biol. 216, 573–577. ( 10.1242/jeb.078790) [DOI] [PubMed] [Google Scholar]
- 41.Thomas DW, Cloutier D. 1992. Evaporative water loss by hibernating little brown bats, Myotis lucifugus. Physiol. Zool. 65, 443–456. [Google Scholar]
- 42.Thomas DW, Geiser F. 1997. Periodic arousals in hibernating mammals: is evaporative water loss involved? Funct. Ecol. 11, 585–591. ( 10.1046/j.1365-2435.1997.00129.x) [DOI] [Google Scholar]
- 43.Vogel VB. 1969. Comparative studies on water metabolism of bats (Rhinopoma, Rhinolophus and Myotis). Z. Vergleichende Physiol. 64, 324–340. ( 10.1007/BF00340550) [DOI] [Google Scholar]
- 44.Hays GC, Webb PI, Speakman JR. 1991. Arrhthmic breathing in torpid pipistrelle bats, Pipistrellus pipistrellus. Respir. Physiol. 85, 185–192. ( 10.1016/0034-5687(91)90060-V) [DOI] [PubMed] [Google Scholar]
- 45.Szewczak JM, Jackson DC. 1992. Apneic oxygen uptake in the torpid bat, Eptisicus fuscus. J. Exp. Biol. 173, 217–227. [DOI] [PubMed] [Google Scholar]
- 46.Levy RI, Schneiderman HA. 1958. An experimental solution to the paradox of discontinuous respiration in insects. Nature 182, 491–493. ( 10.1038/182491a0) [DOI] [PubMed] [Google Scholar]