Abstract
The Drosophila pheromone cis-11-octadecenyl acetate (cVA) is used as pheromone throughout the melanogaster group and fulfils a primary role in sexual and social behaviours. Here, we found that Drosophila suzukii, an invasive pest that oviposits in undamaged ripe fruit, does not produce cVA. In fact, its production site, the ejaculatory bulb, is atrophied. Despite loss of cVA production, its receptor, Or67d, and cognate sensillum, T1, which are essential in cVA-mediated behaviours, were fully functional. However, T1 expression was dramatically reduced in D. suzukii, and the corresponding antennal lobe glomerulus, DA1, minute. Behavioural responses to cVA depend on the input balance of Or67d neurons (driving cVA-mediated behaviours) and Or65a neurons (inhibiting cVA-mediated behaviours). Accordingly, the shifted input balance in D. suzukii has reversed cVA's role in sexual behaviour: perfuming D. suzukii males with Drosophila melanogaster equivalents of cVA strongly reduced mating rates. cVA has thus evolved from a generic sex pheromone to a heterospecific signal that disrupts mating in D. suzukii, a saltational shift, mediated through offsetting the input balance that is highly conserved in congeneric species. This study underlines that dramatic changes in a species' sensory preference can result from rather ‘simple’ numerical shifts in underlying neural circuits.
Keywords: Drosophila suzukii, pheromone, evolution, ejaculatory bulb, fruitless, sensory drive
1. Introduction
Melanogaster-clade species generically use male-produced cis-11-octadecenyl acetate (cVA) as sex pheromone [1]. The underlying cVA circuitry is arguably the best-studied pheromone communication system, from production to detection, and from processing to resulting muscle output [2–4]. Different from ‘typical’ sex pheromones in insects, cVA acts at short range and not only fulfils a role in orientation and sexual attraction, but also most prominently in a variety of sexual and social behaviours in Drosophila melanogaster: it increases male mate acceptance by females [5,6], reduces attractiveness of newly mated females, reduces male–male courtship, while increasing aggression between males [7,8]. cVA is a sex pheromone found throughout and basal to the melanogaster group species, such as in the obscura and immigrans group species [1], underlining its primal role in Drosophila.
The fact that all melanogaster group species studied thus far use cVA as volatile sex pheromone [1] would preclude a role of cVA in species recognition, a function that is otherwise typical for insect sex pheromones [9]. Moths in particular are well known for their species-specific pheromone blends, which are used to discriminate between conspecific and heterospecifics [10]. Whereas males are finely tuned to their conspecific female-produced pheromone, they often also display acute sensitivity to pheromones of other, often sympatric, species. In the latter case, such pheromones elicit responses on sensory neurons that mediate behavioural antagonism, and typically disrupt orientation to conspecifics [11].
Perhaps owing to the complex role that cVA fulfils in a range of sexual and social behaviours in Drosophila, evolution of the signal to convey species-specificity may be constrained. Besides, other, non-volatile Drosophila pheromones fulfil a function in species recognition instead [12]. These are non-volatile pheromones, produced by enocytes and embedded in the cuticular hydrocarbons (CHs), and sensed through the taste sensilla on the legs and proboscis [13–15].
However, in spite of the evolutionary constraints cVA may have, here we report how this conserved pheromone has undergone a radical functional reversal in Drosophila suzukii, a melanogaster group species. Drosophila suzukii does not produce cVA. Here, we describe the changes that are at the basis of the inversion of the role of cVA from a broadly used sex pheromone into a behavioural antagonist in this species.
2. Material and methods
(a). Flies
A Drosophila suzukii colony was established in 2010 from around 1000 individuals emerging from infested blueberries and raspberries collected in Valsugana, Trentino Province, Italy. The US strain of D. suzukii was derived from a colony established by D. Walsh (Washington State University, Prosser, WA, USA). Drosophila biarmipes (14023-0361.02) and Drosophila subpulchrella (14023-0401.01) were obtained from the San Diego stock centre. For D. melanogaster, we used the wild-type Dalby strain [16,17]. Flies were reared in a quarantine facility on a standard cornmeal–yeast–agar medium at 21°C under 12 L : 12 D conditions.
(b). Behavioural assays
Sexes of D. melanogaster and D. suzukii (Trentino strain) were separated within 1 h post emergence and placed in groups of five (females) and seven (males). They were placed in standard food vials and flipped after three days to new food vials. After four days, seven males were placed into the vial containing five conspecific females. We used groups of individuals to increase the mating incidence of D. suzukii, which had a lower mating rate compared with D. melanogaster. The higher ratio of males to females was there to offset any potentially negative effect of the perfuming of the flies (see §2c, below) on the ‘availability’ of male mates, although no clear negative effects of the experimental procedures were observed. No individuals died during the mating experiments. As individuals could not be reliably recognized and followed during the course of the experiments, we scored mating rates only. Matings were noted every 15 min. We observed the mating behaviour for both species during five consecutive hours. A total of 21 and 30 groups of 12 flies (five females, seven cVA-perfumed males) were perfumed D. melanogaster and D. suzukii, respectively, against a total of 19 and 26 control groups, respectively. Flies only mated once during our assay.
(c). Perfuming flies
Three millilitre vials were coated on the inside with a total of 50 μg cVA. The procedures were roughly similar to those described in Billeter et al. [13]. Fifty microlitres of hexane with or without 1 μg μl−1 of cVA were pipetted into a 3-ml glass screw cap vial. The hexane was slowly evaporated while the vial was placed in a horizontal position and slowly rotated. Seven males were briefly immobilized on ice (1 min) and placed in a 3-ml treatment or control vial. Vials, coated with cVA or hexane-treated only and containing seven flies, were placed on a rotator and rotated at 4500 rotations min−1 for 2 min, in a cycle of 6 s on, 4 s off. In D. melanogaster, cVA deposits are mostly found on the abdominal segments. However, perfuming the fly with cVA results in more equal distribution of pheromone across the body. We therefore increased the total amount of cVA slightly to compensate for lower local concentrations on the fly. After shaking, the group of seven treatment (+cVA) or seven control (−cVA) male flies, both shaken on the rotator, were introduced into the food vial containing five virgin females.
(d). Chemical analysis
Pheromones were extracted from the fly cuticle (n = 5, 6 for male and female D. suzukii, respectively) by leaving individual flies in 100 μl of hexane for 5 min at room temperature. One hundred nanograms of heptadecenyl acetate were added as an internal standard. These extracts were analysed on a gas chromatograph coupled with a mass spectrometer (GC-MS; 6890 GC and 5975 MS, Agilent technologies Inc., Santa Clara, CA, USA). Extracts were concentrated to ca 10 μl, and 2 μl were injected into a HP-5MS silica capillary column (30 m × 0.25 mm × 0.25 μm film thickness; Agilent technologies Inc.) that was temperature-programmed from 50°C (2 min), 8°C min−1 to 300°C (5 min).
Compounds of interest were identified based on their mass spectrum, retention time, comparison with previously published works on D. melanogaster CHs [18] and injection of synthetic corresponding compound (for cVA). They were quantified by peak integration and comparison with the response of the internal standard. CH extracts of both the Trentino and US strains of D. suzukii were analysed (indicated in §3).
(e). Sensory physiology
Using a strong airflow, flies were pushed head-first into a truncated pipette tip. The pipette tip was cut distally from the head and the fly was gently pushed forward until the head protruded from the narrow end. The pipette tip was placed on a wax surface on a microscope slide, and using a glass micropipette the right antenna was gently bent backwards and stably positioned on a coverslip. The fly was placed under a microscope (Olympus BX51W1), with a magnification less than or equal to 1500×. Via a glass tube, a 1 l min−1 charcoal-purified and humidified airflow was constantly blown over the fly head. Tungsten microelectrodes, sharpened in a KNO2-solution, were used for recording of action potentials of antennal sensory neurons. A motor-controlled micromanipulator (Märzhauser DC-3K, Wetzlar, Germany) equipped with a piezo unit (Märzhauser PM-10) was used for fine positioning. A reference electrode was inserted into the eye with a manually controlled micromanipulator (Narishige MM33, Tokyo, Japan). Touch stimulation was performed using an additional piezo unit to move the electrode at micrometre scale towards the sensillum. A glass electrode drawn to a sharp tip was dipped briefly in a 1 μg μl−1 cVA solution, air dried and used for stimulation. After A/D conversion (Syntech IDAC PCI card), spikes were visualized and stored on a PC. Analysis was done using AutoSpike v. 3.2 software (Syntech, Kirchzarten, Germany). For our sensory physiological analysis of D. suzukii, we used the Trentino strain. For each species, six individual replicates were used.
(f). Counts of sensilla trichodea
Antenna of WT Dalby (n = 8) and D. suzukii (n = 9, Trentino strain) were mounted using spacer rings (Secure-Seal TM imaging spacers, Sigma-Aldrich) and Vectashield mounting medium Hard Mount (Vector Laboratories, Burlingame, CA, USA). High-resolution confocal scans (Zeiss LSM 510 confocal microscope, Carl Zeiss, Jena, Germany), using a 20× objective, were made through the antennae. The contrast of the relatively thick-walled trichoid sensilla permitted identification and counting of trichoid sensilla on the entire antennal surface.
(g). Ejaculatory bulb
Drosophila suzukii (Trentino strain) and D. melanogaster (WT Dalby) adult males were collected two days after emergence. For each species, a total of 10 males were selected. After brief anaesthetization in the freezer, individuals were dissected in phosphate-buffered saline (PBS). The abdomen was clipped and immersed in 5% KOH for 5 h to remove soft tissues and expose intact hard cuticular structures, washed in distilled water and partly dehydrated in 70% ethanol. Afterwards, the ejaculatory bulb (EB [19,20]) was separated from the rest of the male genitalia and mounted on a glass slide with glycerine. Observations were made with microscope (Leica LMD7000, Wetzlar, Germany). The EB dimensions were measured using a Leica LMD7000 microscope with Leica Application Suite Image Analysis Software (n = 10). As individuals within and among species differed in size and in order to obtain values comparable from one animal to the others, the estimates of EB dimensions of D. melanogaster were compensated for by the smaller body length compared to D. suzukii (multiplication of the dimensions by the ratio (average D. suzukii length/average D. melanogaster body length)). Volumes were calculated by assuming a sphere (4/3 × π × {w/2} × {h/2} × {d/2}). Measurements from 10 individuals were averaged.
(h). Immunocytochemistry
We verified antennal lobe projection patterns of T1 neurons in D. suzukii (Trentino strain and US strain, as indicated in §3) using anterograde-neurobiotin (Molecular Probes, Carlsbad, CA, USA) backfills. Neurobiotin is readily taken up by neurons and transported throughout the neuron, including its axonal targets in the antennal lobes. A glass microelectrode with a 0.25 M KCl + 2% neurobiotin was placed over the tip of a T1 neuron. Neurobiotin was allowed to diffuse into the sensillum and taken up by the neuron for 1 h. Preparations were then fixed in 0.1 M PBS with 0.25% Triton-X + 4% formaldehyde for 3.5 h at 4°C, dissected, washed 3× with PBS containing 0.25% Triton-X (PBST) and incubated with fluorescein-avidin 488. Ten per cent mouse α-synapsin antibody (Hybridoma, University of Iowa, Iowa, IA, USA) was included to identify targeted glomeruli in the antennal lobes. After 24 h at room temperature on the rotator, brains were washed 3× with PBST and incubated with anti-mouse conjugated with Alexafluor 546 (Molecular Probes). After another 24 h on the rotator at room temperature, brains were washed 3× with PBST and mounted in Vectashield Hard Mount (Vector Laboratories), 0.12 mm thick, using spacer rings (Secure-Seal TM imaging spacers, Sigma-Aldrich). The above-described technique for mouse α-synapsin antibody staining was also used for overview stainings and reconstructions of antennal lobes of D. suzukii, D. biarmipes and D. subpulchrella.
(i). Confocal microscopy and reconstructions
Whole-mount brains were viewed in a Zeiss LSM 510 confocal microscope (Carl Zeiss) equipped with a 40×, 1.4 oil-immersion DIC objective lens. Structures labelled with Fluorescein Avidin were excited with an Argon laser at 488 nm with detection of reflected light in the range of 505–515 nm. Alexa 546-labelled structures were excited with a HeNe laser at 543 nm and detected using a 560 nm long pass filter. Stacks of 50–200 confocal images were scanned and the images were stored at a size of 1024 × 1024 pixels. The three-dimensional reconstructions, volumetric measurements of the glomeruli were done using AMIRA v. 3.0 software (Visage Imaging, Berlin, Germany). In every optical section, the contours of glomeruli were demarcated by hand (i.e. image segmentation) and interpolated. Volumes were obtained from Amira, based on reconstructed images.
(j). Bioinformatics and phylogenetics
Orthologues searches and assembly. We downloaded various types of genome data (gene, transcript and protein sequences) for odorant receptors and other cVA-related genes (desaturases, elongases, Fruitless, Transformer) of D. melanogaster, Drosophila simulans, Drosophila sechellia, Drosophila erecta and D. ananassae from Flybase [21] and OrthoDB [22] repositories. For D. biarmipes and D. suzukii, the protein sequences of D. melanogaster were used for queries to identify the corresponding orthologues through exhaustive blast searches. First, TBlastN (BLOSUM 62 matrix with an e-value threshold of 10−5 [23]) was applied for preliminary search of all the gene families. For the elongases superfamily alone, orthologues were searched using HMMER (http://hmmer.janelia.org/ v3.1b1, using an e-value threshold of 10−5) and the following specifications: profile ‘hmmbuild’ was constructed from GNS1/SUR4 (ELO;PF01151) HMM profile of the PFAM database [24], the result of which was run against D. suzukii and D. biarmipes genomes using ‘hmmsearch’; the output was manually checked for all the positive hits, specifically looking for the presence of elo-specific domain HXHH and hydrophobic transmembrane regions. For fruitless, we underwent a complex manual curation directly from D. suzukii genome contigs, using the 11 main isoforms of D. melanogaster to construct putative exons in D. suzukii. This is because the fru gene spans over a long genome region, contains long introns and codes for various different isoforms, which are unlikely to be present in transcriptomic data or being correctly annotated in gene models.
(k). Phylogenetics
For each of the gene families (Desaturases, Elongases, Fruitless and Odorant receptors), we constructed nucleotide datasets and checked for the correct reading frame. The translated protein datasets were aligned using MUSCLE v. 3.8.31 [25], and preliminary tree searches were done to assess the actual orthology of genes. These preliminary analyses led to recognition of a few wrong orthologies (false positives owing to gene loss) and characterization of new orthologues in unannotated genomes. After this manual curation, a second round of MUSCLE alignment using option—refine was done followed by phylogenetic reconstruction using PHYML [26] and employing 100 non-parametric bootstrap replicates and the LG + G model [27] of replacement.
(l). Statistics
Behavioural data (mating rate) were analysed using a Kaplan–Meier survival probability estimator and curves were statistically compared using a log-rank test. Correlation between cVA depletion after application (measured through GC-MS analysis, see above) and the relative mating rate in D. suzukii were analysed with a Pearson product–moment correlation coefficient, with the relative mating rate of cVA-perfumed D. suzukii being the fraction of the mating rate of cVA perfumed over control flies. Anatomical data (EB and glomerular volumes) were analysed using independent two-sample t-tests. Sensory physiological data (spiking rate) were analysed with a one-way ANOVA, followed by Tukey's HSD post hoc test.
3. Results and discussion
(a). cVA production is lost in Drosophila suzukii, and its production site atrophied
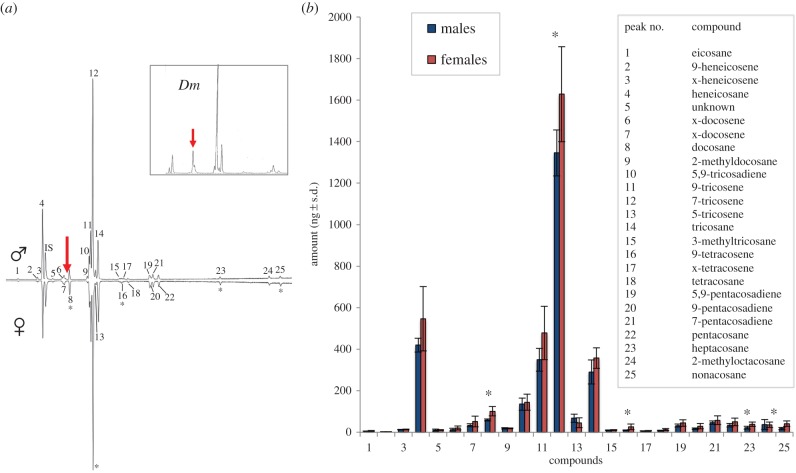
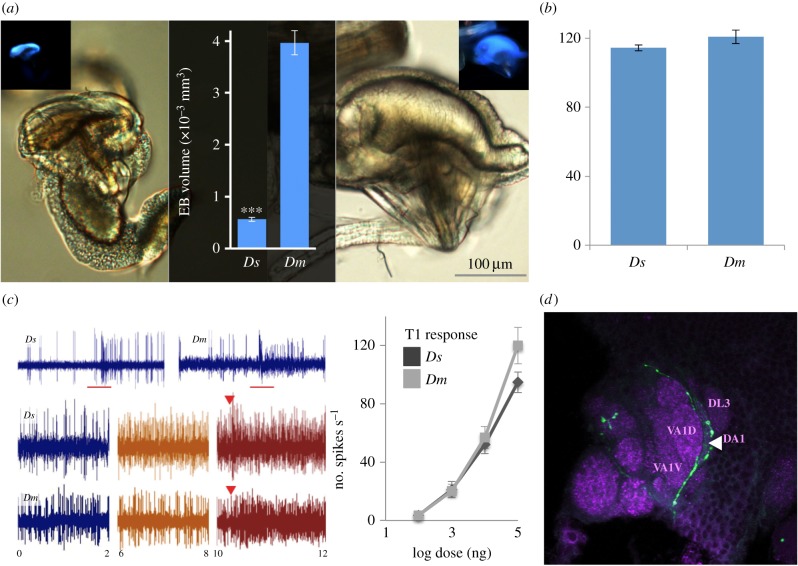
Using gas chromatography-coupled mass spectrometry (GC-MS), we analysed the CH profile of D. suzukii. Unlike the CH profile of D. melanogaster, D. suzukii's CH profile was isomorphic, with just small quantitative differences between sexes (figure 1a,b). This was also true for its sibling species D. biarmipes and D. subpulchrella (electronic supplementary material, figure S1c,d). We note that D. suzukii and D. biarmipes lacked the desaturase desat2 and the elongases eloF, which have been implied in the biosynthesis of species-specific and sexually dimorphic CH profiles in Drosophila ([28,29]; electronic supplementary material, figure S2). However, more striking was the lack of cVA in the chemical profile of D. suzukii (figure 1a, arrow, verified with D. suzukii from the USA: electronic supplementary material, figure S1d). The lack of cVA in D. suzukii casts the question of whether this pheromone might have been replaced by another compound similar to cVA. However, no other cVA-type compounds (including the C20 variant present in some species [1]) were found in D. suzukii cuticular extractions (figure 1a,b and electronic supplementary material, figure S1d). In fact, the EB, the production site of cVA [30], was dramatically reduced in volume in D. suzukii compared with that of D. melanogaster (by a factor of 7.0, p < 0.001, unpaired t-test, n = 10, d.f. = 18, t = 10.1, d = 3.22, figure 2a). Intact, conserved homologues of corresponding genes for cVA production, elo68 and desat1, were nevertheless present in the genome of D. suzukii (table 1 and electronic supplementary material, figure S2), as were miRNA-124 binding sites upstream of transformer, a factor involved in cVA production in male D. melanogaster (electronic supplementary material, figure S3e).
Figure 1.
(a) Chromatograms showing female and male D. suzukii CHs. Arrow indicates retention time where cVA would elute. Inset: chromatogram of D. melanogaster with the arrow indicating cVA (see also electronic supplementary material, figure S1). Note the dominance of tricosenes in both sexes. Tricosenes are more abundant in male D. melanogaster's CH profile. IS internal standard (heptadecenyl acetate, 17:OAc). Asterisks (*) indicate significantly higher amount in females (p < 0.05). (b) Comparison of the CH profile of male and female D. suzukii (n = 6 and n = 5, respectively). Stars indicate significant differences between males and females (Mann–Whitney test, α < 0.05). x = unknown double bond position. (Online version in colour.)
Figure 2.
(a) Micrographs of the EB, lateral view, light and autofluorescence microscopy (insets). Overlay: volumetric estimates of the EB of D. suzukii (Ds) and D. melanogaster (Dm) (n = 10 for each species, independent t-test p < 0.001). ***, p < 0.001. (b) Number of sensilla trichoidea and sensilla intermedia in D. suzukii and D. melanogaster females. (c) Top: sample trace of T1 sensillum responses to 0.5 s 1 μg cVA stimulation. Bottom: sample traces of T4 sensilla in D. suzukii and D. melanogaster to cVA, using the ‘touch’ stimulation [24], with the time (s) indicated at the bottom of the traces. Blue: before stimulation, orange: just prior to contact, red: contact. Side panel: dose response curves of T1 sensilla in D. suzukii and D. melanogaster to 0.5 s cVA stimulation (red bar, n = 6 per species). (d) Neurobiotin backfill of T1 neuron (a spurious fill of a neighbouring AB7 neuron toVM5v is also visible). Letters indicate various anterior trichoid glomeruli. Arrowhead indicates DA1.
Table 1.
Olfactory receptor, desaturase and elongase genes are conserved and under purifying selection. Values are Ka/Ks (dN/dS) with the actual value of the ratio (omega) in brackets. Values have been calculated using http://services.cbu.uib.no/tools/kaks under a maximum-likelihood framework providing input trees according to trees from figure 3 and electronic supplementary material, figures S2 and S3. In all cases, D. suzukii has an omega substantially lower than 1, suggesting purifying selection and therefore conserved function. Asterisk (*) denotes values obtained using topology: (((mel1,(ere1,ere2)),(bia,(suz1,suz2))),ana); similar results were obtained when using an alternative topology: ((((mel1,ere1),(bia,suz1)),ana),(suz2,ere2)); compare with tree A of electronic supplementary material, figure S2.
| DesatF | Desat1 | Or65a | Or67d | Elo68a | EloC2* | Elop | |
|---|---|---|---|---|---|---|---|
| D. suzukii | 0.013/0.288 (0.044) |
0.008/0.124 (0.062) |
0.033/0.140 (0.234) |
0.024/0.181 (0.135) |
0.016/0.114 (0.1447) |
0.034/0.121 (0.280) |
0.045/0.142 (0.317) |
| D. biarmipes | 0.014/0.115 (0.124) |
0.005/0.07 (0.074) |
0.037/0.166 (0.225) |
0.021/0.156 (0.136) |
0.026/0.113 (0.233) |
not present | 0.043/0.075 (0.575) |
(b). The relative volume of glomeruli tuned to cVA is reversed in Drosophila suzukii
We then determined how loss of cVA might have influenced its corresponding olfactory circuitry. In D. melanogaster, the trichoid sensillum T1 and its cognate Or67d-expressing neuron [5] are key in cVA-mediated behaviours. T1s project to a large glomerulus, DA1 [31,32]. T1s are the most abundant sensillum type in D. melanogaster [33]. Although T1s were fully functional (figure 1c), and its receptor, Or67d, highly conserved (table 1 and figure 3), T1 sensilla were rare in D. suzukii antenna: we identified only around 7–10 T1s per individual, compared to 55–60 found in D. melanogaster [33]. Accordingly, the relative volume of DA1 was minute in both sexes of D. suzukii when compared with D. melanogaster (p < 0.0001, two-tailed independent t-test, t = 10.5, n = 8, d.f. = 6, d = 3.7, figure 4 and electronic supplementary material, figure S4).
Figure 3.
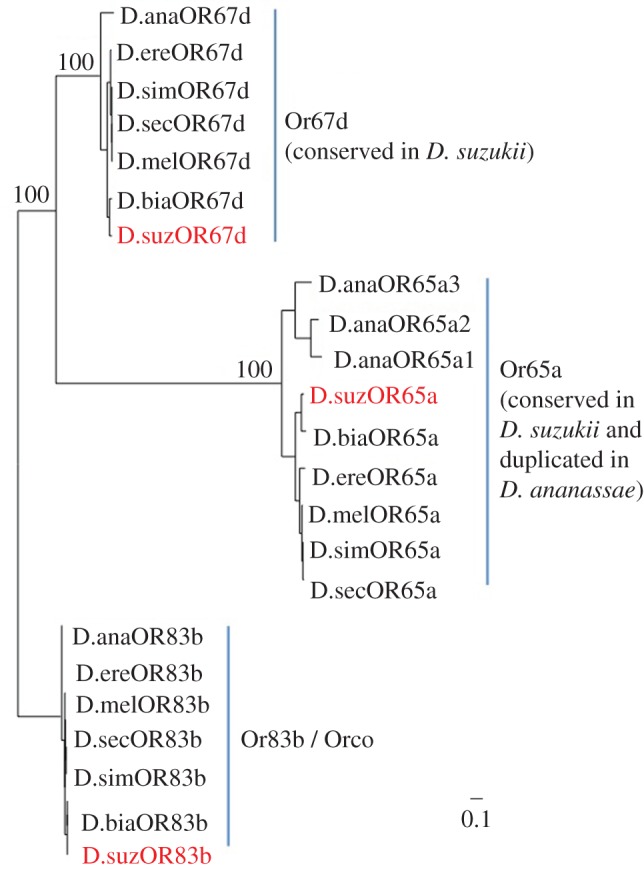
cVA odorant receptors are conserved in D. suzukii. Phylogenetic tree of Or67d and Or65a in D. suzukii and other Drosophila. These genes are extremely conserved in D. suzukii and the other species sampled. This suggests that these genes are indeed expressed in neurons of T1 and T4 sensilla and are structurally constrained to recognize cVA, as indicated by the electrophysiological reponses. The tree is rooted with the Or coreceptor Orco. Abbreviations and supports are as in electronic supplementary material, figure S2. (Online version in colour.)
Figure 4.
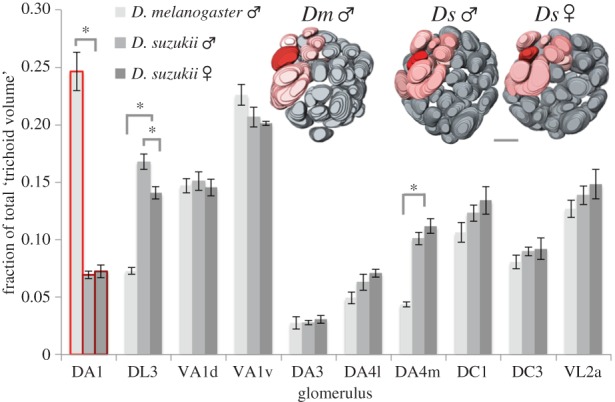
Volume of DA1 and other trichoid glomeruli, relative to the total volume of all glomeruli receiving input from sensilla trichodea and intermedia. VL2a, which receives input from coeloconic Ac4a neurons, is included as it is part of the fru circuitry. Red-outlined bars: DA1 of D. melanogaster and D. suzukii ♂ and ♀. Insets are reconstructions of the antennal lobes, with DA1 in bright red, and other glomeruli that receive input from sensilla trichodea and sensilla intermedia neurons in light red. *, p < 0.05. Scale bar, 20 μm.
At close range, cVA also induces responses in Or65a-expressing OSNs housed in antennal trichoid T4 [32,34,35]. Or65a neurons counteract behaviour induced through Or67d OSNs, and reduce cVA-mediated male–male aggression [8], as well as male attraction to recently mated females [35]. In D. suzukii, Or65a was highly conserved (table 1), and T4 sensilla were abundantly present and responded to cVA touch stimulation [34] (figure 2c). The neuron may be slightly more sensitive to cVA in D. suzukii than in D. melanogaster, responding prior to contact, unlike in D. melanogaster (less than 1 mm distance). The cognate antennal lobe glomerulus, DL3, which receives input from Or65a neurons, was enlarged in D. suzukii (two-tailed independent t-test, t = 12.9, d.f. = 6, p < 0.001, d = 4.6, figure 4) and 19% larger in male than female D. suzukii (two-tailed independent t-test, t = 3.13, d.f. = 6, p = 0.01, d = 1.1). Other glomeruli innervated by sensilla trichodea and sensilla intermedia neurons [32,33] were similar in volume between the two species (except for DA4m) and sexually isomorphic (figure 4). The total number of trichoid sensilla was similar between species (figure 2b).
(c). Glomerular volume and reversal from pheromone to antagonist
Or65a and DL3 provide context-dependent suppression of cVA responses in D. melanogaster, putatively by suppressing output of DA1 via antennal lobe inhibitory interneuronal connections [8,17]. We therefore hypothesized that the reversal of glomerular volume ratios in D. suzukii's cVA circuit (DA1/DL3 from 3.3 in D. melanogaster to 0.41 in D. suzukii, figure 4) would favour Or65a-mediated behavioural responses to cVA, generating opposite behavioural outputs to those observed in D. melanogaster. We tested this by applying cVA to male D. suzukii cuticle with doses equivalent to those found on male D. melanogaster and assaying the effect on conspecific courtship behaviour. Because mating rates are low in D. suzukii, and because applied cVA rapidly decreases over time on the cuticle (figure 5, red line), we grouped seven virgin males and five virgin females to ensure sufficiently high courtship and mating rates in the bioassay. As we predicted, application of cVA on male D. suzukii strongly suppressed mating (figure 5 insets, Kaplan–Meier estimator, Z = 4.45, p < 0.001, n = 150). By contrast, perfuming male D. melanogaster with cVA did not affect the mating rate (figure 5 insets, Kaplan–Meier, Z = 0.33, p = 0.36, n = 110, [5,6]). Following an initially nearly complete shutdown of mating in D. suzukii, when cVA doses were high, mating rates slowly increased over experimental time (figure 5, blue line, the relative mating rate of perfumed or non-perfumed flies). This was significantly correlated with a gradual loss of cVA on the cuticle (figure 5, Pearson's r = 0.93, t = 4.38, p < 0.025). The down-regulation of T1 sensilla expression, the volume decrease of DA1 and the suppression of mating in cVA-perfumed D. suzukii support a model in which Or65a and DL3 suppress Or67d-mediated behaviours similar to observations in D. melanogaster [7,8,17,35]. Apparently, in D. suzukii, the balance of DA1 and DL3 input and output has shifted through evolution, resulting in a chronic suppression of cVA-induced behaviours in this species, when compared with other melanogaster group species. This effectively has reversed the role of cVA from a pheromone to a heterospecific signal that inhibits mating.
Figure 5.
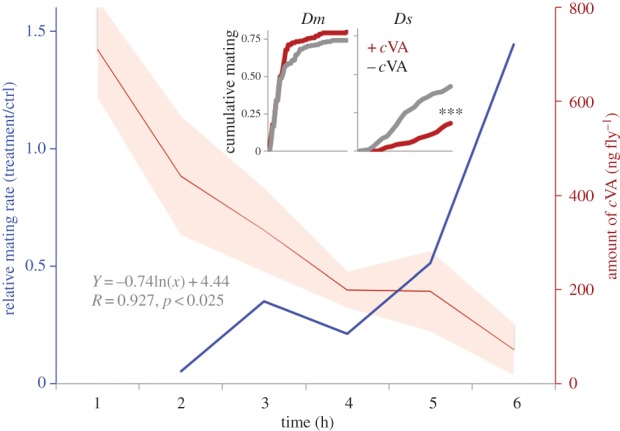
Effect of cVA perfuming on mating in D. suzukii and D. melanogaster. The relative mating rate increased (blue line, n values see below) with a decrease in cVA levels on the male flies over time (n = 5 per data point). Insets: cumulative mating in Dm and Ds in response to the perfuming with cVA (+cVA, red lines, n = 21, 30 for Dm and Ds, respectively) or hexane (control, −cVA, grey lines, n = 19, 26 for Dm and Ds, respectively). ***, p < 0.001.
The concurrent miniaturization of DA1 volume and the behavioural shift in response to cVA are reminiscent of observations in Drosophila's coding of general odour preference [36,37] and preference coding of pheromones in moths [38]. In these studies, changes in relative glomerular volume were associated with shifts in olfactory preference. Increased glomerular volume is associated with an increased preference for the ligand of these glomeruli. In this study, however, we observed the opposite: a severe reduction in glomerular volume converts attraction into inhibition, suppressing the cascade of behaviours associated with its ligand (figure 6a).
Figure 6.
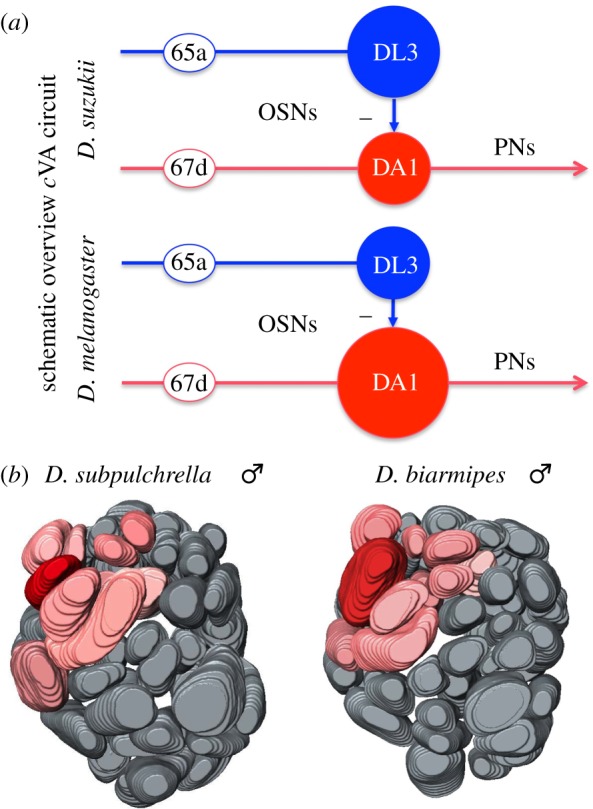
(a) Schematic overview of the change in the circuitry of D. suzukii compared to D. melanogaster. Based on earlier work [6], we conclude that reduction in volume of DA1 in D. suzukii lead to the observed chronically depresses DA1 output and the observed inhibition of sexual behaviour. OSNs, olfactory sensory neurons; PNs, antennal lobe projection neurons. (b) Reconstructions of the antennal lobes of D. subpulchrella and D. biarmipes. In bright red DA1, which received input from T1 neurons, and in light red other glomeruli receiving input from sensilla trichodea neurons. Note: the small volume of DA1 in D. subpulchrella, which was comparable to D. suzukii (see figure 4).
What factors underlie the reduced T1 expression and diminution of DA1 are unknown. An important factor in driving sexual behaviours in D. melanogaster is the transcription factor fruitless (fru). A male-specific splicing variant of fru, FruM, causes sex-specific neuronal growth, targeting and corresponding behaviour [31,39–42]. Our gene annotations show nevertheless that the fru region, spanning 100 kb of genomic DNA, contains all putative exons to build the various isoforms found in D. melanogaster, including FruM (electronic supplementary material, figure S3; [31]). Other well-known genes involved in sexual dimorphism of the olfactory circuitry, such as sexlethal (sxl [43,44]), transformer (tra [43,45]) and doublesex (dsx [33,34]), were also well conserved in D. suzukii (electronic supplementary material, figure S3). However, we noted that the volume of DA1 is sexually isomorphic in D. suzukii (figure 4). This contrasts with D. melanogaster, where Fru causes a substantial dimorphism in volume and behaviour between males and females [31,42]. Similarly, the two other glomeruli receiving input from sensory neurons in the fru circuitry, VA1v and VL2a, were also sexually isomorphic (figure 4). However, DL3 was 19% enlarged in male compared with female D. suzukii (see above), whereas DL3 is isomorphic in D. melanogaster [31]. This may indicate an altered expression of Fru in the olfactory circuitry of D. suzukii males and females. This notion of an altered expression pattern in D. suzukii is substantiated by the observation that Fru is translated in brains of both sexes of D. suzukii, whereas in D. melanogaster only in males [46].
(d). Loss of cVA is associated with the species' shift to undamaged fruit
The loss of cVA as sex pheromone in D. suzukii may be an adaptation associated with its new niche, or reflect this species' higher reliance on visual cues (males have a conspicuous wing spot) over olfactory cues in sex discrimination. It may also be simply the result of drift, although the presence of cVA throughout the melanogaster group may render this a less likely scenario. We therefore compared D. suzukii to the other two suzukii group species, D. subpulchrella and D. biarmipes, males of which also have conspicuous wing spots [47]. Whereas both species were found to have sexually monomorphic CH profiles (electronic supplementary, material figure S1), D. biarmipes, a species that oviposits on rotten fruit, produced large amounts of cVA, whereas D. subpulchrella, a species with a similar ovipositor and oviposition preference to D. suzukii, lacked cVA. As expected, the relative volume of DA1 in D. subpulchrella was much reduced and comparable to DA1 in D. suzukii, whereas that of D. biarmipes was similar to D. melanogaster (figure 6b). Apparently, loss of cVA is not associated with the use of a conspicuous wing spot. Instead, our data suggest that the loss co-occurs with the shift to fresh fruit.
Although the above results demonstrate that cVA (i) has been lost in D. suzukii and (ii) reduces mating rates in D. suzukii, the selection pressures that have led to these shifts are unknown. Loss of cVA production to increase species recognition seems unlikely, as CHs, and not cVA, appear paramount in mate preference and species recognition of drosophilids [12]. The fact that cVA reduces mating in D. suzukii may thus be a consequence rather than a cause.
Further, it is uncertain whether reduction of T1 abundance preceded loss of cVA production or the other way around. The first scenario would support the sensory drive hypothesis, whereby the sensory characteristics of the detection system drive the evolution of intraspecific signalling [48]. For instance, T1 loss may have been selected for to suppress, e.g. oviposition in the presence of interspecific competitors (i.e. other Drosophila spp. that use cVA deposits for oviposition aggregation [1]). cVA loss would then have followed the adaptation in the detection circuit.
Alternatively, cVA production may have been lost first. A variety of unrelated factors may, alone or in combination, have caused loss of cVA. For instance, cVA production may have been lost owing to pressure of natural enemies cueing in to cVA in habitats where the species evolved [49]. Or, the shift in ecology to fresh fruit, along with a change in oviposition behaviour (single ovipositions in undamaged fruit), may have obliterated the need for cVA as oviposition aggregation pheromone.
It should be noted that cVA production constitutes a large cost for male Drosophila: a male D. melanogaster carries approximately 1 μg cVA, which is roughly 1/200th of the dry weight of a male Drosophila fly [50]. If uncountered by other fitness consequences, loss of cVA would be highly selected for. Be it as it may, further work on the evolutionary forces that have led to the change in pheromone communication in D. suzukii is needed.
4. Conclusion
Sex pheromones are intraspecific signals involved in influencing behaviours of conspecifics during sexual communication. cVA is a sex pheromone produced by male Drosophila species and fulfils a complex role in intraspecific communication. We show that, in spite of the fact that cVA signalling fulfils a primal role and is highly conserved in the melanogaster group, it has been lost in D. suzukii. We furthermore demonstrate that its underlying circuit can rapidly evolve to serve a radically opposite behavioural and ecological function, mediated by offsetting the balance of sensory input. Although this study does not exclude the existence of other volatile pheromones in D. suzukii, the CH extracts and the ‘relictual’ size of the EB indicate otherwise.
The results are of significance for our understanding of how sensory circuits can mediate, through numerical changes, radical changes in preference that may support the speciation process. In more practical terms, loss of cVA in this pestiferous species may be used in designing new control tools.
Supplementary Material
Acknowledgements
We thank O. Hansson, M. Biolchini, J. Abraham and V. Rossi Stacconi for their help during the experiments. We acknowledge the two anonymous reviewers for their suggestions that helped improve the manuscript. Amino acid alignments are available for download at http://dx.doi.org/10.6084/m9.figshare.865654. Further supplementary material is available at http://dx.doi.org/10.1098/rspb.2014.3018.
Author contributions
Conceived the idea: T.D. Experimental design and data acquisition: T.D., S.M., S.R., S.Ra, S.L., P.G.B., S.A., S.L., O.R.-S., G.A. Data analysis and interpretation: T.D., S.M., S.R., S.Ra, O.R.-S., G.A. Data analysis and interpretation: T.D., S.R., S.Ra, S.L., P.G.B., S.A., O.R.-S., G.A.. First draft of manuscript: T.D. Comments and revision: T.D., S.M., S.R., S.Ra, S.L., P.G.B., S.A., O.R.-S., G.A.
Funding statement
This work was supported by the FORMAS 2007-1491 (IC-E3 to the division of Chemical Ecology), 220-2007-1491 (T.D.) and 2011-390 (P.G.B.), SLU funding on Invasive Species (T.D., S.R., S.M.), The Nilsson's Minnesfond (T.D., S.M.), the Autonomous Province of Trento (Accordo di Programma 2012–2013) (S.R., O.R.-S., S.R., G.A.) and the Free University of Bozen-Bolzano internal funds (S.A.).
Conflict of interests
The authors declare no conflicting interests.
References
- 1.Symonds MRE, Wertheim B. 2005. The mode of evolution of aggregation pheromones in Drosophila species. J. Evol. Biol. 18, 1253–1263. ( 10.1111/j.1420-9101.2005.00971.x) [DOI] [PubMed] [Google Scholar]
- 2.Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. 2010. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20, 1602–1614. ( 10.1016/j.cub.2010.08.025) [DOI] [PubMed] [Google Scholar]
- 3.Dahanukar A, Ray A. 2011. Courtship, aggression and avoidance: pheromones, receptors and neurons for social behaviors in Drosophila. Fly 5, 58–63. ( 10.4161/fly.5.1.13794) [DOI] [PubMed] [Google Scholar]
- 4.Dickson BJ. 2008. Wired for sex: the neurobiology of Drosophila mating decisions. Science 322, 904–909. ( 10.1126/science.1159276) [DOI] [PubMed] [Google Scholar]
- 5.Kurtovic A, Widmer A, Dickson BJ. 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546. ( 10.1038/nature05672) [DOI] [PubMed] [Google Scholar]
- 6.Weng R, Chin JSR, Yew JY, Bushati N, Cohen SM. 2013. miR-124 controls male reproductive success in Drosophila. eLife 2, 1–16. ( 10.7554/eLife.00640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Anderson DJ. 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231. ( 10.1038/nature08678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Liang X, Gong J, Yang Z, Zhang Y-H, Zhang J-X, Rao Y. 2011. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosci. 14, 896–902. ( 10.1038/nn.2836) [DOI] [PubMed] [Google Scholar]
- 9.Symonds MRE, Elgar MA. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228. ( 10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 10.Löfstedt C. 1993. Moth pheromone genetics and evolution. Phil. Trans. R. Soc. Lond. B 340, 167–177. ( 10.1098/rstb.1993.0055) [DOI] [Google Scholar]
- 11.Baker TC. 2008. Balanced olfactory antagonism as a concept for understanding evolutionary shifts in moth sex pheromone blends. J. Chem. Ecol. 34, 971–981. ( 10.1007/s10886-008-9468-5) [DOI] [PubMed] [Google Scholar]
- 12.Ferveur J-F. 2005. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35, 279–295. ( 10.1007/s10519-005-3220-5) [DOI] [PubMed] [Google Scholar]
- 13.Billeter J-C, Atallah J, Krupp JJ, Millar JG, Levine JD. 2009. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991. ( 10.1038/nature08495) [DOI] [PubMed] [Google Scholar]
- 14.Billeter J-C, Jagadeesh S, Stepek N, Azanchi R, Levine JD. 2012. Drosophila melanogaster females change mating behaviour and offspring production based on social context. Proc. R. Soc B 279, 2417–2425. ( 10.1098/rspb.2011.2676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto T, Amrein H. 2008. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11, 874–876. ( 10.1038/nn.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becher P, Bengtsson M, Hansson BS, Witzgall P. 2010. Flying the fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 36, 599–607. ( 10.1007/s10886-010-9794-2) [DOI] [PubMed] [Google Scholar]
- 17.Lebreton S, Grabe V, Omondi AB, Ignell R, Becher PG, Hansson SS, Witzgall P. 2014. Love makes smell blind: mating suppresses pheromone attraction in Drosophila females via Or65a olfactory neurons. Sci. Rep. 4, 7119 ( 10.1038/srep07119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everaerts C, Farine J-P, Cobb M, Ferveur J-F. 2010. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5, e9607 ( 10.1371/journal.pone.0009607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bairati A. 1968. Structure and ultrastructure of the male reproductive system in Drosophila melanogaster Meig. 2: the genital duct and accessory glands. Monit. J. Zool. 2, 105–182. [Google Scholar]
- 20.Chen PS. 1984. The functional morphology and biochemistry of insect male accessory glands and their secretions. Annu. Rev. Entomol. 29, 233–255. ( 10.1146/annurev.en.29.010184.001313) [DOI] [Google Scholar]
- 21.Marygold SJ, et al. 2013. FlyBase: improvements to the bibliography. Nucleic Acids Res. 41, D751–D757. ( 10.1093/nar/gks1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterhouse RM, Tegenfeldt F, Li J, Zdobnov EM, Kriventseva EV. 2012. OrthoDB: a hierarchical catalog of animal, fungal and bacterial orthologs. Nucleic Acids Res. 41, D358–D365. ( 10.1093/nar/gks1116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol 215, 403–410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 24.Punta M, et al. 2012. The Pfam protein families database. Nucleic Acids Res. 40, D290–D301. ( 10.1093/nar/gkr1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. ( 10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guindon S, Dufayardi J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. ( 10.1093/sysbio/syq010) [DOI] [PubMed] [Google Scholar]
- 27.Le SQ, Gascuel O. 2008. An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320. ( 10.1093/molbev/msn067) [DOI] [PubMed] [Google Scholar]
- 28.Chertemps T, Duportets L, Labeur L, Ueda R, Takahashi K, Saigo K, Wicker-Thomas C. 2007. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 104, 4273–4278. ( 10.1073/pnas.0608142104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang S, Ting C-T, Lee C-R, Chu K-H, Wang C-C, Tsaur S-C. 2009. Molecular evolution and functional diversification of fatty acid desaturases after recurrent gene duplication in Drosophila. Mol. Biol. Evol. 26, 1447–1456. ( 10.1093/molbev/msp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brieger G, Butterworth FM. 1970. Drosophila melanogaster: identity of male lipid in reproductive system. Science 167, 1262 ( 10.1126/science.167.3922.1262) [DOI] [PubMed] [Google Scholar]
- 31.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. 2005. Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807. ( 10.1016/j.cell.2005.04.026) [DOI] [PubMed] [Google Scholar]
- 32.Couto A, Alenius M, Dickson BJ. 2005. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547. ( 10.1016/j.cub.2005.07.034) [DOI] [PubMed] [Google Scholar]
- 33.Shanbhag SR, Müller B, Steinbrecht RA. 1999. Atlas of olfactory organs of Drosophila melanogaster: 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28, 377–397. ( 10.1016/S0020-7322(99)00039-2) [DOI] [Google Scholar]
- 34.Van der Goes van Naters W, Carlson JR. 2007. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612. ( 10.1016/j.cub.2007.02.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ejima A, Smith BPC, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. 2007. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 17, 599–605. ( 10.1016/j.cub.2007.01.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. 2006. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling D. sechellia. Curr. Biol. 16, 101–109. ( 10.1016/j.cub.2005.11.075) [DOI] [PubMed] [Google Scholar]
- 37.Ibba I, Angioy AM, Hansson BS, Dekker T. 2010. Macroglomeruli for fruit odors change blend preference in Drosophila. Naturwissenschaften 97, 1059–1066. ( 10.1007/s00114-010-0727-2) [DOI] [PubMed] [Google Scholar]
- 38.Kárpáti Z, Olsson S, Hansson BS, Dekker T. 2010. Inheritance of central neuroanatomy and physiology related to pheromone preference in the male European corn borer. BMC Evol. Biol. 10, 286 ( 10.1186/1471-2148-10-286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta SR, et al. 2008. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452, 473–477. ( 10.1038/nature06808) [DOI] [PubMed] [Google Scholar]
- 40.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. 2010. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468, 686–690. ( 10.1038/nature09554) [DOI] [PubMed] [Google Scholar]
- 41.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. 2010. Sexual dimorphism in the fly brain. Curr. Biol. 20, 1589–1601. ( 10.1016/j.cub.2010.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demir E, Dickson BJ. 2005. fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794. ( 10.1016/j.cell.2005.04.027) [DOI] [PubMed] [Google Scholar]
- 43.Billeter JC, Rideout EJ, Dornan AJ, Goodwin SF. 2006. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr. Biol. 16, R766–R776. ( 10.1016/j.cub.2006.08.025) [DOI] [PubMed] [Google Scholar]
- 44.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. 2010. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466. ( 10.1038/nn.2515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández de la Paz M, Chan Y, Yew J. 2010. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 8, e1000541 ( 10.1371/journal.pbio.1000541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto D, Usui-Aoki K, Shima S. 2004. Male-specific expression of the fruitless protein is not common to all Drosophila species. Genetica 120, 267–272. ( 10.1023/B:GENE.0000017648.15038.84) [DOI] [PubMed] [Google Scholar]
- 47.Rota-Stabelli O, Blaxter M, Anfora G. 2013. Drosophila suzukii. Curr. Biol. 23, R8–R9. ( 10.1016/j.cub.2012.11.021) [DOI] [PubMed] [Google Scholar]
- 48.Boughman JW. 2002. How sensory drive can promote speciation. Trends Ecol. Evol. 17, 571–577. ( 10.1016/S0169-5347(02)02595-8) [DOI] [Google Scholar]
- 49.Hedlund K, Vet LEM, Dicke M. 1996. Generalist and specialist parasitoid strategies of using odours of adult drosophild flies when searching for larval hosts. Oikos 77, 390–398. ( 10.2307/3545929) [DOI] [Google Scholar]
- 50.Khazaeli A, Van Voorhies W, Curtsinger JW. 2005. The relationship between life span and adult body size is highly strain-specific in Drosophila melanogaster. Exp. Gerontol. 40, 377–385. ( 10.1016/j.exger.2005.02.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.