Abstract
Across animals and plants, numerous metabolic and defensive adaptations are a direct consequence of symbiotic associations with beneficial microbes. Explaining how these partnerships are maintained through evolutionary time remains one of the central challenges within the field of symbiosis research. While genome erosion and co-cladogenesis with the host are well-established features of symbionts exhibiting intracellular localization and transmission, the ecological and evolutionary consequences of an extracellular lifestyle have received little attention, despite a demonstrated prevalence and functional importance across many host taxa. Using insect–bacteria symbioses as a model, we highlight the diverse routes of extracellular symbiont transfer. Extracellular transmission routes are unified by the common ability of the bacterial partners to survive outside their hosts, thereby imposing different genomic, metabolic and morphological constraints than would be expected from a strictly intracellular lifestyle. We emphasize that the evolutionary implications of symbiont transmission routes (intracellular versus extracellular) do not necessarily correspond to those of the transmission mode (vertical versus horizontal), a distinction of vital significance when addressing the genomic and physiological consequences for both host and symbiont.
Keywords: symbiosis, symbiont transmission, mutualism stability, host–microbe coevolution
1. Introduction
Through a variety of interactions, resident microorganisms have played a significant role in the origin and evolution of animals [1]. Among animals, insects serve as excellent models to elucidate the functional importance of these interactions, because they engage in a particularly wide range of mutualisms with bacteria and fungi [2].
The remarkable diversity in form and function of insect-microbial interactions, however, can only be rivalled by the variety of symbiont transmission structures and behaviours that contribute towards the fixation, persistence and evolution of such partnerships [2]. These adaptations ensure that beneficial microbes can be transferred across host generations directly from parent to offspring (vertical mode), indirectly from con- or heterospecific host individuals as well as from the environment (horizontal mode), or through a combination of transmission mechanisms (mixed-mode) [3].
Some of the best-studied insect-bacterial mutualisms (e.g. aphids and Buchnera, carpenter ants and Blochmannia, tsetse flies and Wigglesworthia) have yielded extensive knowledge on the transmission ecology and the ensuing evolutionary consequences of mutualistic interactions between insects and intracellularly localized symbionts [4–12]. These obligate mutualists can be transmitted in a number of ways during oogenesis or embryogenesis [4]. For example, in carpenter ants, Blochmannia is vertically transmitted via an acute intracellular infection of the ovaries and subsequent incorporation into the eggs [9]. Similarly, in aphids, Buchnera is transovarially transferred to the developing eggs via a highly selective mechanism at the ovariole tips [10]. As for tsetse flies, their B vitamin-supplementing symbiont Wigglesworthia is transmitted via milk gland secretions as the larva develops in utero [11]. While many intracellular symbionts are maternally transmitted to the offspring [4], a recent study [12] has demonstrated both maternal and paternal vertical transmission of Rickettsia in leafhoppers.
The evolutionary implications of an intracellular lifestyle coupled with the strict vertical transmission mode of many insect symbionts have been the focus of considerable attention [5–8]. Nonetheless, research efforts of the past few decades have resulted in a steadily increasing body of knowledge on the diversity, function and evolutionary history of extracellularly localized and transmitted symbionts in insects (electronic supplementary material, table S1). Given this wealth of recent data, it is now feasible to assess the fundamental ecological and evolutionary implications of these types of transmission routes for both host and microbe.
In addition to providing an overview of extracellularly transmitted bacterial symbioses in insects, we discuss the evolutionary origin of such associations and the factors influencing their persistence. We also emphasize the impact of symbiont transmission on the coevolutionary trajectory of the symbioses, and on the genomic and metabolic signatures of the bacterial partners. By illustrating that some extracellularly localized and transmitted bacterial symbionts can exhibit similar patterns of metabolic integration and host-microbe coevolution as strictly intracellular mutualisms, we stress that the mode of symbiont transmission (vertical versus horizontal) is a more accurate indicator of mutualism stability and integrative potential than the stage at which microbes are transmitted (prenatal versus postnatal).
2. Overview of extracellular transmission routes of insect symbionts
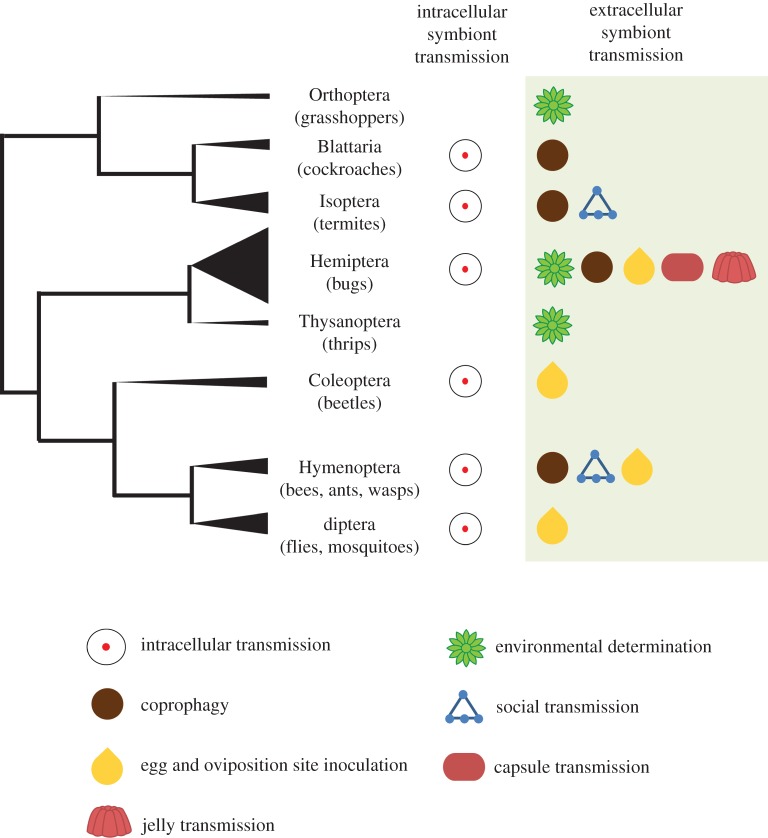
Despite broad functional and taxonomic diversity (figure 1; electronic supplementary material, table S1), extracellularly transmitted symbionts can be unified by the ability to survive outside of their host for part, or all, of their lifetime. This feature markedly differentiates them from the majority of intracellular symbionts, where survival outside the host is no longer possible [5].
Figure 1.
Cladogram depicting the diversity of insect orders with reported extracellularly transmitted bacterial symbionts (as listed in the electronic supplementary material, table S1). Symbols indicate extracellular transmission routes. Terminal branch thickness is proportional to the number of families within the order that have been reported to rely on an extracellular route for symbiont transfer. Orders featuring taxa with an intracellular symbiont transmission route are designated with a symbol as well (per electronic supplementary material, table S3). (Online version in colour.)
In insects, extracellular transmission routes for bacterial symbionts include environmental determination, coprophagy, smearing of brood cell or egg surface, social acquisition, capsule transmission or infection via jelly-like secretions (figure 2).
Figure 2.
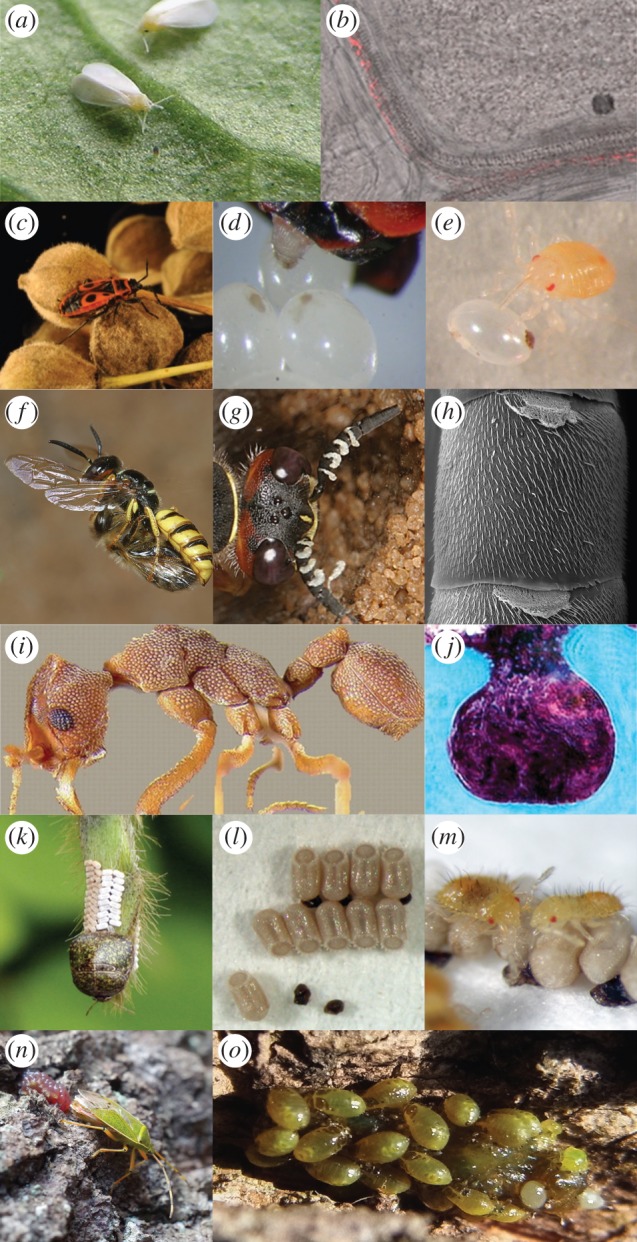
Extracellular symbiont transmission routes in insects. Horizontal transmission of Rickettsia among whiteflies (Bemisia tabaci) (a) involves the use of the insect's host plant [13] (b). Transmission of beneficial gut symbionts in the European firebug (c) relies on secretions that are smeared over the egg surface following oviposition (d,e) [14]. Beewolves (f) cultivate the defensive symbiont ‘Candidatus Streptomyces philanthi’ in specialized antennal gland reservoirs (g,h) and transmit it via the brood cell [15]. Fungus-growing ants harbour defensive bacteria in specialized regions of their cuticle (i,j) that are transmitted via social behaviour among nest-mates [16]. Beneath their egg mass, plataspid stinkbugs (k) deposit brown symbiont-bearing capsules (l) that are ingested by newly hatched nymphs (m) to initiate infection with the gut symbiont. An adult female of Urostylis westwoodii depositing egg-encapsulating, symbiont-containing jelly (n) that is later ingested by newly hatched nymphs (o). (Online version in colour.)
(a). Environmental determination
In animals, the acquisition of specific beneficial microbes from the environment is particularly prevalent in marine invertebrates including tubeworms and luminescent squids [17,18]. However, recent studies examining the microbial symbionts of several broad-headed bug species and whiteflies demonstrate that terrestrial environments can also be a suitable source for the acquisition of beneficial microbes by insect hosts [13,19].
Bean bugs (Riptortus pedestris), as well as many other species within the Lygaeoidea and Coreoidea superfamilies, harbour environmentally acquired Burkholderia symbionts that localize primarily within crypts along their posterior midgut section [20]. The environmental dimension of the symbionts' transmission route was first established following the inadvertent generation of developmentally regressed, aposymbiotic (symbiont-free) R. pedestris when the bugs were reared in sterile bottles. In fact, eggs laid in sterile laboratory settings by Burkholderia-infected individuals were also completely devoid of symbionts, strongly suggesting that the bugs acquired their free-living symbionts every generation from the environment, particularly the soil [19]—not unlike well-established plant–microbe partnerships involving rhizobia [21].
Environmental symbiont acquisition can also occur in associations with predominantly vertical transmission. In addition to their primary endosymbiont Portiera, whiteflies (Bemisia tabaci) also harbour a number of secondary symbionts, including a widely occurring Rickettsia sp. [22]. While Rickettsia has been demonstrated to be primarily transmitted vertically via the eggs during embryogenesis [22], significant inconsistencies were nonetheless observed between the phylogenies of hosts and symbionts, suggesting that the microbe probably undergoes substantial horizontal exchange between whiteflies [23]. Caspi-Fluger et al. [13] confirmed this by demonstrating that the symbiont can be transmitted among B. tabaci via the host plant, as demonstrated by the detection of Rickettsia in the phloem of cotton, basil and black nightshade plants following feeding by an infected whitefly. Additionally, Rickettsia-free individuals were successfully re-infected with the symbionts when allowed to feed on the same leaf (despite physical separation) as Rickettsia-infected B. tabaci [13]. However, the subsequent vertical transmission of horizontally acquired Rickettsia to the whitefly progeny has not yet been demonstrated. While the predominant route for symbiont acquisition in this system is vertical (via the egg), such findings suggest that plants may also serve as sources and sinks for symbiont inoculants in herbivorous insects.
(b). Coprophagy
Acquisition of beneficial bacteria through conspecific probing of faeces has been described as a predominant route of symbiont transmission for several insect groups, including Hemiptera (true bugs), Blattaria (cockroaches) and Isoptera (termites) (electronic supplementary material, table S1). The symbionts usually reside in the insect gut, where they are shed alongside the gut lumen and excreted in faeces [24]. Symbiont acquisition by aposymbiotic individuals then requires direct contact with faeces during or after excretion.
Interestingly, coprophagic symbiont transfer has been suggested to provide the opportunity for biological control of the reduviid bug Rhodnius prolixus, an important insect vector of the Chagas disease-causing parasite, Trypanosoma cruzi [25]. Despite near ubiquitous infection of adult R. prolixus with the actinobacterial symbiont Rhodococcus rhodnii in natural populations, newly hatched nymphs are aposymbiotic until they acquire the symbiont by probing conspecific faeces [25]. The route of symbiont transfer, coupled with the bacterium's amenability for genetic transformation, could facilitate biological control via paratransgenesis, i.e. the introduction and expression of exogenous trypanocidal genes via the symbionts [25,26]. Such findings highlight the potential for manipulation of extracellularly transmitted symbioses to control vector-borne diseases.
(c). Social acquisition
Advanced social behaviour in insects imposes different parameters for the transmission of microbial partners. A central feature of many social and subsocial insects is the intimate interaction of conspecifics through behaviours such as trophallaxis, the transfer of food or other fluids through mouth-to-mouth (stomodeal) or anus-to-mouth (proctodeal) feeding [27]. These behaviours can facilitate exchange of microbes among nest members, thereby contributing to maintenance of a beneficial microbiota, as has been demonstrated in ants [16,28,29], termites [30] and bees [31,32]. In fact, it has been speculated that the evolution of complex social forms could be reinforced, among other factors, by the convenience of acquiring beneficial microbes through recurring contact with conspecifics [33].
Recent examination of the gut microbiota of different bee species suggests that sociality plays an integral role in maintenance of the distinctive microbial communities within the Apoidea superfamily [31,34]. While the majority of solitary bee microbiota examined by Martinson et al. [35] seem to be indiscriminately dominated by Burkholderia or Wolbachia, the social corbiculate clade (including Bombus and Apis) carry a largely conserved microbiota that may have co-evolved with the hosts as a byproduct of eusocial behaviour. In honeybees (Apis mellifera), workers lack this distinctive microbial community upon eclosion, and recent findings demonstrate that they acquire the most dominant members of the microbiota either through social contact with nest-mates (trophallaxis), specifically nurses, or via contact with the hive components (e.g. combs and honey) [32,36]. As for bumblebees, molecular analyses carried out across three host species (B. sonorus, B. impatientis and Bombus sp.) show that two of the most dominant bacterial strains (Snodgrassella alvi and Gilliamella apicola) are transmitted vertically from the mother colony to daughter queens, and that social contact among nest-mates following pupal emergence is required for intra-colony transmission [31].
Social transmission of beneficial symbionts has also been described for fungus-farming ants (Attini: Formicidae) in their association with the defensive mutualist Pseudonocardia [28]. Most attine ant genera extracellularly harbour the symbiont in specialized cuticular crypts [16] (figure 2i and j), and the presence of Pseudonocardia on foundress queens during their mating flight suggests a vertical transmission route linking parent and offspring colonies [16,28]. The singular association of each nest to individual Pseudonocardia strains further implies that the symbionts proliferate among nest members via social behaviour [37,38], which was confirmed in a recent study by Marsh et al. [29]. Here, the ants were found to only acquire Pseudonocardia following contact with nest-mates within the first 2 h after emerging from their pupal cases.
(d). Egg and oviposition site inoculation
Smearing bacteria over the surface of newly deposited eggs is one of the most commonly described routes of extracellular symbiont transfer and has been reported for various insect orders, including Diptera, Coleoptera, Hymenoptera and Hemiptera (figure 1; electronic supplementary material, table S1). Successful infection primarily depends on the ability of nymphs or larvae to acquire their bacterial symbionts shortly after hatching, usually through active probing of egg or brood cell surfaces.
Within the Hemiptera, numerous studies have reported that egg smearing by the mother leads to successful transmission of beneficial microbes, particularly among bugs of the Pentatomomorpha infraorder (e.g. firebugs, stinkbugs, shield bugs, etc.) (electronic supplementary material, table S1). For example, firebugs (Pyrrhocoridae) deposit excretion droplets that are later taken up from the egg surface by young nymphs, ensuring successful transfer of the two co-occurring actinobacterial symbionts Coriobacterium glomerans and Gordonibacter sp. [14] (figure 2c–e). Similarly, in shield bugs of the family Acanthosomatidae, a γ-proteobacterial symbiont is harboured in cavities that are sealed off from the midgut main tract, as well as in a pair of lubricating organs associated with the female ovipositor [39]. It is through these specialized organs that the symbionts are vertically transmitted via egg surface contamination. Across both insect groups, disruption of the symbiont transmission route through the surface sterilization of newly laid eggs results in aposymbiotic individuals that suffer retarded growth, higher mortality and lower reproductive success [39,40].
By contrast, transmission of defensive Streptomyces symbionts of solitary digger wasps (Philanthus, Trachypus and Philanthinus spp.) relies not on surface contamination of eggs but rather of brood cells where eggs are deposited [15] (figure 2f–h). Prior to oviposition, female wasps secrete a symbiont-containing white substance from their antennal glands and onto the ceiling of brood cells [15]. During cocoon spinning, larvae then take up the bacteria, which confer protection against pathogenic fungi in the brood through production of antibiotic substances on the cocoon [41].
(e). Capsule and jelly transmission
Plataspid and urostylidid bugs use two of the most specialized mechanisms for extracellular symbiont transmission at the oviposition site [42–44]. In Plataspidae, adult females produce symbiont-enclosing ‘capsules', which they deposit among their newly laid egg masses to ensure the successful vertical transmission of their γ-proteobacterial midgut mutualist ‘Candidatus Ishikawaella capsulata’ (figure 2k–m) [42]. Infection of newly hatched nymphs is associated with capsule feeding [42]. In addition to the adverse fitness effects associated with aposymbiosis (e.g. high juvenile mortality and slow development), capsule removal causes bugs to wander from the egg masses rather than rest in aggregation—the typical behaviour associated with capsule feeding [45]. This suggests that insect behaviour may be linked to ensuring successful symbiont acquisition, as has been demonstrated in social insects.
In Urostylis westwoodii (Urostylididae), jelly-like secretions deposited by mothers over newly laid egg masses represent a remarkable adaptation with versatile biological roles [44]. While the sugar- and amino acid-rich jelly allows the newly hatched nymphs to withstand the nutritional burdens of overwintering underground (in the absence of their natural food source of plant sap), Kaiwa et al. [44] have also implicated the gelatinous structure in ensuring the successful transmission of the gut symbiont ‘Candidatus Tachikaweaea gelatinosa’ following ingestion (figure 2n,o).
3. Transition from a free-living state to symbiosis
Symbiont transmission mechanisms represent adaptations to ensure the maintenance of mutualisms. It is imperative to understand the factors that initially contributed towards the emergence of these mutualisms, and the evolutionary transitions that have shaped their history. Insects that rely on extracellular routes for transmission of their beneficial microbes present us with excellent systems to address such topics, given the diversity in evolutionary states of the symbioses, ranging from facultative and horizontally acquired, to obligate and vertically transmitted.
The origin of bacterial mutualisms, however, remains one of the most elusive questions within the field of symbiosis [46]. Traditionally, several hypotheses have been suggested for the initial evolution of microbial symbioses [47–49]. Recent phylogenetic analyses demonstrate the plausibility and occurrence of all these scenarios and suggest that the evolution of bacterial mutualists from environmental strains may represent a particularly common occurrence [50].
For extracellularly transmitted symbionts, there are several systems consistent with environmentally acquired bacteria providing immediate benefits to their hosts upon establishment. In leguminous plants, the nitrogen-fixing ability of soil rhizobia coupled with the gain of a core set of symbiosis loci as selected for by access to a metabolically stable environment (i.e. the host), enabled the establishment and maintenance of a cosmopolitan mutualistic partnership [21]. Analogous mutualisms that exemplify this dynamic have also been described for insects. Within the bean bug's (R. pedestris) association with Burkholderia, recent findings by Kikuchi et al. [51] demonstrate that under certain conditions, the environment can select for the optimal symbiont; which, in turn, can inadvertently align the bilateral benefits of the host and symbiont without the prerequisite of strict vertical transmission.
However, for such mutualisms to be evolutionarily stable, the benefits of investing in a vertical or horizontal symbiont transmission mechanism by the host must outweigh a couple of significant costs: the risk of not acquiring the symbiont and/or acquiring an antagonistic partner (e.g. pathogens, parasites or cheaters). In other words, insects that rely on environmentally acquired symbionts [19,20] probably run a higher risk of aposymbiosis as well as pathogen exposure than mutualisms featuring adaptations that ensure faithful vertical transmission of the microbial partner [43,44].
For example, for R. pedestris and several other bug species, failure to pick up specific bacterial symbionts during a short development window significantly affects fitness [52]. Similarly, the uptake of a suboptimal symbiont also poses risks for the host in terms of competitively subsisting on resources in a specific niche [51]. Interestingly, across the true bugs, there are multiple independent origins of Burkholderia symbioses, many, and possibly all, of which are presumed to be dependent on environmental acquisition [20]. These bug–Burkholderia partnerships are interspersed among other systems that use vertical transmission through both internal and external mechanisms. One possibility is that environmental acquisition is selected for in fluctuating environments when the symbiont genotype that is most optimal varies significantly across space or time. Thus, being able to switch partners, and possibly to actively select the optimal partner or to allow potential partners to outcompete one another, may supersede the costs of risking the failure to obtain an optimal partner.
This raises interesting questions relating to the initial stages of symbiont colonization; specifically, what are the mechanisms that mediate the recognition and uptake of the right symbiont and how does the host select for these microbes while eliminating less beneficial ones?
Mechanisms could be behavioural, in which insects actively seek out symbionts with certain traits, or physiological, in which insects take in a diversity of microbes and then actively winnow down associations to a narrow few. There has been little exploration of the former, but there is increasing evidence for the latter. As previously discussed, R. pedestris possess remarkably efficient symbiont detection and uptake mechanisms, where a mere 80 Burkholderia cells in a gram of soil are sufficient for successful infection [52] during a highly specific developmental window of acquisition [53]. The efficient establishment of only a small subset of the diverse microbes that these bugs encounter in soil is mediated, at least in part, through complex immunological processes that prevent the growth of other bacteria and may tightly regulate proliferation of the symbionts [54–57]. Antimicrobial peptides isolated from the haemolymph of the Burkholderia-harbouring coreoid Alydus calcaratus, for example, can suppress growth of some soil-dwelling Gram-negative bacteria (e.g. Escherichia coli) but not Burkholderia symbionts [57]. Comparative transcriptomic analyses of the midguts of symbiont-containing and aposymbiotic R. pedestris revealed an upregulation of cysteine-rich antimicrobial peptides, which could inhibit growth of some bacteria and also regulate symbiont populations [56]. These findings are complemented by evidence suggesting that Burkholderia symbiont establishment and proliferation requires the bacteria to have several genes necessary for combating host-induced stress [54,55]. This suggests that the host's physiology and immune system may be under selection to suppress proliferation of non-symbionts while allowing for regulated growth of symbiont populations. Burkholderia may have become the primary symbionts because they possessed the necessary features to facilitate establishment (e.g. motility, resistance to host antimicrobial activity) prior to, and independent of, any host-mediated selection.
Another factor that could favour the evolution of environmental acquisition would be the ubiquity of the microbe in the environment. Common occurrence of beneficial symbionts in the environment could relax selection to maintain vertical transmission mechanisms. To date, we have little data on the prevalence of beneficial symbionts in environmental reservoirs in systems where environmental acquisition is known (but see [13,19,57,58]). Further insight into factors favouring the evolution of environmentally acquired mutualisms will require such environmental sampling as well as characterization of symbiosis mechanisms across groups of insects, such as has been started, to some extent, for the true bugs [20].
4. Evolutionary transitions among transmission routes
Given the diverse mechanisms of extracellular transmission and their varying ecological and evolutionary implications, it is tempting to speculate on likely scenarios of evolutionary transitions between transmission routes. Owing to the high selective pressures of endowing beneficial symbionts efficiently to offspring, the majority of specific mutualistic insect–bacteria interactions appear to transition to vertical or mixed-mode transmission routes over the course of evolution.
Extracellular nutritional symbionts are usually localized within the gut, and significant numbers of cells are often shed and excreted along with faecal matter [14,24]. Thus, transmission via faeces (i.e. coprophagy and proctodeal trophallaxis) constitutes a simple transitory step from environmental acquisition to vertical transmission, as it does not require any specialized morphological adaptations of the host [2]. In taxa with social interactions between parents and offspring or within groups of related or unrelated conspecifics, direct transfer of faeces by proctodeal trophallaxis also ensures symbiont transmission along with the provisioning of enzymes that may facilitate digestive processes in immature individuals [30]. Non-social insects without direct contact between symbiotic and aposymbiotic individuals, on the other hand, can increase the probability of successful transmission to the offspring by applying symbiont-containing faeces to locations that have high chances of being frequented and probed by the hatching larvae [2]. The egg surface is the most commonly used and reliable place of symbiont application and uptake (e.g. [2,39,59]), but in special cases with locally confined developmental conditions, the brood cell surface can be equally suitable [15].
Coincident with, or subsequent to, an increased fidelity in vertical symbiont transmission, several insects with extracellular symbionts have evolved specialized structures to house and transmit symbionts to the offspring (e.g. [42,44]). As discussed for the capsule and jelly transmitted symbionts of plataspid and urostylidid bugs, these structures can serve to protect the symbionts from abiotic stresses during egg development [44], as well as allow for enhanced control over the identity and number of symbiont cells allocated to offspring, thereby ensuring that the progeny are endowed with sufficient numbers of viable symbionts for successful colonization [60]. While speculative, such adaptations may also mitigate the chances of co-transmitting potentially detrimental microbes, which is consistent with theoretical predictions implicating the restriction of symbiont migration as a mechanism adopted by the host in order to reduce virulent tendencies arising from competition between heterospecific symbiont lineages [61].
Finally, the highest level of integration between host and symbiont occurs when symbiont transmission is internalized within the host's body. Starting out with an extracellular symbiosis, however, this can be achieved through a shift in the symbiont's lifestyle to intracellular maintenance and transmission to the developing oocyte or embryo (e.g. aphids–Buchnera, carpenter ants–Blochmannia) [4].
5. Implications of transmission ecology for symbiont genome evolution
Obligate mutualisms can have a strong effect on the evolution of the bacterial partner's genome. As exemplified by Buchnera and Sulcia—the primary intracellular endosymbionts of pea aphids and sharpshooters, respectively—as well as many others, symbiont genomes can undergo strikingly convergent patterns of degradation and reduction [5]. These small genomes exhibit extensive AT nucleotide enrichment and undergo accelerated molecular evolution [6,7]. Such features are presumed to be driven by gene loss resulting from a combination of strong genetic drift in small populations undergoing severe bottlenecks during transmission, and relaxed selection to no longer maintain genes necessary for an extracellular lifestyle [7].
As a result, many of the aforementioned genomic features became consequential hallmarks of an intracellular lifestyle within animal hosts [8]. Thus, it seemed unlikely that extracellularly transmitted and localized symbionts would undergo similar patterns of reductive genome evolution, considering that these microbes reside outside of the insect host for part, or all, of their life cycle, where they can readily undergo recombination to offset gene loss due to genetic drift, and generally need to retain a larger set of genes to survive in less stable environmental conditions and to move between various habitats (e.g. different host tissues and outside of hosts).
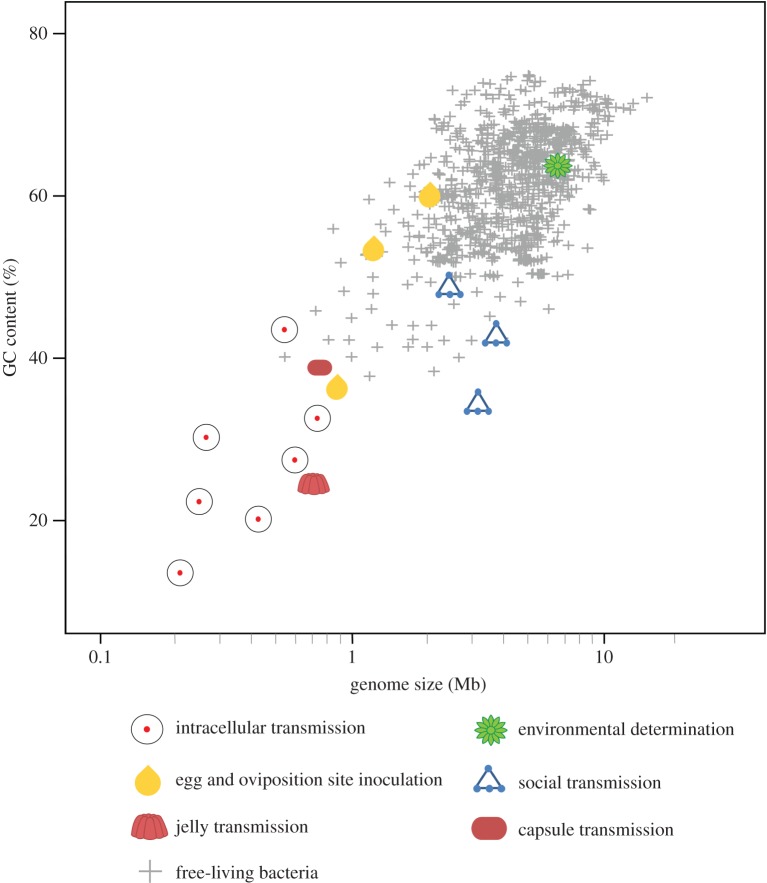
Nonetheless, studies examining genomic features of some extracellularly transmitted symbionts indicate that similar evolutionary processes can occur in these symbionts as those restricted to a strict intracellular lifestyle (figure 3 and electronic supplementary material, table S4).
Figure 3.
Relationship between genome size and GC content for a representative subset of intra- as well as all extracellularly transmitted bacterial symbionts in insects (per electronic supplementary material, table S2), as compared to free-living bacteria. Symbols indicate symbiont transmission route and biotic condition (symbiotic versus free-living). (Online version in colour.)
Specifically, extracellularly transmitted symbionts of plataspid (capsule transmission), acanthosomatid (egg smearing) and urostylidid (jelly transmission) bugs possess reduced genomes (estimated sizes around 0.7–0.9 Mb) [39,43,44,62]. Additionally, Ishikawaella symbionts isolated from the plataspid M. punctatissima exhibit other genomic features reminiscent of intracellular symbiotic bacteria (i.e. AT nucleotide bias and few mobile elements) [62]. Examination of Ishikawaella's metabolic potential reveals that despite significant gene loss, the bacterium retains the ability to synthesize almost all essential amino acids, in addition to some vitamins and cofactors [62]. This is consistent with the suggested benefit of the symbiont for M. punctatissima, whose plant diet is poor in essential amino acids and certain vitamins.
Despite exhibiting similar patterns of reductive genome evolution, pairwise comparisons of gene profiles between Ishikawaella and a range of intracellularly localized and transmitted symbionts revealed a number of important discrepancies that may reflect their different ecologies [62]. Most prominent was the complete retention of genes involved in the Krebs cycle, as well as many other genes underlying energy production and conversion. This was attributed to the more stringent metabolic requirements of an extracellular lifestyle, where access to metabolic intermediates in the host cytoplasm is not an option, unlike for many obligate intracellular symbionts. Additionally, Ishikawaella possesses a greater number of genes involved in the synthesis of amino acids and cofactors than Buchnera (aphids), Blochmannia (ants) or Wigglesworthia (tsetse flies), a condition that implies a broader metabolic repertoire for supplementation, and/or a younger coevolutionary history with its host [62].
The genomic features of these symbionts provide insight into the evolutionary forces driving genome reduction in obligate microbial mutualists [39,43,44,62] by demonstrating that these convergent traits are not strictly a consequence of an intracellular lifestyle but rather are more likely due to increased impact of genetic drift associated with a host-restricted lifestyle. This highlights small population sizes and strong bottlenecks promoted by spatial isolation, prior to and/or during transmission, as important factors for genome evolutionary patterns in heritable symbionts.
These findings, coupled with analyses of a broad range of bacterial genomes demonstrating a clear inverse correlation linking genome size and the incidence of genetic drift [63] further support the concept that reductive genome evolution can be associated with intracellularity but is not necessarily derived from it.
6. Host–symbiont coevolutionary dynamics
Acquisition of complex traits and adaptations by insects to ensure that their progeny are endowed with beneficial microbes often results in symbiotic systems that are evolutionarily stable and mutually obligatory. The high fidelity exhibited by these partnerships can be quantified (and visualized) through the congruent branching patterns of host and symbiont phylogenies in what is commonly referred to as co-cladogenesis [5]. Strict co-cladogenesis has been demonstrated in a number of insect-bacterial mutualisms featuring intracellular symbionts, for example, among sap-feedings insects (e.g. aphids, sharpshooters, etc.) [4].
With co-cladogenesis as a measure for mutualism fidelity, inferring phylogenetic relationships between symbiotic bacteria relative to their insect hosts can provide insights into the evolutionary implications of different routes of symbiont transmission. Bugs of the Pentatomomorpha infraorder—whose bacterial partners colonize similar gut regions but use different transmission routes and modes to initiate infection—provide a point of comparison for consequences of transmission routes on host–symbiont evolution (figure 4). In members of this group that acquire beneficial bacteria via the environment (e.g. soil), there are numerous discrepancies between symbiont and host phylogenies [20] (figure 4a). Some host species harbour different symbiont genotypes, while others share a single identical symbiont [20,57]. This lack of fidelity is driven by the bugs acquiring symbionts from a potentially diverse, shared environmental reservoir. Similarly, there are instances where vertical transmission of symbionts via egg smearing does not result in clear co-cladogenesis between host and microbe, as has been demonstrated in pentatomid [65] and pyrrhocorid bugs (figure 4b) [64]. In such instances, it is presumed that while a mechanism exists for the microbes to be transmitted directly from mother to offspring, significant exchange of symbionts within and between host species is also taking place.
Figure 4.
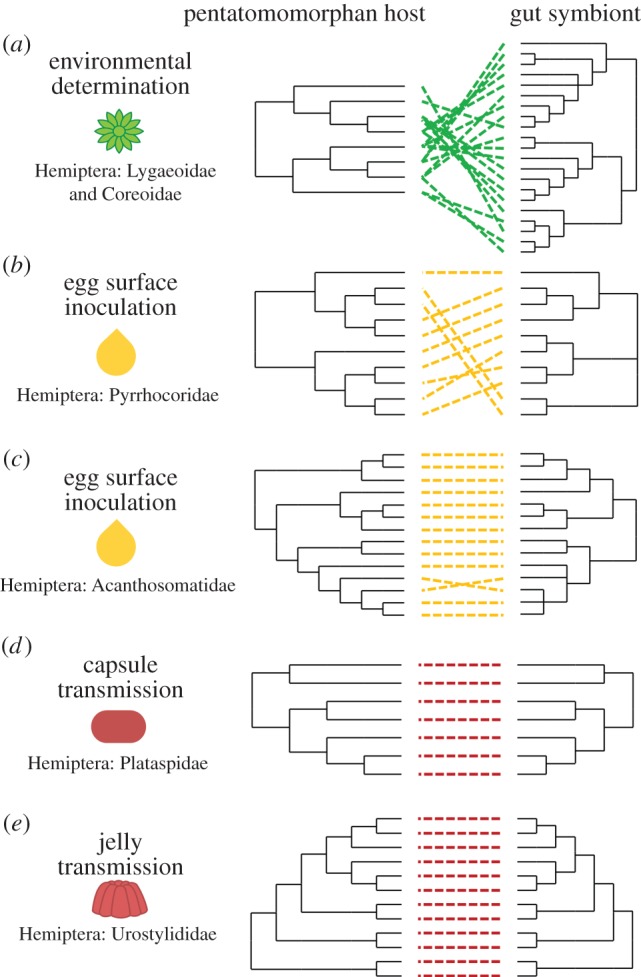
Comparison of evolutionary relationships between bugs and their gut symbionts as it relates to the symbionts' extracellular transmission routes. These relationships were established for (a) Lygaeoidea and Coreoidea and their environmentally acquired Burkholderia symbionts [20], (b) Pyrrhocoridae [64] and (c) Acanthosomatidae [39] relying on egg smearing, as well as (d) Plataspidae [43] and (e) Urostylididae [44] using symbiont capsules and jelly, respectively, for the transmission of their gut symbionts. (Online version in colour.)
Conversely, in instances where gut symbionts are transferred directly from mother to progeny in a monoclonal manner, aided by symbiont-bearing structures and/or intimate behavioural responses, a remarkably convergent evolutionary history is often observed between host and microbe, irrespective of whether the symbionts are transmitted intra- or extracellularly [39,43,44]. For example, symbionts of Acanthosomatidae, Plataspidae and Urostylididae bugs, which are transmitted via egg smearing, symbiont capsules and jelly secretions, respectively, exhibit near strict co-cladogenesis with their hosts [39,43,44] (figure 4c–e), as has been shown for a multitude of intracellular symbionts [4].
Thus, despite the consistent localization of symbionts in similar midgut environments across many bugs, differences in extracellular transmission mechanisms alter patterns of co-cladogenesis and, more broadly, probably have important consequences for coevolution. Specifically, in cases of strict vertical transmission, fitness of host and symbiont are aligned, even in the absence of intracellular maintenance and transmission. However, when symbionts are either occasionally or frequently environmentally acquired, there is reduced host–symbiont fidelity and reduced alignment of host and bacterial interests. In the former case, we can expect that both host and symbionts are evolving in response to one another. In the latter case, much of the bacteria's adaptation may be shaped by forces external to a given host.
7. Conclusion and future perspectives
In addition to providing insights into evolutionary aspects of symbiosis, insect-bacterial mutualisms that rely on extracellular mechanisms for symbiont transmission present excellent opportunities to elucidate functional aspects of these partnerships. Given a transiently aposymbiotic phase during the early stages of insect development, alongside the ability of the microbe to survive outside of the host's body for part of its lifetime—two conditions universally shared across the aforementioned systems—it is in many cases experimentally feasible to physically separate both partners by disrupting the transmission cycle (e.g. [31,39,40,43,59]). Such experiments have been successfully employed to elucidate symbiont contributions towards host fitness, to assess host–symbiont specificity, and to detail the effects of symbiont replacement on host ecology (e.g. [66,67]).
Furthermore, the extracellular nature of the symbionts contributes to the likelihood that they can be cultured and genetically manipulated, which is not possible for most intracellular symbionts. In vitro cultivation and manipulation can facilitate introduction of genetically modified symbionts into their insect hosts. Thereby, the importance of candidate symbiont genes for establishment or maintenance of a mutualistic association, as well as for the fitness benefits conferred to the host, can be directly assessed [54,55]. Additionally, this strategy may prove valuable to manage agricultural pest species or disease vectors by modification of their symbionts [25]. Combining symbiont manipulation with targeted knock-down of host genes potentially involved in mediating symbiosis will undoubtedly provide unprecedented opportunities to study host–symbiont molecular interactions and investigate the genomic and physiological underpinnings of these associations.
Supplementary Material
Acknowledgements
We thank Aileen Berasategui, Christian Kost and Peter Biedermann for critical comments on the manuscript. Additionally, we would like to thank Takema Fukatsu, Takahiro Hosokawa, Nahomi Kaiwa, Ayelet Caspi-Fluger and Gudrun Herzner for sharing pictures; James van Leuven and John McCutcheon for compiling the list of bacterial genomes used in figure 3, and Yoshitomo Kikuchi for providing host sequences to complete figure 4. Owing to space restrictions, we apologize to our colleagues for not being able to include all possible references.
Author contributions
H.S. and M.K. conceived of the study, and all authors contributed to drafting the manuscript.
Funding statement
We gratefully acknowledge financial support from the Max Planck Society (to H.S., L.F. and M.K.), the German Science Foundation (DFG KA2846/2–1 to M.K.) and the National Science Foundation (IOS-1149829 to N.G.).
Conflict of interest
The authors declare they have no competing interests.
References
- 1.McFall-Ngai M, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. New York, NY: Interscience. [Google Scholar]
- 3.Ebert D. 2013. The epidemiology and evolution of symbionts with mixed-mode transmission. Annu. Rev. Ecol. Evol. Syst. 44, 623–643. ( 10.1146/annurev-ecolsys-032513-100555) [DOI] [Google Scholar]
- 4.Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59, 155–189. ( 10.1146/annurev.micro.59.030804.121041) [DOI] [PubMed] [Google Scholar]
- 5.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Ann. Rev. Genet. 42, 165–190. ( 10.1146/annurev.genet.41.110306.130119) [DOI] [PubMed] [Google Scholar]
- 6.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407, 81–86. ( 10.1038/35024074) [DOI] [PubMed] [Google Scholar]
- 7.McCutcheon JP, Moran NA. 2007. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc. Natl Acad. Sci. USA 104, 19 392–19 397. ( 10.1073/pnas.0708855104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran NA, Wernegreen JJ. 2000. Lifestyle evolution in symbiotic bacteria: insights from genomics. Trends Ecol. Evol. 15, 321–326. ( 10.1016/S0169-5347(00)01902-9) [DOI] [PubMed] [Google Scholar]
- 9.Feldhaar H, Straka J, Krischke M, Berthold K, Stoll S, Mueller MJ, et al. 2007. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 5, 48 ( 10.1186/1741-7007-5-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga R, Meng XY, Tsuchida T, Fukatsu T. 2012. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte–embryo interface . Proc. Natl Acad. Sci. USA 109, E1230–E1237. ( 10.1073/pnas.1119212109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. 2008. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J. Insect. Physiol. 54, 1236–1242. ( 10.1016/j.jinsphys.2008.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K, Yukuhiro F, Matsuura Y, Fukatsu T, Noda H. 2014. Intrasperm vertical symbiont transmission. Proc. Natl Acad. Sci. USA 111, 7433–7437. ( 10.1073/pnas.1402476111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspi-Fluger A, et al. 2012. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. R. Soc. B 279, 1791–1796. ( 10.1098/rspb.2011.2095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaltenpoth M, Winter SA, Kleinhammer A. 2009. Localization and transmission route of Coriobacterium glomerans, the endosymbiont of pyrrhocorid bugs. FEMS Microbiol. Ecol. 69, 373–383. ( 10.1111/j.1574-6941.2009.00722.x) [DOI] [PubMed] [Google Scholar]
- 15.Kaltenpoth M, Gottler W, Herzner G, Strohm E. 2005. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 15, 475–479. ( 10.1016/j.cub.2004.12.084) [DOI] [PubMed] [Google Scholar]
- 16.Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311, 81–83. ( 10.1126/science.1119744) [DOI] [PubMed] [Google Scholar]
- 17.Dubilier N, Bergin C, Lott C. 2008. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat. Rev. Microbiol. 6, 725–740. ( 10.1038/nrmicro1992) [DOI] [PubMed] [Google Scholar]
- 18.Nyholm S, McFall-Ngai M. 2004. The winnowing: establishing the squid-Vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642. ( 10.1038/nrmicro957) [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi Y, Hosokawa T, Fukatsu T. 2007. Insect–microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 73, 4308–4316. ( 10.1128/AEM.00067-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikuchi Y, Hosokawa T, Fukatsu T. 2011. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 5, 446–460. ( 10.1038/ismej.2010.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond J, Siefert JL, Staples CR, Blankenship RE. 2004. The natural history of nitrogen fixation. Mol. Biol. Evol. 21, 541–554. ( 10.1093/molbev/msh047) [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb Y, et al. 2006. Identification and localization of a Rickettsia sp in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 72, 3646–3652. ( 10.1128/AEM.72.5.3646-3652.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinert LA, Welch JJ, Jiggins FM. 2009. Conjugation genes are common throughout the genus Rickettsia and are transmitted horizontally. Proc. R. Soc. B 276, 3619–3627. ( 10.1098/rspb.2009.0875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourtzis K, Miller T. 2006. Insect symbiosis 2. Boca Raton, FL: CRC Press. [Google Scholar]
- 25.Beard CB, Cordon-Rosales C, Durvasula RV. 2002. Bacterial symbionts of the triatominae and their potential use in control of Chagas disease transmission. Annu. Rev. Entomol. 47, 123–141. ( 10.1146/annurev.ento.47.091201.145144) [DOI] [PubMed] [Google Scholar]
- 26.Durvasula RV, et al. 1997. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc. Natl Acad. Sci. USA 94, 3274–3278. ( 10.1073/pnas.94.7.3274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson EO. 1971. Social insects. Science 172, 406 ( 10.1126/science.172.3981.406) [DOI] [PubMed] [Google Scholar]
- 28.Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA 96, 7998–8002. ( 10.1073/pnas.96.14.7998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh SE, Poulsen M, Pinto-Tomás A, Currie CR. 2014. Interaction between workers during a short time window is required for bacterial symbiont transmission in Acromyrmex leaf-cutting ants. PLoS ONE 9, e103269 ( 10.1371/journal.pone.0103269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hongoh Y. 2010. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci. Biotechnol. Biochem. 74, 1145–1151. ( 10.1271/bbb.100094) [DOI] [PubMed] [Google Scholar]
- 31.Koch H, Schmid-Hempel P. 2011. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl Acad. Sci. USA 108, 19 288–19 292. ( 10.1073/pnas.1110474108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell JE, Martinson VG, Urban-Mead K, Moran NA. 2014. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 80, 7378–7387. ( 10.1128/AEM.01861-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardo MP. 2008. Access to mutualistic endosymbiotic microbes: an underappreciated benefit of group living. Behav. Ecol. Sociobiol. 62, 479–497. ( 10.1007/s00265-007-0428-9) [DOI] [Google Scholar]
- 34.Cox-Foster DL, et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318, 283–287. ( 10.1126/science.1146498) [DOI] [PubMed] [Google Scholar]
- 35.Martinson VG, Danforth BN, Minckley RL, Rueppell O, Tingek S, Moran NA. 2011. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 20, 619–628. ( 10.1111/j.1365-294X.2010.04959.x) [DOI] [PubMed] [Google Scholar]
- 36.Vasquez A, et al. 2012. Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS ONE 7, e33188 ( 10.1371/journal.pone.0033188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen SB, Hansen LH, Sapountzis P, Sorensen SJ, Boomsma JJ. 2013. Specificity and stability of the Acromyrmex–Pseudonocardia symbiosis. Mol. Ecol. 22, 4307–4321. ( 10.1111/mec.12380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulsen M, Cafaro M, Boomsma JJ, Currie CR. 2005. Specificity of the mutualistic association between actinomycete bacteria and two sympatric species of Acromyrmex leaf-cutting ants. Mol. Ecol. 14, 3597–3604. ( 10.1111/j.1365-294X.2005.02695.x) [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi Y, Hosokawa T, Nikoh N, Meng XY, Kamagata Y, Fukatsu T. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7, 2 ( 10.1186/1741-7007-7-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salem H, Kreutzer E, Sudakaran S, Kaltenpoth M. 2013. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae). Environ. Microbiol. 15, 1956–1968. ( 10.1111/1462-2920.12001) [DOI] [PubMed] [Google Scholar]
- 41.Kroiss J, et al. 2010. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 6, 261–263. ( 10.1038/nchembio.331) [DOI] [PubMed] [Google Scholar]
- 42.Fukatsu T, Hosokawa T. 2002. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl. Environ. Microbiol. 68, 389–396. ( 10.1128/AEM.68.1.389-396.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4, e337 ( 10.1371/journal.pbio.0040337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiwa N, et al. 2014. Symbiont-supplemented maternal investment underpinning host's ecological adaptation. Curr. Biol. 24, 2465–2470. ( 10.1016/j.cub.2014.08.065) [DOI] [PubMed] [Google Scholar]
- 45.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2008. Symbiont acquisition alters behaviour of stinkbug nymphs. Biol. Lett. 4, 45–48. ( 10.1098/rsbl.2007.0510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szathmary E, Smith JM. 1995. The major evolutionary transitions. Nature 374, 227–232. ( 10.1038/374227a0) [DOI] [PubMed] [Google Scholar]
- 47.Doolittle WF. 1998. You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends Genet. 14, 307–311. ( 10.1016/S0168-9525(98)01494-2) [DOI] [PubMed] [Google Scholar]
- 48.Morris BE, et al. 2013. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37, 384–406. ( 10.1111/1574-6976.12019) [DOI] [PubMed] [Google Scholar]
- 49.Ewald PW. 1987. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. NY Acad. Sci. 503, 295–306. ( 10.1111/j.1749-6632.1987.tb40616.x) [DOI] [PubMed] [Google Scholar]
- 50.Sachs JL, Skophammer RG, Regus JU. 2011. Evolutionary transitions in bacterial symbiosis. Proc. Natl Acad. Sci. USA 108, 10 800–10 807. ( 10.1073/pnas.1100304108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Fukatsu T. 2012. Symbiont-mediated insecticide resistance. Proc. Natl Acad. Sci. USA 109, 8618–8622. ( 10.1073/pnas.1200231109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kikuchi Y, Yumoto I. 2013. Efficient colonization of the bean bug Riptortus pedestris by an environmentally transmitted Burkholderia symbiont. Appl. Environ. Microbiol. 79, 2088–2091. ( 10.1128/AEM.03299-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikuchi Y, Hosokawa T, Fukatsu T. 2011. Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl. Environ. Microbiol. 77, 4075–4081. ( 10.1128/AEM.00358-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JK, et al. 2013. Bacterial cell wall synthesis gene uppP is required for Burkholderia colonization of the stinkbug gut. Appl. Environ. Microbiol. 79, 4879–4886. ( 10.1128/AEM.01269-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JK, et al. 2013. Polyester synthesis genes associated with stress resistance are involved in an insect–bacterium symbiosis. Proc. Natl Acad. Sci. USA 110, E2381–E2389. ( 10.1073/pnas.1303228110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Futahashi R, et al. 2013. Gene expression in gut symbiotic organ of stinkbug affected by extracellular bacterial symbiont. PLoS ONE 8, e64557 ( 10.1371/journal.pone.0064557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia JR, et al. 2014. Partner associations across sympatric broad-headed bug species and their environmentally acquired bacterial symbionts. Mol. Ecol. 23, 1333–1347. ( 10.1111/mec.12655) [DOI] [PubMed] [Google Scholar]
- 58.Kost C, Lakatos T, Bottcher I, Arendholz WR, Redenbach M, Wirth R. 2007. Non-specific association between filamentous bacteria and fungus-growing ants. Naturwissenschaften 94, 821–828. ( 10.1007/s00114-007-0262-y) [DOI] [PubMed] [Google Scholar]
- 59.Prado SS, Rubinoff D, Almeida RP. 2006. Vertical transmission of a pentatomid caeca-associated symbiont. Ann. Entomol. Soc. Am. 99, 577–585. ( 10.1603/0013-8746(2006)99[577:VTOAPC]2.0.CO;2) [DOI] [Google Scholar]
- 60.Hosokawa T, Kikuchi Y, Fukatsu T. 2007. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect–bacterium mutualism? Mol. Ecol. 16, 5316–5325. ( 10.1111/j.1365-294X.2007.03592.x) [DOI] [PubMed] [Google Scholar]
- 61.Frank SA. 1996. Host–symbiont conflict over the mixing of symbiotic lineages. Proc. R. Soc. Lond. B 263, 339–344. ( 10.1098/rspb.1996.0052) [DOI] [PubMed] [Google Scholar]
- 62.Nikoh N, Hosokawa T, Oshima K, Hattori M, Fukatsu T. 2011. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 3, 702–714. ( 10.1093/gbe/evr064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo CH, Moran NA, Ochman H. 2009. The consequences of genetic drift for bacterial genome complexity. Genome Res. 19, 1450–1454. ( 10.1101/gr.091785.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sudakaran S, Retz F, Kikuchi Y, Kost C, Kaltenpoth K. Submitted Evolutionary transition in symbiotic syndromes enables adaptive radiation of phytophagous insects on an imbalanced diet. ISME J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prado SS, Almeida RPP. 2009. Phylogenetic placement of pentatomid stink bug gut symbionts. Curr. Microbiol. 58, 64–69. ( 10.1007/s00284-008-9267-9) [DOI] [PubMed] [Google Scholar]
- 66.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc. R. Soc. B 274, 1979–1984. ( 10.1098/rspb.2007.0620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salem H, Bauer E, Strauss AS, Vogel H, Marz M, Kaltenpoth M. 2014. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. R. Soc. B 281, 20141838 ( 10.1098/rspb.2014.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.